Abstract
Human immunodeficiency virus 1 and its envelope protein gp120 reduce synaptodendritic complexity. However, the mechanisms contributing to this pathological feature are still not understood. The proneurotrophin brain-derived neurotrophic factor promotes synaptic simplification through the activation of the p75 neurotrophin receptor (p75NTR). Here, we have used gp120 transgenic (gp120tg) mice to investigate whether p75NTR has a role in gp120-mediated neurotoxicity. Old (~10 months) gp120tg mice exhibited an increase in proneurotrophin brain-derived neurotrophic factor levels in the hippocampus as well as a decrease in the number of dendritic spines when compared to age-matched wild type. These effects were not observed in 3- or 6-month-old mice. To test if the reduction in spine density and morphology is caused by the activation of p75NTR, we crossed gp120tg mice with p75NTR null mice. We found that deletion of only 1 copy of the p75NTR gene in gp120tg mice is sufficient to normalize the number of hippocampal spines, strongly suggesting that the neurotoxic effect of gp120 is mediated by p75NTR. These data indicate that p75NTR antagonists could provide an adjunct therapy against synaptic simplification caused by human immunodeficiency virus 1.
Keywords: Aging, Hippocampus, HIV, p75NTR, proBDNF, TrkB
1. Introduction
Despite the introduction of combination antiretroviral therapy (Ellis et al., 2007; Everall et al., 2009), older human immunodeficiency virus 1 (HIV)-positive adults are at a high risk for developing mild to severe cognitive abnormalities, termed HIV-associated neurocognitive disorders (HANDs). Therefore, new therapeutic approaches must be developed to prevent the neurologic deficits observed in these individuals. The key to access a new therapy is a better understanding of the molecular mechanisms underlying HIV neurotoxicity.
HIV positive, cognitively impaired subjects exhibit synaptic pruning and neuronal injury (Ellis et al., 2007). Loss of synapses in HAND subjects does not result from direct intrinsic viral infection given that neurons are not infected but, most likely, is caused by neurotoxins (Haughey et al., 2004) or viral proteins released by infected microglia (Kaul et al., 2001). Indeed, some HIV subjects develop HIV encephalitis (HIVE), a neuroinflammatory condition characterized by the presence of activated and infected microglia (Persidsky et al., 1997). Nevertheless, since the introduction of antiretroviral therapies, only a small percent of HIV-positive patients develop HIVE (Gelman, 2015), suggesting that inflammation alone may not be sufficient to cause synaptic pruning and justifying the investigation for additional mechanisms of decreased synaptic plasticity.
Cognitive alterations may be promoted by conditions that alone affect cognitive functions such as aging or cardiovascular and cerebrovascular diseases. In addition, aging may have an additive effect on HAND neuropathology (Canizares et al., 2014) because aging (Driscoll et al., 2012; Erickson et al., 2010) and HAND subjects (Bachis et al., 2012) both exhibit low levels of brain-derived neurotrophic factor (BDNF), a potent prosurvival neurotrophic factor that plays a role in hippocampal plasticity (Kang and Schuman, 1995; Minichiello et al., 2002; Patterson et al., 1996) including spine morphology (Tyler and Pozzo-Miller, 2003). Moreover, BDNF is decreased by an acute injection of gp120 into the brain (Nosheny et al., 2004) or in a mouse model of HAND (Lee et al., 2013), in which the HIV protein gp120 is chronically overexpressed under an astrocytic promoter (Toggas et al., 1994). It is then plausible to suggest that the neurological features observed in HAND subjects could be due to a lack of a proper supply of BDNF and other neurotrophic factors to neurons that could accelerate the aging process of the brain.
BDNF binds and activates a receptor complex composed of the tropomyosin-related kinase receptor B (TrkB), and the p75 neurotrophin receptor (p75NTR). This receptor complex organizes the signaling cascade events that promote synaptic plasticity (Chao et al., 2006). However, TrkB and p75NTR can also exert an opposing functional action on neurons. In fact, while TrkB promotes neuronal survival and maturation of dendritic spines, p75NTR inhibits axonal growth (Kaplan and Miller, 2003) and mediates apoptosis (Teng et al., 2005). An intriguing level of complexity for p75NTR was recently added when it was discovered that proneurotrophin brain-derived neurotrophic factor (proBDNF), the larger glycosylated precursor of BDNF, has higher affinity for p75NTR than BDNF and, therefore, it is considered a neuronal proapoptotic ligand (Teng et al., 2005; Volosin et al., 2006; Woo et al., 2005). proBDNF is increased in the brain of HIV-positive subjects as well as in rat cortical cultures exposed to gp120 (Bachis et al., 2012). Moreover, p75NTR inhibitors block the gp120-mediated terminal retraction as well as neuronal apoptosis (Bachis et al., 2012). Thus, gp120’s neurotoxic profile appears to involve its indirect ability to activate p75NTR. However, these data were obtained in vitro. In this study, we aim at establishing whether gp120 promotes the increase in proBDNF, which, in turn, activates p75NTR in vivo. We used gp120 transgenic (gp120tg) mice, which exhibit impaired cognitive behavior and synaptic simplification (Toggas et al., 1994) and other pathological features similar to HAND. These animals were intercrossed with p75NTR null mice to obtain p75+/− gp120tg mice. Here, we report that the reduction of p75NTR expression significantly decreases the neurotoxic effect of gp120.
2. Materials and methods
2.1. Animals
Breeding pairs of gp120tg mice were received from E. Masliah (University of California San Diego, San Diego, CA). These mice, which express gp120 under the control of a modified glial fibrillary acidic protein promoter, have been previously characterized in terms of behavior and neuropathology (Toggas et al., 1994). Gp120tg females were intercrossed with C57BL/6 male p75NTR−/− mice (The Jackson Laboratory, Bar Harbor, ME) to generate males and females p75+/− gp120tg. Wild-type (WT) littermates (gp120 null/p75+/+) were generated from these colonies and used as controls. Mice were maintained in our facility for up to 12 months. For these studies, 3- to 4-month-old, 6- to 7-month-old, and 9- to 12-month-old mice (both gender) were used. Mice were euthanized at different time points either by intracardial exsanguination or cervical dislocation. All studies were carried out following the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the US National Institutes of Health and approved by the Georgetown University Animal Care and Use Committee.
2.2. Human samples
Frozen human hippocampal samples of HIV-positive subjects were obtained from the National NeuroAIDS Tissue Consortium. A total of 6 HIV-positive cases with no neurocognitive impairment and 6 HIV-positive cases with HIV-associated dementia were used. The characteristics of these samples are described in Table 1.
Table 1.
Characteristics of study samples
| Sample number | HIV −1 status | Age | Gender | ARV use | Drug abuse | CNS pathologies | Neurocognitive diagnosis | CSF VL (copies/ml) |
|---|---|---|---|---|---|---|---|---|
| 1 | + | 53 | Male | Yes | None | NA | HAD | 50 |
| 2 | + | 40 | Male | No | None | NA | HAD | 400 |
| 3 | + | 34 | Female | Yes | NA | Minimal abnormalities | HAD | NA |
| 4 | + | 57 | Male | No | None | Atherosclerosis of the brain | HAD | 2747 |
| 5 | + | 34 | Male | No | None | Minimal abnormalities | HAD | NA |
| 6 | + | 40 | Male | No | NA | Minimal abnormalities | HAD | NA |
| 7 | + | 46 | Male | Yes | Past Cocaine | No known pathology | Neurocognitive normal | 2355 |
| 8 | + | 45 | Male | Yes | None | Focal infarct, contusion | Neurocognitive normal | 11,405 |
| 9 | + | 51 | Male | NA | Past cocaine | Bacterial parenchymal infection | Neurocognitive normal | 53,215 |
| 10 | + | 37 | Male | Yes | NA | Cryptococcus | Neurocognitive normal | 400 |
| 11 | + | 34 | Female | Yes | NA | Other noninfectious path | Neurocognitive normal | 50 |
| 12 | + | 39 | Male | Yes | NA | Minimal abnormalities | Neurocognitive normal | NA |
Key: ARV, antiretroviral therapy; CNS, central nervous system; CSF/VL, cerebrospinal fluid viral load; HAD, HIV-associated dementia; HIV, human immunodeficiency virus 1; NA, not available.
2.3. Western blot
Lysates were prepared by using gentle sonication of mouse hippocampi or human frozen tissues in lysis buffer composed of 1× Tris-buffered saline (TBS), 1% NP-40, 1% Triton-100, 1-mM phenylmethylsulfonyl fluoride, 10% glycerol, and protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO, USA) on ice. The homogenates were incubated for 5–10 minutes on ice and then centrifuged at 14,000g for 5 minutes at 4 °C. Supernatants were collected and stored at −80 °C. Total protein content was determined by Bradford Coomassie Blue colorimetric assay. Lysates were loaded onto a NuPAGE 4%–12% Bis-Tris gel (Invitrogen, Carlsbad, CA, USA) and transferred to a polyvinylidene difluoride membrane using the iBlot device (Invitrogen). Membranes were blocked with 5% milk in phosphate-buffered saline and 0.05% Tween and incubated with antibodies specific for either p75NTR (1:100, a gift from Bruce Carter, Vanderbilt University) or TrkB (1:3000, Cell Signaling, Danvers, MA, USA). Membranes were stripped and reprobed with mouse monoclonal anti β-actin antibody (1:10,000, Sigma-Aldrich) to control for artifacts due to loading. Immune complexes were detected by the corresponding secondary antibody and chemiluminescence reagent (Thermo Fisher Scientific Inc). The intensity of immunoreactive bands was quantified using Quantity One 1-D (Bio-Rad Laboratories, Inc, Hercules, CA, USA) and expressed in arbitrary units after normalization to β-actin immunoreactivity.
2.4. Proneurotrophin brain-derived neurotrophic factor
ProBDNF levels were analyzed as described previously (Bachis et al., 2012). In brief, hippocampal lysates, prepared as above, were precleared using agarose resin according to the manufacturer’s instructions (Thermo Scientific Pierce, Rockford, IL, USA) and then incubated with an anti-proBDNF antibody (5 µg, Sigma-Aldrich) for 18 hours at 4 °C. Samples were centrifuged at 3000g for 2 minutes at 4 °C to collect immunoprecipitated complexes. Beads were washed in lysis buffer, and total protein content was determined by Bradford Coomassie Blue colorimetric assay. The same amount of immune complexes was loaded onto sodium dodecyl sulfate polyacrylamide gel electrophoresis for separation. Proteins were transferred to a polyvinylidene difluoride membrane (Bio-Rad) and blocked with TBS-T (25-mM Tris and 1% Tween) containing 5% milk powder. Blots were then incubated overnight with an anti-proBDNF antibody (1:2500, Sigma). After 3 washes with TBS-T, the blots were incubated with peroxidase-conjugated secondary antibody (dilution 1:1000; Santa Cruz Biotec, Inc, Santa Cruz, CA, USA) for 1 hour at room temperature. Immunoreactivity was detected by enhanced chemiluminescence (Thermo Scientific Pierce).
2.5. Enzyme-linked immunosorbent assay
Levels of BDNF, furin, tissue plasminogen activator (tPA), interleukin-1β (IL-1β), IL-6, and tumor necrosis factor α (TNFα) in the hippocampus were determined using enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions with minor modifications described elsewhere (Bachis et al., 2010, 2012). Furin, ILs, and TNFα DuoSet ELISA kits were from R&D System (Minneapolis, MN, USA); tPA ELISA from Molecular Innovations, Inc (Peary Court, Novi, MI, USA), and BDNF Emax from Promega Corporation (Madison, WI, USA). The ELISA for BDNF has a negligible cross reactivity with proBDNF (~2.5%) as determined by running in parallel a standard curve with proBDNF.
2.6. Golgi staining
Golgi-Cox staining was performed using FD Rapid GolgiStain Kit according to the manufacturer instructions (FD Neurotechologies, Inc, Columbia, MD, USA). Measurements of spine count were performed in fully impregnated hippocampal neurons from the cornus ammonis 1 (CA1), CA2, CA3, and dentate gyrus (DG) subregions. All analyzed neurons displayed a dendritic tree without obvious truncations and impregnation without breaks. To minimize errors during the analysis, the measurement of dendritic branch was done on fragments that were straight, in the same focus plane, and had a length of at least 30 µm. Two separate counts were performed—dendritic spines on basal shaft and dendritic spines on the apical oblique (AO), which project off the apical dendrite. Spines in the basal shaft dendrites, which project directly off the cell soma, were counted along 30-µm sections of the shaft (between 30–100 µm from the soma). Spines in the primary AO dendrites were counted in a 30-µM section of the primary AO, 100 µm away from the soma. Images were coded, and dendritic spines were counted in a blinded fashion using Nikon imaging software. We averaged a total of 32 neurons per animal from a total of 6 mice per group.
2.7. Immunohistochemistry
Immunohistochemistry for microglia was performed as described with minor alterations (Campbell et al., 2015). Briefly, fixed brains were transferred to 30% sucrose, and serial sections (30 µm) throughout the hippocampus were prepared by a sliding microtome (Microm International, Heidelberg, Germany). Sections were blocked in phosphate saline–blocking buffer (1% bovine serum albumin, 0.2% Triton X-100) for 1 hour at room temperature prior to primary antibody incubation. To detect microglia, slices were incubated with an antibody against ionized calcium-binding adapter molecule 1 (Iba-1, 1:1000, Wako Chemicals USA, Inc, Richmond, VA, USA) overnight at 4 °C followed by AlexaFluor 594 goat anti-rabbit secondary antibody (1:2000, Invitrogen) for 1 hour at room temperature. Sections were washed with 1× phosphate-buffered saline with 0.2% Triton X-100. Slices were then incubated with 4′, 6-diamidino-2-phenylindole (1:10,000, Sigma) for 10 minutes at room temperature and then mounted using Fluoro- Gel with TES buffer (Electron Microscopy Sciences, Hatfield, PA, USA). Images were analyzed with a Nikon Eclipse Ni microscope at 450-nm and 594-nm wavelengths.
2.8. Statistical analysis
Statistical analyses were performed using GraphPad Prism software (GraphPad Software, Inc). Comparison of more than 2 groups was done using analysis of variance followed by Tukey post hoc test for multiple comparisons. Results are presented as mean ± standard error of mean. p values of <0.05 indicate statistical significance.
3. Results
3.1. Gp120tg mice exhibit an increase in proBDNF
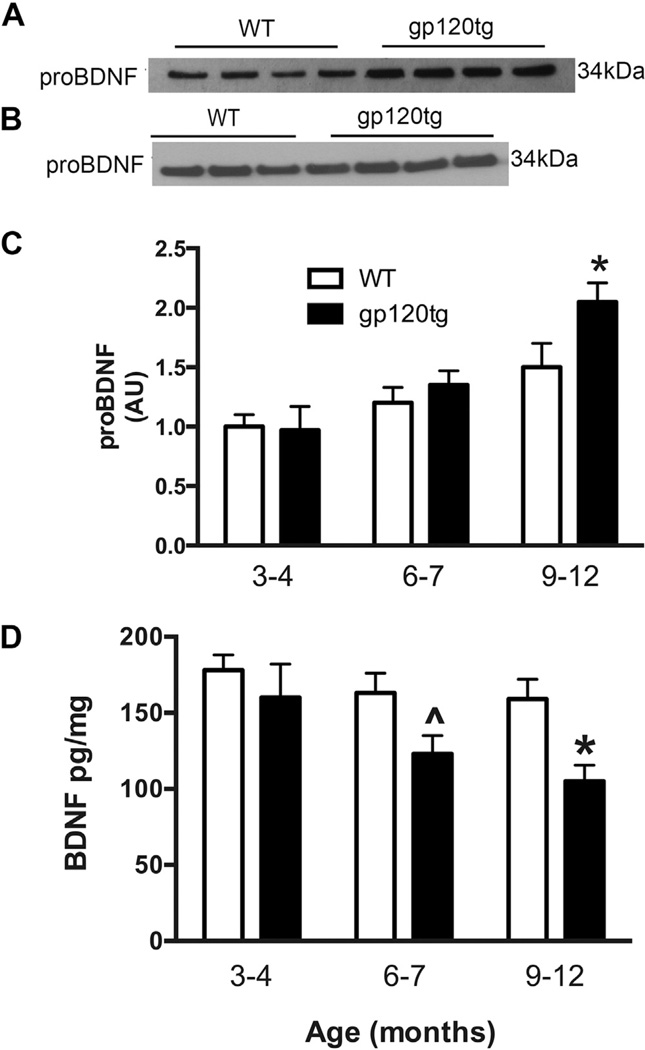
Gp120 increases proBDNF in cultured neurons in vitro (Bachis et al., 2012). To ascertain whether gp120 exerts the same effect in vivo, we measured proBDNF immunoreactivity in lysates of hippocampi from gp120tg mice by Western blot analysis following immunoprecipitation with an anti-proBDNF antibody. WT littermates were used as controls. We started with 9- to12-month-old mice because gp120tg mice exhibit age-dependent impaired cognitive behaviors (Toggas et al., 1994). Incubation of the membranes with an antibody against proBDNF revealed an immunoreactive band of ~34 KDa in both WT and gp120tg mice (Fig. 1A). When compared to age-matched WT, the intensity of proBDNF immunoreactivity was stronger in gp120tg mice (Fig. 1A and C). We then analyzed hippocampal lysates from 3- to 4-month-old and 6- to 7-month-old mice to ascertain whether the increase in proBDNF is age related. We found no difference in proBDNF levels between gp120 and WT mice at these age points (Fig. 1B and C).
Fig. 1.
Gp120 causes an age-dependent increase of hippocampal proBDNF. Hippocampal lysates from 3- to 4-month-old, 6- to 7-month-old, and 9- to 12-month-old WT and gp120tg mice were analyzed for proBDNF and total BDNF. (A and B) Examples of Western blots used to determine proBDNF levels in lysates (after immunoprecipitation) from the hippocampi of 9- to 12-month-old and 6- to 7-month-old mice, respectively (see Section 2). (C) Semiquantitative analysis of proBDNF immunoreactivity. Levels of proBDNF are expressed as arbitrary units. Data are the mean ± SEM of 6 samples per group each time point. *p < 0.01 versus age-matched WT. (D) Total hippocampal BDNF was determined by ELISA. Data are the mean ± SEM of 6 samples per group.^p < 0.05, *p < 0.01 versus age-matched WT. Abbreviations: BDNF, brain-derived neurotrophic factor; ELISA, enzyme-linked immunosorbent assay; gp120tg, gp120 transgenic; proBDNF, proneurotrophin brain-derived neurotrophic factor; SEM, standard error of mean; WT, wild type.
Previous studies have shown that gp120 decreases the release of BDNF in vitro by reducing its processing from proBDNF (Bachis et al., 2012). To confirm this result in vivo, we measured the levels of BDNF by ELISA in hippocampal lysates from WT and age-matched gp120tg mice. We found that gp120 evokes an age-dependent decrease in the levels of BDNF, starting at 6–7 months. This decrease was greater in 9- to 12-month-old gp120tg mice (Fig.1D), suggesting that gp120 affects the processing of proBDNF in vivo as well.
3.2. Gp120 changes processing enzymes
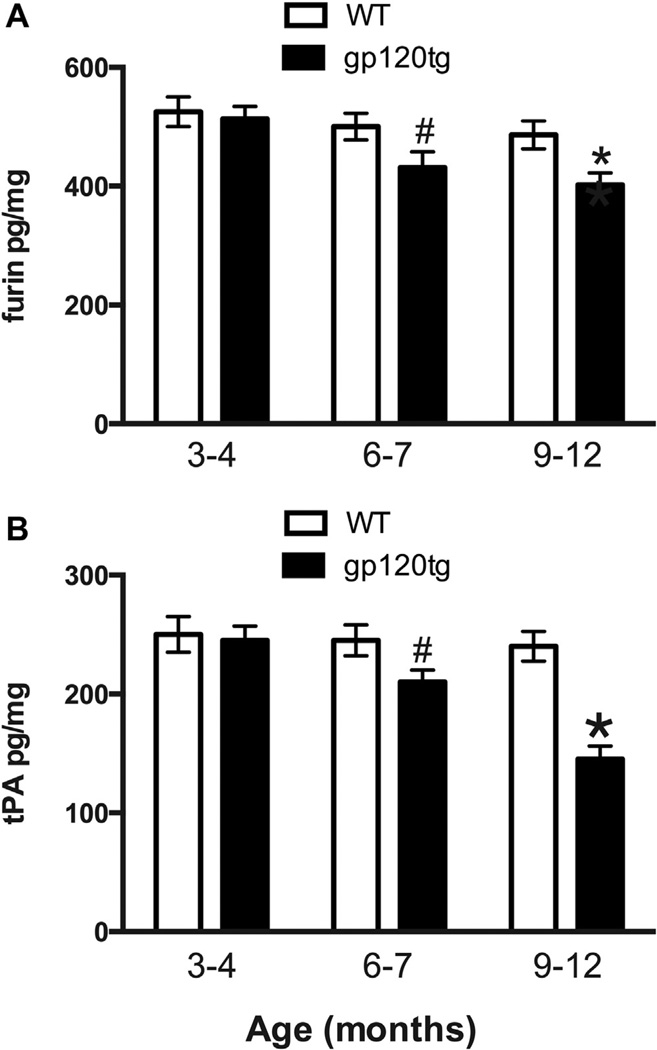
ProBDNF is processed to mature BDNF by several enzymes including furin and the tPA and/or plasmin system (Seidah et al., 1996). Our earlier study has shown that HIV and gp120 decrease the levels of these enzymes in human postmortem brain and neuronal cultures, respectively (Bachis et al., 2012). Therefore, we determined the level of furin and tPA in WT and gp120tg mice. To provide a correlation between the age-dependent changes in proBDNF and BDNF, furin and tPA levels were determined in the 3 groups of mice. We found a significant age-dependent decrease in the levels of furin (Fig. 2A) as well as tPA levels (Fig. 2B) in gp120tg mice when compared to WT. This decrease correlated with the age-dependent reduction of BDNF levels (Fig. 1C) because the levels of either furin or tPA were greatly diminished in 9- to 12-month-old gp120tg when compared to 6- to 7-month-old mice (Fig. 2).
Fig. 2.
gp120 decreases furin and tPA levels. Hippocampal levels of furin (A) and tPA (B) were determined by ELISA in gp120tg and age-matched WT. Data are the mean ± SEM of 6 mice per each group. #p < 0.05, *p < 0.01 versus age-matched WT. Abbreviations: ELISA, enzyme-linked immunosorbent assay; gp120tg, gp120 transgenic; SEM, standard error of mean; tPA, tissue plasminogen activator; WT, wild type.
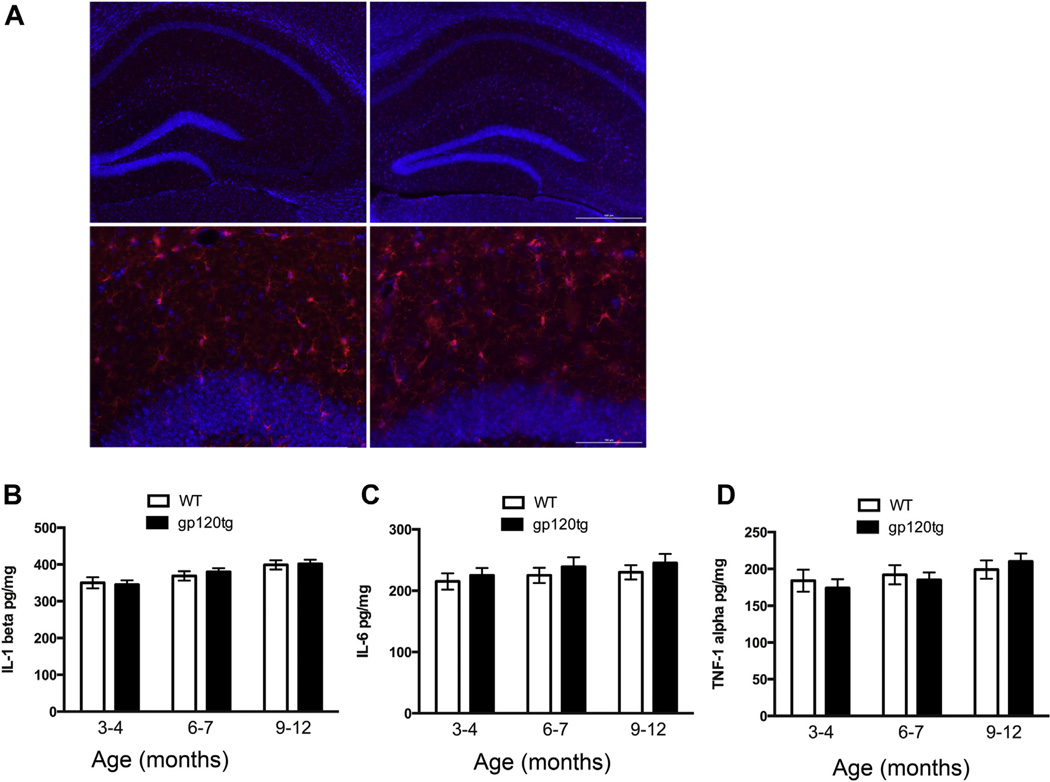
Inflammation has been reported as one of the main events responsible for HIV-induced neuronal degeneration (Lisi et al., 2012; Persidsky and Gendelman, 2002; Tong et al., 2000). Therefore, to examine whether proBDNF is an event that may occur concomitantly with inflammation, we analyzed Iba-1-positive cells (microglia) in the hippocampus by immunohistochemistry, as well as the levels of proinflammatory cytokines IL-1β, IL-6, and TNFα, by ELISA. These cytokines are activated in the brain of HIV-positive subjects (Persidsky et al., 1997). We found no significant differences between WT and gp120tg mice in the number of Iba-1-positive cells (Fig. 3A) or in IL-1β (Fig. 3B), IL-6 (Fig. 3C), or TNFα (Fig. 3D) in any age examined.
Fig. 3.
Microglia and proinflammatory cytokines are not altered in gp120tg mice. (A) Example of Iba-1 immunoreactivity (red) in hippocampal sections from 10-month-old WT (left panels) and gp120 tg mice (right panels). 4′, 6-diamidino-2-phenylindole (blue) was used to visualize nuclei. Lower panels = higher magnification (×20) of the area delimited by the white squares. Upper panel scale bar = 500 µm; lower panel scale bar = 100 µm. (B–D) IL-1β, IL-6, and TNFα levels were determined in the hippocampus of WT and gp120tg mice at the indicated ages by ELISA. Data are the mean ± SEM (n = 6 per group). Abbreviations: ELISA, enzyme-linked immunosorbent assay; gp120tg, gp120 transgenic; SEM, standard error of mean; TNFα, tumor necrosis factor α; tPA, tissue plasminogen activator; WT, wild type. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. Gpg120 tg mice exhibit a decrease in the number of spines
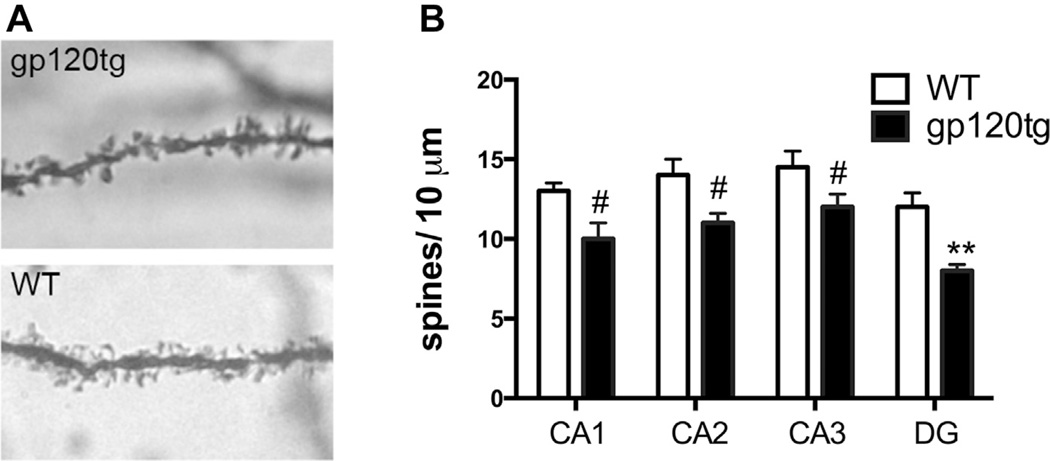
BDNF has been shown to increase spine density of hippocampal neurons (Chapleau and Pozzo-Miller, 2012). Conversely, proBDNF negatively regulates spine formation (Koshimizu et al., 2009). To determine whether the increase in proBDNF correlates with a reduction in spine density, we analyzed the number of spines in individual hippocampal neurons by Golgi staining (Fig. 4A). Spines were analyzed throughout the hippocampus, including the CA1–3 and DG regions. The number of spines was not significantly different between the 6-month-old WT and gp120tg mice (not shown). However, throughout various hippocampal regions, neurons of 9- to 12-month-old gp120tg mice exhibited a significant decrease in spine numbers when compared to WT (Fig. 4B).
Fig. 4.
Spine count is decreased in old gp120tg mice. Golgi staining was used to label hippocampal spines in WT and 9- to 12-month-old gp120tg mice. (A) Representative images of dendritic spines in hippocampal sections taken from the CA1 region. (B) The number of spines was determined in the indicated hippocampal regions (DG) as described in Section 2 on an average of 32 neurons per animal. #p < 0.05, **p < 0.01 versus WT (n = 6 animals per group). Abbreviations: DG, dentate gyrus; gp120tg, gp120 transgenic; TNFα, tumor necrosis factor α; WT, wild type.
3.4. TrkB but not p75NTR is decreased in gp120tg mice
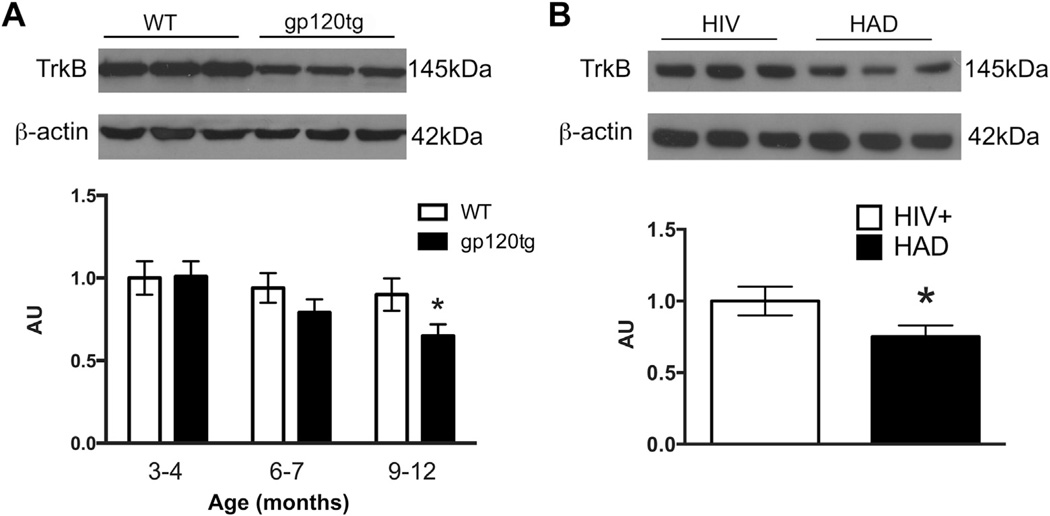
The number of hippocampal spines is increased and maintained by TrkB (Ji et al., 2005; von Bohlen und Halbach et al., 2008), the receptor tyrosine kinase TrkB, which is rapidly activated upon binding to BDNF (Soppet et al., 1991). To determine whether gp120 affects TrkB levels, we measured TrkB immunoreactivity in hippocampal lysates from WT and gp120tg mice (Fig. 5A). Western blot analysis revealed an age-dependent decrease in the levels of TrkB in gp120tg mice. In fact, we found a ~50% reduction of TrkB immunoreactive band in 9- to 12-month-old gp120tg mice when compared to age-matched WT mice (Fig. 5A). Although this result was obtained in an experimental animal model of HIV dementia, it may be also significant for human subjects. In fact, we have found that the hippocampal levels of TrkB are decreased in HIV-positive subjects with dementia (Table 1) when compared to HIV-positive individuals with no cognitive impairment (Fig. 5B).
Fig. 5.
TrkB levels are decreased in gp120tg mice and HAD. Hippocampal lysates were prepared from WT and gp120tg mice or frozen human samples and analyzed by Western blot. (A and B) Examples of blots of lysates from mice (A) or human tissues (B) using a TrkB antibody that recognizes both mice and human TrkB. Semiquantitative determination of TrkB levels was done by densitometric analysis using β-actin as loading control (see Section 2). Data, expressed in arbitrary units, are the mean ± SEM of 6 mice each group or 5 human samples per condition. (A) *p < 0.01 versus WT. (B) HIV+ = subjects with no cognitive impairment, HAD = HIV+ subjects with dementia. *p < 0.05 versus HIV+. Abbreviations: gp120tg, gp120 transgenic; HAD, HIV-associated dementia; HIV, human immunodeficiency virus 1; SEM, standard error of mean; TrkB, tropomyosin-related kinase receptor B; WT, wild type.
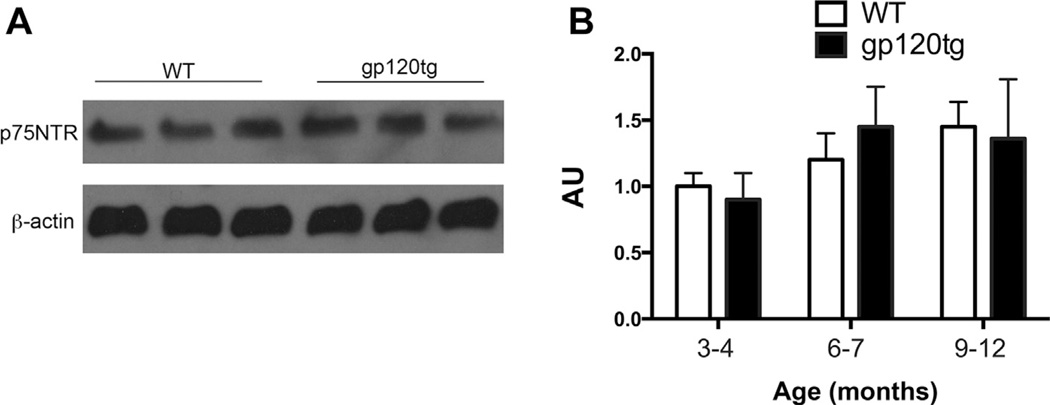
Adult hippocampal spines are also modulated by p75NTR (Zagrebelsky et al., 2005). Because this receptor is upregulated in the hippocampus by seizure-induced brain damage (Volosin et al., 2008), we examined whether overexpression of gp120 modifies the levels of p75NTR expression. Lysates from WT and gp120tg mice were analyzed for p75NTR immunoreactivity by Western blot (Fig. 6A). There were no differences in the levels of p75NTR between WT and gp120tg mice at any age examined (Fig. 6B). Therefore, it appears that gp120 decreases TrkB without affecting the expression of p75NTR.
Fig. 6.
p75NTR does not change in gp120tg mice. Hippocampal lysates from WT and gp120tg mice were analyzed for p75NTR by Western blot. (A) Example of a blot of lysates from 9- to -12-month-old mice. β-actin was used as loading control. (B) Levels of p75NTR were determined by densitometry and expressed in arbitrary units (AU). Data are the mean ± SEM of 5 samples each group per condition. Abbreviations: p75NTR, p75 neurotrophin receptor; SEM, standard error of mean; WT, wild type.
3.5. Reduction of p75NTR rescues gp120-mediated spine loss
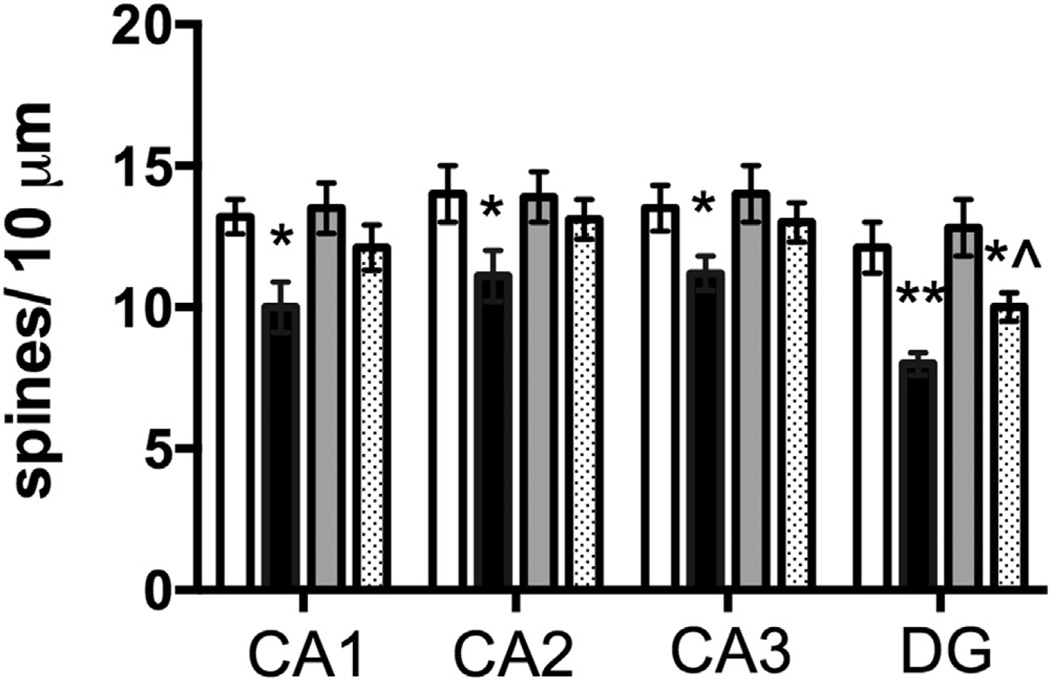
We have previously shown that a p75NTR-blocking antibody inhibits gp120-mediated synaptic pruning in vitro (Bachis et al., 2012), suggesting that activation of p75NTR is crucial for the neurotoxic effect of gp120. However, TrkB deficiency can lead to a reduction of synapses and spine density in the hippocampus (Otal et al., 2005). To determine the role of p75NTR, we have inter-crossed p75NTR null mice with gp120tg mice to obtain 9- to 12-month-old mice transgenic for gp120 and with only 1 allele for p75NTR (p75+/− gp120tg). We did not use p75NTR null mice because these animals exhibit a profound deficit in cognitive and motor activities accompanied by loss of neurons (Peterson et al., 1999). Spine counts of hippocampal sections revealed that the removal of 1 copy of p75NTR alone does not affect spine density (Fig. 7) but rescues the reduction of spine density observed in the CA1–3 regions of gp120tg mice (Fig. 7), suggesting that the lack of 1 p75NTR allele is sufficient to confer neuroprotection against gp120.
Fig. 7.
Reduction of p75NTR rescues gp120-mediated loss of hippocampal spines. All groups of mice were maintained for 9–12 months. Spine count was performed in hippocampal sections containing the indicated subregions as described in Section 2. Data are the mean ± SEM of 6 animals per group. *p < 0.05 versus WT; **p < 0.01 versus WT; *^p < 0.05 versus gp120tg. Abbreviations: DG, dentate gyrus; p75NTR, p75 neurotrophin receptor; SEM, standard error of mean; WT, wild type.
Analyses of spines in the DG revealed a different scenario. In fact, the DG of p75+/− gp120tg mice exhibited more spines when compared to that of gp120tg mice (Fig. 7) but contained fewer spines than WT or p75NTR+/− mice (Fig. 7). Thus, it appears that a lower expression of p75NTR may be neuroprotective against gp120 in the CA regions, but it may only partially prevent gp120 toxicity in the DG.
4. Discussion
Disruption of neurocognitive functioning is one of the most frequent complications in patients infected with HIV and appears to be linked to hippocampal dysfunction (Maki et al., 2009). The complexity of dendrites and synapses is compromised in HAND subjects (Masliah et al., 1997). Several mechanisms have been proposed to explain this pathological scenario, including an increased inflammatory environment. However, only a fraction of HAND patients (~20%) suffer from HIVE (Gelman, 2015). Thus, we have used transgenic mice overexpressing gp120 that exhibit synaptodendritic simplifications (Toggas et al., 1994) and impaired neurogenesis (Lee et al., 2013), to study the mechanisms whereby HIV decreases synaptic plasticity. These mice have cognitive decline as measured by Morris water maze testing (Toggas et al., 1994). Moreover, they exhibit a significant deficit in long-term potentiation in the hippocampal CA1 area (Krucker et al., 1998), suggesting that the memory circuitry involving the hippocampus is compromised. In this study, we report that long-term overexpression of gp120, such as that obtained in aged mice, reduces spine density in the hippocampus.
We have also tested the hypothesis that dendrite spine density, which is controlled by neurotrophins (Tanaka et al., 2008), is decreased by a lack of trophic support. We have found that, in the hippocampus of gp120tg mice, the total levels of BDNF as well as its tyrosine kinase receptor TrkB, undergo an age-dependent decline, whereas proBDNF, which inhibits synaptic plasticity by activating p75NTR (Woo et al., 2005), is increased. Our data suggest that gp120 promotes an environment that is conducive for the activation of p75NTR. This mechanism, when associated with aging, could be a risk factor for promoting synaptic simplifications seen in HAND. In fact, we have discovered that the reduction of p75NTR expression is sufficient to inhibit the neurotoxic effect of the viral protein even in old animals. Overall, our data support the notion that increasing the endogenous production of BDNF could be a therapeutic strategy to reverse the toxic effect of gp120 (Lee et al., 2013) and perhaps to slow down the progression of premature aging seen in HAND subjects (Canizares et al., 2014; Chang et al., 2013).
One important finding reported in our study is the reduction of furin and tPA levels in old gp120tg mice. This result is in line with the previous data showing reduced furin levels in the cortex and striatum of HIV-associated dementia subjects as well as a decrease in tPA levels in vitro by gp120 (Bachis et al., 2012). Furin is an endoprotease that participates in the conversion of proBDNF into mature BDNF intracellularly (Mowla et al., 2001). tPA, upon release, activates the protease plasmin extracellularly, which cleaves proBDNF to mature BDNF. This conversion is critical for long-term potentiation (Pang et al., 2004). Gp120tg mice exhibited an aged-dependent reduction of tPA levels when compared to WT. Because the total levels of BDNF are decreased in aged gp120tg mice, it appears that gp120 interferes with the physiological processing of proBDNF by reducing significantly the levels of key enzymes necessary for its cleavage. How gp120 reduces furin and tPA levels is still a matter of speculation. Furin has been shown to traffic between the trans-Golgi network to early endosomes to be recycled (Molloy et al., 1998). Gp120 is also transported in endosomes (Bachis et al., 2006). Thus, gp120 might change furin trafficking and help promote furin degradation, perhaps through ubiquitination. tPA is contained in secretory granules that are axonally transported (Lochner et al., 1998). Gp120 binds to microtubules (Avdoshina et al., 2016; Bachis et al., 2006) and may disrupt their function, including axonal transport. On the other hand, the decrease in tPA levels may reflect the loss of synapses that is seen in gp120tg mice (Toggas et al., 1994). Clearly, more experiments are needed to support these hypotheses.
Most of the initial data supporting a role for BDNF in neuronal differentiation and axonal outgrowth have been obtained from the developing central nervous system (Klein et al., 1993; McAllister et al., 1995; Zagrebelsky et al., 2005). Evidence has shown that BDNF also modulates synaptic plasticity and spine density in the adult hippocampus (Otal et al., 2005; Yacoubian and Lo, 2000). Moreover, recent results have shown that there is a functional antagonism between TrkB and p75NTR and suggested that p75NTR is crucial for the initial dendritic spine formation whereas TrkB participates in spine maintenance (Chapleau and Pozzo-Miller, 2012). In our study, we observed a significant decrease in TrkB in the hippocampus of aged gp120tg mice concomitantly with an increase in proBDNF, suggesting that while TrkB signaling is decreased, that of p75NTR is increased. Unlike mature BDNF, proBDNF decreases synaptic plasticity in the hippocampus (Yang et al., 2014), suggesting that gp120 creates an imbalance between prosurvival and anti-survival signaling cascades that favors the inhibition of synaptic plasticity and causes the loss of spines. However, we cannot exclude the role that TrkB plays in spine formation. A decrease in TrkB may reflect the synaptic simplification previously reported in these animals (Toggas et al.,1994). On the other hand, we should also consider the outcome of previous in vitro data showing that either an intracellular inhibitor of p75NTR or a blocking antibody for p75NTR reverses and/or prevents the pruning property of gp120 (Bachis et al., 2012). To better refine the role of p75NTR, we intercrossed p75 null mice with gp120tg and found that the neurotoxic effect of gp120 on spine number is greatly diminished when only 1 p75NTR allele is present. Overall, our data suggest a scenario in which gp120, by increasing proBDNF levels, indirectly activates p75NTR.
The gp120tg mouse is one of the animal models used to study the mechanisms underlying the neuropathological and behavioral abnormalities seen in HAND. These animals exhibit loss of synapses that are commonly seen in HAND (Toggas and Mucke, 1996) and other neurodegenerative diseases such as Parkinson’s disease (PD) and Alzheimer’s disease (AD). Functional magnetic resonance imaging has shown that HIV promotes a pattern of degradation in brain function similar to aging (Thomas et al., 2013). However, HAND subjects are on average younger than AD or PD patients. There are several mechanisms that could explain how HIV promotes neuronal injury in aging, including metabolic and inflammatory processes, cardiovascular diseases, and immune senescence. However, data presented here and elsewhere have shown that gp120tg mice develop pathological abnormalities later in life but prior to 1 year of age, suggesting that gp120, like HIV, accelerates the aging of the brain, most likely by reducing the neurotrophic factor environment. This hypothesis has been suggested to explain the neuropathological alterations seen in PD, AD, and HAND because synaptic loss correlates with a significant decline in BDNF expression seen in these diseases (Bachis et al., 2012; Connor et al., 1997; Kunugi et al., 2001; Nagatsu et al., 2000).
5. Conclusions
We have observed an age-dependent decrease of the trophic factor environment in favor of a proapoptotic setting in an animal model of HIV infection of the brain. This phenomenon can lead to the synaptic simplification seen in HAND subjects. Because the neurotoxic effect of gp120 is greatly diminished in p75NTR heterozygous mice, blocking p75NTR function could mitigate the neurotoxic effect of HIV.
Acknowledgments
This work was supported by HHS grants NS079172 and NS074916. Special thanks to Dr. Eliezer Masliah (University of California San Diego, San Diego, CA) for the breading pairs of gp120tg mice, to Dr. Bruce Carter (Vanderbilt University, Nashville, TE) for the gift of the p75NTR antibody, and Lino Tessarollo (NCI-Frederick, MD) for discussions and advice with the animal studies. The authors are grateful to the National NeuroAIDS Tissue Consortium for providing human brain tissues.
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose.
References
- Avdoshina V, Taraballi F, Dedoni S, Corbo C, Paige M, Saygideger Kont Y, Uren A, Tasciotti E, Mocchetti I. Identification of a binding site of the human immunodeficiency virus envelope protein gp120 to neuronal specific tubulin. J. Neurochem. 2016;137:287–298. doi: 10.1111/jnc.13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Aden SA, Nosheny RL, Andrews PM, Mocchetti I. Axonal transport of human immunodeficiency virus type 1 envelope protein glycoprotein 120 is found in association with neuronal apoptosis. J. Neurosci. 2006;26:6771–6780. doi: 10.1523/JNEUROSCI.1054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Avdoshina V, Zecca L, Parsadanian M, Mocchetti I. Human immunodeficiency virus type 1 alters brain-derived neurotrophic factor processing in neurons. J. Neurosci. 2012;32:9477–9484. doi: 10.1523/JNEUROSCI.0865-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Cruz MI, Mocchetti I. M-tropic HIV envelope protein gp120 exhibits a different neuropathological profile than T-tropic gp120 in rat striatum. Eur. J. Neurosci. 2010;32:570–578. doi: 10.1111/j.1460-9568.2010.07325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LA, Avdoshina V, Day C, Lim ST, Mocchetti I. Pharmacological induction of CCL5 in vivo prevents gp120-mediated neuronal injury. Neuropharmacology. 2015;92:98–107. doi: 10.1016/j.neuropharm.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canizares S, Cherner M, Ellis RJ. HIV and aging: effects on the central nervous system. Semin. Neurol. 2014;34:27–34. doi: 10.1055/s-0034-1372340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Holt JL, Yakupov R, Jiang CS, Ernst T. Lower cognitive reserve in the aging human immunodeficiency virus-infected brain. Neurobiol. Aging. 2013;34:1240–1253. doi: 10.1016/j.neurobiolaging.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV, Rajagopal R, Lee FS. Neurotrophin signalling in health and disease. Clin. Sci. (Lond.) 2006;110:167–173. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- Chapleau CA, Pozzo-Miller L. Divergent roles of p75NTR and Trk receptors in BDNF’s effects on dendritic spine density and morphology. Neural Plast. 2012;2012:578057. doi: 10.1155/2012/578057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor B, Young D, Yan Q, Faull RL, Synek B, Dragunow M. Brain-derived neurotrophic factor is reduced in Alzheimer’s disease. Brain Res. Mol. Brain Res. 1997;49:71–81. doi: 10.1016/s0169-328x(97)00125-3. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Martin B, An Y, Maudsley S, Ferrucci L, Mattson MP, Resnick SM. Plasma BDNF is associated with age-related white matter atrophy but not with cognitive function in older, non-demented adults. PLoS One. 2012;7:e35217. doi: 10.1371/journal.pone.0035217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat. Rev. Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M, Pence BD, Martin SA, Vieria VJ, Woods JA, McAuley E, Kramer AF. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J. Neurosci. 2010;30:5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall I, Vaida F, Khanlou N, Lazzaretto D, Achim C, Letendre S, Moore D, Ellis R, Cherner M, Gelman B, Morgello S, Singer E, Grant I, Masliah E National Neuro, A.T.C. Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J. Neurovirol. 2009;15:360–370. doi: 10.3109/13550280903131915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB. Neuropathology of HAND with suppressive antiretroviral therapy: encephalitis and neurodegeneration reconsidered. Curr. HIV/AIDS Rep. 2015;12:272–279. doi: 10.1007/s11904-015-0266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, Mattson MP. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann. Neurol. 2004;55:257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- Ji Y, Pang PT, Feng L, Lu B. Cyclic AMP controls BDNF-induced TrkB phosphorylation and dendritic spine formation in mature hippocampal neurons. Nat. Neurosci. 2005;8:164–172. doi: 10.1038/nn1381. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Axon growth inhibition: signals from the p75 neurotrophin receptor. Nat. Neurosci. 2003;6:435–436. doi: 10.1038/nn0503-435. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL, Barbacid M. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell. 1993;75:113–122. [PubMed] [Google Scholar]
- Koshimizu H, Kiyosue K, Hara T, Hazama S, Suzuki S, Uegaki K, Nagappan G, Zaitsev E, Hirokawa T, Tatsu Y, Ogura A, Lu B, Kojima M. Multiple functions of precursor BDNF to CNS neurons: negative regulation of neurite growth, spine formation and cell survival. Mol. Brain. 2009;2:27. doi: 10.1186/1756-6606-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krucker T, Toggas SM, Mucke L, Siggins GR. Transgenic mice with cerebral expression of human immunodeficiency virus type-1 coat protein gp120 show divergent changes in short- and long-term potentiation in CA1 hippocampus. Neuroscience. 1998;83:691–700. doi: 10.1016/s0306-4522(97)00413-2. [DOI] [PubMed] [Google Scholar]
- Kunugi H, Ueki A, Otsuka M, Isse K, Hirasawa H, Kato N, Nabika T, Kobayashi S, Nanko S. A novel polymorphism of the brain-derived neurotrophic factor (BDNF) gene associated with late-onset Alzheimer’s disease. Mol. Psychiatry. 2001;6:83–86. doi: 10.1038/sj.mp.4000792. [DOI] [PubMed] [Google Scholar]
- Lee MH, Amin ND, Venkatesan A, Wang T, Tyagi R, Pant HC, Nath A. Impaired neurogenesis and neurite outgrowth in an HIV-gp120 transgenic model is reversed by exercise via BDNF production and Cdk5 regulation. J. Neurovirol. 2013;19:418–431. doi: 10.1007/s13365-013-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisi L, Tramutola A, De Luca A, Navarra P, Dello Russo C. Modulatory effects of the CCR5 antagonist maraviroc on microglial pro-inflammatory activation elicited by gp120. J. Neurochem. 2012;120:106–114. doi: 10.1111/j.1471-4159.2011.07549.x. [DOI] [PubMed] [Google Scholar]
- Lochner JE, Kingma M, Kuhn S, Meliza CD, Cutler B, Scalettar BA. Real-time imaging of the axonal transport of granules containing a tissue plasminogen activator/green fluorescent protein hybrid. Mol. Biol. Cell. 1998;9:2463–2476. doi: 10.1091/mbc.9.9.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Cohen MH, Weber K, Little DM, Fornelli D, Rubin LH, Perschler P, Gould F, Martin E. Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women. Neurology. 2009;72:1661–1668. doi: 10.1212/WNL.0b013e3181a55f65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC group. The HIV Neurobehavioral Research Center. Ann. Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- Minichiello L, Calella AM, Medina DL, Bonhoeffer T, Klein R, Korte M. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron. 2002;36:121–137. doi: 10.1016/s0896-6273(02)00942-x. [DOI] [PubMed] [Google Scholar]
- Molloy SS, Thomas L, Kamibayashi C, Mumby MC, Thomas G. Regulation of endosome sorting by a specific PP2A isoform. J. Cell Biol. 1998;142:1399–1411. doi: 10.1083/jcb.142.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, Murphy RA. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J. Biol. Chem. 2001;276:12660–12666. doi: 10.1074/jbc.M008104200. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson’s disease. J. Neural Transm. Suppl. 2000;60:277–290. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Bachis A, Acquas E, Mocchetti I. Human immunodeficiency virus type 1 glycoprotein gp120 reduces the levels of brain-derived neurotrophic factor in vivo: potential implication for neuronal cell death. Eur. J. Neurosci. 2004;20:2857–2864. doi: 10.1111/j.1460-9568.2004.03764.x. [DOI] [PubMed] [Google Scholar]
- Otal R, Martinez A, Soriano E. Lack of TrkB and TrkC signaling alters the synaptogenesis and maturation of mossy fiber terminals in the hippocampus. Cell Tissue Res. 2005;319:349–358. doi: 10.1007/s00441-004-1020-5. [DOI] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Buttini M, Limoges J, Bock P, Gendelman HE. An analysis of HIV-1-associated inflammatory products in brain tissue of humans and SCID mice with HIV-1 encephalitis. J. Neurovirol. 1997;3:401–416. doi: 10.3109/13550289709031186. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Gendelman HE. Murine models for human immunodeficiency virus type 1-associated dementia: the development of new treatment testing paradigms. J. Neurovirol. 2002;8(Suppl 2):49–52. doi: 10.1080/13550280290167993. [DOI] [PubMed] [Google Scholar]
- Peterson DA, Dickinson-Anson HA, Leppert JT, Lee KF, Gage FH. Central neuronal loss and behavioral impairment in mice lacking neurotrophin receptor p75. J. Comp. Neurol. 1999;404:1–20. doi: 10.1002/(sici)1096-9861(19990201)404:1<1::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Benjannet S, Pareek S, Chretien M, Murphy RA. Cellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proprotein convertases. FEBS Lett. 1996;379:247–250. doi: 10.1016/0014-5793(95)01520-5. [DOI] [PubMed] [Google Scholar]
- Soppet D, Escandon E, Maragos J, Middlemas DS, Reid SW, Blair J, Burton LE, Stanton BR, Kaplan DR, Hunter T, Nikolics K, Parada LF. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell. 1991;65:895–903. doi: 10.1016/0092-8674(91)90396-g. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GC, Kasai H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319:1683–1687. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J. Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JB, Brier MR, Snyder AZ, Vaida FF, Ances BM. Pathways to neurodegeneration: effects of HIV and aging on resting-state functional connectivity. Neurology. 2013;80:1186–1193. doi: 10.1212/WNL.0b013e318288792b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Toggas SM, Mucke L. Transgenic models in the study of AIDS dementia complex. Curr. Top Microbiol. Immunol. 1996;206:223–241. doi: 10.1007/978-3-642-85208-4_12. [DOI] [PubMed] [Google Scholar]
- Tong N, Perry SW, Zhang Q, James HJ, Guo H, Brooks A, Bal H, Kinnear SA, Fine S, Epstein LG, Dairaghi D, Schall TJ, Gendelman HE, Dewhurst S, Sharer LR, Gelbard HA. Neuronal fractalkine expression in HIV-1 encephalitis: roles for macrophage recruitment and neuroprotection in the central nervous system. J. Immunol. 2000;164:1333–1339. doi: 10.4049/jimmunol.164.3.1333. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller L. Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones. J. Physiol. 2003;553:497–509. doi: 10.1113/jphysiol.2003.052639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volosin M, Song W, Almeida RD, Kaplan DR, Hempstead BL, Friedman WJ. Interaction of survival and death signaling in basal forebrain neurons: roles of neurotrophins and proneurotrophins. J. Neurosci. 2006;26:7756–7766. doi: 10.1523/JNEUROSCI.1560-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volosin M, Trotter C, Cragnolini A, Kenchappa RS, Light M, Hempstead BL, Carter BD, Friedman WJ. Induction of proneurotrophins and activation of p75NTR-mediated apoptosis via neurotrophin receptor-interacting factor in hippocampal neurons after seizures. J. Neurosci. 2008;28:9870–9879. doi: 10.1523/JNEUROSCI.2841-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Minichiello L, Unsicker K. TrkB but not trkC receptors are necessary for postnatal maintenance of hippocampal spines. Neurobiol. Aging. 2008;29:1247–1255. doi: 10.1016/j.neurobiolaging.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat. Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- Yacoubian TA, Lo DC. Truncated and full-length TrkB receptors regulate distinct modes of dendritic growth. Nat. Neurosci. 2000;3:342–349. doi: 10.1038/73911. [DOI] [PubMed] [Google Scholar]
- Yang J, Harte-Hargrove LC, Siao CJ, Marinic T, Clarke R, Ma Q, Jing D, Lafrancois JJ, Bath KG, Mark W, Ballon D, Lee FS, Scharfman HE, Hempstead BL. proBDNF negatively regulates neuronal remodeling, synaptic transmission, and synaptic plasticity in hippocampus. Cell Rep. 2014;7:796–806. doi: 10.1016/j.celrep.2014.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagrebelsky M, Holz A, Dechant G, Barde YA, Bonhoeffer T, Korte M. The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons. J. Neurosci. 2005;25:9989–9999. doi: 10.1523/JNEUROSCI.2492-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]