Abstract
Objective
To examine the degree to which non-medical factors explain additional variance in parent proxy-report and child self-report health related quality of life (HRQOL) among newly diagnosed children with JIA after accounting for medical factors.
Method
Parents (of children ages 2–16 years; n = 230) and patients (>5 years; n = 180), diagnosed within the previous 6 months completed surveys to assess medical (clinical parameters, functional status) and non-medical (self-efficacy, coping, barriers to adherence, social support, parental distress, access to care) factors and HRQOL (PedsQL Generic Core Scales). Physician-rated global assessment of disease activity, active joint count, and select laboratory variables (rheumatoid factor, antinuclear antibodies and erythrocyte sedimentation rate) were recorded.
Results
Non-medical factors, including self-efficacy, coping with pain, barriers to adherence, social support and parental distress, explained additional variance in HRQOL Total, Physical Functioning, and Psychosocial Functioning scales (R2 increase of 6%, 1%, 13% for parent proxy-report and 16%, 7%, and 30% for self-report). Parental distress was uniquely associated with parent proxy-report HRQOL, while child self-efficacy and social support were uniquely associated with self-report HRQOL.
Conclusion
Non-medical factors are associated with HRQOL in newly diagnosed patients with JIA, after accounting for medical variables, particularly for psychosocial functioning.
Health-related quality of life (HRQOL), an individual’s perceptions of their physical, psychosocial, and role functioning with respect to their health, is a key outcome in clinical care and clinical trials for children with Juvenile Idiopathic Arthritis (JIA), the most common pediatric autoimmune disease affecting the musculoskeletal organ system. The importance of HRQOL as a primary outcome is echoed by the Food and Drug Administration (1, 2), the Centers for Disease Control and Prevention (3), and the World Health Organization (WHO)(4). Implicit in the measurement of HRQOL as a JIA outcome is the notion that medical interventions, such as drug therapies, can affect not only clinical parameters (pain, joint count, and functional status), but also more distal outcomes, such as HRQOL.
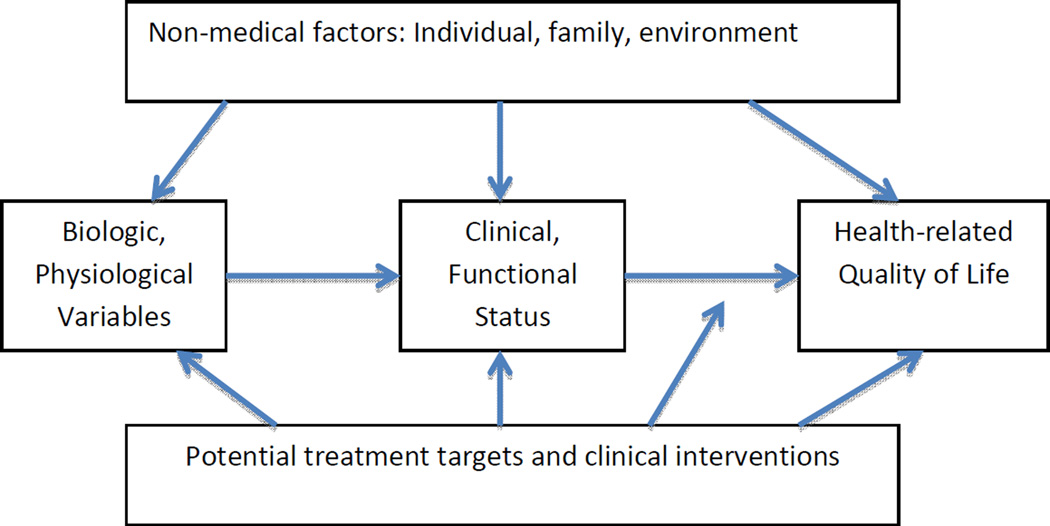
Early research examined the etiologic role of psychosocial factors in juvenile arthritis (5). Varni and colleagues, in the mid-1980’s, were among the first to document the effect of clinical and psychosocial factors on children’s adjustment to juvenile arthritis and to put forth a conceptual framework linking both clinical and psychosocial factors to HRQOL (6, 7). Wilson and Cleary (8) further specified a causal pathway from physiological variables to clinical status to functional outcomes and thus to HRQOL. Figure 1, based on these models, posits a causal cascade from biological and physiological variables to clinical status and functional status to HRQOL. Figure 1 also suggests a causal role, both direct and indirect, for non-medical variables on HRQOL and, potentially, an expanded set of targets for clinical intervention.
Fig 1.
The square partial correlation for each domain is adjusted for the preceding domain of variables. Figure 1: Conceptual framework showing causal cascade of medical factors affecting health-related quality of life, the hypothesized non-medical factors, and potential treatment targets and clinical interventions. Adopted from Varni JW, Wilcox KT, Hanson V (1988) and Wilson and Cleary (1995).
While much research has focused on understanding how biological and physiological variables affect clinical outcomes, far less is known about how clinical parameters are related to HRQOL in JIA patients and how characteristics of the child, family, or environment affect HRQOL in the context of ongoing treatment. Lack of such knowledge is an important problem. Researchers have documented worse HRQOL for children with JIA compared to healthy controls (9–11) and have found that, even with excellent disease control through the use of biologic medications, about 50% of children with polyarticular JIA continue to have a lower HRQOL than healthy children (12). This finding suggests that without a thorough understanding of the determinants of HRQOL and identification of potential modifiers, many children with JIA, even when treated with biologics and despite well-controlled arthritis, will continue to experience sub-optimal HRQOL.
Given the growing understanding of HRQOL as an important outcome, the traditional focus of therapeutics on medical outcomes, and the relative lack of knowledge regarding how clinical parameters and therapeutic interventions affect HRQOL in children with JIA, there is a need to understand parent and child perceptions of HRQOL and their relationship to medical and non-medical factors. We therefore examine the degree to which medical variables (biological, physiological, clinical, physical function) and non-medical characteristics of the child (coping, self-efficacy, social support, adherence), family (parental distress, family climate), and environment (socioeconomic status, access to care) relate to HRQOL in newly diagnosed children with JIA. We hypothesized that both medical and non-medical variables will be significantly associated with patient HRQOL.
Methods
Participants
In this study we considered reports from children with JIA and their primary caregivers. Eligible index patients were male or female patients, ages 2 to 16 years of any race or ethnicity who had been diagnosed within the last 6 months with any type of JIA as per the International League of Associations for Rheumatology (ILAR)(13), irrespective of the specific JIA category. Patients who carried the diagnosis of a medical condition that would otherwise severely impair their HRQOL (e.g., cerebral palsy, spina bifida, severe mental retardation, fibromyalgia) were excluded from study participation. All participants gave assent or informed consent to participate in the study.
Study Design
The study was a prospective new onset patient cohort study design. Two centers were involved in this study, Cincinnati Children’s Hospital Rheumatology clinic and the University of Louisville Pediatric Rheumatology clinic. Participants were recruited from October 2008 through August 2012. During the study period, consecutive patients eligible for the study were approached as soon as a diagnosis of JIA was established. The study was reviewed and approved by the Institutional Review Boards of each of the two participating centers: Cincinnati Children’s Hospital Medical Center and University of Louisville.
Measures: Explanatory Variables
Biological and physiological variables
Biological variables included age, gender, race, and disease duration with JIA. Physiological variables considered were rheumatoid factor (RF; positive/negative), antinuclear antibodies (ANA; positive/negative), and erythrocyte sedimentation rate (ESR; normal range: 0–10 mm/hr), and JIA category (systemic JIA; polyarticular RF negative JIA, polyarticular RF positive, extended oligoarticular JIA, oligoarticular JIA, psoriatic JIA; undifferentiated JIA), active joint count, and number of joints with limited range of motion (LROM).
Clinical and functional status
Clinical variables included proxy- and self-reported patient pain, measured using a visual analog scale (anchors: 0 = no pain, 10 = very severe pain) (24) and the PedsQL Rheumatology module (11, 14, 15) Pain Subscale (scaled 0–100 with higher value indicating less pain). We also measured overall physician global assessment of disease activity (anchors: 0 = inactive disease, 10 = very active disease). Physical function was measured using the PedsQL Rheumatology module (11, 15, 16) Functioning Subscale also scaled 0–100 with higher value indicating better functioning and the revised version of the Childhood Health Assessment Questionnaire (CHAQ) disability index (not including aides and help items) (16, 17). This index is calculated as the unweighted average of 30 questions in 8 domains covering major aspects of daily living over a one-week period and yields a score between 0 (no disability) and 3 (most severe disability).
Non-medical characteristics of the child
Non-medical characteristics of the child are assessed by age appropriate questionnaires completed by the child or, for younger children, by their parents..
Coping was measured using the Waldron/Varni Pediatric Pain Coping Inventory (PPCI) (18). The PPCI is a 41-item measure used to identify strategies children use to cope with pain. Respondents are asked to rate, on a three-point response scale, how frequently coping takes the form of: 1) cognitive self-instruction, 2) seek social support, 3) strive to rest and be alone, 4) cognitive refocusing, and 5) problem-solving/self-efficacy. Higher scores indicate more frequent use of the particular coping strategy. The PPCI was completed by children ages 5 and older and by parents.
Self-efficacy was measured using the Children’s Arthritis Self-Efficacy Scale (CASE) (19). The CASE is an 11-item scale that measures children’s self-efficacy with respect to managing symptoms, emotional consequences, and activities, each with score ranges from 0 to 4, with higher scores indicating more self-efficacy. Although validated for children ages 7–17 years, we used it with children ages 5–17 years.
Social support was measured using the Harter Social Support Scale for Children (SSSC) (20). The 21-item SSSC was designed to assess social support from parents, classmates, teachers and friends, with each score ranging from 1 to 4, with higher value indicating stronger social support. The SSSC has been shown to moderate the effect of stress (daily hassles) on depression in children with rheumatic disease (21). It was completed by participants aged 5–16 years.
Adherence was measured via the Medication Adherence Self-Report Inventory (MASRI). The 5-item MASRI is validated for pediatric rheumatic (22) and other chronic disease (23). Parents of children ages 5 to 11 years and patients 12 to 16 years completed the MASRI. In addition, we used the PedsQL Rheumatology module (15) Treatment Problems Subscale (0–100, with higher value indicating fewer barriers) to measure barriers to adherence.
Non-medical characteristics of the family
The primary caregiver reported on non-medical characteristics of the family. Parental distress was measured using the Symptom Checklist 10 (SCL-10), a 10-item self-report measure of psychological symptoms rated on a 5-point Likert scale (24). The SCL-10 ranges between 0–4, with higher value indicating higher stress. Family climate was assessed via the Family Environment Scale (FES). The FES (25) consists of 90 items rated as “true” or “false” to assess family climate. In this study, the Family Relationship Index subscore was used due to its extensive use in previous research (26). The FES yields a standardized T-score centered at 50 with standard deviation of 10.
Non-medical characteristics of the socioeconomic and financial environment
To measure socioeconomic status (SES), mother’s education level, ZIP code, and insurance type (private or publicly financed) were used as proxies. Financial access to care was measured by parents’ report of whether their child has health insurance and parents’ report of the degree to which cost was a problem (i.e., ‘a big problem,’ ‘a small problem,’ ‘not a problem.’) (27). Realized access was measured through parents’ reports of foregone care (28), - any time when the child should get medical care, but did not.
Response Variable: Health-related quality of life (HRQOL)
HRQOL was measured using the Pediatric Quality of Life Inventory (PedsQL) Generic Core Scales (29). The PedsQL consists of parallel forms for children (ages 5–18 years) to report on their own HRQOL and for parents of children 2–18 years to report on their child’s HRQOL. We measured the Total Scale, Physical Functioning Subscale and Psychosocial Functioning Subscale scores. Scale scores range from 0–100 with higher scores indicating better HRQOL.
Procedures
Upon consent, the participants aged 5–16 completed the CASE, SSSC, PPCI, PedsQL, and PedsQL rheumatology module. Participant aged 12–16 also completed the MASRI survey. Children unable to complete the survey by themselves were read the surveys by the research coordinator or a parent. Accompanying parents or guardians completed the PPCI, PedsQL, PedsQL Rheumatology module, FES, SCL-10, CHAQ, and the non-medical characteristics for children aged 2–16 and completed the MASRI for children aged 2–11. The biological, physiological and clinical function data from the same visit were obtained from the clinical record.
Statistical Analyses
The goal of the study was to examine the effect of nonmedical factors, after accounting for medical factors and functional status, on HRQOL in newly diagnosed children with JIA. The primary outcome is PedsQL Total score, and secondary outcomes are PedsQL Physical and Psychosocial subscale scores. Examination of the distributions of the study outcomes showed reasonable fit with a normal distribution assumption. We ran parallel linear regression analyses for child self-report and parent proxy-report HRQOL. In the first stage (Table 3), multivariate linear regression analyses were performed for each set of explanatory variables in the conceptual framework (Figure 1) separately (i.e. biological, physiological, clinical, functional status, child, family, and environmental variables). From each of these sets of explanatory variables, only those that remained statistically significant were selected and entered into the next stage of analyses. The rationale for this first stage of analyses is that it allows us to identify the strongest independent variables to represent a set of potentially correlated variables within the same domain. At the second stage (Table 4), the variables selected from the first stage analyses are entered into a linear regression analysis. The biological variables of age, gender, race, and JIA disease onset age are always included in linear regression modeling, regardless of their statistical significance. Stepwise selection method was used to select the best subset of the variables that maximize the R-square, and only the variables remaining significant at 0.05 levels are retained in the final model. Finally, the type I squared partial correlations are assessed for each domain of variables remaining in the final model, following the order of causal chain as specified in the conceptual model, i.e. biologic and physiologic domain followed by clinical and functional status, then followed by non-medical characteristics. The analyses for parent proxy-report PedsQL were performed both with and without parents of children younger than 5 years old. This was done to examine the sensitivity of the study results to the inclusion and exclusion of the younger age children, and to be able to compare the results between parent proxy-report and self-report for children 5 years old and older.
Table 3.
Regression Beta Coefficients for Parameters Predicting HRQOL, Adjusted for age, gender, race, age of onset and JIA subtypes
| Parameters | Parent | Child | |||||
|---|---|---|---|---|---|---|---|
| Total | Physical | Psychosocial | Total | Physical | Psychosocial | ||
| Clinical Variables | |||||||
| S-R PedsQL Rheum Pain | 0.23*** | 0.29*** | 0.11** | ||||
| P-R PedsQL Rheum Pain | 0.25*** | 0.42*** | 0.14*** | 0.23*** | |||
| Functional Status | |||||||
| CHAQ | −3.04** | −7.14*** | |||||
| S-R PedsQL Rheum Activities | 0.14** | 0.24*** | |||||
| P-R PedsQL Rheum Activities | 0.19*** | 0.21** | 0.19*** | ||||
| Individual: Arthritis Self-efficacy | |||||||
| Managing Emotional Consequence | 1.49* | 1.85* | 1.47* | ||||
| Individual: Coping with Pain | |||||||
| S-R Cognitive Self-Instruction | 0.72* | ||||||
| S-R Coping via Catastophizing | −0.83* | ||||||
| P-R Cognitive Self-Instruction | 1.49*** | ||||||
| P-R Copying via Distraction | −1.04** | ||||||
| P-R Coping via Catastophizing | −1.01*** | −1.50*** | |||||
| Individual: Adherence | |||||||
| S-R PedsQL Treatment Problem | 0.25*** | 0.14* | 0.32*** | ||||
| P-R PedsQL Treatment Problem | 0.13*** | 0.15** | 0.12** | ||||
| Individual: Social Support | |||||||
| Social Support Classmates | 5.20*** | 6.94*** | |||||
| Social Support Parent | 4.38** | 6.26*** | |||||
| Family: Parental Distress | |||||||
| Parent Emotional Distress | −4.32** | −6.54*** | |||||
Note: S-R = Self-report, P-R = Proxy-Report;
Each column represents separate models for the corresponding outcomes specified by the column header.
P<.05;
P<.01;
P<.001;
Table 4.
Total R-square from the final model* and the type I square partial correlation corresponding to each domain
| Type I Square Partial Correlation** |
Parent | Child | ||||
|---|---|---|---|---|---|---|
| Total | Physical | Psychosocial | Total | Physical | Psychosocial | |
| Biologic and Physiologic | 0.19 | 0.14 | 0.20 | 0.11 | 0.15 | 0.07 |
| Clinical and Functional Status | 0.44 | 0.51 | 0.27 | 0.35 | 0.50 | 0.27 |
| Non-Medical Characteristics | 0.07 | 0.01 | 0.13 | 0.30 | 0.07 | 0.30 |
| Full model | 0.70 | 0.66 | 0.59 | 0.75 | 0.73 | 0.65 |
Results
Sample characteristics
316 children screened eligible for the study, 262 families were approached, and 230 participated, yielding a 73% participation rate and an 88% response rate. Of the 230 families, 180 included children old enough to complete surveys. Table 1 shows biological and demographic characteristics of the sample (n=230). Patient age ranged from 2–16, and was distributed fairly consistently. The patients were predominantly girls (69.1%) and White (92.6%). Table 1 also displays JIA subtype and physiological variables. Polyarticular RF negative and oligoarticular subtypes were most common, and most patients in the study were ANA (67.8%) and RF (92.6%) negative. Baseline clinical, functional status, HRQOL, individual, and family characteristics are summarized in Table 2.
Table 1.
Biological, demographic, JIA subtype and Physiological characteristics of the sample
| Biological Variables | Mean (SD) | Med (Q1–Q3) | |
|---|---|---|---|
| Age (years) | 9.42 (4.49) | Disease Duration (Days since diagnosis) |
57 (35–105) |
| Age of Onset of Symptoms (years) |
8.43 (4.41) | Days between Symptoms & Diagnosis | 126 (65–328) |
| Age Group | N (%) | N (%) | |
| 2–4 | 49 (21.3%) | 8–12 | 81 (35.2%) |
| 5–7 | 39 (17.0%) | 13–18 | 61 (26.5%) |
| Female | 159 (69.1%) | ||
| White | 213 (92.6%) | ||
| JIA subtype | N (%) | N (%) | |
| Poly-articular JIA, RF + | 17 (7.4%) | Undifferentiated | 3 (1.3%) |
| Polyarticular JIA, RF− | 85 (37%) | Systemic JIA | 12 (5.2%) |
| Oligoarticular JIA | 82 (35.7%) | Enthesitis | 10 (4.3%) |
| Psoriatic | 16 (7%) | Other | 5 (2.2%) |
| Demographic | N (%) | N (%) | |
| Parental Education | |||
| Less than College | 61 (27.0%) | College or More | 165 (73.0%) |
| Insurance Status | |||
| No Insurance | 4 (1.8%) | Public Insurance | 47 (20.7%) |
| Private Insurance | 162 (71.3%) | Both Private and Public Insurance | 14 (6.2%) |
| Problem with Costs of Care |
|||
| Yes | 114 (50.7%) | No | 111 (49.3%) |
| Physiological variables | N (%) | N (%) | |
| Rheumatoid Factor | Antinuclear Antibodies | ||
| Positive | 17 (7.4%) | Positive | 74 (32.2%) |
| Negative | 162 (70.4%) | Negative | 136 (59.1%) |
| No Result | 51 (22.2%) | No Result | 20 (8.7%) |
| Median (Q1– Q3) |
Median (Q1 – Q3) | ||
| Erythrocyte Sedimentation Rate (ESR) |
11.5 (7.0–22.5) | ||
| Active Joint Count (AJC) | 2 (0–5) | Number of Joints with Limited Range of Motion (LROM) |
1 (0–4) |
Table 2.
Clinical, Functional Status, Health-Related Quality of Life, Individual, and Family Characteristics of the Sample
| Proxy* Report N=230 |
Self** Report N=180 |
||
|---|---|---|---|
| Variables (Unite or Range) | Mean (SD) | Mean (SD) | |
| Clinical Variables | |||
| Pain VAS (1–10) | 2.96 (2.70) | ||
| Physician’s Global Assessment VAS (1–10) | 2.91 (2.52) | ||
| PedsQL Rheumatology Pain (0–100) | 60.51 (25.43) | 61.64 (25.94) | |
| Functional Status | |||
| Childhood Health Assessment Questionnaire (CHAQ) | 0.53 (0.70) | ||
| PedsQL Rheumatology Module Activities (0–100) | 88.99 (17.22) | 89.59 (16.61) | |
| Health-Related Quality of Life | |||
| PedsQL Generic Total Score (0–100) | 77.57 (17.23) | 75.88 (15.74) | |
| Individual Characteristics | |||
| Child Arthritis Self-Efficacy (CASE) | |||
| Activity (0–4) | 2.50 (1.22) | ||
| Symptom (0–4) | 1.90 (1.13) | ||
| Emotion (0–4) | 2.47 (1.29) | ||
| Pediatric Pain Coping Inventory (PPCI) | |||
| Cognitive Self-Instruction (0–7) | 4.78 (2.93) | 6.33 (3.10) | |
| Problem Solving (0–10) | 8.18 (3.41) | 7.83 (3.53) | |
| Coping via Distraction (0–9) | 6.90 (3.43) | 7.86 (3.48) | |
| Seeks Social Support (0–9) | 7.56 (3.32) | 6.50 (3.62) | |
| Coping via Catastophizing/Helplessness (0–6) | 5.28 (2.56) | 4.68 (2.45) | |
| Adherence | |||
| Medication Adherence Self-Report Inventory (MASRI***) (0 – 100) | 91.18 (13.90) | ||
| PedsQL Rheumatology Module Treatment Problems (0–100) | 68.02 (21.89) | 69.88 (20.39) | |
| Social Support Scale for Children (Harter SSSC) | |||
| Parent (1–4) | 3.68 (0.48) | ||
| Classmates (1–4) | 3.39 (0.54) | ||
| Teacher (1–4) | 3.45 (0.55) | ||
| Friend (1–4) | 3.51 (0.58) | ||
| Parent Emotion Distress Symptom Check List (SCL-10) (0 –4) | 0.44 (0.54) | ||
| Family Environment Scale (FES T-Score) | 56.59 (10.01) | ||
Proxy Report is by parent except as noted;
Self Report by patients ages 5 and older;
MASRI is self-report for patient’s aged 12 years and older, proxy-report for patients less than 12 years old. Only Item E is used.
First stage analyses
Table 3 shows the regression coefficients for the variables that significantly predicted parent proxy-report and child self-report HRQOL – Generic Core Total Scale, Physical Subscale, and Psychosocial Subscale. Pain, activity limitations, coping with pain, barriers to adherence, and parental emotional distress predicted parent proxy-report HRQOL. Pain, functional status, self-efficacy, coping with pain, barriers to adherence, and social support predicted patient self-report HRQOL.
Second stage analysis
Table 4 presents the percent of variance (R-square) accounted for by each set of explanatory variables, after accounting for the domain of variables entered earlier, where the ordering of domains are based on the conceptual model (Figure 1),predicting parent proxy-report and child self-report HRQOL – Generic Core Total Scale, Physical Subscale, and Psychosocial Subscale. These models include biologic variables and JIA subtype, as well as the variables from the domain specific analyses that remained significant in the final model. Collectively, biologic and physiological variables, clinical and functional status, and non-medical characteristics explained a 70% and 75% of variance in parent proxy-report and child self-report HRQOL. After adjusting for biological and physiologic domain, clinical and functional status explained 44% of proxy-report and 35% of self-report total score for HRQOL. After adjusting for biologic and physiologic variables and clinical and functional status, non-medical characteristics explained only 7% of proxy-report total HRQOL, but 30% of self-report total HRQOL. This is reflected in the relative larger proportion of variance explained by non-medical characteristics for self-report (30%) versus proxy-report (13%) psychosocial HRQOL. The same results hold true when excluding children younger than 5 years old.
Discussion
We examined medical (biological, physiological, clinical, functional status) and non-medical (individual, family, environmental) explanatory variables of HRQOL in a cohort of children newly diagnosed with JIA. Consistent with earlier conceptual frameworks and our hypotheses, results suggest that non-medical factors are significant explanatory variables of HRQOL and that parent proxy-report and child self-report HRQOL explanatory variables overlap incompletely.
Our first hypothesis was that non-medical factors are significant explanatory variables for HRQOL. We tested this by step-wise inclusion of sets of potential explanatory variables in regression models to predict HRQOL. Non-medical factors did explain additional variance in overall HRQOL after accounting for medical variables, more so for self-reported HRQOL (an additional 30% of explained variance) than for parent proxy-reported HRQOL (an additional 7%). In the sub-domain of HRQOL related to psychosocial functioning, the size and difference were even more striking – non-medical variables accounted for an additional 30% of self-report and 13% of proxy-report variance in psychosocial functioning. In contrast, when all sets of potential explanatory variables were entered, no biological or physiological variables explained significant variance, nor did physician ratings of global disease assessment. Explaining the vast majority (e.g. 70–75%) of variance in a construct like HRQOL is quite unusual and corresponds to a very large effect size (30).
Our second hypothesis was that the set of explanatory variables significant for parent proxy-report and child self-report HRQOL would overlap incompletely. We found support for this hypothesis as well. For the PedsQL Total Scale score, for example, reports of pain and functioning explained both proxy- and self-report, but proxy-report HRQOL was explained additionally by parent emotional distress, while self-report HRQOL was predicted strongly by classmate and parent social support, as well as self-efficacy.
Together, these results complement an emerging literature on the relationship between clinical factors and HRQOL in children with JIA. Seid et al. (12) showed significant variation in HRQOL despite excellent symptom control, and Haverman et al. (31) showed that perceived difficulty with treatment regimen and missed school were additional predictors of HRQOL. This study adds to the literature by demonstrating that psychosocial factors such as parental distress and children’s perception of social support are strong predictors of HRQOL in newly diagnosed patients. Of note, the point-change estimate for PedsQL Total Scale score associated with a one point difference in classmate (5.2) and parent (4.4) social support and with parent emotional distress (−4.3) is similar to the minimally important difference of the PedsQL Total Scale (approximately 4.5) (32). This suggests that changes in social support and parent emotional distress are related to changes in HRQOL that are not only statistically significant, but also clinically important.
This study has limitations. We sampled patients from only two clinical centers, limiting our ability to generalize. This is a cross-sectional analysis of baseline data from a cohort of new onset patients. The results are applicable to the early stage of the new onset patients. It is not clear whether the results will hold the same at later stages of the disease course. The small variance contributed by non-medical variables after accounting for medical variables could suggest a mediated pathway, as presented in our conceptual model. However, these data are cross-sectional and the casual direction of effect is unclear. The fact that parent-report explanatory variable and self-report explanatory variables were significantly related to proxy-report and self-report HRQOL, respectively, raises the possibility of a rater effect – that the relationship found is due to the same person completing the surveys rather than to whether the constructs are related. As well, we used a simple linear regression and did not account for either higher-order regression or for mediating or moderating variables. Future research should sample additional care centers, examine longitudinal effects, and explore the role of mediating and moderating variables.
Nevertheless, this study improves our understanding of possible determinants of HRQOL in children with JIA and raises the possibility of additional intervention targets for clinicians to consider.
Significance/Innovation.
This study examines the degree to which medical and non-medical variables explain variance in health-related quality of life (HRQOL) in children newly diagnosed with JIA.
Results suggest that non-medical variables – parent emotional distress, social support, self-efficacy - are important explanatory variables in HRQOL for these children, particularly for psychosocial functioning.
These results can be used to develop clinical interventions to help improve HRQOL in JIA.
Acknowledgments
Research funding provided by a grant from the National Institutes of Health (NIH), 5P60AR047784-07.
Footnotes
No authors have financial disclosures to declare.
References
- 1.Bren L. The importance of patient-reported outcomes… It's all aout the patients. Washington, DC: FDA; 2006. [cited 2007 May 11]. Available from: http://www.fda.gov/fdac/features/2006/606_patients.html. [PubMed] [Google Scholar]
- 2.Food and Drug Administration. Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Rockville, MD: FDA; 2006. [cited 2007 May 11]. Available from: http://www.fda.gov/cber/gdlns/prolbl.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zahran HS, Kobau R, Moriarty DG, Zack MM, Holt J, Donehoo R. Health-related quality of life surveillance--United States, 1993–2002. MMWR Surveill Summ. 2005;54(4):1–35. PubMed PMID: 16251867. [PubMed] [Google Scholar]
- 4.World Health Organization. Constitution of the World Health Organization basic document. Geneva, Switzerland: World Health Organization; 1948. [Google Scholar]
- 5.Henoch MJ, Batson JW, Baum J. Psychosocial factors in juvenile rheumatoid arthritis. Arthritis and rheumatism. 1978;21(2):229–233. doi: 10.1002/art.1780210209. Epub 1978/03/01. PubMed PMID: 637889. [DOI] [PubMed] [Google Scholar]
- 6.Varni JW, Jay SM. Biobehavioral factors in juvenile rheumatoid arthritis: Implications for resarch and practice. Clin Psychol Rev. 1984;4:543–560. [Google Scholar]
- 7.Varni JW, Wilcox KT, Hanson V. Mediating effects of family social support on child psychological adjustment in juvenile rheumatoid arthritis. Health psychology. 1988;7(5):421–431. doi: 10.1037//0278-6133.7.5.421. Epub 1988/01/01. PubMed PMID: 3215154. [DOI] [PubMed] [Google Scholar]
- 8.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273(1):59–65. Epub 1995/01/04. PubMed PMID: 7996652. [PubMed] [Google Scholar]
- 9.Oliveira S, Ravelli A, Pistorio A, Castell E, Malattia C, Prieur AM, et al. Proxy-reported health-related quality of life of patients with juvenile idiopathic arthritis: the Pediatric Rheumatology International Trials Organization multinational quality of life cohort study. Arthritis and rheumatism. 2007;57(1):35–43. doi: 10.1002/art.22473. PubMed PMID: 17266064. [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez-Suarez R, Pistorio A, Cespedes Cruz A, Norambuena X, Flato B, Rumba I, et al. Health-related quality of life of patients with juvenile idiopathic arthritis coming from 3 different geographic areas. The PRINTO multinational quality of life cohort study. Rheumatology (Oxford, England) 2007;46(2):314–320. doi: 10.1093/rheumatology/kel218. PubMed PMID: 16877459. [DOI] [PubMed] [Google Scholar]
- 11.Varni JW, Seid M, Smith Knight T, Burwinkle T, Brown J, Szer IS. The PedsQL™ in pediatric rheumatology: Reliability, validity, and responsiveness of the Pediatric Quality of Life Inventory™ Generic Core Scales and Rheumatology Module. Arthritis and rheumatism. 2002;46(3):714–725. doi: 10.1002/art.10095. [DOI] [PubMed] [Google Scholar]
- 12.Seid M, Opipari L, Huang B, Brunner HI, Lovell DJ. Disease control and health-related quality of life in juvenile idiopathic arthritis. Arthritis and rheumatism. 2009;61(3):393–399. doi: 10.1002/art.24477. Epub 2009/02/28. PubMed PMID: 19248113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–392. Epub 2004/02/05. doi: 0315162X-31-390 [pii]. PubMed PMID: 14760812. [PubMed] [Google Scholar]
- 14.Brunner HI, Klein-Gitelman MS, Miller MJ, Barron A, Baldwin N, Trombley M, et al. Minimal clinically important differences of the childhood health assessment questionnaire. J Rheumatol. 2005;32(1):150–161. PubMed PMID: 15630741. [PubMed] [Google Scholar]
- 15.Brunner HI, Taylor J, Britto MT, Corcoran MS, Kramer SL, Melson PG, et al. Differences in disease outcomes between medicaid and privately insured children: possible health disparities in juvenile rheumatoid arthritis. Arthritis and rheumatism. 2006;55(3):378–384. doi: 10.1002/art.21991. PubMed PMID: 16739206. [DOI] [PubMed] [Google Scholar]
- 16.Brunner HI, Johnson AL, Barron AC, Passo MH, Griffin TA, Graham TB, et al. Gastrointestinal symptoms and their association with health-related quality of life of children with juvenile rheumatoid arthritis: validation of a gastrointestinal symptom questionnaire. J Clin Rheumatol. 2005;11(4):194–204. doi: 10.1097/01.rhu.0000173616.81928.44. PubMed PMID: 16357756. [DOI] [PubMed] [Google Scholar]
- 17.Singh G, Athreya BH, Fries JF, Goldsmith DP. Measurement of health status in children with juvenile rheumatoid arthritis. Arthritis and rheumatism. 1994;37(12):1761–1769. doi: 10.1002/art.1780371209. PubMed PMID: 7986222. [DOI] [PubMed] [Google Scholar]
- 18.Varni JW, Waldron SA, Gragg RA, Rapoff MA, Bernstein BH, Lindsley CB, et al. Development of the Waldron/Varni pediatric pain coping inventory. Pain. 1996;67(1):141–150. doi: 10.1016/0304-3959(96)03077-1. Epub 1996/09/01. PubMed PMID: 8895242. [DOI] [PubMed] [Google Scholar]
- 19.Barlow JH, Shaw KL, Wright CC. Development and preliminary validation of a children's arthritis self-efficacy scale. Arthritis and rheumatism. 2001;45(2):159–166. doi: 10.1002/1529-0131(200104)45:2<159::AID-ANR169>3.0.CO;2-2. Epub 2001/04/28. PubMed PMID: 11324780. [DOI] [PubMed] [Google Scholar]
- 20.Harter S. Manual for the Social Support Scale for Children. Denver, CO: 1985. [Google Scholar]
- 21.von Weiss RT, Rapoff MA, Varni JW, Lindsley CB, Olson NY, Madson KL, et al. Daily hassles and social support as predictors of adjustment in children with pediatric rheumatic disease. Journal of pediatric psychology. 2002;27(2):155–165. doi: 10.1093/jpepsy/27.2.155. Epub 2002/02/01. PubMed PMID: 11821499. [DOI] [PubMed] [Google Scholar]
- 22.Koneru S, Shishov M, Ware A, Farhey Y, Mongey AB, Graham TB, et al. Effectively measuring adherence to medications for systemic lupus erythematosus in a clinical setting. Arthritis and rheumatism. 2007;57(6):1000–1006. doi: 10.1002/art.22898. Epub 2007/08/01. PubMed PMID: 17665465. [DOI] [PubMed] [Google Scholar]
- 23.Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. 2002;16(2):269–277. doi: 10.1097/00002030-200201250-00017. Epub 2002/01/25. PubMed PMID: 11807312. [DOI] [PubMed] [Google Scholar]
- 24.Rosen CS, Drescher KD, Moos RH, Finney JW, Murphy RT, Gusman F. Six- and ten-item indexes of psychological distress based on the Symptom Checklist-90. Assessment. 2000;7(2):103–111. doi: 10.1177/107319110000700201. Epub 2000/06/27. PubMed PMID: 10868247. [DOI] [PubMed] [Google Scholar]
- 25.Moos R, Moos B. Family environment scale manual. Palo Alto, CA: Consulting Psychologists Press; 1986. [Google Scholar]
- 26.Kronenberger WG, Thompson RJ. Dimensions of Family Functioning in Families with Chronically Ill Children - a Higher-Order Factor-Analysis of the Family Environment Scale. J Clin Child Psychol. 1990;19(4):380–388. PubMed PMID: ISI:A1990EP04700010. [Google Scholar]
- 27.Agency for Healthcare Research and Quality. CAHPS(tm) 2.0 Questionnaires: Agency for Healthcare Research and Quality. 1998 Available from: http://www.ahrq.gov/qual/cahps/cahpques.htm.
- 28.Ford CA, Bearman PS, Moody J. Foregone health care among adolescents. JAMA. 1999;282(23):2227–2234. doi: 10.1001/jama.282.23.2227. PubMed PMID: ISI:000084138600030. [DOI] [PubMed] [Google Scholar]
- 29.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–812. doi: 10.1097/00005650-200108000-00006. Epub 2001/07/27. PubMed PMID: 11468499. [DOI] [PubMed] [Google Scholar]
- 30.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 31.Haverman L, Grootenhuis MA, van den Berg JM, van Veenendaal M, Dolman KM, Swart JF, et al. Predictors of health-related quality of life in children and adolescents with juvenile idiopathic arthritis: results from a Web-based survey. Arthritis care & research. 2012;64(5):694–703. doi: 10.1002/acr.21609. Epub 2012/01/13. PubMed PMID: 22238240. [DOI] [PubMed] [Google Scholar]
- 32.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL™ 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3(6):329–341. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. PubMed PMID: 14616041. [DOI] [PubMed] [Google Scholar]