Abstract
Introduction
There is growing concern around the effects of concussion and sub-concussive impacts in sport. Routine game-play in soccer involves intentional and repeated head impacts through ball heading. Although heading is frequently cited as a risk to brain health, little data exist regarding the consequences of this activity. This study aims to assess the immediate outcomes of routine football heading using direct and sensitive measures of brain function.
Methods
Nineteen amateur football players (5 females; age 22 ± 3 y) headed machine-projected soccer balls at standardized speeds, modelling routine soccer practice. The primary outcome measure of corticomotor inhibition measured using transcranial magnetic stimulation, was assessed prior to heading and repeated immediately, 24 h, 48 h and 2 weeks post-heading. Secondary outcome measures were cortical excitability, postural control, and cognitive function.
Results
Immediately following heading an increase in corticomotor inhibition was detected; further to these electrophysiological alterations, measurable reduction memory function were also found. These acute changes appear transient, with values normalizing 24 h post-heading.
Discussion
Sub-concussive head impacts routine in soccer heading are associated with immediate, measurable electrophysiological and cognitive impairments. Although these changes in brain function were transient, these effects may signal direct consequences of routine soccer heading on (long-term) brain health which requires further study.
Keywords: Sports concussion, Transcranial magnetic stimulation, Sub-concussion, Traumatic brain injury
Highlights
-
•
Standard soccer heading results in immediate and measurable alterations in brain function.
-
•
Changes in short and long term memory function and corticomotor inhibition are detectable immediately after soccer heading.
-
•
These changes in brain function after just a single session of heading appear transient.
-
•
These data provide direct evidence of immediate brain functional impairment associated with soccer heading.
Questions have been raised over whether soccer heading might have an effect on a player's brain, with particular worry over the proposed link between brain injury and increased risk of dementia. However, until now there have been no studies of the immediate effects of heading in soccer directly on brain function. This study found that just a single session of heading practice resulted in temporary impairment of short and long term memory function and in electrophysiological function of the brain. Whether these effects remain temporary after repeated soccer heading exposure and their long-term consequences on brain health must now be investigated.
1. Introduction
With increased awareness of immediate and late complications of head injuries in sport, in particular the proposed association between exposure to repetitive concussion and late neurodegenerative disease (Hay et al., 2016), there have been considerable efforts to reduce risk of injury and better manage concussions when they do arise (McCrory et al., 2013). Soccer (association football) is acknowledged as the most popular participation sport globally, with routine game-play in soccer involving intentional and repeated head impacts through heading the ball; a skill regularly included in training sessions and from a young age. Therefore, although rates of concussion are relative low in soccer compared to other contact sports such as rugby union or American football (Pfister et al., 2016), participation rates and the incidence of intentional sub-concussive impacts through heading in training and match play are such that the safety of heading in soccer has been questioned in some quarters (Patlak and Joy, 2002).
Though accepted as part of routine game-play, emerging evidence suggests exposure to repeated sub-concussive impacts in soccer may be associated with measurable changes in brain structure and function, and perhaps with late neurodegenerative disease. Rotational headers may prove of particular interest as they are often performed in training drills and matches (i.e. corner kicks) and are believed to be more injurious compared to linear accelerations (Cantu and Hyman, 2012). Imaging studies over the course of a season in active soccer players report evidence of white matter microstructural changes with associated impaired cognition (Lipton et al., 2013). Further, imaging of former professional soccer players aged 40–65 demonstrates evidence of cortical thinning, again with associated cognitive impairment (Koerte et al., 2015). Regarding longer term outcomes, recent identification of a form of dementia known as chronic traumatic encephalopathy (CTE) in athletes from a range of contact sports (for review see Smith et al., 2013) including soccer (Geddes et al., 1999, McKee et al., 2014) has drawn attention to the possibility that head impacts in soccer might be associated with increased risk of neurodegenerative disease.
Nevertheless, despite growing evidence on risks from the cumulative effects of sport-related head impacts and anxieties around the safety of ball-heading, little data exists demonstrating direct consequences of heading on brain function. Transcranial magnetic stimulation (TMS) can be used to assess a variety of indices of function in the brain to muscle pathway (Goodall et al., 2014). Further, TMS has demonstrated utility in quantifying electrophysiological changes in concussion (Major et al., 2015). The most consistent TMS marker of concussion (both acutely and longitudinally) appears to be corticomotor inhibition (Major et al., 2015, Pearce et al., 2015, Miller et al., 2014), expressed by a longer period of electromyographic silence (cortical silent period – cSP), after a motor evoked potential (MEP) is delivered to the primary motor cortex during contraction. Given the apparent high sensitivity to identify alterations in brain function, TMS could potentially be used to detect acute changes in brain function following sub-concussive head impacts. The relative novelty of TMS used in this context makes interpretation in terms clinically meaningful effects difficult, but its appeal is sensitivity in detecting direct brain changes (De Beaumont et al., 2007). It potentially highlights neurochemical changes that can be used to direct routes of investigation into the effects of sub-concussive impacts on the brain. Therefore, the aim was to study the use of TMS corticomotor inhibition in the lower limbs as primary outcome measure to detect acute changes in brain function from repetitive sub-concussive head impacts simulating routine soccer heading. We hypothesized that there would be a (transient) increase in corticomotor inhibition following a standardized bout of soccer heading, which may be accompanied by measurable changes in other established but less sensitive or less objective indexes of changes to brain function and brain injury as secondary outcome measures such as cognitive tests.
2. Methods
2.1. Approvals and Recruitment
The study was approved by the local Research Ethics Committee and procedures conformed to the guidelines set out by the Declaration of Helsinki. Written informed consent was obtained from all participants, prior to taking part. Twenty-three healthy, amateur football players (5 females; age 22 ± 3 y; weight 72.9 ± 8.3 kg; height 175.4 ± 10.2 cm) were recruited for study via advertisement on university noticeboards and meetings with local football clubs. Participants were excluded from taking part if they presented with any of the following: 1) history of brain injury resulting in loss of consciousness; 2) history of a neurological condition; 3) history of concussion in the 12 months prior to taking part; 4) family history of epilepsy; 5) use of psychoactive recreational or prescription drugs. Data from one participant could not be analyzed and three more participants withdrew from the study for personal reasons. The final cohort included a total of nineteen participants.
2.2. Study Design
Participants were asked to refrain from vigorous physical activity, consuming alcohol and caffeine or smoking for 24 h prior to each study session. Furthermore, participants were required to present to the laboratory fasted where they were provided with a standardized breakfast. At the first experimental session baseline measures for cognitive function, postural control, corticospinal excitability and corticomotor inhibition were recorded in this order; assessments thereafter recorded in the same order at each time point during study. Following baseline testing, participants underwent the heading protocol and then repeated the measures at 4 follow-up time points (taking measures in reverse order from the baseline order, starting with the TMS primary outcome measure); immediately post-heading and at 24 h, 48 h and 14 days following the heading protocol. The decision to include the 48 h follow-up was to assess the transient nature of the effects of heading, and the 14 day follow-up was intended as a time point at which complete “wash out” would have occurred following heading impact. Prior to commencement of study data collection participants attended the laboratory for a familiarization session, during which they completed all outcome measures to acquaint them with the assessment procedures and minimize later learning effects.
2.3. Heading Protocol
The heading protocol consisted of heading a standard football (400 g; 70 cm circumference; 8 psi) projected at a speed of 38·7 ± 2.1 kph from a football delivery device (JUGS sports, Tualatin, USA) positioned 6 m from participants, simulating routine soccer game-play (Haran et al., 2013, Broglio et al., 2004). Participants were instructed to perform a rotational header, redirecting the football perpendicularly to the initial trajectory, with each session consisting of 20 consecutive head impacts over a 10 min period, replicating typical heading practice. A custom-built accelerometer placed at the back of the participant's head recorded linear g-force of the head during impact. Ball speed was determined based on the participants' perceived ability to head the ball with a minimum speed of 30 kph and maximum speed of 50 kph.
2.4. Transcranial Magnetic Stimulation
Motor evoked potentials (MEPs) were elicited in the rectus femoris of the dominant leg via single pulse TMS and assessed using electromyographic (EMG) recordings (see below). Single magnetic stimuli of 1 ms duration where applied over the contralateral primary motor cortex using a magnetic stimulator (Magstim 2002 unit, The Magstim Company Ltd., Whitland, UK) and a 110 mm double cone coil. Optimal coil location for generating MEPs was determined by placing the coil over the motor cortex, laterally to the vertex; the area where the largest MEP peak-to-peak amplitudes occurred was identified and marked on the scalp with ink (Goodall et al., 2009). The active motor threshold for the quadriceps femoris was determined by increasing stimulator output from 10% by 5% increments, while the participant held a 10% maximal voluntary contraction (MVC) isometric contraction until discernible MEPs were visible (Wilson et al., 1995). Once this individual level was established, subsequent stimulations were delivered at 130% of active motor threshold.
MEPs, alongside all other EMG measures, were recorded with participants sitting with their dominant leg secured to a calibrated load cell of an isokinetic dynamometer (Kin-Com, Chattecx Corp, Chattanooga Group Inc., Tennessee). Knee angle was set at 60° (0° being fully extended limb) and the arm of the dynamometer was set such that the axis of rotation was aligned with the participant's lateral femoral condyle.
To assess the primary outcome measure corticomotor inhibition participants were required to perform maximal knee extensor voluntary contractions (MVCs) of 5 s duration while a single TMS stimulation was delivered over the motor cortex. This was repeated three times with a minute's rest between each contraction, as is common practice. Corticomotor inhibition was quantified as the cortical silent period (cSP) duration, taken from the stimulation artefact to the resumption of discernible, uninterrupted EMG activity from the muscle (Fig. 1). By measuring cSP at MVC, even though this limits the number of repetitions feasible, we ensure to recruit a motor unit pool large enough to show an effect. Measuring cSP at lower intensity may not be as sensitive since a smaller pool of motor units is recruited, reducing the relative effect size of GABA inhibitory mechanisms on the EMG signal. In turn, making the cSP measurements less sensitive in detecting subtle and transient cortico-spinal changes. During the assessment of secondary outcome measure corticospinal excitability, participants maintained a 20% MVC isometric contraction while 20 single TMS pulses, separated by 6 s, were delivered over the motor cortex. Corticospinal excitability was determined as the average MEP amplitude normalized to the maximal response elicited by motor nerve stimulation (%Mmax, see below). We chose to assess cortical excitability and inhibition in the lower limbs rather than in the upper limbs because of its functional relevance; in soccer, changes in lower limb may be more valid as they relate directly to gait and performance.
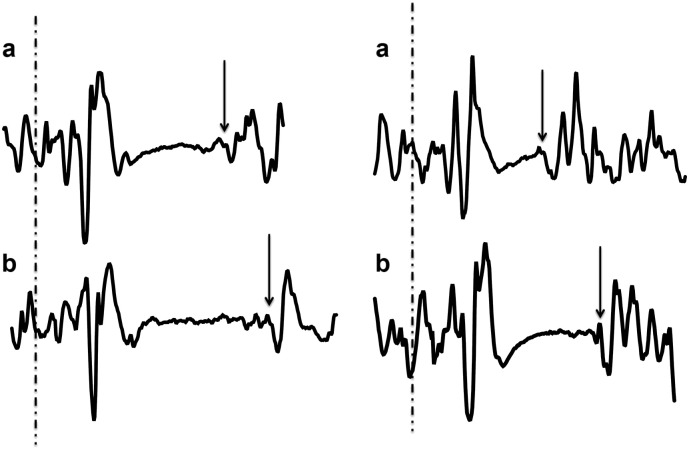
Fig. 1.
Snapshots of the cortical silent period (cSP) of two participants measured by TMS before (a) and immediately after heading (b) illustrating a typical lengthening in cSP immediately following heading. The cSP was quantified as the period of time between the delivered TMS pulse (dashed line) and the resumption of uninterrupted EMG activity (arrows).
2.5. Electromyography and Femoral Nerve Stimulation
Electromyographic activity was recorded using a wireless system (Biopac Systems, Inc. Goleta, CA, USA). Data were sampled at 2 kHz, and filtered using 500 Hz low and 1.0 Hz high band filters. Signals were analyzed offline (Acqknowledge, v3.9.1.6, Biopac Systems, Inc. Goleta, CA, USA). EMG activity was assessed using Ag/AgCl surface electrodes (Vermed, Devon, UK) with an intra-electrode distance of 2 cm positioned over rectus femoris; prior to electrode placement, the area of interest was shaved and abraded as per Surface Electromyography for the Non-Invasive Assessment of Muscles (SENIAM) guidelines. The position of each electrode was marked with permanent ink to ensure consistent placement during subsequent visits.
Peripheral stimulation of the femoral motor nerve was administered using an electrical stimulator (Biopac Systems, Inc.). The stimulation site was identified by locating the femoral artery and placing a self-adhesive surface electrode (cathode) lateral to it, high over the femoral triangle, with the anode on the buttock. Single stimuli were delivered to the muscle while participants maintained a 20% MVC isometric contraction, and the intensity of stimulation was increased until a plateau in twitch amplitude and rectus femoris M-wave (Mmax) occurred. Supramaximal stimulation was delivered by increasing the final stimulator output intensity by a further 30%.
2.6. Cognitive Function
Secondary outcome measure cognitive function was assessed in a quiet room using the Cambridge Neuropsychological Test Automated Battery (CANTAB), a computer based cognitive assessment tool and neuropsychological standard. The following CANTAB tasks were included: Reaction Time (RTI; divided attention); Paired Associate Learning (PAL; long-term memory); Spatial Working Memory (SWM; short-term memory); Attention Switching Task (AST; executive function); and Rapid Visual Processing (RVP; sustained attention).
2.7. Postural Control
Secondary outcome measure postural control was assessed using the Biodex Balance System SD (BBS; Biodex Medical Systems, Inc. New York, USA). Participants stood on a circular dynamic platform and average sway score was determined by measuring the degree of tilt on anterior-posterior and medial-lateral axes during three, 20 s trials (using dedicated Biodex software, v1.08, Biodex Inc.).
2.8. Statistical Analysis
Immediate post-heading responses were analyzed using a paired t-test, comparing measures before and immediately after the heading protocol. Recovery was analyzed for individual growth curves using the SPSS MIXED model with the restricted maximum likelihood method in keeping with standards for analysis of longitudinal data (Singer and Willett, 2003, Peugh and Enders, 2005). To achieve this, individual curves were analyzed to examine change over time. The following time points were included: immediate post, 24 h post, 48 h post, two weeks post. Data was checked for skewness and kurtosis and three cognitive measures (SWM, PAL, and RVP) and the balance variable were normalized using log transformation. The 95% lower and upper confidence intervals (CIs) were also calculated from difference of the mean values. Effect sizes (ES) were calculated for non-transformed differences using Cohen's d formula and were quantified as follows: 0.2 = small; 0.5 = medium; 0.8 = large. Statistical significance was set at p ≤ 0.05. Each measure was separate in relation to the hypotheses and therefore no correction for multiple comparisons was necessary. Data are expressed as means (± standard deviation) unless otherwise stated. The funding source had no input in the study other than suggesting at the design stage to add a full recovery assessment time-point.
3. Results
Overall, each participant performed 20 headers, achieving a mean force of impact of 13.1 ± 1.9 g (Table 1), with a coefficient of variance of 18%(± 3%).
Table 1.
Mean impact values for each individual recorded using a linear accelerometer. Data for 2 participants was not recorded due to hardware malfunction.
| Force of head impact (g) for each participant (mean ± SD) |
|---|
| 12.7 ± 2.02 |
| 11.9 ± 2.1 |
| 13.9 ± 2.1 |
| 15.3 ± 3.2 |
| 12.7 ± 2.1 |
| 14.6 ± 2.4 |
| 11.6 ± 2.6 |
| 11.3 ± 1.7 |
| 10.5 ± 1.8 |
| 12.3 ± 1.7 |
| 16.9 ± 4.0 |
| 11.7 ± 2.9 |
| 11.9 ± 2.1 |
| 12.3 ± 2.6 |
| 11.8 ± 1.9 |
| 14.6 ± 2.5 |
| 16.7 ± 4 |
3.1. Effect of Heading on Corticomotor Inhibition and Corticospinal Excitability
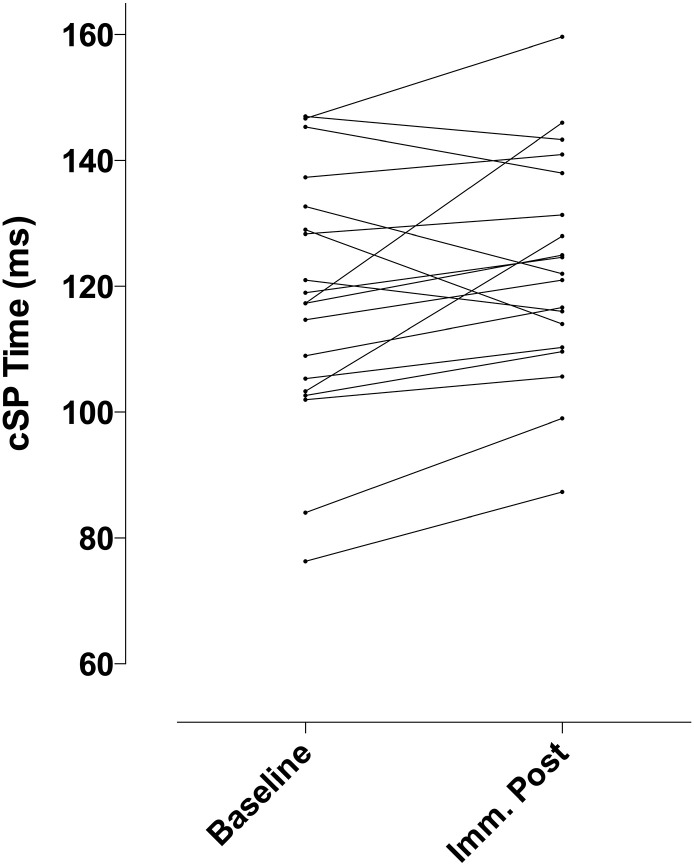
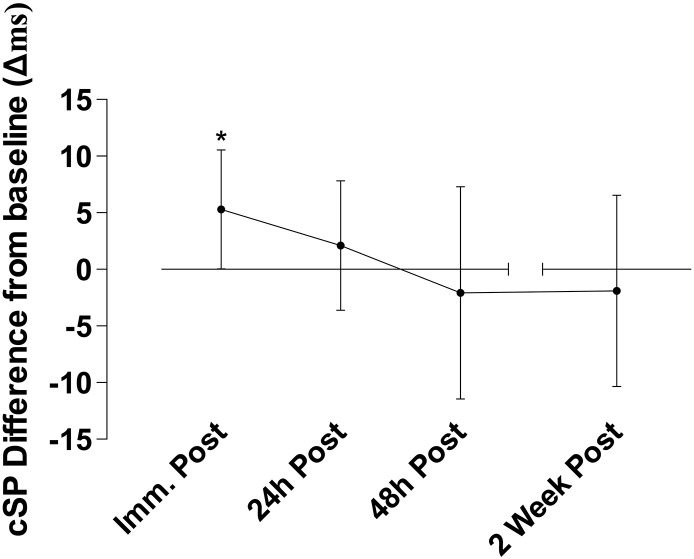
Immediately after ball heading there was a measurable increase in the primary outcome measure cSP within 74% (14 out of 19) of participants (Fig. 2). The cSP duration increased from 117.8(± 19.8)ms at baseline to 123.1(± 17.6)ms (t(18) = − 2.11, p = 0.049; ES 0.28), representing an average increase of 5.4(± 4.8)% in cSP duration, compatible with increased corticomotor inhibition. This increase in cSP proved transient with apparent normalization to baseline in subsequent follow-up assessments at 24 h, 48 h and 14 days (F(1,18) = 4.23, p = 0.04) (Fig. 3). There was a moderate, but not significant, relationship between these acute increases in cSP and g-force on impact with the ball (r = 0.37, p = 0.07 one-tailed).
Fig. 2.
Change in cortical silent period (cSP) duration for each participant from baseline to immediately following the heading protocol.
Fig. 3.
Difference in cSP in ms after heading relative to baseline. Immediately after heading cSP duration increased on average by 5.3(± 5.7)ms (*p < 0.05) which within participants is an 5.4(± 4.8)% average increase from baseline values. This increase detectable immediately after heading normalized over the four follow-up timepoints (p < 0.05) with values apparently returning to baseline level. Error bars indicate the 95% confidence intervals.
To determine the reproducibility of the primary outcome measure the intraclass correlation coefficient (ICC) was calculated between the baseline and the 2 weeks post-measure. ICC for the two measures was 0.764, suggesting excellent agreement (Cicchetti, 1994). Furthermore, no within-participant differences were found between the two time points using a paired t-test (t(18) = − 0.47, p < 0.63; ES 0.09; CI − 10.36 to 6.53).
No changes were found on the secondary TMS outcome measure corticospinal excitability; MEP amplitude demonstrated no change in the acute phase immediately after ball heading, nor in the follow-up assessment time-points (Table 2). There was no notable change in knee extensor MVC after heading the ball (Table 2), suggesting the participants did not experience significant muscular fatigue that might interfere with TMS measurement.
Table 2.
Mean (standard deviation) values for each of the outcome measures: corticomotor inhibition (cortical silent period in ms) and corticospinal excitability (MEP amplitude normalized to femoral nerve M-wave, %Mmax), Spatial Working Memory (SWM errors), Paired Associate Learning (PAL errors), Rapid Visual Processing (RVP A′ score), Attention Shifting Task (AST median corrected latency), Reaction Time (Choice RTI decision times) and Postural control (Balance, SI stability index deviation from the horizontal baseline) measured at each time point, and 95% lower and upper confidence intervals (CIs) for the difference in means before and immediately after heading.
*p < 0.05 Baseline v Imm. Post; §p < 0.05 change over time growth curve analysis.
| Variable | Assessment time post-heading exposure |
|||||
|---|---|---|---|---|---|---|
| Baseline | Immediately | 24 h | 48 h | 2 weeks | ∆ mean Pre v Imm Post (95% CI) | |
| TMS | ||||||
| Inhibition (ms) | 117.8 ± 19.8 | 123.0 ± 17.6* | 119.9 ± 19.8 | 115.7 ± 20.6 | 115.9 ± 19.7§ | 5.28 (0.017 to 10.54) |
| Excitability (%Mmax) | 44.1 ± 20.6 | 47.4 ± 22.3 | 47.9 ± 24.0 | 44.4 ± 22.5 | 46.5 ± 22.1 | 3.34 (− 5.03 to 11.72) |
| Cognitive function | ||||||
| SWM (log_errors) | 0.79 ± 0.59 | 1 ± 0.51* | 0.77 ± 0.62 | 0.72 ± 0.57 | 0.69 ± 0.57§ | 0.2 (0.016 to 0.40) |
| PAL (log_errors) | 0.38 ± 0.41 | 0.65 ± 0.29* | 0.49 ± 0.32 | 0.51 ± 0.32 | 0.35 ± 0.32§ | 0.26 (0.08 to 0.44) |
| RVP A′ | 0.952 ± 0.052 | 0.959 ± 0.040 | 0.958 ± 0.044 | 0.971 ± 0.028 | 0.962 ± 0.038 | 0.0007 (− 0.005 to 0.021) |
| AST (ms) | 396 ± 58 | 376 ± 67* | 369 ± 64 | 370 ± 66 | 373 ± 82 | − 19.11 (− 35.01 to − 3.20) |
| RTI (ms) | 295 ± 29 | 301 ± 35 | 295 ± 33 | 297 ± 32 | 297 ± 31 | 6 (− 6.13 to 19.24) |
| Postural control | ||||||
| Balance (SI) | 0.76 ± 0.36 | 0.71 ± 0.21 | 0.67 ± 0.23 | 0.63 ± 0.25 | 0.72 ± 0.18 | − 0.06842 (− 0.164 to 0.028) |
3.2. Altered Cognitive Function Following Heading
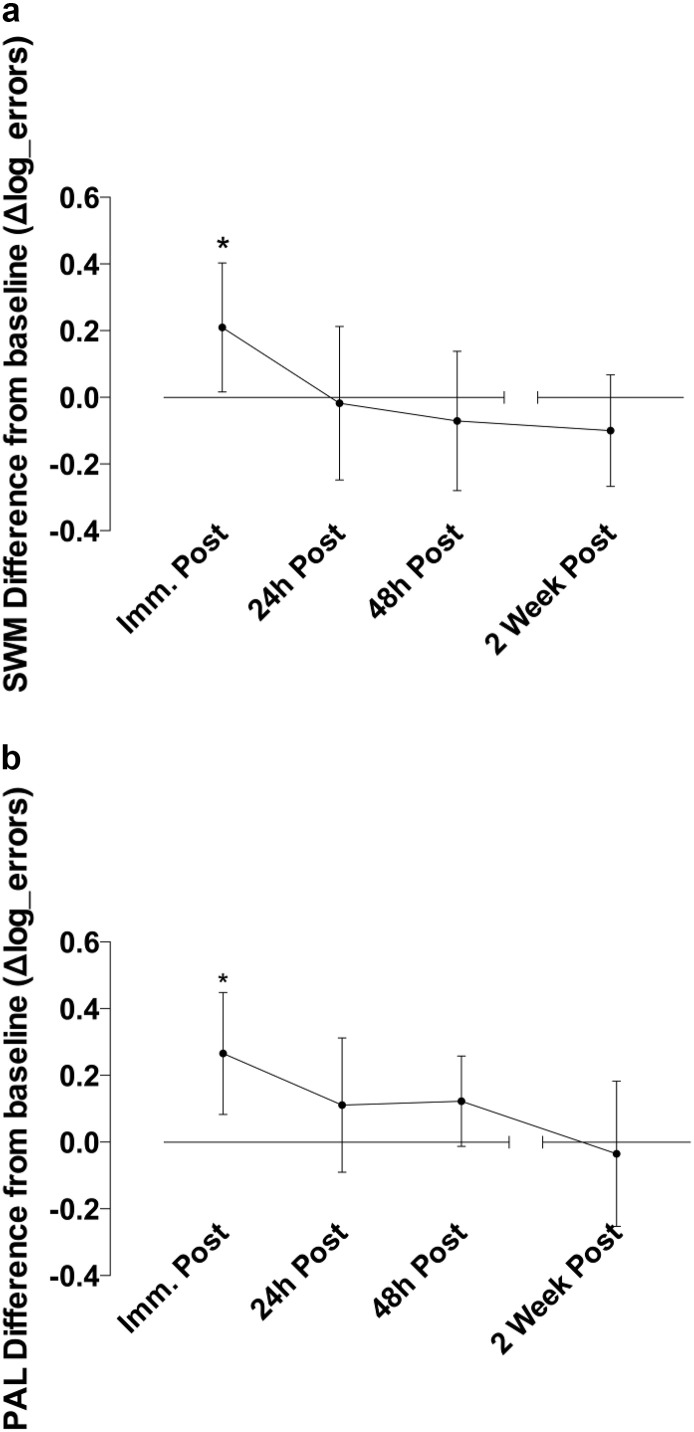
Immediately after the heading protocol there was a reduced performance compared to baseline in two CANTAB sub-tasks assessing accuracy on different aspects of memory. Specifically, Spatial Working Memory (SWM) error scores were significantly higher (t(18) = − 2.28, p = 0.03, ES 0.3) immediately after the heading protocol, compatible with impairment in short-term memory (Fig. 4a). Furthermore, total adjusted error score on the Paired Associated Learning (PAL) task immediately after heading increased by 67% (t(18) = − 3.05, p = 0.007, ES 0.5), compatible with a reduced long-term memory function (Fig. 4b). These disturbances in short- and long-term memory proved transient, with normalization to baseline performance in SWM (F(1,18) = 10.28, p = 0.002) and PAL (F(1,18) = 11.14, p = 0.002) in the subsequent follow-up assessments at 24 h, 48 h and 14 days (Table 2).
Fig. 4.
Difference in memory performance (log transformed error score difference) after heading relative to baseline. Immediately after heading, errors were higher compared to baseline on both the (a) Spatial Working Memory SWM (*p < 0.05) and (b) Paired Associated Learning PAL (*p < 0.01) tasks. This increase evident immediately after heading normalized over the four follow-up timepoints (p < 0.01) with error scores apparently returning to baseline level. Error bars indicate the 95% confidence intervals.
Heading only significantly affected memory function; the remaining CANTAB tasks assessing aspects of attention and processing speed did not show significant heading-associated decrements compared to baseline assessments (Table 2). No change was detected on the Rapid Visual Processing task, with RVP A′ scores close to ceiling/maximum, making the measure insensitive to change. There was a marginal improvement on the median corrected latency scores of the executive function Attention Shifting Task (t(18) = 2.52, p = 0.021), possibly due to practice. On the Choice RTI measure there was no effect of heading on decision times (t(18) = 0.69, p = 0.5) (Table 2).
4. Discussion
Following a standardized session of football heading designed to simulate routine soccer practice our data demonstrate immediate alterations in brain electrophysiological and cognitive function compared to baseline assessments in a cohort of healthy, young soccer players. Specifically, using TMS we found a measurable increase in cortical silent period (cSP) after just 20 consecutive headers. Furthermore, in cognitive assessments, our data demonstrate evidence of decreases in measures of both short- and long-term memory immediately following heading. Notably, in this single exposure paradigm, these alterations in brain corticomotor inhibition and cognitive function appeared short-lived; the effects apparently normalizing in follow-up assessments from 24 h onwards. In contrast to previous studies in athletes and patients with confirmed concussion or mild TBI (De Beaumont et al., 2007, Chistyakov et al., 2001, Bernabeu et al., 2009, Livingston et al., 2010) these novel observations demonstrate, for the first time, detectable alterations in brain function in footballers exposed to ‘routine’ head impacts not associated with clinically recognizable brain injury.
The prolonged silent period of neuromuscular recruitment found in this study is a sign of increased inhibition in the motor system and is thought to reflect GABA activity (Inghilleri et al., 1993, McDonnell et al., 2006) which is the most powerful inhibitor in the motor system. Although the mechanisms behind corticomotor inhibition are not fully understood (Chen et al., 1999), increased inhibition following repeated sub-concussive head impact may reflect protective mechanisms against minor injury. What is a concern however is that such protective mechanisms could become maladaptive when stimulated repeatedly, as occurs during soccer heading practice. Albeit apparently transient, the acute increases in corticomotor inhibition following football heading could trigger a pathological process damaging brain health through the accumulative effect of sub-concussive head impact. Increased corticomotor inhibition silent period has been found to be associated with pathophysiology in brain damage suggesting a link between functional deficits and hyperactivity of cortical inhibitory interneurons (Classen et al., 1997). Further study into the dynamic metabolic processes as a direct result of soccer heading is required. When we understand the complex interplay between functional, metabolic, and structural brain changes following repeated sub-concussive head impact, we can establish the link to accumulative and long-term consequences. At present, the current findings at least suggest acute brain changes occur as a direct consequence of soccer heading.
As well as increased corticomotor inhibition, parameters of memory function were altered following the heading protocol, consistent with a recent report of a relationship between memory function and history of heading in soccer (Lipton et al., 2013). Furthermore, a study of retired Australian Rules footballers found that elite players performed more poorly on the Paired Associate Learning test than amateurs (Pearce et al., 2014). Practical limitations of cognitive-based tests to detect impairment in athletes are due to reliability: in high performance sports athletes have been recognized to purposely produce low baseline performances on cognitive tests to allow them to avoid removal from play, or to reduce return to play intervals (Erdal, 2012).
For completeness postural control (balance) was included as a secondary, albeit indirect, outcome measure as concussion has been shown to result in impaired balance (McCrory et al., 2013, Powers et al., 2014), yet the participants in the current study were able to maintain their balance despite an increased level of corticomotor inhibition. And while one study has shown a decrease in postural control following bouts of soccer heading (Haran et al., 2013), another study has not (Broglio et al., 2004); and now our own show no change in postural control.
Secondary TMS outcome measure cortical excitability has previously been shown to decrease following TBI (De Beaumont et al., 2007, Chistyakov et al., 2001, Bernabeu et al., 2009, Livingston et al., 2010), yet we demonstrated no such change following ball heading. The reason why changes were seen in cortical inhibition and not cortical excitability may be due to the different levels of muscle contractile force applied during recording of the two parameters (20% MVC for excitability vs. 100% MVC for inhibition, see the introduction for its justification). Furthermore, it should be noted that measuring cortical excitability is a less straightforward procedure than corticomotor inhibition as it requires MEP normalization to maximal motor nerve response (Goodall et al., 2009). Primary outcome measure TMS corticomotor inhibition was thought to be most sensitive to quantifying electrophysiological changes based on a recent systematic review (Major et al., 2015), and is a direct measure of changes to brain function.
Future work should include a control activity, such as body movement without head impact. However, the current pattern of results leaves little doubt that the changes in brain function were related to head impact rather than physical activity. The force of maximal knee contraction was not reduced after heading, therefore the absence of a physical exercise control group it is highly unlikely to explain the effect on corticomotor inhibition or memory function. Nevertheless, a future extension of this work can focus on the acute effects of heading now that the transience of the effect has been established, and would be well placed to reveal the mechanisms underlying these brain changes through a cross-over design that includes a control activity. Furthermore, because it is likely that sub-concussive impacts are more general in nature (i.e. they do not affect single muscles) future work should assess corticomotor inhibition in a larger number of muscles, possibly encompassing both upper and lower limbs. Further study into the dynamic metabolic processes as a direct result of soccer heading is required. Implementing the use of magnetic resonance spectroscopy in future studies could help determine short-term alterations in GABA and glutamate responses. With regard to changes in GABA, because of the use of single-pulse TMS in this study, we were only able to report on the activity of GABAB, while the use of paired-pulse TMS in future work can distinguish modulation of GABAA and GABAB. Critically, however, the sensitivity of the current primary outcome measure suggests that corticomotor inhibition through future dose-response studies has the potential to provide the evidence-base to guide safe engagement in contact-sports, such as soccer.
5. Conclusion
The current study is the first to show direct evidence for acute changes to corticomotor function and changes to memory function following routine soccer heading. It is furthermore the first study to show that corticomotor inhibition, measured by TMS, is able to detect acute transient changes in brain function following sub-concussive head impacts. And although the magnitude of the acute changes observed was small, it is the presence of the effect that is of interest. This measure was previously shown to be altered in confirmed concussion, but the acute changes in corticomotor inhibition, accompanied by cognitive changes, following the sub-concussive impact of football heading raise concerns that this practice, routine in soccer, may affect brain health.
Funding and Acknowledgements
This work was supported by the National Institute for Health Research (NIHR) Brain Injury Healthcare Technology Cooperative. This work was supported by existing funding awarded to L.W. as part of Framework 7 programme of the European Union (CENTER-TBI, Grant number: 602150-2). The work made use of a TMS coil to which the company Smartfish contributed £1500 for purchase of. T.DiV.'s postgraduate study is support by the research office of Stirling University. W.S. is supported by a NHS Research Scotland Career Researcher Fellowship. D.I.D. and M.I. are members of SINAPSE – see www.sinapse.ac.uk.
The funders had no input in the conception or design of the study, other than NIHR suggesting, at the design stage, to add a full recovery assessment time-point. The funders also had no input in the interpretation or presentation of the results.
Declaration
None of the authors have a competing interest to declare.
Author Contributions
TDV, A.H., L.W., W.S., D.I.D, and M.I. conceived of the study, TDV, A.H., L.W., W.S., S.G., G.H., D.I.D, and M.I. designed the study; TDV executed the study under the guidance of A.H., L.W., S.G., G.H., and M.I.; TDV analyzed data; TDV, A.H., L.W., W.S., S.G., G.H., D.I.D, and M.I. interpreted the results; TDV prepared figures; TDV, A.H. and M.I. drafted manuscript; TDV, A.H., L.W., W.S., S.G., G.H., D.I.D, and M.I. edited and revised manuscript; TDV, A.H., L.W., W.S., S.G., G.H., D.I.D, and M.I. approved final version of manuscript.
References
- Bernabeu M., Demirtas-Tatlidede A., Opisso E. Abnormal corticospinal excitability in traumatic diffuse axonal brain injury. J. Neurotrauma. 2009;26:2185–2193. doi: 10.1089/neu.2008.0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglio S.P., Guskiewicz K.M., Sell T.C. No acute changes in postural control after soccer heading. Br. J. Sports Med. 2004;38:561–567. doi: 10.1136/bjsm.2003.004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantu R.C., Hyman M. Houghton Mifflin Harcourt; Boston: 2012. Concussions and Our Kids: America's Leading Expert on How to Protect Young Athletes and Keep Sports Safe. [Google Scholar]
- Chen R., Lozano A.M., Ashby P. Mechanisms of the silent period following transcranial magnetic stimulation. Exp. Brain Res. 1999;128:539–542. doi: 10.1007/s002210050878. [DOI] [PubMed] [Google Scholar]
- Chistyakov A.V., Soustiel J.F., Hafner H. Excitatory and inhibitory corticospinal responses to transcranial magnetic stimulation in patients with minor to moderate head injury. J. Neurol. Neurosurg. Psychiatry. 2001;70:580–587. doi: 10.1136/jnnp.70.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D.V. Guidelines, criteria and rules of thumb for evuating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994;6(4):284–290. [Google Scholar]
- Classen J., Schnitzler A., Binkofski F. The motor syndrome associated with exaggerated inhibition within the primary motor cortex of patients with hemiparetic. Brain. 1997;120:605–619. doi: 10.1093/brain/120.4.605. [DOI] [PubMed] [Google Scholar]
- De Beaumont L., Lassonde M., Leclerc S. Long-term and cumulative effects of sports concussion on motor cortex inhibition. Neurosurgery. 2007;61(2):329–336. doi: 10.1227/01.NEU.0000280000.03578.B6. [DOI] [PubMed] [Google Scholar]
- Erdal K. Neuropsychological testing for sports related concussion: how athletes can sandbag their baseline testing without detection. Arch. Clin. Neuropsychol. 2012;27:473–479. doi: 10.1093/arclin/acs050. [DOI] [PubMed] [Google Scholar]
- Geddes J.F., Vowles G.H., Nicoll J.A., Revesz T. Neuronal cytoskeletal changes are an early consequence of repetitive head injury. Acta Neuropathol. 1999;98:171–178. doi: 10.1007/s004010051066. [DOI] [PubMed] [Google Scholar]
- Goodall S., Romer L.M., Ross E.Z. Voluntary activation of human knee extensors measured using transcranial magnetic stimulation. Exp. Physiol. 2009;94(9):995–1004. doi: 10.1113/expphysiol.2009.047902. [DOI] [PubMed] [Google Scholar]
- Goodall S., Howatson G., Romer L.M. Transcranial magnetic stimulation in sport science: a commentary. Eur J Sport Sci. 2014;14:332–340. doi: 10.1080/17461391.2012.704079. [DOI] [PubMed] [Google Scholar]
- Haran F.J., Tierney R., Wright W.G. Acute changes in postural control after soccer heading. Int. J. Sports Med. 2013;34(4):350–354. doi: 10.1055/s-0032-1304647. [DOI] [PubMed] [Google Scholar]
- Hay J., Johnson V.E., Smith D.H., Stewart W. Chronic traumatic encephalopathy: the neuropathological legacy of traumatic brain injury. Annu. Rev. Pathol.: Mech. Dis. 2016;11:21–45. doi: 10.1146/annurev-pathol-012615-044116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M., Berardelli A., Cruccu G. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J. Physiol. 1993;466:521–534. [PMC free article] [PubMed] [Google Scholar]
- Koerte I.K., Lin A.P., Willems A. A review of neuroimaging findings in repetitive brain trauma. Brain Pathol. 2015;25:318–349. doi: 10.1111/bpa.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton M.L., Kim N., Zimmerman M.E. Soccer heading is associated with white matter microstructural changes and cognitive abnormalities. Radiology. 2013;268(3):850–857. doi: 10.1148/radiol.13130545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston S.C., Saliba E.N., Goodkin H.P. A preliminary investigation of motor evoked potential abnormalities following a sport-related concussion. Brain Inj. 2010;24(6):904–913. doi: 10.3109/02699051003789245. [DOI] [PubMed] [Google Scholar]
- Major B.P., Rogers M.A., Pearce A.J. Using transcranial magnetic stimulation to quantify electrophysiological changes following concussive brain injury: a systematic review. Clin. Exp. Pharmacol. Physiol. 2015;42:394–405. doi: 10.1111/1440-1681.12363. [DOI] [PubMed] [Google Scholar]
- McCrory P., Meeuwisse W.H., Aubry M. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br. J. Sports Med. 2013;47:250–258. doi: 10.1136/bjsports-2013-092313. [DOI] [PubMed] [Google Scholar]
- McDonnell M.N., Orekhov Y., Ziemann U. The role of GABAb receptors in intracortical inhibition in the human motor cortex. Exp. Brain Res. 2006;173:86–93. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- McKee A.C., Daneshvar D.H., Alvarez V.E. The neuropathology of sport. Acta Neuropathol. 2014;127:29–51. doi: 10.1007/s00401-013-1230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N.R., Yasen A.L., Maynard L.F., Chou L., Howell D., Christie A.D. Acute and longitudinal changes in motor cortex function following mild traumatic brain injury. Brain Inj. 2014;28(10):1270–1276. doi: 10.3109/02699052.2014.915987. [DOI] [PubMed] [Google Scholar]
- Patlak M., Joy J.E. Is Soccer Bad for Children's Heads? Summary of the IOM Workshop on Neuropsychological Consequences of Head Impact in Youth Soccer. National Academy Press; Washington DC: 2002. Board on neuroscience and behavioural health. [PubMed] [Google Scholar]
- Pearce A.J., Hoy K., Rogers M.A., Corp D.T., Maller J.J., Drury H.G., Fitzgerald P.B. The long-term effects of sports concussion on retired Australian football players: a study using transcranial magnetic stimulation. J. Neurotrauma. 2014;31:1139–1145. doi: 10.1089/neu.2013.3219. [DOI] [PubMed] [Google Scholar]
- Pearce A.J., Hoy K., Rogers M.A. Acute motor, neurocognitive and neurophysiological change following concussion injury in Australian amateur football. J. Sci. Med. Sport. 2015;18:500–516. doi: 10.1016/j.jsams.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Peugh J.L., Enders C.K. Using the SPSS Mixed procedure to fit hierarchical linear and growth trajectory models. Educ. Psychol. Meas. 2005;65:811–835. [Google Scholar]
- Pfister T., Pfister K., Hagel B. The incidence of concussion in youth sports: a systematic review and meta-analysis. Br. J. Sports Med. 2016;50(5):292–297. doi: 10.1136/bjsports-2015-094978. [DOI] [PubMed] [Google Scholar]
- Powers K.C., Kalmar J.M., Cinelli M.E. Recovery of static stability following a concussion. Gait & Posture. 2014;39:611–614. doi: 10.1016/j.gaitpost.2013.05.026. [DOI] [PubMed] [Google Scholar]
- Singer J.D., Willett J.B. Oxford University Press; New York: 2003. Applied Longitudinal Data Analysis: Modelling Change and Event Occurrence. [Google Scholar]
- Smith D.H., Johnson V.E., Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat. Rev. Neurol. 2013;9:211–221. doi: 10.1038/nrneurol.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S.A., Thickbroom G.W., Mastaglia F.L. Comparison of the magnetically mapped corticomotor representation of a muscle at rest and during low-level voluntary contraction. Electroencephalogr. Clin. Neurophysiol. 1995;97(5):246–250. doi: 10.1016/0013-4694(95)00052-z. [DOI] [PubMed] [Google Scholar]