Abstract
Tumor necrosis factor (TNF) is a key cytokine in HIV replication and pathogenesis. For reasons that are not entirely clear, the cytokine remains upregulated despite anti-retroviral therapy (ART). Here we demonstrate that HIV Nef induces an alternative TNF secretion mechanism that remains active in chronic infection. Ingestion of Nef-containing plasma extracellular vesicles (pEV) from ART patients by primary immune cells, but also Nef expression, induced intracellular proTNF cleavage and secretion of vesicular TNF endosomes. Key event was the Nef-mediated routing of the TNF-converting enzyme ADAM17 into Rab4 + early endosomes and the Rab27 + secretory pathway. Analysis of lymph-node tissue by multi-epitope-ligand-cartography (MELC) confirmed a vesicular TNF secretion phenotype that co-localized with persistent Nef expression, and implicated Notch1 as an essential co-factor. Surprisingly Notch1 had no transcriptional effect but was required for the endosomal trafficking of ADAM17. We conclude that Nef expression and Nef-containing pEV mobilize TNF from endosomal compartments in acute and chronic infection.
Keywords: HIV Nef, ADAM17, Plasma extracellular vesicles, Endosomal secretion, Notch1
Highlights
-
•
Nef/ADAM17 containing extracellular vesicles induce an endosomal TNF secretion type in primary target cells.
-
•
The mechanism required the shuttling of ADAM17 into Rab4 + endosomal compartments in a Notch1-dependent manner.
-
•
The mechanism could be demonstrated in tissue by multi-epitope-ligand-cartography (MELC) technology.
Despite antiviral therapy, plasma levels of TNF remain upregulated and likely play a role in many comorbidities seen in chronic HIV infection. We found that this is due to high levels of HIV-induced plasma extracellular vesicles (pEV) containing the TNF processing ADAM17 protease. Interestingly these vesicles induced a different TNF secretion type. Whereas TNF is usually shed from the plasma membrane, pEV mobilized intracellular TNF storage compartments, secreting endosomal vesicles. We could confirm this mechanism analyzing lymph node tissue sections by a novel immunostaining technology. Our report supports our previous publication implying ongoing viral activity despite successful antiretroviral therapy.
1. Introduction
Early in HIV research it was demonstrated that pro-inflammatory cytokines, in particular TNF, drive HIV replication and pathogenesis (Reddy et al., 1988, Fauci, 1996). However, despite efficient ART, TNF plasma levels remain elevated and soluble forms of the TNF receptors (TNFR-I and II) even increase (Aukrust et al., 1999, Wada et al., 2015). As both, proTNF cleavage and TNFR shedding, require the activation of TNF-alpha converting enzyme (TACE/ADAM17), the activation of this protease seems at least in part unaffected by ART. Reaffirming a role of TNF in chronic HIV infection, treatment with TNF inhibitors improves CD4 counts and reduces immune activation (Gallitano et al., 2016, Kumar et al., 2013).
To explain chronic immune activation, it is suggested that direct and indirect effects of the infection, as for example microbial translocation, viral co-infections and HIV expression itself including exosomal TAR RNA, stimulate the innate immune sensing system leading to immune cell stimulation and TNF production (Sandler and Douek, 2012, Iwasaki, 2012, Lawn, 2004, Sampey et al., 2016). In addition a direct stimulation of cytokine production has been reported for Vpr, Tat and Nef, however, most of these studies were done with recombinant viral proteins (Kumar et al., 2013).
Since viral replication is greatly reduced in chronic infection, current concepts do not consider a major role of viral proteins in persistent immune activation. This includes the viral Nef protein, an essential cofactor of HIV replication in vivo (Gorry et al., 2007). Therefore, the role of Nef appears to be restricted to augment viral replication in a yet poorly understood manner that seems to include receptor trafficking and stimulation of T cell signaling (Abraham and Fackler, 2012, Baur, 2011).
We have previously reported that Nef activates and shuttles activated ADAM17 into extracellular vesicles (EV) (Lee et al., 2013). This occurred through interaction with a multifaceted protein complex termed the Nef-associated kinase complex (NAKC). In more recent results we demonstrated that Nef/ADAM17-containing plasma extracellular vesicles (HIV-pEV) persist in high concentrations despite ART and correlate with low CD4 counts (Lee et al., 2016). Together these findings suggested that Nef has a direct role in HIV immune activation and in AIDS pathogenesis. To substantiate this assumption we were looking for a mechanistic link between the effects of Nef and HIV plasma EV (pEV) and the release of TNF in chronic infection.
To this end we analyzed TNF secretion in vitro and in/with human material from ART patients. In addition to current concepts, which describe a plasma membrane (PM)-associated shedding of soluble TNF, we found that the larger pool of proTNF is cleaved intracellularly and secreted through vesicular endosomes. This unusual mechanism required the translocation of ADAM17 into Rab4 + compartments, where the protease converged with its substrate. Analysis of human tissue confirmed these findings and implicated Notch1 as a crucial co-factor in trafficking of ADAM17. We conclude that HIV evolved a powerful TNF-mobilizing mechanism, which persists in ART-patients and may contribute to chronic immune activation.
2. Material and Methods
2.1. Cell Lines, Antibodies, Reagents and Plasmids
293Tcells were cultured in DMEM (Lonza), 10% (v/v) fetal bovine serum (FBS) with Penicillin-Streptomycin (P/S) at 37 °C, 5% CO2. following antibodies and reagents were purchased: α-Nef (JR6) and α-ADAM17 (Abcam); α-mouse Alexa555 (Life Technologies); TAPI-1 (Life Technologies); PMA (Sigma Aldrich); BrefeldinA and Wortmannin (Becton Dickinson); GMCSF (Miltenyi Biotech); Ly294002 (Selleck Chemicals); YO-01027 (Cellagen Technology). Plasmids for ADAM17, GFP-proTNF-RFP and HIV NL4-3 with SF2 nef and NL4.3 delta nef were described recently (Fackler et al., 2006). The PI3-kinase regulatory domain p85 was described in (Wolf et al., 2001). Rab-GFP (4, 11, 27a and 27b), RFP-ADAM17, TNFalpha-RFP and fusion proteins were constructed by overlapping PCR-based cloning technique. The Nef mutants Nef.LL/AA and Nef.ED/AA and were obtained from Warner Greene (Bresnahan et al., 1998) and Matija Peterlin (Lu et al., 1998) respectively. For the MELC analysis the following antibodies were used: CD4 (13B8.2, Beckman Coulter); ADAM10 (SHM14, Biolegend, PE); TNFα (D1G2; Cell Signaling, FITC); CD107a (H4A3 FITC, BD Pharmingen); CD45 (5B1 FITC, Miltenyi); HLA DR (Immu-357 FITC, Beckman Coulter); HLA ABC (B9.12.1 FITC, Beckman Coulter); Notch1 (527425 PE, BioLegend); Propidium iodide (Genaxxon). Anti-Nef and anti-gag p24 (both sheep polyclonal) were provided by NEXT Biomed, Helsinki.
2.2. Transfections and Protein Assays
Transient transfections into 293T cells were performed as described previously (Wolf et al., 2008) and harvested/analyzed usually 24–36 h after transfection. Macrophages (4 × 104 cells/well) were seeded on glass slides in a 12well plate in 800 μl RPMI substituted with 1% human serum, P/S and 800 U/ml GMCSF. After adherence, cells were transfected using the FuGene HD reagent (Promega, Mannheim, Germany) according to manufacturer's protocol.
For pulse chase analysis of ADAM17, 12 × 105 293T cells were seeded onto glass slides in a 12 well dish and transfected with ADAM17 and Rab27a or Rab27b. 16 h after transfection cells were gently washed and incubated with PBS at 4 °C. After 10 min a non-conjugated α-ADAM17 (mouse) antibody was added and incubated for 30 min at 4 °C. Subsequently cells were gently washed and either incubated with media containing PMA, PMA and Monensin or PMA and Brefeldin A for another 30 min at 4 or 37 °C. Finally, cells were fixed for 30 min with 4% PFA and stained with a secondary antibody (goat α-mouse).
ADAM activity in EV was measured using the SensoLyte®520 ADAM17 (α-Secretase) Activity Assay Kit from AnaSpec according to the manufacturer's procedures. Plasma-derived EV (pEV) were purified as described below and 10 μl of a pEV-containing PBS solution, corresponding to 1 ml plasma, were used according to the manufacturers procedures.
To assess TNF secretion from PBMC, cells (1 × 105) were placed in 96-well-U-bottom plates in a total volume of 100 μl (RPMI) and 10 ng of p24 (10 μl) or 10 μl EV corresponding to 1 ml plasma were incubated. When indicated, 10 μl PHA (positive control) or 50 μM TAPI-1 (Peptides International), were used. TNF in the supernatant (100 μl) was measured after 16 h using the CBA (Cytometric Bead Array) Human Soluble Protein Flex Set System (BD Biosciences).
2.3. Plasmid Microinjection
Primary human macrophages were grown on cover glasses and microinjected into their nuclei with an AIS 2 microinjection apparatus using pulled borosilicate glass capillaries in principle as reported (Schmidt et al., 2011). Plasmid encoding Nef wt or pcDNA3.1(+) as control and the G-proTNF-R indicator plasmid were mixed in water at concentrations of 10 ng/μl. Dibenzazepine (YO-01027), or DMSO as control, was added approx. 1,5 h before microinjection. Following microinjection, cells were cultured for 6 h to allow protein expression. At the indicated time point cells were fixed with PBS 4% paraformaldehyde and subjected to microscopic analysis.
2.4. Isolation and Purification of pEV
Plasma EV purification was performed as previously described (Muratori et al., 2009). Briefly, 12 ml blood plasma was diluted with 12 ml PBS and centrifuged for 30 min at 2000 g, 45 min at 12,000g and ultra-centrifuged for 2 h at 110,000g. Pellets were washed in 32 ml PBS and pEV were ultra-centrifuged for 1 h at 110,0007g. Pellets were resuspended in a final volume of 120 μl, resulting in an equivalent of 1 ml plasma in 10 μl pEV-suspension.
2.5. Patients, Tissue and Primary Cells
Blood was drawn from patients and healthy donors after an informed consent, approved by the local ethics committee, was signed. At the time of blood sampling, all HIV-1 patients were under HAART treatment, showing no detectable levels of viral load (below 20 copies/ml blood). The axillary lymph nodes were obtained (04/2008) from a 42 year old male HIV patient treated since 2005, suppressing his viral load to non-detectable levels. Despite treatment, his CD4 count dropped in 2007/8 to 200–300 helper T cells/μl and he developed non-viremic AIDS and died in 2008.
For isolation of PBMCs, EDTA blood samples were diluted 1:1 with PBS and loaded on a 15 ml cushion of Lymphoprep (Axis Shield, Heidelberg, Germany) and centrifuged at 1.500 rpm for 30 min. The obtained cell layers were diluted in cold PBS, spun down at 1150 rpm/4 °C and washed 2-times with PBS. For the Cytokine release assays (Fig. 1b), cells were suspended in RPMI 10% FCS in a concentration of 1 × 106 PBMC/ml. For the generation of macrophages, PBMC were seeded in a density of 15 × 107 cells/20 ml RPMI for 1 h in a T175 flask. After adherence, cells were thoroughly washed and, over a 2 weeks period, repeatedly supplied with fresh RPMI containing 1% human sera and 800 U/ml GMCSF.
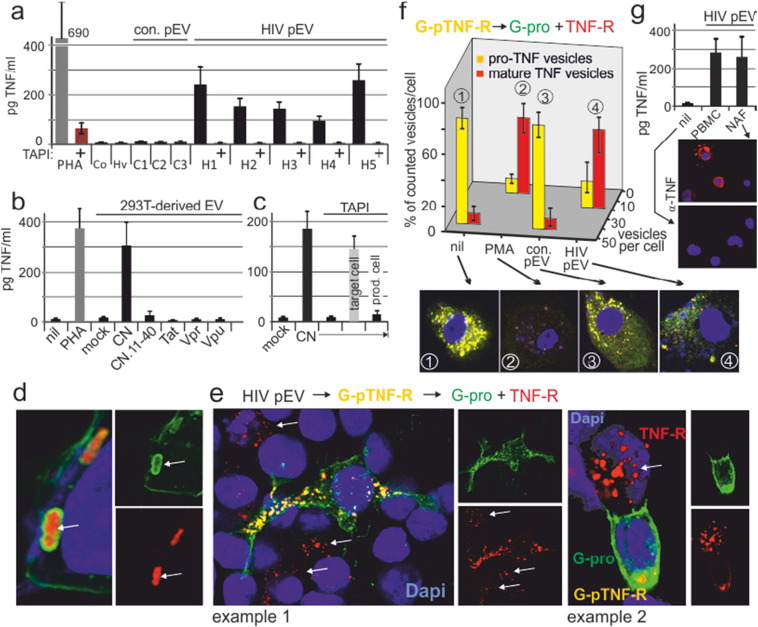
Fig. 1.
HIV pEV induce endosomal proTNF cleavage. (a) HIV pEV from ART patients induce TNF secretion in PBMC. Resting PBMC were incubated with purified pEV (equivalent to 1 ml of plasma) for 12 h w/wo TAPI-1 before culture supernatants were assayed for TNF by CBA (pg/ml). H1–H5: HIV patients 1–5. C1–3: healthy controls 1–3. One PBMC aliquot was stimulated with PHA. For control, input aliquots of HIV patients (Hv) and healthy control (Co) were pooled and analyzed for TNF. Error bars were calculated on the basis of triplicates of a single experiment, performed 3 times with different donor PBMC. (b) Induction of proTNF cleaving EV is Nef-dependent. EV were purified from 293 T cell culture supernatants transfected with CN (CD8.Nef), CN.11-40, Tat, Vpr or Vpu and incubated with PBMC and analyzed as in (A). Error bars indicate standard deviation of the mean from three transfections. (c) Nef-induced EV obtain their proTNF cleaving ability in the produced cell. Same experimental setup as in (b) transfecting CN; however in one aliquot the EV-producing cells were incubated with TAPI, in another aliquot the target PBMC. (d) Spatial orientation of G-pTNF-R in endosomes. 293 T cells were transfected with G-pTNF-R and analyzed by confocal microscopy after 24 h. (e) HIV pEV induce a vesicular secretion mechanism. G-pTNF-R transfected 293T cells (12 h) were incubated with HIV pEV (1 ml plasma equivalent pooled from different donors) for 8 h, mixed with non-transfected cells (1:4; 12 h) and analyzed by confocal microscopy. (f) HIV pEV induce proTNF cleavage in macrophages. Macrophages were incubated (16 h) with pEV-aliquots as in (a) before yellow (proTNF) and red (mature TNF) vesicular compartments were quantified in % of total vesicles, counted on one confocal level (examples at the bottom) of 20 randomly selected cells for each condition. Error bars indicate standard deviation of the mean of 20 cells. (g) HIV pEV induce TNF release in the not-adherent PBMC fraction (NAF: T/B cells). PBMC and the NAF fraction of the same PBMC were incubated with HIV pEV as in (a). In addition, cells were stained for TNF by confocal microscopy as indicated. Error bars were calculated on the basis of triplicates.
2.6. Immunofluorescence and Confocal Microscopy
Immunostainings were performed as described previously (Muratori et al., 2009). Slides were analyzed either on LEICA TCS SP5 laser scanning microscope equipped with the LAS-AF software (Leica Microsystems, Mannheim, Germany) and, due to the purchase of another system, on a Zeiss Laser Scanning Microscope LSM780 equipped with the ZEN software (Carl Zeiss AG, Oberkochen, Germany).
2.7. MELC Technology
The MELC technology has been described previously (Schubert et al., 2006, Baur et al., 2013). Briefly, a slide with two tissue specimens were placed side by side on an inverted wide-field fluorescence microscope (Leica) fitted with fluorescence filters for FITC and PE. Fluorochrome conjugated antibodies and wash solutions were added to both samples simultaneously and removed robotically under temperature control, avoiding any displacement of the samples and objective. In each staining cycle, an antibody was added; phase contrast and fluorescence images were recorded by a high-sensitivity cooled CCD camera; the sample was washed with PBS and bleached at the excitation wavelengths. Data acquisition was fully automated.
2.8. HIV Replication in PBMCs
Peripheral blood mononuclear cells (PBMCs) from healthy blood donors were purified by Ficoll-Hypaque gradient centrifugation. 2 × 106 cells were plated in 200 μl medium without stimuli per well in 96well V-bottom plates. HIV-1NL4.3 SF2 nef (Wt) or HIV-1 NL4.3 ∆ nef (Fackler et al., 2006) virus was concentrated using Centricon® Plus-70 spin columns (Millipore, Billerica, MA) from the supernatant of infected MT-4 cells and used for infection (10 ng or 100 ng p24 per well). After wash-out of input virus 3 h post infection, cultures were kept in the presence of solvent control or 10 ng/μl YO-01027 and the cell culture supernatant was analyzed for TNF content by CBA (24 h p.i.) or HIV reverse transcription activity by the SG-PERT assay (over 16 days p.i.) (Trotard et al., 2016).
2.9. Image Quantifications of Immunoblotting
All image quantifications were performed with ImageJ software (NIH). The quantified data were analyzed using Excel 2010 (Microsoft) for statistical analysis.
2.10. Statistical Analyses
Selected significance analyses in bar diagrams show the means and standard deviations for 3 independent experiments. Statistical comparison was performed using the student t-test or Mann-Whitney U tests (SPSS Inc.; Chicago; USA). The level of significance was set at p < 0.05.
3. Results
3.1. Plasma Extracellular Vesicles (pEV) from HIV ART Patients Induce TNF Cleavage in Endosomes
Plasma EV from HIV ART patients contain Nef and TNF converting enzyme (TACE/ADAM17) (Lee et al., 2016). We asked whether and how these vesicles would induce TNF cleavage and release in target cells. Vesicles were purified from 5 HIV-infected individuals under ART with no plasma viral load and 3 healthy controls. Aliquots equivalent to 1 ml plasma containing Nef and ADAM17 (Fig. S1a) were incubated with resting PBMC. After 12 h TNF was detected in all HIV pEV/PBMC incubations but not in controls (Fig. 1a). TNF secretion was blocked by the addition of TAPI-1, an established ADAM17 inhibitor. ProTNF cleavage was Nef-dependent as purified EV from transfected 293T cells revealed that only Nef (represented by a CD8.Nef (CN) fusion protein), but not Tat, Vpr or Vpu induced EV with the capacity to cleave proTNF in target PBMC (Fig. 1b). Similarly, a CD8.Nef mutant (CN.11-40), unable to interact with the Nef-associated kinase complex (NAKC) and stimulate secretion (Muratori et al., 2009), failed to induce EV with proTNF cleavage ability (Fig. 1b). Preincubation of EV producer cells (293T), but not target cells (PBMC), with TAPI-1 abolished proTNF cleavage, implying that the processing protease originated from the EV producing cell (Fig. 1c). Consistent with this result, aliquots of the HIV pEV samples revealed strong ADAM17 activity as assessed by a commercial assay (SensoLyte®, AnaSpec) (Fig. S1b), which correlated with the presence ADAM17 protein (Fig. S1c).
Since resting PBMC have little to none proTNF on their cell surface, we asked whether TNF was secreted from an intracellular compartment. To test this assumption we transfected 293T cells with an indicator plasmid for proTNF cleavage (GFP-proTNF-RFP or G-pTNF-R). The fusion protein, which is expressed at the plasma membrane and in endosomes (see Fig. 4c) gives a yellow color under UV light, whereas its cleavage produces a red (TNF-RFP) and green moiety (GFP-pro) (Lee et al., 2013). The C-terminal TNF part (red) of endosomal G-proTNF-R was directed towards the lumen, while the pro-domain (green) was anchored in the membrane (Fig. 1d, arrows). Incubation of individual HIV pEV samples (1 ml plasma-equivalent) with G-pTNF-R-transfected cells induced an intracellular TNF cleavage, evidenced by the appearance of red (TNF-RFP) and disappearance of yellow vesicles (Fig. S1d). Strikingly, these TNF-RFP containing vesicular structures were transferred to non-transfected bystander cells (Fig. 1e, white arrows). A similar result was obtained when G-pTNF-R-transfected macrophages were incubated with HIV-pEV (Fig. 1f). Also Macrophages seemingly secreted the red TNF vesicles, suggested by the strong decrease of their relative number per cell. Comparable TNF secretion was observed when the PBMC non-adherent fraction (NAF; mainly T and B cells) were incubated with HIV pEV, showing that not only monocyte-derived cells secreted TNF (Fig. 1g, see also Fig. 5). Taken together, pEV from HIV-patients under ART had a strong capacity to induce proTNF cleavage in endosomes, leading to an unusual vesicular secretion mechanism.
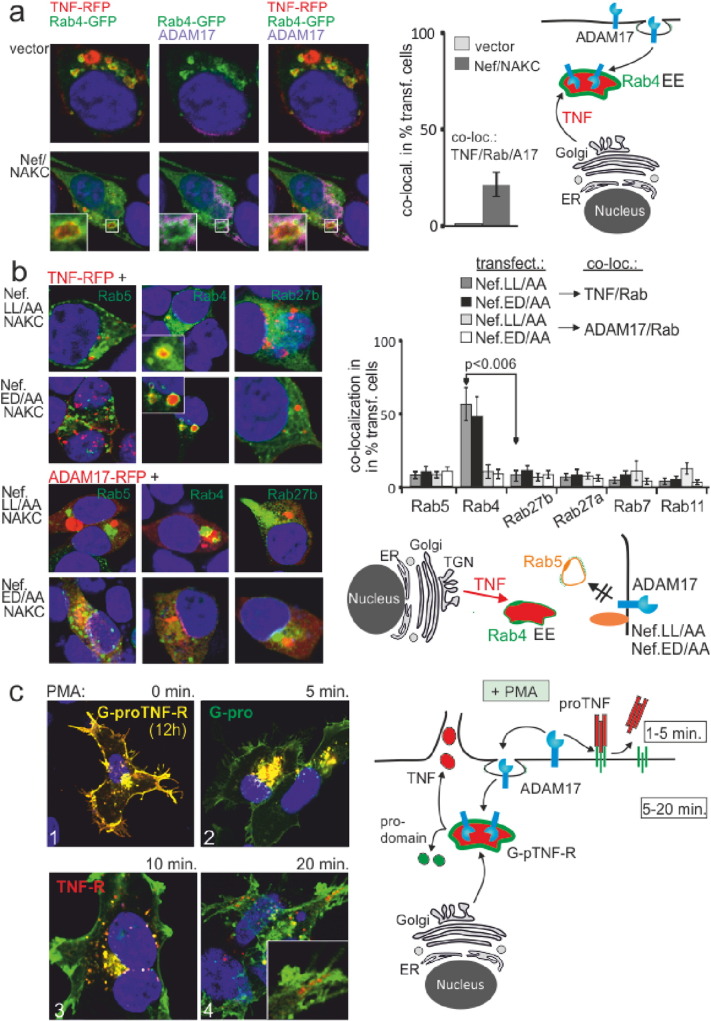
Fig. 4.
ProTNF cleavage in Rab4 + EE is induced by the recruitment of ADAM17. (a) TNF and ADAM17 colocalize in Rab4 + compartments after Nef/NAKC expression. ADAM17, TNF-RFP and Rab4-GFP were cotransfected w/wo Nef/NAKC. After 24 h cells were stained for ADAM17 and analyzed for ADAM17/TNF/Rab4 colocalization by confocal microscopy. Analysis and quantification (bar diagram) of the phenotype (ADAM17/Rab/TNF co-localization) as in Fig. 2. (b) Nef mutants abolish TNF secretion and ADAM17 trafficking. Nef mutants Nef.LL/AA and Nef.ED/AA were co-transfected with Rab-GFP proteins, TNF-RFP and NAKC and subsequently analyzed for TNF/Rap colocalization (upper two panels). Nef.LL/AA and Nef.ED/AA were cotransfected with NAKC and ADAM17-RFP (instead of TNF-RFP; lower panels). Analysis and quantification of the phenotype (TNF/Rab and ADAM17/Rab co-localization) as in Fig. 2. (c) ProTNF is cleaved in two different subcellular compartments. G-pTNF-R transfected 293T cells were stimulated with PMA for indicated time intervals and analyzed by confocal microscopy.
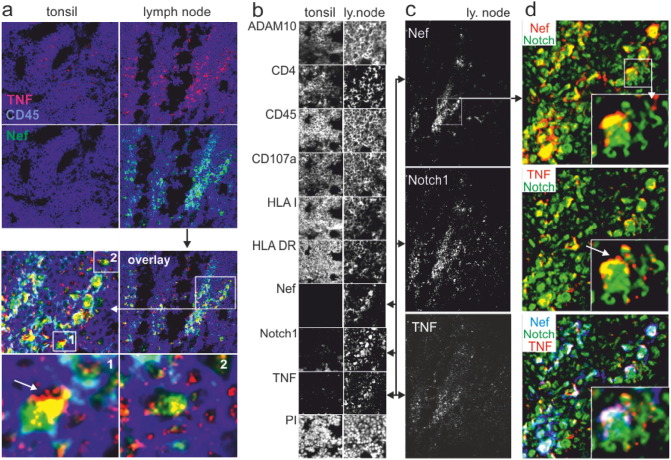
Fig. 5.
Colocalization of Nef, TNF and Notch1 in lymph node tissue. (a) Costaining of Nef, TNF and CD45 in healthy tonsil tissue and a lymph node obtained from a non-viremic HIV-infected individual. The white arrow (bottom left panel) depicts the speckled TNF staining pattern suggesting vesicular TNF secretion. (b) MELC analysis of the HIV lymph node and healthy tonsil reveal different staining patterns and expression levels of antigens. All panels show the same section of tonsil or lymph node tissue, stained with nine different antibodies and Propidiumiodid (PI). (c, d) TNF, Notch1 and Nef colocalize in cells with budding membrane structures (white arrows in (d)).
3.2. PMA, Nef and HIV pEV Trigger TNF Trafficking into Rab4 + and Rab27 + Compartments
To detail this mechanism, we analyzed the trafficking of a TNF-RFP fusion protein in 293T cells using a panel of Rab-GFP proteins (Fig. S2). The transfected cells were stimulated with PMA, by expression of Nef and Nef cofactors (Nef-associated kinase complex (NAKC), see (Lee et al., 2013)) or by incubation with HIV pEV from ART patients.
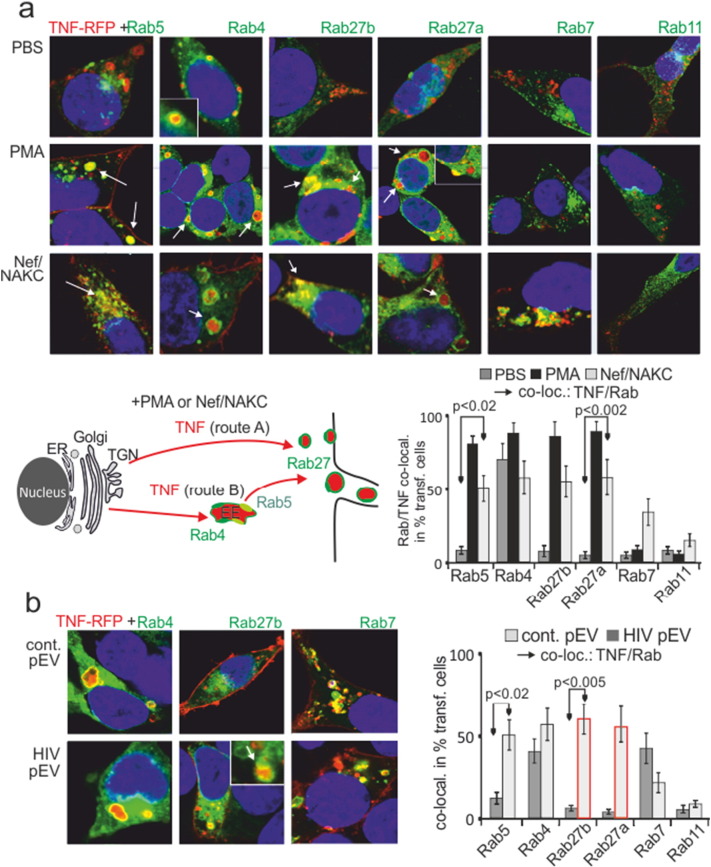
Without stimulation (PBS) TNF-RFP was only seen in Rab4 + early endosomes (EE), likely translocating from the Golgi (Stenmark, 2009) (Fig. 2a, quantification by bar diagram). Upon PMA stimulation or expression of Nef/NAKC, TNF-RFP appeared in Rab4/Rab5 + compartments (likely Rab4/Rab5 double positive EE) and in Rab27a/b + secretory vesicles (Fig. 2a, white arrows). This suggested two possible TNF secretion routes, namely from the ER/TGN directly into the secretion pathway (Fig. 2a, cartoon, route A), or through Rab4 + compartments (route B). In contrast to some cell surface receptors that are downregulated by Nef (Chaudhry et al., 2008, Madrid et al., 2005), TNF was not seen in Rab11 + recycling endosomes (RE). However, some of the cytokine appeared in Rab7 + late endosomes (LE) or lysosomes, where Nef and associated receptors were also found (Schaefer et al., 2008). Monensin or Brefeldin A blocked or reduced the appearance of TNF-RFP in Rab4 + and Rab27 + endosomes (Fig. S3), confirming that TNF-RFP was trafficking through the Golgi/TGN to EE as suggested previously (Stow and Murray, 2013).
Fig. 2.
PMA, Nef/NAKC and HIV pEV shuttle TNF from Rab4 + EE into the p27 + secretory pathway. (a) TNF is constitutively present in Rab4 + EE and mobilized by PMA or Nef/NAKC (NAKC: PKCδ, hnRNPK, Lck) into Rab27 + secretory compartments. TNF-RFP and Rab-GFP transfected 293T cells were stimulated with PMA, or co-transfected with Nef/NAKC and analyzed for TNF/Rab colocalization by confocal imaging. Representative images are shown for each condition. The positive phenotype (TNF/Rab colocalization) was quantified (bar diagrams) in % of 15 transfected and randomly selected cells in three independent experiments. Error bars indicate standard deviation of the mean from three transfections. The cartoon depicts the two possible secretion routs (red arrows, a and b) of TNF under stimulating conditions (PMA or Nef/NAKC). (b) HIV pEV shuttle TNF into the secretion pathway. TNF-RFP and Rab-GFP proteins were cotransfected into 293T cells and incubated with purified HIV and control pEV-aliquots (1 ml plasma equivalent, as in 1D) for 16 h before being analyzed for TNF/Rab colocalization. Analysis and quantification of the phenotype (TNF/Rab co-localization) as in (a).
HIV pEV incubation with target cells induced a similar effect as expression of Nef/NAKC, and TNF-RFP was shuttled into Rab27 + secretion compartments (Fig. 2b, white arrow, red bars). Under these conditions a considerable amount of TNF was also found in late endosomes (Rab7 +). Taken together, PMA, Nef/NAKC and HIV pEV induced a similar routing and secretion of TNF.
3.3. HIV pEV and Nef/NAKC Shuttle ADAM17 into Rab4 + Compartments for proTNF Cleavage
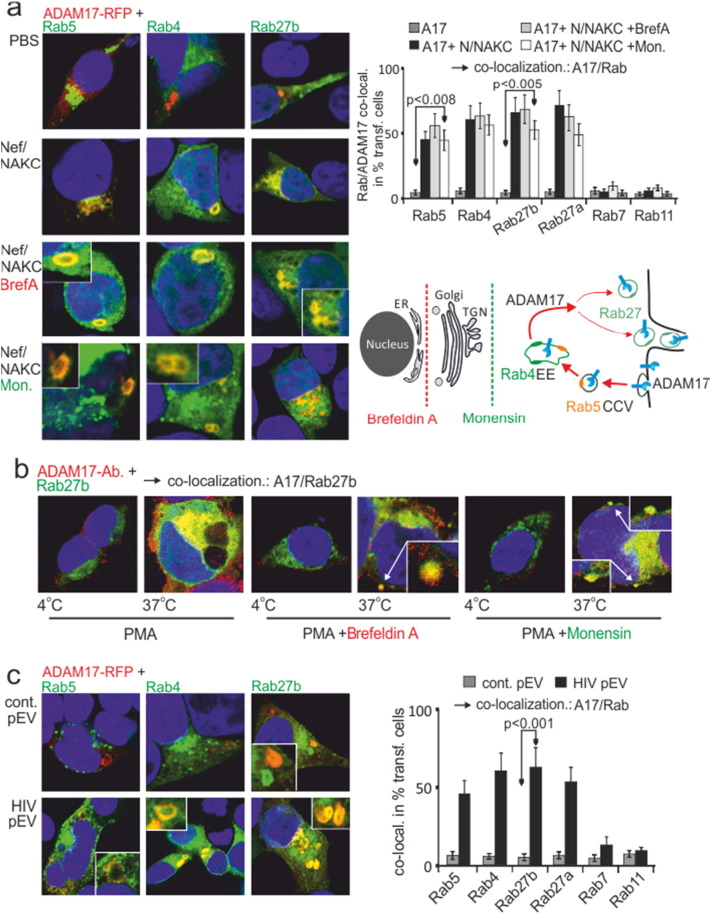
Next we asked where endosomal proTNF was cleaved by ADAM17. Under non-stimulated conditions (PBS), an ADAM17-RFP fusion-protein was not detected in any of the compartments labelled by the transfected Rab proteins (Fig. 3a). Conversely, upon Nef/NAKC expression a strong, often doughnut-shaped (see inserts) colocalization of ADAM17 with Rab5, Rab4 and Rab27a/b was seen, implying that the protease was shuttled into the secretory pathway via Rab4 + EE (Fig. 3a).
Fig. 3.
PMA, Nef/NAKC and HIV pEV shuttle ADAM17 from the PM into secretory pathway. (a) ADAM17 is mobilized by Nef/NAKC into Rab4 + EE and Rab27 + secretory compartments. ADAM17-RFP and Rab-GFP proteins were cotransfected w/wo Nef/NAKC and analyzed for ADAM17/Rab colocalization (upper two image panels). In addition, Nef/NAKC cotransfected cells were stimulated with Brefeldin A or Monensin (lower two image panels). The cartoon depicts the secretion rout of ADAM17 upon Nef/NAKC expression and Monensin and Brefeldin A treatment. Analysis and quantification (bar diagram) of the phenotype (ADAM17/Rab co-localization) as in Fig. 2. (b) PMA-activated ADAM17 is shuttled from the PM into Rab4 + EE and the Rab27 + secretory pathway. Pulse/chase of ADAM17 cotransfected with Rab27b. After 16 h a non-conjugated α-ADAM17 antibody was added (pulse) and chased for 30 min either in the presence of PMA, PMA and Monensin or PMA and Brefeldin A as indicated. Then cells were stained with a conjugated secondary antibody and analyzed for ADAM17/Rab colocalization. (c) HIV pEV shuttle ADAM17 into the secretion pathway. ADAM17-RFP and Rab-GFP proteins were co-transfected into 293T cells, incubated with purified HIV- and control pEV-aliquots and analyzed for ADAM17/Rab colocalization. Analysis and quantification (bar diagram) of the phenotype (ADAM17/Rab co-localization) as in Fig. 2.
Monensin and Brefeldin A did not inhibit the routing of ADAM17 (Fig. 3a, lower panels), suggesting that the protease was shuttled from the PM into Rab4 + EE but not from the ER/Golgi (see cartoon). For confirmation we performed a pulse chase experiment. Surface ADAM17 was pulsed and chased at 37 °C for 30 min in the presence of PMA. Indeed, ADAM17 colocalized with Rab27a/b and this was not inhibited by Monensin or Brefeldin A (Fig. 3b). We also detected budding structures containing ADAM17 that resembled the EV-clusters described in our previous report (see arrows in lower right panels) (Muratori et al., 2009).
Upon incubation of HIV pEV with target cells similar doughnut-shaped Rab4 and Rab27 colocalizations were observed as with Nef/NAKC expression (Fig. 3c). The strong effect implied that HIV pEV induced endosomal circulation of ADAM17. These data also suggested that activated ADAM17 converged with proTNF in EE, placing TNF in secretion route B (see cartoon in Fig. 2a).
3.4. TNF and ADAM17 Converge in Early Endosomes for Intracellular proTNF Cleavage and Secretion
To confirm the last conclusion, ADAM17, TNF-RFP, Rab4-GFP and Nef/NAKC were co-expressed. After 24 h we observed an onion layer-like colocalization of ADAM17, Rab4 and TNF in EE, but only in presence of Nef/NAKC (Fig. 4a, lower panels). We then asked whether the shuttling of ADAM17 required the interaction of Nef with the endocytosis machinery. Two Nef mutants were analyzed that disrupt interactions of Nef with the adaptor protein complexes and the vacuolar ATPase (Nef.LL/AA and ED/AA) (Bresnahan et al., 1998, Lu et al., 1998), of which Nef.ED/AA is also defective for assembly of NAKC (Witte et al., 2004).
As expected, both mutants blocked the internalization of Nef by preventing the formation of Nef-containing clathrin coated vesicles (CCV) (Fig. S4). In addition, the secretion of TNF was halted at the level of the Rab4 + EE (Fig. 4b). This was likely due to the blocked trafficking of ADAM17, as both Nef mutants inhibited the internalization of the protease (Fig. 4b).
These results did not prove that proTNF was processed in EE, as it is generally assumed that the cytokine is shuttled via the Rab11 + recycling endosomes (RE) to the cell surface to be cleaved by ADAM17 (Stow and Murray, 2013). For clarification we analyzed proTNF cleavage and secretion using PMA stimulation. Expression of G-pTNF-R in 293T cells revealed two distinct proTNF pools, namely at the PM and the perinuclear region (ER/Golgi) (Fig. 4c-1). Five minutes after PMA stimulation the PM pool was processed, leaving behind the GFP-pro (green) cleavage product (Fig. 4c-2). In the remaining perinuclear pool, proTNF cleavage started after 10 min, visible by the intracellular appearance of red vesicles perpendicular around yellow endosomal compartments (Fig. 4c-3). The process was completed after 20 min leaving behind only red vesicles with mature TNF-RFP transported to the periphery of the cell (Fig. 4c-4). Together these data suggested that ADAM17 was activated at the PM, cleaving first PM-associated proTNF. The protease was then internalized into endosomal compartments cleaving the intracellular TNF pool (see cartoon Fig. 4c).
3.5. Expression of Nef, TNF and Notch1 Correlate in Lymph Node Tissue
To test whether Nef-induced TNF secretion can be observed in tissue, we analyzed two kryo-preserved lymph nodes from a non-viremic HIV-infected individual under ART (one sample shown). Healthy tonsil tissue served as control. For this analysis we used the multi-epitope-ligand-cartography (MELC) technology (Schubert et al., 2006), allowing the staining of single tissue sections with multiple antibodies.
The lymph node of the HIV patient was negative for Gag p24 (data not shown) but revealed a distinct presence of Nef that co-localized with an increased TNF expression (Fig. 5a). TNF was secreted in large vesicular structures and was seemingly transferred to bystander cells, as judged by the overall speckled TNF staining pattern and budding membrane structures (Fig. 5a, lower panels, Fig. 5d middle panel, white arrows). The lymph node also revealed a lower and speckled expression of HLA class I, HLA DR, CD107a and CD4, and a higher expression of ADAM10 (Fig. 5b). Although, these latter signals were not restricted to Nef-containing cells, they were consistent with an increased secretion activity in this tissue.
Interestingly, we noted a strong appearance of Notch1 (Fig. 5b), which is a substrate of ADAM17/10. Notch1 appearance correlated with Nef and TNF staining patterns (Fig. 5c and d) and decreased radially around Nef-expressing cells (Fig. 5c). This suggested a connection between Nef, Notch signaling and TNF secretion.
3.6. Notch1 Is Required for Nef-mediated Endocytosis of ADAM17
In lymph-node cells Notch1 appeared to have a cytoplasmic localization (Fig. 5d). In line with this result, expression of Nef/NAKC in 293T cells induced a cytoplasmic colocalization of Nef and endogenous Notch1 (Fig. 6a). A nuclear translocation of the Notch1 intracellular domain as a consequence of this interaction seemed unlikely, as the transcriptional effect of Nef/NAKC-expression on a Notch1-responsive (CSL)4-luc reporter plasmid was marginal (Fig. S5a). Thus we assumed a role of Notch1 in endocytosis and trafficking.
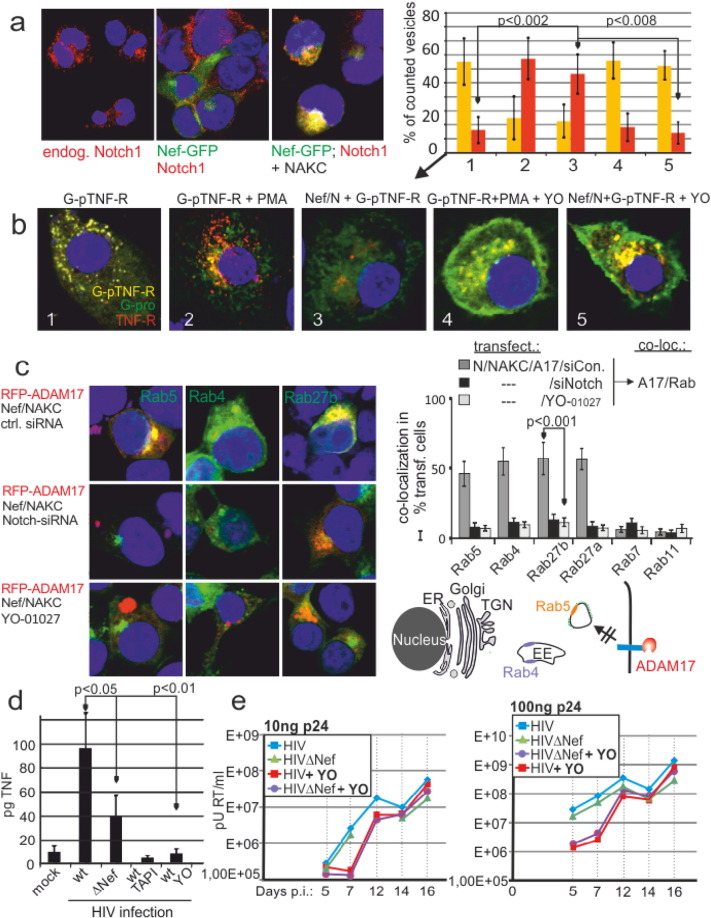
Fig. 6.
Notch1 is required for ADAM17 trafficking into Rab4 + EE and proTNF cleavage. (a) Nef and Notch1 colocalize after NAKC coexpression. Nef-GFP and NAKC were transfected into 293T cells and analyzed for Nef colocalization with endogenous Notch1. (b) The Notch1 inhibitor YO-01027 blocks proTNF cleavage in primary macrophages. G-pTNF-R transfected macrophages were co-transfected with Nef/NAKC (N) or stimulated with PMA in the presence or absence of YO-01027 as indicated. The bar diagram represents the relative percentage of yellow and red vesicles per cell similar as described for Fig. 1. Error bars indicate standard deviation of the mean of 10 cells. (c) Inhibition of Notch1 blocks ADAM17 trafficking. Rab-GFP/RFP-ADAM17/ Nef/NAKC-transfected cells were co-transfected with Notch1 siRNA or scrambled siRNA or incubated with YO-01027 as indicated and analyzed for Rab/ADAM17 co-localization. Analysis and quantification (bar diagram) of the phenotype (ADAM17/Rab co-localization) as in Fig. 2. (d) Nef-augmented TNF-release from infected PBMC is blocked by TAPI and YO-01027. Resting PBMC (1 × 105) were incubated with 10 ng p24 + infectious inoculum of a nef + (wt) or nef − (∆ Nef) HIV virus for 12 h before TNF was measured in the culture supernatant by CBA. Aliquots of the wt cultures were treated with TAPI or YO-01027 as indicated. (e) The Notch1 inhibitor YO-01027 delays HIV replication in PBMC. Non-stimulated PBMC (2 × 106) were infected with 10 or 100 ng p24 + infectious inoculum of a wt and a nef-negative (∆ Nef) HIV infectious clone and reverse transcriptase activity was measured over 16 days as indicated. Aliquots of the cultures were treated with the Notch1 inhibitor YO-01027.
We blocked Notch1 signaling by Dibenzazepine (YO-01027) and analyzed PMA- and Nef-induced cleavage of the G-pTNF-R indicator plasmid. Consistent with our assumption, Dibenzazepine blocked mainly cleavage of the intracellular pool of proTNF in Nef-transfected, PMA-stimulated (Fig. 6b) or plasmid-injected primary macrophages (Fig. S6). This implied that ADAM17 did not reach proTNF containing endosomes under these conditions. This was confirmed when ADAM17 trafficking was studied under Dibenzazipine treatment or Notch1 siRNA-mediated knock down: under either condition, Nef-activated ADAM17 was not internalized/routed into CCV, EE or Rab27a/b compartments (Fig. 6c). Together this suggested that Notch1 was required for the trafficking of activated ADAM17.
We reasoned that HIV-induced TNF secretion was relevant for replication in non-stimulated/resting PBMC. We infected non-stimulated PBMCs with a wild type and a Nef-deleted virus. The infection induced TNF-release after 6 h in both cases. However, more TNF was secreted when Nef was present (Fig. 6d). This depended on ADAM17, as the ADAM inhibitor TAPI blocked the cytokine release. In addition, inhibition of Notch1 reduced TNF release significantly. For further confirmation non-stimulated PBMC were infected with two different infectious doses of wt and ∆ Nef HIV (10 and 100 ng p24) and replication was measured (reverse transcriptase) over 2 weeks. In this setting both virus variants did not show a significant difference in replication. However, in the presence of the Notch1 inhibitor viral replication was markedly reduced and lagged 5–10-fold behind untreated cultures in the first 12 days. Collectively this confirmed an important role for Notch1 in the HIV life cycle.
4. Discussion
The here presented results describe the mechanism of HIV-induced TNF secretion in host and bystander cells. This mechanism was induced by Nef expression or ingestion of HIV-pEV and activated a vesicular secretion type. Our work implies that HIV directly stimulates cytokine release in acute and chronic non-viremic infection.
We have previously demonstrated a transfer of Nef and Nef-associated signaling mechanisms to bystander cells by means of vesicle transfer and trogocytosis (Muratori et al., 2009). We speculated that this transfer of infected cell signaling (TOS) serves to prepare/activate the resting bystander cell for viral infection. The here described vesicular secretion of TNF appears to be part of this function. This type of secretion is mechanistically different from TNF surface shedding as cleaved TNF is kept in the lumen of endosomal vesicles (see Fig. 1d). This excludes a surface to surface interaction, e.g. vesicle to plasma membrane, for TNF receptor stimulation. Hence, this secretion type, which we detailed with electron micrographs in our previous work (Muratori et al., 2009), is different from microvesicle (Cocucci et al., 2009) and Exosome release (Thery et al., 2002) and may be termed endosomal or vesicular secretion.
Based on our confocal analysis we suggest that pEV are endocytosed by target cells, possibly in a clathrin-dependent manner. Subsequently they may fuse with endosomes, for example containing inward directed TNF receptor molecules for sustained signaling. Although this is speculation and requires further analysis, we summarized these events in a model, which includes findings from our previous work and the literature (Fig. S7) (Muratori et al., 2009, Gonzalez-Gaitan, 2003, Lee et al., 2013).
ADAM17 needed to be internalized into Rab4 + positive EE, in order to converge with its substrate proTNF. This routing was initiated by Nef, most likely through its well described induction of clathrin coated vesicles (CCV) (Mangasarian et al., 1997). The CCV probably transformed into, or fused with, pre-existing Rab4 + and proTNF-containing compartments. These events suggested a spatially separated TNF secretion mechanism, which is not necessarily connected to TNF surface shedding. We speculate that activation stimuli that include internalization signals for ADAM17 (e.g. Nef or PMA) will induce endosomal proTNF cleavage and secretion, whereas signals confined to the plasma membrane will cause primarily TNF shedding from the cell surface.
We found that Nef association with signaling molecules (NAKC) was required for endosomal TNF processing, suggesting that Nef-induced endocytosis and Nef signaling serve a common purpose. This function is characterized by two tightly linked steps. First, ADAM17 is activated by a Nef-induced signaling process (Percario et al., 2015, Lee et al., 2013) and, in a second step, shuttled to the proTNF pool in Rab4 + EE. In host cells this is initiated by Nef expression, in bystander cells via Nef EV-ingestion. By which mechanism incoming Nef pEV induce internalization of cell-resident ADAM17 (as in Fig. 3) is not clear. Based on our data we could envision a scenario by which incoming pEV fuse with endosomes containing cellular ADAM17 (Fig. S7).
Our in vitro findings were confirmed by the MELC analysis of two lymph nodes. The latter confirmed a predominantly vesicular secretion mechanism for TNF. In addition the analysis suggested a role of Notch1 in TNF secretion. For its activation Notch1 requires the processing by ADAM proteases (Musse et al., 2012) and thus could be a downstream effector of Nef. Notch function is usually linked to the nuclear translocation of its intracellular domain (NICD) (Baron, 2012). However, we could not confirm a transcriptional effect of Nef/NAKC via Notch1. A ligand-independent role of Notch1 is now recognized in endosomal trafficking (Palmer and Deng, 2015), shuttling surface proteins into endosomal compartments (Waters et al., 2012). Furthermore, the protein was found to be required for cytokine secretion in T cells (Benson et al., 2005, Manaster et al., 2010). Thus the receptor could have a role in ADAM17 trafficking. Our results are seemingly in contrast to a previous finding in a mouse model, describing HIV-mediated transcriptional activation of Notch1 and 4 in kidney cells (Sharma et al., 2010). Potentially HIV-activated Notch1 has cell-type dependent transcriptional effects.
The here proposed Nef function raises the question of why the rather complex generation of ADAM17-containing EV evolved over a direct stimulation of transcription? We speculate that HIV-pEV induce a radial perpetuation of TNF secretion around infected cells leading to bystander cell activation. In fact, early dissemination of the virus in vaginal tissue (SIV) occurs in such a radial manner (Li et al., 2005). This conclusion is also supported by our observations in tissue, demonstrating the presence of TNF and also Notch1 in a seemingly radial gradient from Nef-expressing cells. Potentially, pEV-associated TNF is particularly potent. Since TNF has to bind TNF receptors as a trimer, this requires higher concentrations of the cytokine, which are easier achieved in closed endosomal compartments, rather than the extracellular space. Taken together a strong pEV-mediated TNF secretion and stimulation mechanism may induce an efficient radial transmission of HIV into resting host cells.
Nef-containing HIV-pEV and the Nef-expressing lymph nodes imply persistent transcription of at least some viral proteins under ART, that may even occur without viral integration (Kelly et al., 2008). Irrespective of the specific mechanism involved, our findings challenge the currently prevailing view that effective ART almost fully suppresses virus replication and protein production. In support of such activity under ART, reports based on different experimental approaches are beginning to emerge that hint at the existence of viral sanctuaries in chronic HIV infection in which virus replication can occur (North et al., 2010, Fukazawa et al., 2015, Lorenzo-Redondo et al., 2016, Lee et al., 2016, Kumar et al., 2016). Identifying these tissue sanctuaries will be of great importance for the treatment of chronic HIV infection.
Conflict of Interest
The authors declare no conflict of interest.
Funding Sources
S.W., JH.L. and A.S.B. were supported by the German Research Foundation (DFG; BA961/4-1) including the Collaborative Research Center grant SFB 643 (Project A9) and by funds from the European Union (HIVERA IRIFCURE). C.O. is supported by the Comprehensive Cancer Center (CCC) Erlangen. T.H. was supported by the Emerging Fields Program of the Friedrich-Alexander-University of Erlangen-Nürnberg. O.T.F acknowledges funding from the Deutsche Forschungsgemeinschaft (TRR83 project 15) and the European Union (HIVERA IRIFCURE).
Author Contributions
The project was conceptualized and coordinated by A.S.B. Substantial contributions to the work were made by C.O. (MELC analysis, transfections, immunofluorescence); S.W. (transfections, confocal imaging); JH.L. (Western Blot); MM.G. (microinjection experiments); N.T. (HIV replication assay); T.H. (plasma and tissue sample analysis); O.F. and G.S. (expertise, feedback and reagents).
Acknowledgement
The anti-Nef antibodies were a generous gift from NEXT Biomed in Helsinki.
Footnotes
Supplementary data to this article can be found online at doi:10.1016/j.ebiom.2016.10.027.
Appendix A. Supplementary data
Supplementary figures
References
- Abraham L., Fackler O.T. HIV-1 Nef: a multifaceted modulator of T cell receptor signaling. Cell Commun. Signal. 2012;10:39. doi: 10.1186/1478-811X-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukrust P., Muller F., Lien E., Nordoy I., Liabakk N.B., Kvale D., Espevik T., Froland S.S. Tumor necrosis factor (TNF) system levels in human immunodeficiency virus-infected patients during highly active antiretroviral therapy: persistent TNF activation is associated with virologic and immunologic treatment failure. J. Infect. Dis. 1999;179:74–82. doi: 10.1086/314572. [DOI] [PubMed] [Google Scholar]
- Baron M. Endocytic routes to Notch activation. Semin. Cell Dev. Biol. 2012;23:437–442. doi: 10.1016/j.semcdb.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Baur A.S. HIV-Nef and AIDS pathogenesis: are we barking up the wrong tree? Trends Microbiol. 2011;19:435–440. doi: 10.1016/j.tim.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Baur A.S., Lutz M.B., Schierer S., Beltrame L., Theiner G., Zinser E., Ostalecki C., Heidkamp G., Haendle I., Erdmann M., Wiesinger M., Leisgang W., Gross S., Pommer A.J., Kampgen E., Dudziak D., Steinkasserer A., Cavalieri D., Schuler-Thurner B., Schuler G. Denileukin diftitox (ONTAK) induces a tolerogenic phenotype in dendritic cells and stimulates survival of resting Treg. Blood. 2013;122:2185–2194. doi: 10.1182/blood-2012-09-456988. [DOI] [PubMed] [Google Scholar]
- Benson R.A., Adamson K., Corsin-Jimenez M., Marley J.V., Wahl K.A., Lamb J.R., Howie S.E. Notch1 co-localizes with CD4 on activated T cells and Notch signaling is required for IL-10 production. Eur. J. Immunol. 2005;35:859–869. doi: 10.1002/eji.200425562. [DOI] [PubMed] [Google Scholar]
- Bresnahan P.A., Yonemoto W., Ferrell S., Williams-Herman D., Geleziunas R., Greene W.C. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr. Biol. 1998;8:1235–1238. doi: 10.1016/s0960-9822(07)00517-9. [DOI] [PubMed] [Google Scholar]
- Chaudhry A., Das S.R., Jameel S., George A., Bal V., Mayor S., Rath S. HIV-1 Nef induces a Rab11-dependent routing of endocytosed immune costimulatory proteins CD80 and CD86 to the Golgi. Traffic. 2008;9:1925–1935. doi: 10.1111/j.1600-0854.2008.00802.x. [DOI] [PubMed] [Google Scholar]
- Cocucci E., Racchetti G., Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19 doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Fackler O.T., Moris A., Tibroni N., Giese S.I., Glass B., Schwartz O., Krausslich H.G. Functional characterization of HIV-1 Nef mutants in the context of viral infection. Virology. 2006;351:322–339. doi: 10.1016/j.virol.2006.03.044. [DOI] [PubMed] [Google Scholar]
- Fauci A.S. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- Fukazawa Y., Lum R., Okoye A.A., Park H., Matsuda K., Bae J.Y., Hagen S.I., Shoemaker R., Deleage C., Lucero C., Morcock D., Swanson T., Legasse A.W., Axthelm M.K., Hesselgesser J., Geleziunas R., Hirsch V.M., Edlefsen P.T., Piatak M., Jr., Estes J.D., Lifson J.D., Picker L.J. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat. Med. 2015;21:132–139. doi: 10.1038/nm.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallitano S.M., McDermott L., Brar K., Lowenstein E. Use of tumor necrosis factor (TNF) inhibitors in patients with HIV/AIDS. J. Am. Acad. Dermatol. 2016;74:974–980. doi: 10.1016/j.jaad.2015.11.043. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gaitan M. Signal dispersal and transduction through the endocytic pathway. Nat. Rev. Mol. Cell Biol. 2003;4:213–224. doi: 10.1038/nrm1053. [DOI] [PubMed] [Google Scholar]
- Gorry P.R., McPhee D.A., Verity E., Dyer W.B., Wesselingh S.L., Learmont J., Sullivan J.S., Roche M., Zaunders J.J., Gabuzda D., Crowe S.M., Mills J., Lewin S.R., Brew B.J., Cunningham A.L., Churchill M.J. Pathogenicity and immunogenicity of attenuated, nef-deleted HIV-1 strains in vivo. Retrovirology. 2007;4:66. doi: 10.1186/1742-4690-4-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A. Innate immune recognition of HIV-1. Immunity. 2012;37:389–398. doi: 10.1016/j.immuni.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J., Beddall M.H., Yu D., Iyer S.R., Marsh J.W., Wu Y. Human macrophages support persistent transcription from unintegrated HIV-1 DNA. Virology. 2008;372:300–312. doi: 10.1016/j.virol.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Abbas W., Herbein G. TNF and TNF receptor superfamily members in HIV infection: new cellular targets for therapy? Mediat. Inflamm. 2013;2013:484378. doi: 10.1155/2013/484378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Abbas W., Colin L., Khan K.A., Bouchat S., Varin A., Larbi A., Gatot J.S., Kabeya K., Vanhulle C., Delacourt N., Pasquereau S., Coquard L., Borch A., Konig R., Clumeck N., De W.S., Rohr O., Rouzioux C., Fulop T., Van L.C., Herbein G. Tuning of AKT-pathway by Nef and its blockade by protease inhibitors results in limited recovery in latently HIV infected T-cell line. Sci. Rep. 2016;6:24090. doi: 10.1038/srep24090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn S.D. AIDS in Africa: the impact of coinfections on the pathogenesis of HIV-1 infection. J. Infect. 2004;48:1–12. doi: 10.1016/j.jinf.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Wittki S., Brau T., Dreyer F.S., Kratzel K., Dindorf J., Johnston I.C., Gross S., Kremmer E., Zeidler R., Schlotzer-Schrehardt U., Lichtenheld M., Saksela K., Harrer T., Schuler G., Federico M., Baur A.S. HIV Nef, paxillin, and Pak1/2 regulate activation and secretion of TACE/ADAM10 proteases. Mol. Cell. 2013;49:668–679. doi: 10.1016/j.molcel.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Schierer S., Blume K., Dindorf J., Wittki S., Xiang W., Ostalecki C., Koliha N., Wild S., Schuler G., Fackler O.T., Saksela K., Harrer T., Baur A.S. HIV-Nef and ADAM17-containing plasma extracellular vesicles induce and correlate with immune pathogenesis in chronic HIV infection. EBioMedicine. 2016;6:103–113. doi: 10.1016/j.ebiom.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Duan L., Estes J.D., Ma Z.M., Rourke T., Wang Y., Reilly C., Carlis J., Miller C.J., Haase A.T. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Redondo R., Fryer H.R., Bedford T., Kim E.Y., Archer J., Kosakovsky Pond S.L., Chung Y.S., Penugonda S., Chipman J.G., Fletcher C.V., Schacker T.W., Malim M.H., Rambaut A., Haase A.T., McLean A.R., Wolinsky S.M. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature. 2016;530:51–56. doi: 10.1038/nature16933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Yu H., Liu S.H., Brodsky F.M., Peterlin B.M. Interactions between HIV1 Nef and vacuolar ATPase facilitate the internalization of CD4. Immunity. 1998;8:647–656. doi: 10.1016/s1074-7613(00)80569-5. [DOI] [PubMed] [Google Scholar]
- Madrid R., Janvier K., Hitchin D., Day J., Coleman S., Noviello C., Bouchet J., Benmerah A., Guatelli J., Benichou S. Nef-induced alteration of the early/recycling endosomal compartment correlates with enhancement of HIV-1 infectivity. J. Biol. Chem. 2005;280:5032–5044. doi: 10.1074/jbc.M401202200. [DOI] [PubMed] [Google Scholar]
- Manaster I., Gazit R., Goldman-Wohl D., Stern-Ginossar N., Mizrahi S., Yagel S., Mandelboim O. Notch activation enhances IFNgamma secretion by human peripheral blood and decidual NK cells. J. Reprod. Immunol. 2010;84:1–7. doi: 10.1016/j.jri.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Mangasarian A., Foti M., Aiken C., Chin D., Carpentier J.L., Trono D. The HIV-1 Nef protein acts as a connector with sorting pathways in the Golgi and at the plasma membrane. Immunity. 1997;6:67–77. doi: 10.1016/s1074-7613(00)80243-5. [DOI] [PubMed] [Google Scholar]
- Muratori C., Cavallin L.E., Kratzel K., Tinari A., De M.A., Fais S., D'Aloja P., Federico M., Vullo V., Fomina A., Mesri E.A., Superti F., Baur A.S. Massive secretion by T cells is caused by HIV Nef in infected cells and by Nef transfer to bystander cells. Cell Host Microbe. 2009;6:218–230. doi: 10.1016/j.chom.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Musse A.A., Meloty-Kapella L., Weinmaster G. Notch ligand endocytosis: mechanistic basis of signaling activity. Semin. Cell Dev. Biol. 2012;23:429–436. doi: 10.1016/j.semcdb.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North T.W., Higgins J., Deere J.D., Hayes T.L., Villalobos A., Adamson L., Shacklett B.L., Schinazi R.F., Luciw P.A. Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. J. Virol. 2010;84:2913–2922. doi: 10.1128/JVI.02356-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer W.H., Deng W.M. Ligand-independent mechanisms of Notch activity. Trends Cell Biol. 2015;25:697–707. doi: 10.1016/j.tcb.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percario Z.A., Ali M., Mangino G., Affabris E. Nef, the shuttling molecular adaptor of HIV, influences the cytokine network. Cytokine Growth Factor Rev. 2015;26:159–173. doi: 10.1016/j.cytogfr.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Reddy M.M., Sorrell S.J., Lange M., Grieco M.H. Tumor necrosis factor and HIV P24 antigen levels in serum of HIV-infected populations. J. Acquir. Immune Defic. Syndr. 1988;1:436–440. [PubMed] [Google Scholar]
- Sampey G.C., Saifuddin M., Schwab A., Barclay R., Punya S., Chung M.C., Hakami R.M., Zadeh M.A., Lepene B., Klase Z.A., El-Hage N., Young M., Iordanskiy S., Kashanchi F. Exosomes from HIV-1-infected cells stimulate production of pro-inflammatory cytokines through trans-activating response (TAR) RNA. J. Biol. Chem. 2016;291:1251–1266. doi: 10.1074/jbc.M115.662171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler N.G., Douek D.C. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat. Rev. Microbiol. 2012;10:655–666. doi: 10.1038/nrmicro2848. [DOI] [PubMed] [Google Scholar]
- Schaefer M.R., Wonderlich E.R., Roeth J.F., Leonard J.A., Collins K.L. HIV-1 Nef targets MHC-I and CD4 for degradation via a final common beta-COP-dependent pathway in T cells. PLoS Pathog. 2008;4:e1000131. doi: 10.1371/journal.ppat.1000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S., Fritz J.V., Bitzegeio J., Fackler O.T., Keppler O.T. HIV-1 Vpu blocks recycling and biosynthetic transport of the intrinsic immunity factor CD317/tetherin to overcome the virion release restriction. MBio. 2011;2 doi: 10.1128/mBio.00036-11. (e00036-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert W., Bonnekoh B., Pommer A.J., Philipsen L., Bockelmann R., Malykh Y., Gollnick H., Friedenberger M., Bode M., Dress A.W. Analyzing proteome topology and function by automated multidimensional fluorescence microscopy. Nat. Biotechnol. 2006;24:1270–1278. doi: 10.1038/nbt1250. [DOI] [PubMed] [Google Scholar]
- Sharma M., Callen S., Zhang D., Singhal P.C., Vanden Heuvel G.B., Buch S. Activation of Notch signaling pathway in HIV-associated nephropathy. AIDS. 2010;24:2161–2170. doi: 10.1097/QAD.0b013e32833dbc31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Stow J.L., Murray R.Z. Intracellular trafficking and secretion of inflammatory cytokines. Cytokine Growth Factor Rev. 2013;24:227–239. doi: 10.1016/j.cytogfr.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Thery C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2002;2 doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- Trotard M., Tsopoulidis N., Tibroni N., Willemsen J., Binder M., Ruggieri A., Fackler O.T. Sensing of HIV-1 infection in Tzm-bl cells with reconstituted expression of STING. J. Virol. 2016;90:2064–2076. doi: 10.1128/JVI.02966-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada N.I., Jacobson L.P., Margolick J.B., Breen E.C., Macatangay B., Penugonda S., Martinez-Maza O., Bream J.H. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS. 2015;29:463–471. doi: 10.1097/QAD.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters A.M., Wu M.Y., Huang Y.W., Liu G.Y., Holmyard D., Onay T., Jones N., Egan S.E., Robinson L.A., Piscione T.D. Notch promotes dynamin-dependent endocytosis of nephrin. J. Am. Soc. Nephrol. 2012;23:27–35. doi: 10.1681/ASN.2011010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte V., Laffert B., Rosorius O., Lischka P., Blume K., Galler G., Stilper A., Willbold D., D'Aloja P., Sixt M., Kolanus J., Ott M., Kolanus W., Schuler G., Baur A.S. HIV-1 Nef mimics an integrin receptor signal that recruits the polycomb group protein Eed to the plasma membrane. Mol. Cell. 2004;13:179–190. doi: 10.1016/s1097-2765(04)00004-8. [DOI] [PubMed] [Google Scholar]
- Wolf D., Witte V., Laffert B., Blume K., Stromer E., Trapp S., D'Aloja P., Schurmann A., Baur A.S. HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti-apoptotic signals. Nat. Med. 2001;7:1217–1224. doi: 10.1038/nm1101-1217. [DOI] [PubMed] [Google Scholar]
- Wolf D., Witte V., Clark P., Blume K., Lichtenheld M.G., Baur A.S. HIV Nef enhances Tat-mediated viral transcription through a hnRNP-K-nucleated signaling complex. Cell Host Microbe. 2008;4:398–408. doi: 10.1016/j.chom.2008.08.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures