Abstract
Background and aims
This review appraises the progression and status of the evidence base for the treatment of compulsive buying disorder (CBD), in order to highlight what currently works and to prompt useful future research.
Methods
Online databases ISI Web of Knowledge, PsycINFO, and PubMed via Ovid were searched at two time points. Two quality checklists and an established model of therapy evaluation (hourglass model) evaluated the quality and progression of both psychotherapy and pharmacotherapy treatments for CBD. Uncontrolled effect sizes were calculated and meta-regression analyses were performed regarding treatment duration.
Results
A total of 29 articles met the inclusion criteria, which were divided into psychotherapy (n = 17) and pharmacotherapy treatments (n = 12). Of the 29 studies, only 5 studies have been tested under conditions of high methodological quality. Both forms of treatment had been evaluated in a haphazard manner across the stages of the hourglass model. Although large effects were demonstrated for group psychotherapy and pharmacotherapy, such evidence of effectiveness was undermined by poor study quality and risk of publication bias. Long-term CBD treatment was associated with improved outcome with pharmacotherapy, but not when delivering psychotherapy.
Discussion
Group psychotherapy currently appears the most promising treatment option for CBD. Poor methodological control and sporadic evaluation of specific treatments have slowed the generation of a convincing evidence base for CBD treatment. Defining the active ingredients of effective CBD treatment is a key research goal.
Keywords: compulsive buying disorder, effectiveness, treatments, meta-analysis, review
Background and Aims
Compulsive buying disorder (CBD) is characterized by excessive or poorly controlled preoccupations, urges, or behaviors regarding shopping and spending, which leads to adverse consequences (Black, 2007). CBD is distinguished by a motivation to feel better, rather than from excessive spending and materialism alone (O’Guinn & Faber, 1989), often creating serious associated impacts on lives, such as substantial debt, relationship problems, elevated risk of criminal behavior, and suicide attempts (Black, 2007; Boundy, 2000; Lejoyeux, Tassain, Solomon, & Adès, 1997; O’Guinn & Faber, 1989).
CBD was included in the earliest attempts at classification of mental disorders as “impulsive insanity” (Bleuler, 1930; Kraepelin, 1915), but has since been largely ignored until the last few decades, when the self-help movement testified to the emotional, financial, and interpersonal impacts of CBD (Benson, 2000; Faber, 2011). Categorization of CBD still remains a debate, reinforced by its omission in the most recent edition of the Diagnostic and Statistical Manual (DSM-5; American Psychiatric Association, 2013). Historically, CBD was classified within the DSM-III-R as an example of an impulse control disorder not elsewhere specified (American Psychiatric Association, 1987). CBD has also been conceptualized as a form of obsessive–compulsive disorder, and thus, CBD has been characterized as existing on the impulsive–compulsive spectrum (Frost, Kim, Morris, Bloss, & Murray-Close, 1998). More recently, research has indicated correlates of behavioral addictions like cue reactivity and cravings (Starcke, Schlereth, Domass, Schöler, & Brand, 2013; Trotzke, Starcke, Pederson, & Brand, 2014), adding further debate to the categorization of CBD.
The development of a clinical screening tool for CBD has supported the progression of epidemiological research (Faber & O’Guinn, 1992). A recent meta-analysis of 49 prevalence estimates from 16 countries produced a pooled prevalence estimate of 4.9% for CBD (Maraz, Griffiths, & Demetrovics, 2016). Early research indicated a higher proportion of females than males meeting criteria (Christenson et al., 1994; Dittmar, 2005), though recent larger studies have evidenced an equal gender distribution (Koran, Faber, Aboujaoude, Large, & Serpe, 2006; Mueller et al., 2010). Epidemiological research has also indicated that CBD is associated with high rates of psychiatric comorbidity with both depression (Mueller et al., 2010) and anxiety (Schlosser, Black, Repertinger, & Freet, 1994), with base rates higher than when compared with the general population (Black, Repertinger, Gaffney, & Gabel, 1998). Steffen and Mitchell (2011) noted that CBD outcome research benefited from the development of the Yale–Brown Obsessive Compulsive Scale-Shopping Version (YBOCS-SV; Monahan, Black, & Gabel, 1996). This is because the YBOCS-SV provides a psychometrically robust and sensitive measure of change during CBD treatment (Black, Gabel, Hansen, & Schlosser, 2000; Black, Monaghan, & Gabel, 1997).
Despite increased clarity regarding the phenomenology of CBD, no evidence-based treatments have emerged (Black, 2007). Lourenço Leite, Pereira, Nardi, and Silva (2014) conducted a qualitative review of psychotherapeutic treatments for CBD, supporting the potential for cognitive behavioral group therapy. However, the effectiveness or quality of the psychotherapy studies was not quantitatively examined in that review. Moreover, pharmacotherapy of CBD constitutes a significant proportion of the treatment evidence base (Aboujaoude, 2014; Steffen & Mitchell, 2011) and this type of intervention was omitted from the Lourenço Leite et al.’s (2014) review. This review therefore sought to gain greater clarity concerning the quality and effectiveness of both psychotherapy and pharmacotherapy treatments of CBD in order to guide clinicians regarding treatment allocation and to stimulate further targeted research.
The “hourglass model” is a recognized framework for supporting the appropriate stage development of treatments and therapies (Salkovskis, 1995) and has previously been used to evaluate a psychotherapy evidence base (see Calvert & Kellett, 2014 for an example). In stage 1 of the hourglass model, small practice-based treatment studies (e.g., small N designs) demonstrate proof of concept. In stage 2, treatments are then tested under controlled conditions with larger samples, strict criteria for inclusion, and standardized measurement. Efficacy research designs (such as randomized controlled and deconstruction trials) at stage 2 refine the focus of key ingredients first found in the exploratory research. In the final stage, large-scale practice-based research evaluates the effectiveness of treatment in routine clinical practice and is conducted across multiple sites. The framework is also purposefully cyclical, to be responsive to any conceptual or treatment limitations unearthed. Due to the relative infancy of CBD outcome research, the hourglass model is also used here to indicate and promote appropriate progression of safe and effective treatments. The specific aims of this review were to (a) assess the quality of CBD outcome research, (b) synthesize the progression of CBD outcome research according to the stages of the hourglass model, (c) compare the effectiveness of CBD treatments, (d) illuminate the developmental areas for CBD models, and (e) define the best practice regarding future research methodologies.
Methods
Literature search
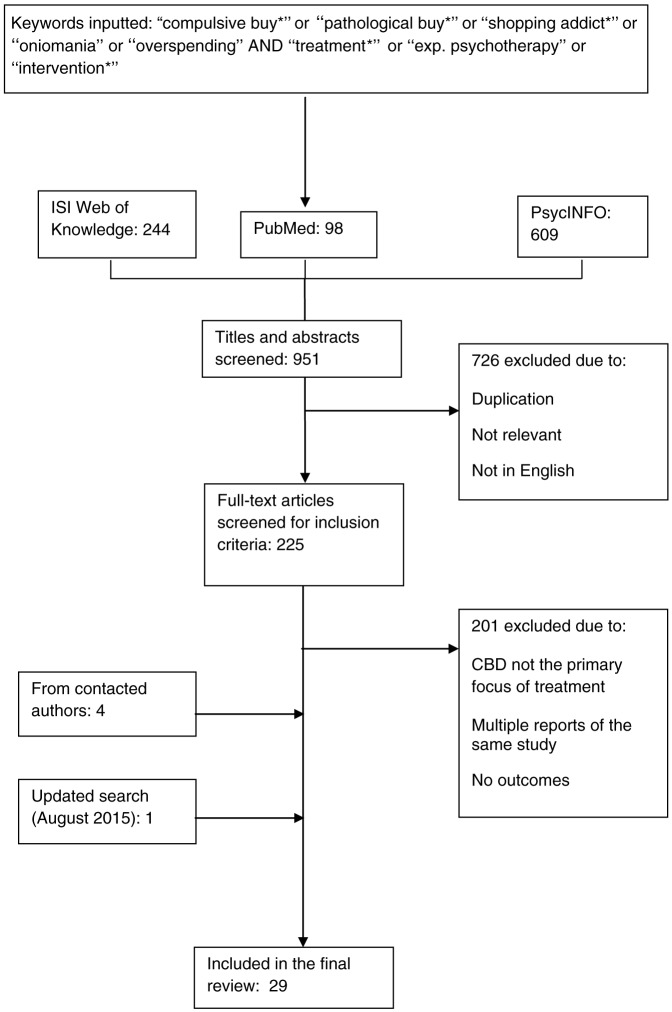
Relevant literature was identified by (a) searching online databases ISI Web of Knowledge, PsycINFO, and PubMed via Ovid search tools on February 1, 2014, (b) searching article reference lists and citation in the extracted articles, and (c) contacting authors for studies in press (Figure 1). The following keywords were used in each database in a range of combinations: “compulsive buying,” “pathological buying,” “shopping addiction,” “oniomania,” “overspending,” and “treatments” or “exp. Psychotherapy” or “interventions.” Moreover, the asterisk function was used to capture the differences in spelling between the UK and the US and also to consider variations (e.g., “buy*” to capture buying and buyers). Initial search titles and abstracts provided 244 studies from ISI Web of Knowledge, 98 studies from PubMed, and 609 studies from PsycINFO. After duplicates were removed and titles were screened for relevance, 225 articles were considered using the following inclusion criteria: (a) treatment was described in the design; (b) treatment primarily targeted compulsive buying; and (c) articles published in English. After screening full articles, 24 studies met the inclusion criteria. No further exclusion filter was imposed due to the low number of CBD treatment studies. A further four studies were included following the correspondence with key authors. An updated search was conducted on August 6, 2015 using the same search criteria, and a further one study was found and included in this review.
Figure 1.
Flowchart of the literature search process
Data synthesis
Both qualitative and quantitative data syntheses were conducted on the extracted articles. First, standardized quality ratings assessed the methodological quality of extracted studies. Second, a narrative synthesis of the outcome research was employed, structured by stages of the hourglass model (Salkovskis, 1995). Third, effect sizes of CBD outcomes were calculated in order to enable effectiveness comparisons, meta-regressions were computed to assess associations between treatment duration and effect size, and funnel plots examined potential publication bias.
Quality ratings. Two quality ratings assessed the methodological quality of studies. First, the Downs and Black (1998) checklist provides a standardized score (0–32) from a list of 27 criteria and is a valid and reliable tool to assess randomized and non-randomized studies (Brouwers et al., 2005; Deeks et al., 2003). As the checklist is difficult to use with case-controlled studies (Higgins et al., 2011), the tool was modified to a 28-criteria scale. Adapted versions of the checklist have been used in previous systematic reviews, where randomized controlled trials (RCTs) are few in number (e.g., MacLehose et al., 2000; Samoocha, Bruinvels, Elbers, Anema, & van der Beek, 2010; Sohanpal, Hooper, Hames, Priebe, & Taylor, 2012). Specifically, the scoring for question 27 dealing with statistical power was simplified to a choice of awarding either 1 or 0 point depending on the presence of a power analysis. A score of 17 points or more identified studies of high methodological quality (Brouwers et al., 2005). Second, the Critical Appraisal Skills Program (CASP UK, 2010) assessed the methodological quality according to specific research design (e.g., RCTs, case-controlled studies, and qualitative studies). For this review, all studies were scored by the first author and 4 of the 29 (14%) studies were selected at random and scored by an independent rater. Good inter-rater reliability was achieved on Downs and Black (1998) checklist ratings (K = 0.67; Altman, 1991), with moderate agreement on CASP ratings (K = 0.51; Altman, 1991).
Calculating and considering effect sizes. Outcome studies are summarized via forest plot analysis to provide a visual representation of the average effect sizes across the studies and enable comparisons of effectiveness between psychotherapy and pharmacotherapy for CBD. Studies were included in the forest plot analysis that (a) reported mean and standard deviations of outcomes at pre- and post-treatment and associated sample sizes, (b) employed the YBOCS-SV (Monahan et al., 1996) as the primary outcome measure, and (c) recruited samples larger than N = 1. Pre–post ES calculations were undertaken using STATA v.10 (StataCorp, 2007) dividing the mean pre- to post-treatment change in YBOCS-SV scores by the pre-treatment standard deviation (Becker, 1988). The YBOCS-SV was the outcome measure of choice because it reports on both distress and behaviors associated with CBD and has been shown to be sensitive to change during CBD treatment (Monahan et al., 1996). Although other CBD assessment measures are available (see Maraz et al., 2015 for examples), these measures were not used here due to their absence in retrieved CBD treatment studies.
Forest plots were then generated on STATA, commanded by Metan (Harris et al., 2008). Tests for heterogeneity were calculated using I2, a statistic that indicates the percentage of variance in a meta-analysis attributable to study heterogeneity (Higgins, Thompson, Deeks, & Altman, 2003). As this review was trying to estimate the combined effect of sets of studies investigating the effectiveness of psychotherapy and pharmacotherapy for CBD, there needed to be a check that the effects found in the individual studies were similar enough that the combined estimate was a meaningful description. The I2 indicated whether there was more heterogeneity than would be expected by the chance alone.
To assess whether treatment duration was associated with YBOCS-SV effect size for each type of treatment (psychotherapy, drug treatment, and placebo), a series of univariate meta-regressions were conducted using the METAREG macro (Wilson, 2005). Funnel plots of YBOCS-SV effect sizes (plotted against the effect size standard error) were used to check for publication bias (Egger, Smith, & Minder, 1997). Three separate funnel plots were produced for each treatment type (psychotherapy, drug treatment, and placebo). Publication bias is indicated by visual asymmetry in funnel plots, the absence of trials in the bottom corner of the plot suggesting inflation of the population effect size estimate (Higgins & Green, 2011).
Results
Study characteristics
Table 1 summarizes the studies (N = 29) extracted for this review, reporting total quality scores. Psychotherapy (n = 17) and pharmacotherapy studies (n = 12) are presented in two sections and studies are arranged by research methodology consistent with identified stages of the hourglass model. Studies that described both psychotherapy and pharmacotherapy treatments (n = 2) were included in the treatment arm that provided the greatest detail in the paper. Table 2 summarizes the CBD treatments (n = 6) that have been tested under conditions of high methodological quality, adjudged by scoring 17 or higher according to the Downs and Black (1998) criteria.
Table 1.
Data extraction table for CBD treatments
| Reference | Treatment | Design (stage of the hourglass model) | Treatment duration (weeks) | Intervention group (N) | Control group (Y/N) | Standardized outcome measures | Downs and Black score | CASP score |
| Bernik et al. (1996) | Behavior therapy | Case report (1) | 4 | 2 | N | – | 3 | 1 |
| Braquehais et al. (2012) | CBT and drug therapy (fluvoxamine/topiramate) | Case report (1) | 4 | 1 | N | – | 8 | 3 |
| Donahue et al. (2011) | Behavior therapy and motivational interviewing | Case report (1) | 12 | 1 | N | – | 5 | 1 |
| Kellett and Bolton (2009) | CBT | Case report (1) | 14 | 1 | N | YBOCS-SV, CBS, BDI, BSI, IIP-32 | 13 | 7 |
| Kellett and Robinson (2009) | CBT and counseling | SCED (1) | 10 | 1 | Y | YBOCS-SV, CBS, BDI, BSI, IIP-32 | 14 | 8 |
| Krueger (1988) | Psychodynamic psychotherapy | Case report (1) | – | 4 | N | – | 0 | 0 |
| Marčinko and Karlović (2005) | CBT and drug therapy (fluvoxamine) | Case report (1) | 52 | 1 | N | YBOCS-SV | 6 | 0 |
| Park et al. (2006) | Family therapy | Qualitative case report (1) | 15 | 1 | N | – | – | 4 |
| Winestine (1985) | Psychoanalytic psychotherapy | Case report (1) | – | 1 | N | – | 0 | 0 |
| Armstrong (2012) | Mindfulness-based stress reduction | Pre–post study (1) | 8 | 8 | N | YBOCS-SV | 11 | 6 |
| Filomensky and Tavares (2009) | Group CBT | Pre–post study (1) | 20 | 9 | N | YBOCS-SV | 6 (9) | 4 (4) |
| Klontz et al. (2008) | Experiential therapy | Pre–post study (1) | <1 | 33 | N | BSI, MAS, FHS | 11 | 4 |
| Mitchell et al. (2006) | Group CBT | Controlled trial (1) | 12 | 28 | Y | YBOCS-SV, CBS | 18a | 9 |
| Benson et al. (2014) | Group psychotherapy | RCT (2) | 12 | 22 | Y | YBOCS-SV | 20a | 7 |
| Mueller et al. (2008) | Group CBT | RCT (2) | 12 | 60 | Y | YBOCS-SV, CBS | 23a | 9 |
| Müller et al. (2013) | Group CBT versus guided self-help | RCT (2) | 12 (CBT) 10 (GSH) | 56 | Y | YBOCS-SV, CBS, BDI | 20a (17a) | 8 (8) |
| Paulsen et al. (1977) | Self-control treatment versus psychoanalytic | RCT (2) | 4 | 16 | N | – | 9 | 5 |
| Grant (2003) | Opioid antagonist (naltrexone) | Case report (1) | 48 | 3 | N | – | 3 | 1 |
| Guzman et al. (2007) | Anticonvulsant (topiramate) | Case report (1) | 12 | 1 | N | BDI | 6 | 2 |
| Kim (1998) | Opioid antagonist (naltrexone) | Case report (1) | 36 | 15 | N | – | 5 | 2 |
| McElroy et al. (1991) | TCA/SSRI antidepressants | Case report (1) | 12 | 3 | N | – | 4 | 2 |
| McElroy et al. (1994) | TCA/SSRI antidepressants | Case report (1) | – | 20 | N | – | 6 | 1 |
| Ye et al. (2014) | Anticonvulsant (topiramate) | Case report (1) | 6 | 1 | N | YBOCS-SV | 4 | 3 |
| Black et al. (1997) | SSRI antidepressant (fluvoxamine) | Pre–post study (1) | 12 | 10 | N | YBOCS-SV, HAM-D | 13 | 7 |
| Grant et al. (2012) | NMDA-receptor agonist (memantine) | Pre–post study (1) | 10 | 9 | N | YBOCS-SV | 13 (14) | 7 (5) |
| Black et al. (2000) | SSRI antidepressant (fluvoxamine) | RCT (2) | 9 | 12 | Y | YBOCS-SV, CBS, HAM-D | 19a | 8 |
| Koran et al. (2003) | SSRI antidepressant (citalopram) | RCT (2) | 9 | 24 | Y | YBOCS-SV, MADRS | 15 | 5 |
| Koran et al. (2007) | SSRI antidepressant (escitalopram) | RCT (2) | 9 | 26 | Y | YBOCS-SV, MADRS | 8 (12) | 4 (5) |
| Ninan et al. (2000) | SSRI antidepressant (fluvoxamine) | RCT (2) | 13 | 23 | Y | YBOCS-SV, HAM-D | 14 | 6 |
Note. SCED = Single-Case Experimental Design; RCT = randomized controlled trial; BDI = Beck Depression Inventory; BSI = Brief Symptom Inventory; CBS = Compulsive Buying Scale; FHS = Financial Health Scale; IIP-32 = Inventory of Interpersonal Problems-32; MADRS = Montgomery–Asberg Depression Rating Scale; MAS = Money Attitude Scale; YBOCS-SV = Yale–Brown Obsessive Compulsive Scale-Shopping Version; GSH = guided self-help; TCA = tricyclic; SSRI = selective serotonin reuptake inhibitor; HAM-D = Hamilton Rating Scale for Depression; NMDA = N-Methyl-D-Aspartate. Scores in parenthesis denote the independent second rating.
Study rated as high quality.
Table 2.
High-quality CBD treatments and outcomes
| CBD treatment (duration; components) | Reference | Sample (n); outcome |
| Group CBT (12 weeks) | Mitchell et al. (2006) | 28; significant pre–post and pre-6-month-follow-up reductions in YBOCS-SV |
| Stopping overshopping group (12-week group program; CBT/MI/DBT/ACT) | Benson et al. (2014) | 11; significant pre–post and pre-6-month-follow-up reductions in YBOCS-SV |
| Group CBT (12 weeks; Mitchell’s group program) | Mueller et al. (2008) | 60; significant pre–post and pre-6-month-follow-up reductions in YBOCS-SV |
| Group CBT (12 weeks) | Müller et al. (2013) | 22; significant reductions in YBOCS-SV compared with wait-list |
| Telephone-guided self-help (12 weeks) | Müller et al. (2013) | 20; significant reductions in YBOCS-SV compared with wait-list |
| SSRI antidepressant fluvoxamine (8 weeks) | Black et al. (2000) | 24; no difference between drug and placebo in YBOCS-SV |
Note. MI = motivational interviewing; DBT = dialectical behavior therapy; ACT = acceptance and commitment therapy.
Nine case reports constituted over half (53%) of the CBD psychotherapy evidence base. Methodological quality was generally poor across each of the quantitative case reports (n = 8, M = 6.3, range 0–14, and 0 of 8 rated as high quality). A notable exception was a cognitive behavioral therapy (CBT) single case experimental design (SCED) that scored on 8 of the 11 quality criteria on the CASP. The qualitative case study on family therapy (n = 1) met only 4 of the 10 quality criteria on the CASP. Four effectiveness studies were of varying quality (M = 11.6, range 6–18, and 1 of 4 rated as high quality) and testing group mindfulness-based stress reduction (n = 1), CBT groups (n = 2), and experiential therapy (n = 1). Four psychotherapy RCTs were identified: (a) group self-control approach (n = 1); (b) group CBT (n = 2); and (c) integrated group therapy (n = 1). RCTs were generally of high quality (M = 18.0, range 9–23, and 3 of 4 rated as high quality; Table 2). No large-scale practice-based research has been conducted (i.e., stage 3 of the hourglass model).
For CBD pharmacotherapy treatment, six case reports (50%) were extracted that tested tricyclic and selective serotonin reuptake inhibitor (SSRI) antidepressants (n = 2), an opioid receptor antagonist (n = 1), an NMDA-receptor antagonist (n =1), and anticonvulsants (n = 2). All case reports were low quality (M = 4.7, range 3–6, and 0 of 6 rated as high quality). Two effectiveness studies were found testing SSRI antidepressant (n = 1) and an NMDA-receptor antagonist treatment (n = 1) and were rated equally in quality (M = 13.0, range 13–13, and 0 of 2 rated as high quality). The four RCTs conducted tested three types of SSRI antidepressants and were of mixed quality (M = 14.0, range 8–19, and 1 of 4 rated as high quality; Table 2). Again, no large practice-based outcome research (stage 3 of the hourglass model) was available.
Synthesis of the CBD psychotherapy evidence base
Case reports (stage 1 of the hourglass model). Ubiquitous positive outcomes for compulsive buyers were reported in case reports describing the psychoanalysis (Winestine, 1985), psychodynamic psychotherapy (Krueger, 1988), behavioral approaches (Bernik, Akerman, Amaral, & Braun, 1996; Donahue, Odlaug, & Grant, 2011), and cognitive-behavioral approaches augmented with antidepressant medication (Braquehais, Del Mar Valls, Sher, & Casas, 2012; Marčinko & Karlović, 2005). Despite the encouraging conclusions, these six case reports had common methodological flaws and omissions, consistently lacking a standardized measure to assess CBD and also an index of treatment adherence. Moreover, all were inadequately described, rendering the research vulnerable to many internal biases.
Of higher quality were a case report (Kellett & Bolton, 2009) and an SCED (Kellett & Robinson, 2009) describing a 10-session cognitive-behavioral intervention that comprised planned avoidance, exposure and response prevention, emotional regulation, and assertiveness training. Clinically significant change was shown on the YBOCS-SV between assessment and termination, with no deterioration at 6-month follow-up. Both reports provided a clear detail on CBT formulation and treatment, with the behavioral measures in the SCED adding objectivity to outcome assessment. Notably, the SCED (Kellett & Robinson, 2009) provided a comparator with counseling, but this within-subject control was undermined by an absence of statistical comparisons between treatment phases. Again, external validity was compromised in both reports by an absence of the source of participant and practitioner qualification. Finally, qualitative evaluation of family therapy provided an appropriate methodology to explore mechanisms of change during CBD treatment (Salkovskis, 1995). Park, Cho, and Seo (2006) evaluated family therapy via grounded theory, in which a clear description of the 15-session treatment is provided. Rigorous analysis was employed on session transcripts, including a validation process by client feedback and then by independent researchers. Conversely, no clear information about the selection of compulsive buyers or a clear statement of outcome was given. As with the other case studies, these omissions compromise the generalizability of the findings.
Effectiveness studies (stage 1 of the hourglass model). All four effectiveness studies considered group treatment of CBD. All studies reported significant reductions in YBOCS-SV scores or distress associated with CBD, but only one group study (Mitchell, Burgard, Faber, Crosby, & de Zwaan, 2006) was rated as high quality. Mitchell et al. (2006) compared female participants assigned (non-randomly) to either group CBT (n = 28) or wait-list (n = 11). Participants were screened using the Compulsive Buying Scale (CBS; Faber & O’Guinn, 1992) and excluded if they had alcohol or drug dependence. CBT comprised 12-weekly sessions covering psychoeducation, cognitive restructuring, financial planning, and exposure techniques, with between-sessions homework. Significant pre–post and pre-follow-up reductions were found in CBD episodes and the money spent on consumer items. However, the considerable attrition rates found at both recruitment (32%) and during treatment (28%) question the acceptability of the treatment. Although selection bias was uncontrolled through a lack of randomization, internal validity was improved by clear sourcing of participants, standardized assessment tools, and intention-to-treat analysis on dropouts. In a smaller CBT group pilot (N = 9), Filomensky and Tavares (2009) delivered the same Mitchell et al. (2006) protocol within an extended 20-week program to more actively target CBD cognitions. Full attendance for the group (100%) and significant reductions in cognitive components of the YBOCS-SV were reported post-treatment. Unlike Mitchell et al. (2006), the authors failed to provide information regarding the participants, the location, or the therapists involved. Inadequate reporting therefore limited the exploration of the results.
Klontz, Bivens, Klontz, Wada, and Kahler (2008) reported intensive 6-day group programs with problem spenders (N = 33), comprising financial planning integrated with the experiential therapy. Results indicated significant improvements in mood and reductions in problematic attitudes toward buying at termination and at 3-month follow-up. Caution must be applied to the findings, as no formal measures were used to determine diagnosis beyond “money-disordered behaviors.” Also, the external validity of the experiential program was questionable, as participants were required to stay at a retreat and engage in over 100 hr of treatment. Armstrong (2012) employed a mixed methods approach to monitor the effectiveness of a small sample (n = 6) undertaking group mindfulness-based stress reduction (MBSR; Kabat-Zinn, 1982). Following the treatment, clinically significant change was found in YBOCS-SV scores of the CBD group receiving MBSR. Interpretative phenomenological analysis also revealed greater awareness of physiological drives to buy, in addition to control over emotional regulation when buying. Despite clear recruitment and treatment procedures, lack of randomization and opportunistic sampling rendered the sample vulnerable to selection bias.
RCTs (stage 2 of the hourglass model). The four RCTs completed also tested group treatment for CBD and were largely (3 of 4) of high quality. The (low quality) exception was the early Paulsen, Rimm, Woodburn, and Rimm (1977) RCT. Participants (N = 19) were randomized to receive either CBT groups that comprised reinforcement principles and practical planning around buying (over 4 weeks) or a placebo condition in which buying was discussed using psychoanalytic constructs. Full attendance in the CBT condition reflected high treatment acceptability. Conclusions, however, are limited to a self-selected and non-clinical sample. The lack of information regarding the recruitment procedure also limits the external validity of the findings.
Two (high quality) RCTs have tested the Mitchell et al. (2006) group CBT approach. First, Mueller et al. (2008) compared the 12-week program to a wait-list condition over the same period. Compulsive buyers were recruited through local advertising and assessed for CBD using a diagnostic interview developed in previous CBD research (McElroy, Keck, Pope, Smith, & Strakowski, 1994). Participants were included only if they were stable on antidepressants for 3 months, but were excluded if they met criteria for manic depression, or had current suicidal intent. Accordingly, only 12% did not meet the inclusion criteria. Eligible participants (N = 60) were randomized to either group CBT or wait-list. Those in the experimental condition showed improvement on the YBOCS-SV and CBS post-treatment and at 6-month follow-up. Müller, Arikian, de Zwaan, and Mitchell (2013) not only employed a similar wait-list RCT design but also used a low-intensity guided self-help (GSH) intervention as an additional active control. Participants randomized to GSH devoted time to reading a manual and completing self-directed tasks (based on Mitchell et al., 2006) and were also supported over the telephone at five time points over a 10-week period. Group CBT (n = 22) and GSH (n = 20) participants showed a marked improvement in YBOCS-SV scores compared with wait-list, with equivalent reliable CBD change rates (45% in GSH group compared with 50% in CBT group). In both of these trials, standardized outcome measures were used and differences in age and severity were controlled for. Equally, intention-to-treat was appropriately employed in both studies, considering attrition rates of 19% (Mueller et al., 2008) and 27% (Müller et al., 2013), respectively. Importantly, Mueller et al. (2008) showed that attendance was a significant predictor of outcome.
Most recently, Benson, Eisenach, Abrams, and van Stolk-Cooke (2014) developed the “stopping overshopping program.” This program integrated CBT (Mitchell et al., 2006; Mueller et al., 2008; Müller et al., 2013), acceptance and commitment therapy, and psychodynamic principles. A 12-week pilot was conducted on a small sample (N = 11), with a comparable recruitment process to the CBT group RCTs. Secondary outcome measures assessed the potential benefit to known comorbid issues associated with CBD. Clinically significant reductions in CBS and YBOCS-SV scores were reported in the CBD group (but not wait-list) at termination, with additional reductions in associated item hoarding. Similar to the CBT group RCTs, the inclusion of a 6-month follow-up period in the Benson et al. (2014) study was a strength of the design, revealing durable gains for compulsive buyers.
In summary, group psychotherapeutic treatment of CBD in terms of delivery of adapted CBT, self-control strategies, and eclectic approaches appears effective in reducing distress and maladaptive buying behavior associated with CBD. The evidence suggests that treatment gains following group intervention are durable. When group psychotherapy outcomes have been compared with a low-intensity intervention (one-to-one telephone GSH for CBD), effects appear comparable.
Synthesis of the CBD pharmacotherapy evidence base
Case reports (stage 1 of the hourglass model). Six case reports describe positive conclusions from treating CBD with tricyclic and SSRI antidepressants (McElroy et al., 1994; McElroy, Satlin, Pope, Keck, & Hudson, 1991), a course of the opioid antagonist, naltrexone with the aim of reducing urges associated with CBD (Grant, 2003; Kim, 1998), and a 3-month treatment of the anticonvulsant topiramate with the rationale that it has shown some efficacy with mood disorders and obsessive and compulsive symptoms (Guzman, Filomensky, & Tavares, 2007; Ye, Kadia, & Lippmann, 2014). The case reports make a poor contribution to CBD pharmacotherapy outcome evidence base, as outcomes in all but one report (Ye et al., 2014) were unsupported by valid or reliable outcome measurement. All case reports had in common a lack of sufficient methodological control and the insufficient detail in general reporting would also greatly limit generalizability and replication. Adverse effects also undermined the effectiveness of each drug (McElroy et al., 1991, 1994).
Effectiveness studies (stage 1 of the hourglass model). Two of the extracted pharmacotherapy studies employed a pre–post design, with varied quality. Black et al. (1997) examined a 9-week course of fluvoxamine (SSRI antidepressant) in an uncontrolled CBD sample (N = 10). Results show significant reductions in YBOCS-SV outcome scores after placebo phase, with further reductions post-treatment. The inclusion of a single-blind placebo phase in the Black et al. (1997) study provided conditions to test the true effect of the drug. However, no comparison was made between improvement pace/rates in each phase, limiting conclusions about continued effect of placebo in active treatment. Grant, Odlaug, Mooney, O’Brien and Kim (2012) completed an open-label study of the effectiveness of memantine (an NMDA-receptor agonist used in the treatment of impulsivity). In the small uncontrolled sample (N = 9), significant improvements in YBOCS-SV scores between baseline and end of treatment were reported. No follow-up data were provided and so restricted any conclusions about durability of memantine and the lack of a control group limited treatment efficacy comparisons. For both studies, inclusion of the YBOCS-SV improved the internal validity of the methodologies used, reflecting a progression from case report methodology. The lack of information regarding the recruitment procedures limits the conclusions concerning generalizability.
RCTs (stage 2 of the hourglass model). Four RCTs have tested the SSRI antidepressants citalopram (Koran, Chuong, Bullock, & Smith, 2003), escitalopram (Koran, Aboujaoude, Solvason, Gamel, & Smith, 2007), and fluvoxamine (Black et al., 2000; Ninan et al., 2000), producing contrasting outcomes. Two comparable placebo-controlled studies tested the efficacy of fluvoxamine to replicate Black et al.’s (1997) findings under stricter methodological conditions. In a high-quality study, Black et al. (2000) recruited compulsive buyers (N = 24), who all first received placebo for 1 week in a ‘‘wash-out’’ phase. Participants were then assigned to either fluvoxamine or placebo for 8 weeks, with weekly check-ups around side effects and dosage. Use of standardized measures of CBD, randomization, and analysis inclusive of dropouts minimized selection bias, improving the internal validity of the findings. No differences were found between fluvoxamine and placebo; the clinically significant change rate (on YBOCS-SV scores) was greater for placebo (55%) than fluvoxamine (17%). Significantly greater symptoms of nausea, insomnia, decreased motivation, and sedation were found in the active drug treatment arm. This method was replicated in a larger university student-based study (N = 42) over a 13-week period (Ninan et al., 2000). No significant differences were found between treatment and placebo in domains of CBD distress, general functioning, and depression. High attrition rates (45%) occurred from recruitment, with a further eight participants (19%) dropping out due to the adverse side effects from taking Fluoxetine. Failure to report the characteristics of these participants limited conclusions about the potential harms of treatment. The initial promising findings for the SSRI fluvoxamine were subsequently not confirmed in high-quality trials, in which experience of side effects appeared common and prominent.
Koran et al. (2003, 2007) completed equivalent double-blind discontinuation trials of the SSRI’s citalopram (N = 24) and escitalopram (N = 26), respectively. Participants were randomized to a 9-week discontinuation phase of placebo or drug treatment, following a 7-week open-label phase. Koran et al. (2003) found reductions in YBOCS-SV scores after open-label treatment. Further improvements were reported in the citalopram group following the discontinuation phase (though non-significant), while YBOCS-SV scores in the placebo group were significantly deteriorated. In the Koran et al. (2007) replication study, findings were reversed for escitalopram. In both trials, weekly consultations monitored drug dosages across study phases. Internal validity was improved from antidepressant case reports (McElroy et al., 1991, 1994) due to the presence of randomization and standardized assessment procedures, outcome monitoring, and the exclusion of participants with comorbid presentations. Substantial relapse rates (defined by scores over 16 on the YBOCS-SV) were found after the discontinuation phase in both the escitalopram arm (63%) and placebo arm (67%). In both studies, a significant number met responder status by the end of open-label treatment, indicating a large placebo effect prior to randomization. Promising findings for citalopram in Koran et al. (2003) requires further study. Conversely, a marked improvement from the open-label phase Koran et al. (2007) failed to confirm true drug effects of escitalopram. Failure to detail safeguards for blinding both the researchers and the participants suggests that vulnerability to these internal biases could account for contrasting outcomes.
Effect of psychotherapy and pharmacotherapy treatments on CBD
Effect sizes were calculated for appropriate CBD treatment studies, with a pre–post effect being employed due to the large proportion of uncontrolled studies (67%). Fourteen CBD treatments met the criteria for inclusion (within 10 CBD studies). Effect sizes were then divided into CBD psychotherapy (n = 6) and CBD pharmacotherapy (n = 8) interventions, with the latter subdivided into active treatment (n = 5) and placebo (n = 3).
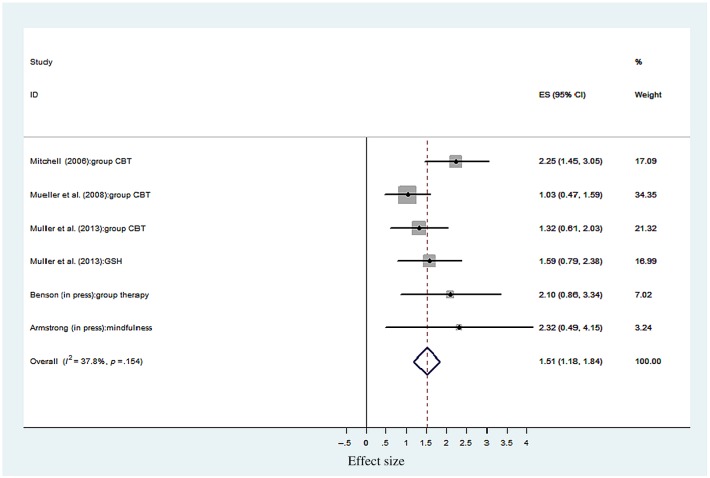
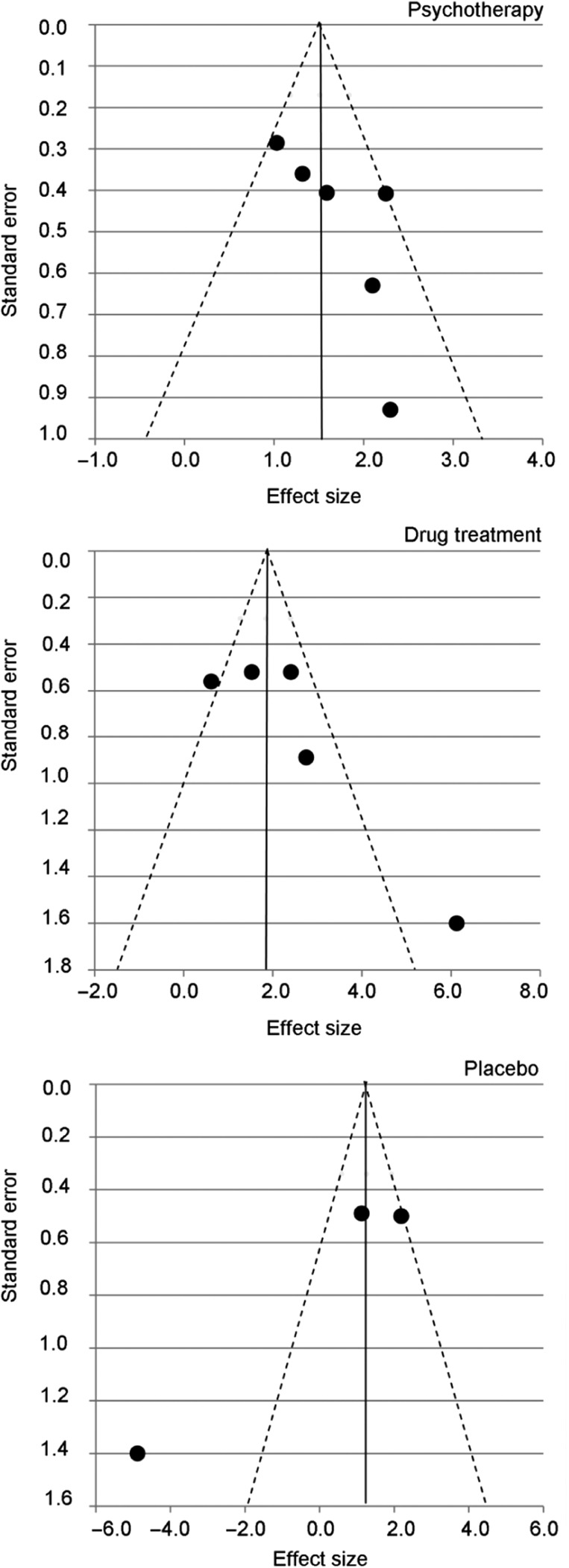
Effect of psychotherapy intervention. Figure 2 illustrates an overall uncontrolled effect size for psychotherapy CBD treatments (n = 6) of d = 1.51 (95% CI = 1.18–1.84), p < .001. Although a range of psychotherapeutic approaches were delivered, tests for heterogeneity showed non-significant differences (I2 = 8.04, df = 5, p = .154), indicating that psychotherapy for CBD were homogenous. Group CBT studies contributed most of the weighting (72.8%) in the large effect size found. The GSH active control arm in the (high quality) Müller et al. (2013) trial produced an equivalent outcome effect to group CBT. Figure 4 (top) shows the funnel plot for the CBD psychotherapy outcome studies. Observed asymmetry indicates that less precise (smaller) psychotherapy studies with non-significant findings may not have been published. This therefore suggests that treatment effect size estimates for psychotherapy for CBD reported may represent an overestimation of the effect. Meta-regression found that duration of psychotherapy was not significantly associated with CBD treatment effects (β = −0.28, z = −1.33, p = not significant).
Figure 2.
Uncontrolled effect sizes for CBD psychotherapy. ES = effect size and 95% CI; % weight = sample size determines the weighting of each study toward the overall ES; GSH = guided self-help (control) condition
Figure 4.
Funnel plots of YBOCS-SV effect sizes for studies included in the forest plot analyses (k = 14), broken down by the treatment type assessed: psychotherapy (top), drug treatment (middle), and placebo (bottom)
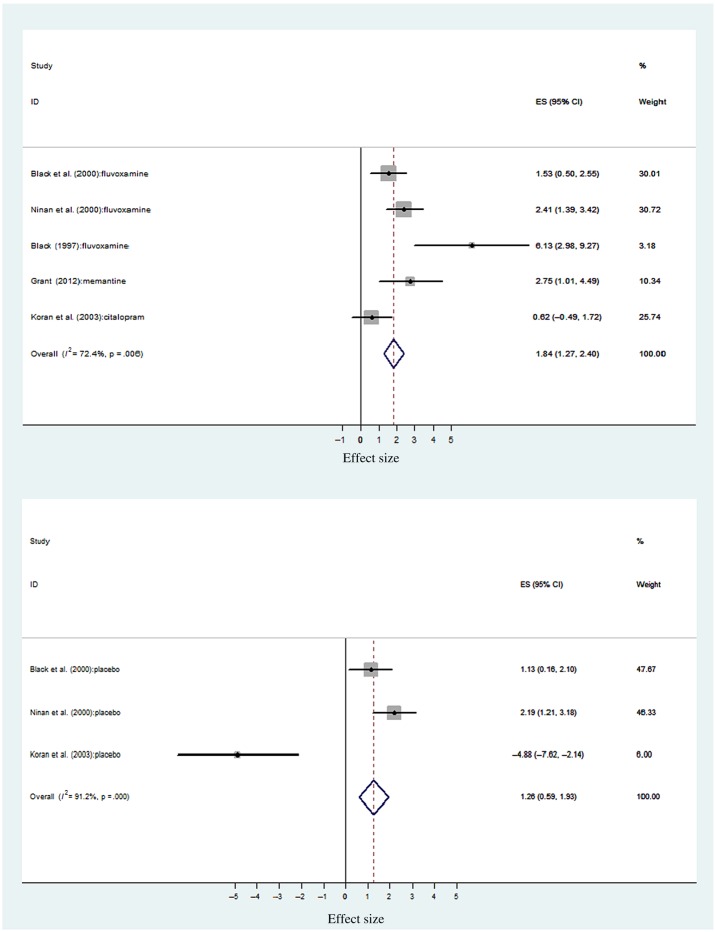
Effect of pharmacotherapy intervention. Figure 3 illustrates the effect sizes for pharmacotherapy CBD treatments and placebo comparisons, respectively. The Koran et al. (2007) study on escitalopram effect size was not calculated due to the lack of reported outcomes in the post-discontinuation phase. Overall, pharmacological treatments (n = 5) produced an uncontrolled effect size of d = 1.84 (95% CI = 1.27–2.40), p < .001. Placebo controls within the same studies (n = 3) produced an equivalent effect of d = 1.26 (95% CI = 0.59–1.93), p < .001. This overlap between drug and placebo confidence intervals suggests a non-significant difference between active and placebo CBD drug treatments. The poor methodological quality of the outcome studies of fluvoxamine (Black et al., 1997; Ninan et al., 2000) and memantine (Grant et al., 2012) undermines the large effect size found. Black et al.’s (2000) trial revealed the comparable effects of placebo to fluvoxamine. However, during the double-blind phase, Koran et al.’s (2003) study effects demonstrate the maintenance of clinical effects with citalopram, and not placebo (Figure 3). The differences in methodological quality, sample size, and intervention contributed to significant heterogeneity being found across active drug treatments (I2 = 14.47, df = 4, p = .006) and placebo (I2 = 22.85, df = 2, p < .001). Asymmetry in the drug treatment funnel plot (Figure 4, middle) indicates that less precise drug treatment studies, with non-significant findings, may not have been published resulting in an overestimation of treatment effects. As expected, given the small number of placebo studies assessed, the corresponding funnel plot (Figure 4, bottom) was also asymmetrical. It is, therefore, likely that publication bias also affected the effect size estimate for CBD placebo studies. Duration of drug treatment was significantly associated with CBD outcome (β = 0.69, z = 7.48, p < .001), as was the duration of the placebo period (β = 0.53, z = 8.64, p < .001). Therefore, longer drug treatments and placebo periods were both associated with improved CBD treatment outcomes.
Figure 3.
Uncontrolled effect sizes for CBD pharmacotherapy and placebo. ES = effect size and 95% CI; % weight = sample size determines the weighting of each study toward the overall ES
In summary, the large effects in studies of high methodological quality for group psychotherapy and also GSH for CBD indicate the promise of such approaches. In contrast, the synthesis and effect sizes for pharmacotherapy CBD treatments highlighted a mismatch between the effectiveness of the intervention and the quality of the study conducted, with the possible exception of Koran et al.’s (2003) citalopram study. Caution is indicated regarding the interpretation of all the calculations across type of CBD intervention as (a) uncontrolled effect sizes are often larger than the controlled effects (Field, 2005) and (b) large effect sizes are potentially compromised once poor acceptability, unknown durability and poor treatment model fidelity are considered.
Discussion
This review assessed the standing, progression, and outcomes of the CBD psychotherapy and pharmacotherapy treatment evidence bases. This review used the Salkovskis (1995) hourglass model as a framework for treatment research progression, whereby externally valid small N practice-based research are the foundation stone for controlled trials whose external validity is then tested again in large N practice-based research. This systematic and meta-analytic review of the CBD outcome research draws the following conclusions: (a) the evidence base for CBD treatments is somewhat undermined by inconsistent study quality and the risk of publication bias; (b) both psychotherapy and pharmacotherapy treatments have been studied somewhat haphazardly and sporadically across the stages of the hourglass model; (c) large pre–post effect sizes in high-quality studies of group psychotherapy show the promise of this approach; (d) large uncontrolled effect sizes for drug treatments are undermined by poor methodological quality; (e) there appears a significant placebo effect when treating CBD with medication; and finally, (f) the lack of large-scale practice-based studies (stage 3 of the hourglass) is appropriate, given the paucity of controlled psychotherapy and pharmacotherapy outcome studies at stage 2 of the hourglass model.
Clinical implications
Group psychotherapy that primarily adopts a cognitive-behavioral approach, or use the cognitive-behavioral approach nested within an eclectic approach appear to be useful and have a durable effect in reducing distress associated with CBD and maladaptive buying behavior. Group treatments have shown to be effective with other impulse control disorders such as pathological gambling (Cowlishaw et al., 2012) and intermittent explosive disorder (McCloskey, Noblett, Deffenbacher, Gollan, & Coccaro, 2008). Recent experimental evidence suggests that impulse control training should be a core component of CBD treatment (Hague, Kellett, & Sheeran, 2016). It is worth noting that attrition rates show that group psychotherapy may not be an acceptable approach for all CBD patients, and that patient choice and suitability are also still important considerations.
Results also need to be considered in light of the stepped care delivery model for psychological interventions ( Bower & Gilbody, 2005). This is because the evidence suggests that a low-intensity GSH approach to treating CBD was comparative to high-intensity group CBT. If patients can be treated with effective, brief, and less intensive psychological intervention first, then this can increase service throughput and efficiency (Firth, Barkham, & Kellett, 2015). Investigation of contemporary interventions such as internet-based therapist-assisted self-help programs also usefully mimic the shift of consumer behavior toward online shopping (Ridgeway, Kukar-Kinney, & Monroe, 2011). Recent research shows that excitability regarding online shopping and CBD are mediated by internet use expectancies (Trotzke, Starcke, Müller, & Brand, 2015). Treatments clearly need to reflect the context within which CBD occurs.
Large effect sizes were found for SSRI antidepressant medication. However, SSRIs did not show significant superiority in terms of efficacy when treating CBD compared with placebo. Interestingly, the SSRI’s citalopram and escitalopram (which share the same active compound) showed contradictory findings. Further high-quality research into the role of SSRIs in treating CBD is required; particularly as these studies currently constitute a large proportion of the current pharmacotherapy evidence base. Greater detail and consistency in reporting outcomes in studies are also highlighted by the lack of effect size calculations in Koran et al.’s (2007) study. Controlled studies of fluvoxamine showed no greater benefit than placebo in treating CBD. The apparent mismatch between large effect sizes and poor quality of SSRI outcome studies particularly emphasizes the importance of consistent utilization of robust outcome methodologies in future CBD pharmacotherapy outcome research. Longer treatments appear associated with improved treatment outcomes when using pharmacotherapy to treat CBD and less when delivering psychotherapy. Why this longitudinal relationship is the case demands further investigation. Harm in terms of side effects and risk of dropout also needs to be carefully considered in relation to the pharmacotherapy of CBD.
A lack of clarity remains in the comparison of psychotherapeutic and pharmacological interventions for CBD. This is because there is a paucity of sufficiently sized trials comparing psychotherapies and pharmacotherapies with themselves and between each other. There is less utility in conducting more passive control wait-list control trials of psychotherapeutic interventions for CBD (more use of active treatment controls is indicated) and conversely the need for more double-blind placebo-controlled trials in pharmacological treatment evidence base. Researchers need to consider randomizing participants to types of psychotherapy following the initial pharmacotherapy (and vice versa). The current CBD evidence base would suggest that clinicians should initially consider a psychotherapeutic treatment option, prior to starting pharmacotherapy. This is because ES metrics should always be considered in the context of the quality of the evidence base. The group psychotherapy effects were found in the context of studies with sufficient methodological quality.
Scientific state of the CBD treatment evidence
Low-quality case reports constitute a worryingly large proportion of CBD treatment evidence base. According to the hourglass model, initial practice-based designs are essential in developing clinical concepts, but are then required to the rigorously tested and refined under strict methodological conditions (Salkovskis, 1995). The CBD evidence base is therefore unbalanced by the number of stage 1 type studies, which have additionally not proven the stimulus or foundation stone for future detailed and controlled inquiry. The small numbers of subsequent stage 2 high-quality studies (i.e., randomized and controlled) means that efficacy of CBD treatments has not been extensively tested. No treatment component analyses have been conducted at stage 2, so that identifying the active ingredients of CBD treatments has been hindered. Due to trials having strict inclusion and exclusion criteria, then CBD participants with comorbid presentations have tended to be excluded. No large-scale stage 3 service evaluations have been attempted and this would seem appropriate given the need for more model-specific stage 1 and 2 evidence as the foundation stone upon which such studies could be based. Future research should endeavor to utilize the hourglass model in order to target treatments and methodologies at appropriate stages to enhance the CBD evidence base. For some psychotherapy modalities (particularly more interpersonal/psychodynamic approaches), it would be a mistake to rush into conducting a trial.
When specific CBD treatments were isolated for analysis, then this review highlights that they have typically been studied sporadically across the stages of the hourglass model. Of the fifteen different treatment modalities extracted, only group CBT and SSRI antidepressant interventions had progressed through more than one stage. The vital importance of the connected progression of outcome research is typified by SSRI antidepressant outcome studies (Black et al., 2000; Koran et al., 2007), where poor efficacy and harm were only highlighted when the complexity of methodological designs were refined and improved. Stage 1 studies can unwittingly and artificially inflate the assumed safety and effectiveness of an intervention. The common inconsistency of study quality indicates the potential presence of a consistent “type-I error” as well as the under-reporting of negative outcomes and the potential for publication bias. All studies and across treatment types need to pay more attention to recording untoward incident and harm rates during treatment. This neglect in the effective sequencing of exploration, refining, and generalizing of CBD treatments has the potential to significantly compromise patient care (Salkovskis, 1995).
Future research
Revisiting the initial stages of the hourglass model is clearly required using mixed methods approaches to enhance the CBD outcome evidence. The potential danger of false-positive outcomes can be addressed with robust outcome measurement and detailed information regarding participants and treatments. SCEDs offer an empirical framework to develop CBD practice-based evidence with minimal restrictions over the service setting (McMillan & Morley, 2010). Moreover, the flexibility inherent to SCED makes it well placed to acknowledge the complexities of CBD (Barkham, Hardy, & Mellor‐Clark, 2010). On comprehensive stage 1 evidence, then future trials at stage 2 need to compare individual versus group psychotherapy for CBD. Qualitative research has the potential to enhance the understanding of high dropout rates evidenced during group psychotherapy of CBD, by exploring the patient experience of treatment. Furthermore, qualitative methodology offers the possibility of defining the common and shared features of CBD treatments (Denzin & Lincoln, 2000). Understanding the active components of effective CBD psychotherapy is a key research goal, using dismantling or additive trial methodologies at the second stage of the hourglass. Robust stages 1 and 2 evidence would enable practice-based research networks to flourish across services (Zarin, West, Placus, & McIntyre, 1996).
The promising findings from the controlled study of citalopram (Koran et al., 2003) and uncontrolled studies of topiramate (Guzman et al., 2007), naltrexone (Grant, 2003; Kim, 1998), and memantine (Grant et al., 2012) require further scrutiny under large sample RCT conditions. This comment also applies to substantiate the initial claims regarding the effectiveness of MBSR for CBD (Armstrong, 2012). More controlled research comparing low- and high-intensity psychological interventions needs to be conducted. Future outcome research needs to consistently use the YBOCS-SV (Monahan et al., 1996) as the primary outcome measure and then consistently report treatment response rates using common clinical and reliable change metrics. The size of future trials needs to be increased in order to reduce the possibility of publication bias and reduce the potential confound of a “small study effect” in future reviews (Rücker, Carpenter, & Schwarzer, 2011). Finally, follow-up across the psychotherapeutic and pharmacological studies tended to be short and so future studies need genuinely long-term follow-up periods to assess the durability of treatment effects.
Limitations
This review had several limitations. First, the large number of poor quality studies failed to use any standardized outcome measurement (38%) and this compromised calculating CBD treatment effects across a wide range of studies. Interpretations regarding the efficacy and effectiveness of CBD treatments were limited by consistently small sample sizes, poor methodological control, a variety of treatment approaches, and risk of publication bias. The inter-rater reliability of the CASP was weak and the lack of a defined quality cutoff compromises its utility. Nevertheless, this review represents a step forward from Lourenço Leite et al.’s (2014) review, due to better consideration of study quality and greater analytical specificity.
Conclusions
The CBD treatment evidence base is clearly a work in progress. Progress has been jointly hindered by the consistent use of poor quality methodologies and the sporadic evaluation of treatments. Greater effort in developing and evaluating interventions in keeping with the hourglass model will improve understanding of the potential benefits and risks of CBD treatments. The promise shown by high-quality group interventions indicate a need to explore what it is about group CBT approach that is particularly useful and a component analysis of group CBT for CBD is particularly indicated. Less-intrusive GSH treatments for CBD appear to hold clinical promise and are suitable for detailed inquiry. SSRI citalopram requires further controlled study to build on promising outcomes. Clearly, CBD remains an under-recognized and challenging clinical disorder to treat. Ensuring and improving the methodological quality of future studies will improve confidence in the initial evidence that compulsive buyers can manage their compulsions to spend through relatively short-term theory-based psychotherapeutic interventions.
Authors’ contribution
The study was conceived and designed by BH and SK. BH and JH conducted the analysis and BH produced the interpretations of the data. SK supervised the study. Final manuscript was approved by all authors.
Conflict of interest
The authors declare no conflict of interest.
Funding Statement
Funding sources: No financial support was received for this study.
References
- Aboujaoude E. (2014). Compulsive buying disorder: A review and update. Current Pharmaceutical Design, 20, 4021–4025. doi:10.2174/13816128113199990618 [DOI] [PubMed] [Google Scholar]
- Altman D. (1991). Practical statistics for medical research. London, UK: Chapman and Hall. [Google Scholar]
- American Psychiatric Association. (1987). Diagnostic and statistical manual of mental disorders (3rd ed., text rev.). Washington, DC: American Psychiatric Publishing. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Armstrong A. (2012). Mindfulness and consumerism: A social psychological investigation (Unpublished doctoral dissertation). University of Surrey, England. [Google Scholar]
- Barkham M., Hardy G., Mellor-Clark J. (2010). Developing and delivering practice-based evidence: A guide for the psychological therapies. Chichester, UK: Wiley. [Google Scholar]
- Becker B. J. (1988). Synthesizing standardized mean-change measures. British Journal of Mathematical and Statistical Psychology, 41, 257–278. doi:10.1111/j.2044-8317.1988.tb00901.x [Google Scholar]
- Benson A. (2000). I shop, therefore I am: Compulsive buying and the search for self. Northvale, NJ: Jason Aronson. [Google Scholar]
- Benson A., Eisenach D., Abrams L., van Stolk-Cooke K. (2014). Stopping overshopping: A preliminary randomized controlled trial of group therapy for compulsive buying disorder. Journal of Groups in Addiction & Recovery, 9, 97–125. doi:10.1080/1556035X.2014.868725 [Google Scholar]
- Bernik M., Akerman D., Amaral J., Braun R. (1996). Cue exposure in compulsive buying. Journal of Clinical Psychiatry, 57, 90. [PubMed] [Google Scholar]
- Black D. (2007). A review of compulsive buying disorder. World Psychiatry, 6, 14–18. [PMC free article] [PubMed] [Google Scholar]
- Black D., Gabel J., Hansen J., Schlosser S. (2000). A double-blind comparison of Fluvoxamine versus placebo in the treatment of compulsive buying disorder. Annals of Clinical Psychiatry, 12, 205–211. doi:10.3109/10401230009147113 [DOI] [PubMed] [Google Scholar]
- Black D., Monaghan P., Gabel J. (1997). Fluvoxamine in the treatment for compulsive buying. Journal of Clinical Psychiatry, 58, 159–163. doi:10.4088/JCP.v58n0404 [DOI] [PubMed] [Google Scholar]
- Black D., Repertinger S., Gaffney G., Gabel J. (1998). Family history and psychiatric comorbidity in persons with compulsive buying: Preliminary findings. American Journal of Psychiatry, 155, 960–963. doi:10.1176/ajp.155.7.960 [DOI] [PubMed] [Google Scholar]
- Bleuler E. (1930). Textbook of psychiatry (A. Brill, Trans.). New York, NY: Macmillan. [Google Scholar]
- Boundy D. (2000). When money is the drug. In Benson A. (Ed.), I shop, therefore I am: Compulsive buying and the search for self (pp. 3–26). Northvale, NJ: Jason Aronson. [Google Scholar]
- Bower P., Gilbody S. (2005). Stepped care in psychological therapies: Access, effectiveness and efficiency. Narrative literature review. Britsh Journal of Psychiatry, 186, 11–17. doi:10.1192/bjp.186.1.11 [DOI] [PubMed] [Google Scholar]
- Braquehais M., Del Mar Valls M., Sher L., Casas M. (2012). Pathological collecting: A case report. International Journal on Disability and Human Development, 11, 81–83. doi:10.1515/IJDHD.2012.001 [Google Scholar]
- Brouwers M. C., Johnston M. E., Charette M. L., Hanna S. E., Jadad A. R., Browman G. P. (2005). Evaluating the role of quality assessment of primary studies in systematic reviews of cancer practice guidelines. BMC Medical Research Methodology, 16, 5–8. doi:10.1186/1471-2288-5-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert R., Kellett S. (2014). Cognitive analytic therapy: A review of the outcome evidence base for treatment. Psychology and Psychotherapy, 87, 253–277. doi:10.1111/papt.12020 [DOI] [PubMed] [Google Scholar]
- CASP UK. (2010). Critical appraisal skills programme: Making sense of evidence about clinical effectiveness: 11 questions to help you make sense of a trial. Retrieved from http://www.casp-uk.net
- Christenson G., Faber R., de Zwaan M., Raymond N., Specker S., Ekern M., Mackenzie T. B., Crosby R. D., Crow S. J., Eckert E. (1994). Compulsive buying: Descriptive characteristics and psychiatric co-morbidity. Journal of Consumer Research, 13, 15–31. [PubMed] [Google Scholar]
- Cowlishaw S., Merkouris S., Dowling N., Anderson C., Jackson A., Thomas S. (2012). Psychological therapies for pathological and problem gambling. Cochrane Database of Systematic Reviews, 11, 1–91. doi:10.1002/14651858.CD008937.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks J., Dinnes J., D’Amico R., Sowden A., Sakarovitch C., Song F., Petticrew M., Altman D. (2003). Evaluating non-randomised intervention studies. Health Technology Assessments, 7, 1–173. doi:10.3310/hta7270 [DOI] [PubMed] [Google Scholar]
- Denzin N., Lincoln Y. (2000). Handbook of qualitative research (2nd ed.). London, UK: Sage Publications. [Google Scholar]
- Dittmar H. (2005). Compulsive buying – a growing concern? An examination of gender, age, and endorsement of materialistic values as predictors. British Journal of Psychology, 96, 467–491. doi:10.1348/000712605X53533 [DOI] [PubMed] [Google Scholar]
- Donahue C., Odlaug B., Grant J. (2011). Compulsive buying treated with motivational interviewing and imaginal desensitization. Annals of Clinical Psychiatry, 23, 226–227. [PubMed] [Google Scholar]
- Downs S., Black N. (1998). The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. Journal of Epidemiology Community Health, 52, 377–384. doi:10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M., Smith D., Minder C. (1997). Bias in meta-analysis detected by a simple, graphical test. British Medical Journal, 315, 629–634. doi:10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber R. (2011). Diagnosis and epidemiology of compulsive buying. In Müller A., Mitchell J. (Eds.). Compulsive buying: Clinical foundations and treatment (pp. 3–18). London, UK: Routledge. [Google Scholar]
- Faber R., O’Guinn T. (1992). A clinical screener for compulsive buying. Journal of Consumer Research, 19, 459–469. doi:10.1086/209315 [Google Scholar]
- Filomensky T., Tavares H. (2009). Cognitive restructuring for compulsive buying. Revista Brasileira de Psiquiatria, 31, 76–81. doi:10.1590/S1516-44462009000100018 [DOI] [PubMed] [Google Scholar]
- Firth N., Barkham M., Kellett S. (2015). The clinical effectiveness of stepped care systems for depression in working age adults: A systematic review. Journal of Affective Disorders, 170, 119–130. doi:10.1016/j.jad.2014.08.030 [DOI] [PubMed] [Google Scholar]
- Frost R., Kim H.-J., Morris C., Bloss C., Murray-Close M. (1998). Hoarding, compulsive buying and reasons for saving. Behavior Research and Therapy, 36, 657–664. doi:10.1016/S0005-7967(98)00056-4 [DOI] [PubMed] [Google Scholar]
- Grant J. (2003). Three cases of compulsive buying treated with Naltrexone. International Journal of Psychiatry in Clinical Practice, 7, 223–225. doi:10.1080/13651500310003219 [Google Scholar]
- Grant J., Odlaug B., Mooney M., O’Brien R., Kim S. W. (2012). Open-label pilot study of memantine in the treatment of compulsive buying. Annals of Clinical Psychiatry, 24, 119–126. [PubMed] [Google Scholar]
- Guzman C., Filomensky T., Tavares H. (2007). Compulsive buying treatment with Topiramate, a case report. Revista Brasileira de Psiquiatria, 29, 380–385. doi:10.1590/S1516-44462007000400020 [DOI] [PubMed] [Google Scholar]
- Hague B., Kellett S., Sheeran P. (2016). Testing the generalizability of impulse control problems in compulsive buying. Journal of Social and Clinical Psychology, 35, 269–288. doi:10.1521/jscp.2016.35.4.269 [Google Scholar]
- Harris R., Bradburn M., Deeks J., Harbord R., Altman D., Sterne J. (2008). Metan: Fixed- and random-effects meta-analysis. The Stata Journal, 8, 3–28. Retrieved from http://www.stata-journal.com/article.html?article=sbe24_2 [Google Scholar]
- Higgins J., Altman D., Gøtzsche P., Jüni P., Moher D., Oxman A., SavoviÄ J., Schulz K. F., Weeks L., Sterne J. (2011). The cochrane collaboration’s tool for assessing risk of bias in randomised trials. British Medical Journal, 343, 1–9. doi:10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P. T., Green S. (Eds.). (2011). Cochrane handbook for systematic reviews of interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration; Retrieved from http://handbook.cochrane.org [Google Scholar]
- Higgins J., Thompson S., Deeks J., Altman D. (2003). Measuring inconsistency in meta-analyses. British Medical Journal, 327, 557–560. doi:10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat-Zinn J. (1982). An out-patient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: Theoretical considerations and preliminary results. General Hospital Psychiatry, 4, 33–47. doi:10.1016/0163-8343(82)90026-3 [DOI] [PubMed] [Google Scholar]
- Kellett S., Bolton J. (2009). Compulsive buying: A cognitive-behavioural model. Clinical Psychology and Psychotherapy, 16, 83–99. doi:10.1002/cpp.585 [DOI] [PubMed] [Google Scholar]
- Kellett S., Robinson L. (2009). The treatment of compulsive buying using CBT. Paper presented at the BABCP Annual Conference, Exeter, UK. [Google Scholar]
- Kim S. W. (1998). Opioid antagonists in the treatment of impulse-control disorders. Journal of Clinical Psychiatry, 59, 159–163. doi:10.4088/JCP.v59n0403 [PubMed] [Google Scholar]
- Klontz B., Bivens A., Klontz P., Wada J., Kahler R. (2008). The treatment of disordered money behaviors: Results of an open clinical trial. Psychological Services, 5, 295–308. doi:10.1037/1541-1559.5.3.295 [Google Scholar]
- Koran L., Aboujaoude E., Solvason B., Gamel N., Smith E. (2007). Escitalopram for compulsive buying disorder: A double-blind discontinuation study. Journal of Clinical Psychopharmacology, 27, 225–227. doi:10.1097/01.jcp.0000264975.79367.f4 [DOI] [PubMed] [Google Scholar]
- Koran L., Chuong H., Bullock K., Smith S. (2003). Citalopram for compulsive shopping disorder: An open-label study followed by double-blind discontinuation. Journal of Clinical Psychiatry, 64, 793–798. doi:10.4088/JCP.v64n0709 [PubMed] [Google Scholar]
- Koran L., Faber R., Aboujaoude E., Large M., Serpe R. (2006). Estimated prevalence of compulsive buying in the United States. American Journal of Psychiatry, 163, 1806–1812. doi:10.1176/appi.ajp.163.10.1806 [DOI] [PubMed] [Google Scholar]
- Kraepelin E. (1915). Psychiatrie (8th ed.). Leipzig, Germany: Verlag Von Johann Abrosius Barth. [Google Scholar]
- Krueger D. (1988). On compulsive shopping and spending: A psychodynamic inquiry. American Journal of Psychotherapy, 42, 574–584. [DOI] [PubMed] [Google Scholar]
- Lejoyeux M., Tassain V., Solomon J., Adès J. (1997). Study of compulsive buying in depressed patients. Journal of Clinical Psychiatry, 58, 169–173. doi:10.1086/209204 [DOI] [PubMed] [Google Scholar]
- Lourenço Leite P., Pereira V. M., Nardi A. E., Silva A. C. (2014). Psychotherapy for compulsive buying disorder: A systematic review. Psychiatry Research, 219, 411–419. doi:10.1016/j.psychres.2014.05.037 [DOI] [PubMed] [Google Scholar]
- MacLehose R., Reeves B., Harvey I., Sheldon T., Russell I., Black A. (2000). A systematic review of comparisons of effect sizes derived from randomised and non-randomised studies. Health Technology Assessments, 4, 1–154. doi:10.3310/hta4340 [PubMed] [Google Scholar]
- Maraz A., Eisinger A., Hende B., Urbán R., Paksi B., Kun B., Kökönyei G., Griffiths M. D., Demetrovics Z. (2015). Measuring compulsive buying behaviour: Psychometric validity of the three different scales and prevalence in the general population and in shopping centres. Psychiatric Research, 225, 326–334. doi:10.1016/j.psychres.2014.11.080 [DOI] [PubMed] [Google Scholar]
- Maraz A., Griffiths M., Demetrovics Z. (2016). The prevalence of compulsive buying: A meta-analysis. Addiction, 111, 408–419. doi:10.1111/add.13223 [DOI] [PubMed] [Google Scholar]
- Marčinko D., Karlović D. (2005). Oniomania – Successful treatment with Fluvoxamine and cognitive-behavioral psychotherapy. Psychiatria Danubina, 17, 97–100. [PubMed] [Google Scholar]
- McCloskey M., Noblett K., Deffenbacher J., Gollan J., Coccaro E. (2008). Cognitive-behavioral therapy for intermittent explosive disorder: A pilot randomized clinical trial. Journal of Consulting and Clinical Psychology, 76, 876–886. doi:10.1037/0022-006X.76.5.876 [DOI] [PubMed] [Google Scholar]
- McElroy S., Keck P.,, Jr., Pope H.,, Jr., Smith J., Strakowski S. (1994). Compulsive buying: A report of 20 cases. Journal of Clinical Psychiatry, 55, 242–248. [PubMed] [Google Scholar]
- McElroy S., Satlin A., Pope H., Keck P., Hudson J. (1991). Treatment of compulsive shopping with antidepressants: A report of three cases. Annals of Clinical Psychiatry, 3, 199–204. doi:10.3109/10401239109147991 [Google Scholar]
- McMillan D., Morley S. (2010). The quantitative single-case design as a research strategy for practice-based evidence In Barkham M., Hardy G. E., Mellor-Clark J. (Eds.), Developing and delivering practice-based evidence: A guide for the psychological therapies (pp. 109–138). Chichester, UK: John Wiley & Sons. [Google Scholar]
- Mitchell J., Burgard M., Faber R., Crosby R., de Zwaan M. (2006). Cognitive behavioural therapy for compulsive buying disorder. Behaviour Research and Therapy, 44, 1859–1865. doi:10.1016/j.brat.2005.12.009 [DOI] [PubMed] [Google Scholar]
- Monahan P., Black D., Gabel J. (1996). Reliability and validity of a scale to measure change in persons with compulsive buying. Psychiatry Research, 64, 59–67. doi:10.1016/0165-1781(96)02908-3 [DOI] [PubMed] [Google Scholar]
- Mueller A., Mitchell J., Crosby R., Gefaller O., Faber R., Martin A., Bleich S., Glaesmer H., Exner C., de Zwaan M. (2010). Estimated prevalence of compulsive buying in Germany and its association with socio demographic characteristics and depressive symptoms. Psychiatric Research, 180, 137–142. doi:10.1016/j.psychres.2009.12.001 [DOI] [PubMed] [Google Scholar]
- Mueller A., Mueller U., Silbermann A., Reinecker H., Bleich S., Mitchell J., de Zwaan M. (2008). A randomized, controlled trial of group cognitive-behavioral therapy for compulsive buying disorder: Posttreatment and 6-month follow-up results. Journal of Clinical Psychiatry, 69, 1131–1138. doi:10.4088/JCP.v69n0713 [DOI] [PubMed] [Google Scholar]
- Müller A., Arikian A., de Zwaan M., Mitchell J. (2013). Cognitive-behavioural group versus guided self-help for compulsive buying disorder: A preliminary study. Clinical Psychology and Psychotherapy, 20, 28–35. doi:10.1002/cpp.773 [DOI] [PubMed] [Google Scholar]
- Ninan P., McElroy S., Kane C., Knight B., Casuto L., Rose S., Marsteller F. A., Nemeroff C. (2000). Placebo-controlled study of fluvoxamine in the treatment of patients with compulsive buying. Journal of Clinical Psychopharmacology, 20, 362–366. doi:10.1097/00004714-200006000-00012 [DOI] [PubMed] [Google Scholar]
- O’Guinn T., Faber R. (1989). Compulsive buying: A phenomenological exploration. Journal of Consumer Research, 16, 147–157. doi:10.1086/209204 [Google Scholar]
- Park T., Cho S., Seo J. (2006). A compulsive buying case: A qualitative analysis by the grounded theory method. Contemporary Family Therapy, 28, 239–249. doi:10.1007/s10591-006-9002-2 [Google Scholar]
- Paulsen K., Rimm D. C., Woodburn L. T., Rimm S. A. (1977). A self-control approach to inefficient spending. Journal of Consulting and Clinical Psychology, 45(3), 433–435. doi:10.1037/0022-006X.45.3.433 [DOI] [PubMed] [Google Scholar]
- Ridgeway N., Kukar-Kinney M., Monroe K. (2011). The measurement of compulsive buying and its application to internet buyers. In Müller A., Mitchell J. (Eds.), Compulsive buying: Clinical foundations and treatment (pp. 51–62). New York, NY: Routledge. [Google Scholar]
- Rücker G., Carpenter J., Schwarzer G. (2011). Detecting and adjusting for small-study effects in meta-analysis. Biometric Journal, 53, 351–368. doi:10.1002/bimj.201000151 [DOI] [PubMed] [Google Scholar]
- Salkovskis P. (1995). Demonstrating specific effects in cognitive and behavioural therapy. In Aveline M., Shapiro D. (Eds.), Research foundations for psychotherapy practice (pp. 191–228). Chichester, UK: Wiley. [Google Scholar]
- Samoocha D., Bruinvels D., Elbers N., Anema J., van der Beek A. (2010). Effectiveness of web-based interventions on patient empowerments: A systematic review and meta-analysis. Journal of Medical Internet Research, 12, e23. doi:10.2196/jmir.1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser S., Black D., Repertinger B., Freet D. (1994). Compulsive buying: Demography, phenomenology, and comorbidity in 46 subjects. General Hospital Psychiatry, 16, 205–212. doi:10.1016/0163-8343(94)90103-1 [DOI] [PubMed] [Google Scholar]
- Sohanpal R., Hooper R., Hames R., Priebe S., Taylor S. (2012). Reporting participation rates in studies of non-pharmacological interventions for patients with chronic obstructive pulmonary disease: A systematic review. Systematic Reviews, 1, 66. doi:10.1186/2046-4053-1-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcke K., Schlereth B., Domass D., Schöler T., Brand M. (2013). Cue reactivity towards shopping cues in female participants. Journal of Behavioral Addictions, 2, 17–22. doi:10.1556/JBA.1.2012.012 [DOI] [PubMed] [Google Scholar]
- StataCorp. (2007). Stata statistical software: Release 10. College Station, TX: StataCorp LP. [Google Scholar]
- Steffen K., Mitchell J. (2011). Overview of treatment for compulsive buying. In Müller A., Mitchell J. (Eds.), Compulsive buying: Clinical foundations and treatment (pp. 129–148). London, UK: Routledge. [Google Scholar]
- Trotzke P., Starcke K., Müller A., Brand M. (2015). Pathological buying online as a specific for of internet addiction: A model-based experimental investigation. PLoS One, 14, 1–17. doi:10.1371/journal.pone.0140296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotzke P., Starcke K., Pederson A., Brand M. (2014). Cue-induced craving in buying: Empirical evidence and clinical implications. Psychosomatic Medicine, 76, 694–700. doi:10.1097/PSY.000000000000126 [DOI] [PubMed] [Google Scholar]
- Wilson D. B. (2005). Meta-analysis macros for SAS, SPSS, and Stata. Retrieved from: http://mason.gmu.edu/~dwilsonb/ma.html
- Winestine M. (1985). Compulsive shopping as a derivative of childhood seduction. Psychoanalytic Quarterly, 54, 70–72. [PubMed] [Google Scholar]
- Ye L., Kadia S., Lippmann S. (2014). Topiramate and compulsive buying disorder. Journal of Clinical Psychopharmacology, 34, 174– 175. doi:10.1097/JCP.0b013e3182aa0139 [DOI] [PubMed] [Google Scholar]
- Zarin D., West J., Placus H., McIntyre J. (1996). The American psychiatric association practice research network. In Sedderer L., Dickey B. (Eds.), Outcomes in Assessment in Clinical Practice (pp. 146–155). Baltimore, MD: Williams & Wilkins. [Google Scholar]