Abstract
Experimental as well as clinical studies demonstrate that the immune system plays a major role in controlling generation and progression of tumors. The cancer immunoediting theory supports the notion that tumor cell immunogenicity is dynamically shaped by the immune system, as it eliminates immunogenic tumor cells in the early stage of the disease and then edits their antigenicity. The end result is the generation of a tumor cell population able to escape from immune recognition and elimination by tumor infiltrating lymphocytes. Two major mechanisms, which affect the target cells and the effector phase of the immune response, play a crucial role in the editing process. One is represented by the downregulation of tumor antigen (TA) processing and presentation because of abnormalities in the HLA class I antigen processing machinery (APM). The other one is represented by the anergy of effector immune infiltrates in the tumor microenvironment caused by aberrant inhibitory signals triggered by immune checkpoint receptor (ICR) ligands, such as programmed death ligand-1 (PD-L1). In this review, we will focus on tumor immune escape mechanisms caused by defects in HLA class I APM component expression and/or function in different types of cancer, with emphasis on head and neck cancer (HNC). We will also discuss the immunological implications and clinical relevance of these HLA class I APM abnormalities. Finally, we will describe strategies to counteract defective TA presentation with the expectation that they will enhance tumor recognition and elimination by tumor infiltrating effector T cells.
Keywords: HLA class I, APM, CTL, Immunoescape
Introduction
The cancer immunoediting theory highlights the notion that tumor cells progressively develop molecular mechanisms to evade immune recognition and elimination by host’s immune system [1]. In this setting, the immune system can readily detect and eliminate malignant cells at initial stages of the disease when they are immunogenic. However, tumors are shaped by selective pressure to downregulate immunogenic stimuli, one of which is diminishing antigen processing and presentation. Tumor antigen presentation is of particular interest among tumor immunologist and clinical oncologists because of the crucial role of this process in the generation of TA-specific adaptive immune responses [2–4], as well as initiating TA antibody-based immunotherapies where monoclonal antibodies (mAbs) enhance immune adaptive responses through upregulating NK cell activation, Th1 cytokine secretion and dendritic cell (DC) TA cross-presentation [5].
Abnormalities in antigen processing and HLA class I mediated antigen presentation are clinically relevant since they have been associated with the clinical course of the disease in many types of cancers including HNC [6,7]. Furthermore, clinical responses to TA-specific T cell-based passive immunotherapies have been limited [2] despite the presence of TA-specific cytotoxic T lymphocytes (CTL) in the tumor microenvironment. These otherwise counterintuitive results are most likely caused by abnormalities in tumor cell APM component expression and/or function [8–11]. Therefore, characterization of the molecular defects in HLA class I APM components becomes important in order to design rational strategies to counteract the mechanisms that tumor cells utilize to evade immune recognition and lysis by host’s immune system.
In this review, we will briefly describe antigen processing and presentation in the normal state and describe its defects in malignancies including HNC, review the APM and HLA class I mutational landscape with particular focus in HNC taking advantage of the large cohort of tumor specimens annotated in The Cancer Genome Atlas (TCGA). We will also comment on the immunological significance of APM defects as a means of resistance to CTL mediated lysis, describe the clinical importance of HLA class I APM defects and finally discuss some approaches to reverse APM-dependent mechanisms of tumor immune evasion.
HLA class I antigen processing and presentation
In normal cellular physiology proteins are degraded by two major pathways: the ubiquitin–proteasome pathway and the lysosomal pathway [12]. While the lysosomal pathway mostly degrades external proteins taken up by endocytosis or recycled internal proteins loading the final peptides onto HLA class II molecules, the proteasomal pathway degrades intracellular proteins through ubiquitination and loads the final peptide product generated by the proteasome onto HLA class I molecules [13,14]. A major exception to this rule is a process called cross-presentation, where external proteins taken up by professional antigen presenting cells entering to the lysosomal pathway get access to the HLA class I pathway [15].
The complex process of ubiquitination is a multi-step tandem enzymatic reaction involving 3 crucial catalytic proteins: E1 (ubiquitin activating enzyme), E2 (ubiquitin conjugating enzyme) and E3 (substrate specific ubiquitin ligase) that covalently link a 76-residue polypeptide to free amino groups on the target protein [16]. Once tagged, the target proteins are degraded by the proteasome. The proteasome is a multimeric protein complex formed by the 20S catalytic core, which has two outer rings of 7 α-subunits (α1–α7) and two inner rings of 7 β-subunits (β1–β7); two regulatory 19S particles sit on both ends of the core. Importantly, 3 β-subunits: β1, β2 and β5 are replaced by the interferon-γ (IFNγ) inducible subunits: low molecular weight protein-2, 7 and 10 (LMP2, LMP7 and LMP10), respectively. The replacement of the above mentioned subunits at the catalytic core form the immunoproteasome [17,18]. This structure generates different antigenic peptides with high affinity for HLA class I alleles [19,20]. Once these immunogenic peptides are generated, they are transported to the endoplasmic reticulum (ER) by transporter of antigen processing (TAP), which is formed by two non-covalently linked subunits TAP1 and TAP2. The assembling of TAP1 and TAP2 forms a pore on the ER membrane allowing the protein to enter the ER lumen [21–23]. Peptides that enter the ER lumen are loaded onto nascent HLA class I heavy chains, which are associated with β2-microglobulin chains, with the assistance of four chaperones: calnexin, ERp57, calreticulin and tapasin. The HLA class I complex is subsequently loaded with the peptide by tapasin (Fig. 1) [24–27]. The stabilized trimeric complex: HLA class I heavy chain, β2-microglobulin and peptide, now transverses the Golgi apparatus, shuttles to the cell membrane and fuses with it so the HLA class I peptide complex is exposed extracellularly and can be recognized by the cognate T cell receptor (TCR) on CD8+ T cells. An intact, stepwise progression of this pathway is required in order for the immunogenic peptide to reach the surface loaded onto HLA class I molecules and interact with CD8+ T cells. If any of the steps is disrupted in tumor cells, antigen presentation does not occur, leading to an impaired TA-specific CTL recognition and subsequent lysis [28].
Fig. 1.
Antigen processing machinery (APM) components. Normal cells process intracellular ubiquitinated proteins tagged for degradation via the proteasome generating peptide fragments that are loaded onto nascent HLA class I molecules inside the endoplasmic reticulum (ER). Antigen presentation on the cell surface requires intact APM machinery in order to stimulate specific CD8+ T cell effector responses.
Defects in HLA class I and APM components in tumor cells
Abnormalities of the APM machinery have been identified in many types of cancer including HNC. Most of them take place at the genetic or epigenetic level, however, there is also evidence of defects at the transcriptional and post-translational level, as follows:
Proteasome defects
Abnormalities in expression and function of the IFNγ inducible subunits LMP2, LMP7 and LMP10 have been described in HNC [11,29] as well as in other cancers such as esophageal [30–32], stomach [33], colorectal [34–36], bladder [37,38], prostate cancer [39] as well as melanoma [40,41]. The molecular basis for these defects has been described for certain types of cancer. For instance, gastric cancer shows microsatellite mutations at the gene encoding LMP7 and single nucleotide polymorphisms for LMP2 and LMP7 have been detected in the case of cervix malignancies [42]. Loss of LMP2 upregulation after IFNγ treatment has been associated with defects in transcription factors such as interferon response factor 1 (IRF1) and signal transducer and activator of transcription (STAT1) binding to promoter sequences [43]. Furthermore, defects in Janus kinase 2 (JAK2) expression have been linked to lack of interferon-mediated upregulation of LMP2 and LMP10 in melanoma [44].
Defects in TAP1, TAP2 and chaperones
Downregulation of TAP1 and TAP2 at the mRNA and protein level in cell lines and primary tumors has been documented for HNC [11,29,45–47] as well as for esophageal [30–32], stomach [33], pancreatic [48], colorectal [34–36], prostate cancers [39] and melanoma [40,41,49–51]. Interestingly, IFNγ treatment restored TAP expression in several cell lines where it was downregulated [52–54]. However, this effect was impaired in those cells with loss of JAK2 expression [44]. Therefore, JAK2 presents as a crucial mediator in IFNγ pathway activation and HLA class I upregulation. Additionally, genetic mutations at the TAP loci that impair normal protein expression or function have been reported in cervix, colorectal, gastric and lung carcinomas [33,34,36,55–57].
As for chaperones expression and function, calnexin, tapasin and ERp57 have been shown to be downregulated in HNC (maxillary sinus and larynx carcinomas) as well as in esophagus, colon, prostate, cervix and breast cancer and melanoma [29,32,34,36,39,42,49,58,59]. Additionally, a defective IFNγ signaling has been associated with low tapasin expression in melanoma cells [44]. However, more interesting is the finding of an irreversible tapasin frameshift mutation in metastatic melanoma cells that is associated with HLA-A3 gene expression selective epigenetic unresponsiveness to IFNγ, which is reversible only after DNA methyltransferase I depletion [60]. Thus, these results suggest the rational use of demethylating agents in order to increase HLA class I antigen presentation and stimulate CTL specific responses.
Defects in HLA class I molecules
Complete absence of HLA class I expression on the cell membrane has been linked with β2-microglobulin mutations and defects in peptide synthesis and transport that are concomitantly found with defects in expression of LMP, TAP and chaperones, leading to a defective peptide loading of HLA molecules and instability of the HLA class I-peptide trimolecular complex. Interestingly, HLA class I and β2-microglobulin defects can only be overcome with gene transfection, and defects in APM components can induce a very marked downregulation in HLA class I expression which can be corrected with IFNγ treatment. Defects in HLA class I expression have been described in HNC, esophagus, gastric and colon carcinomas as well as in melanoma [31,33,36,61–67]. Likewise, partial mutations in the HLA loci have been detected in laryngeal cancer [68,69] colon, cervical carcinoma and melanoma [56,65,70].
Mutational landscape of HLA class I and APM components in human tumors
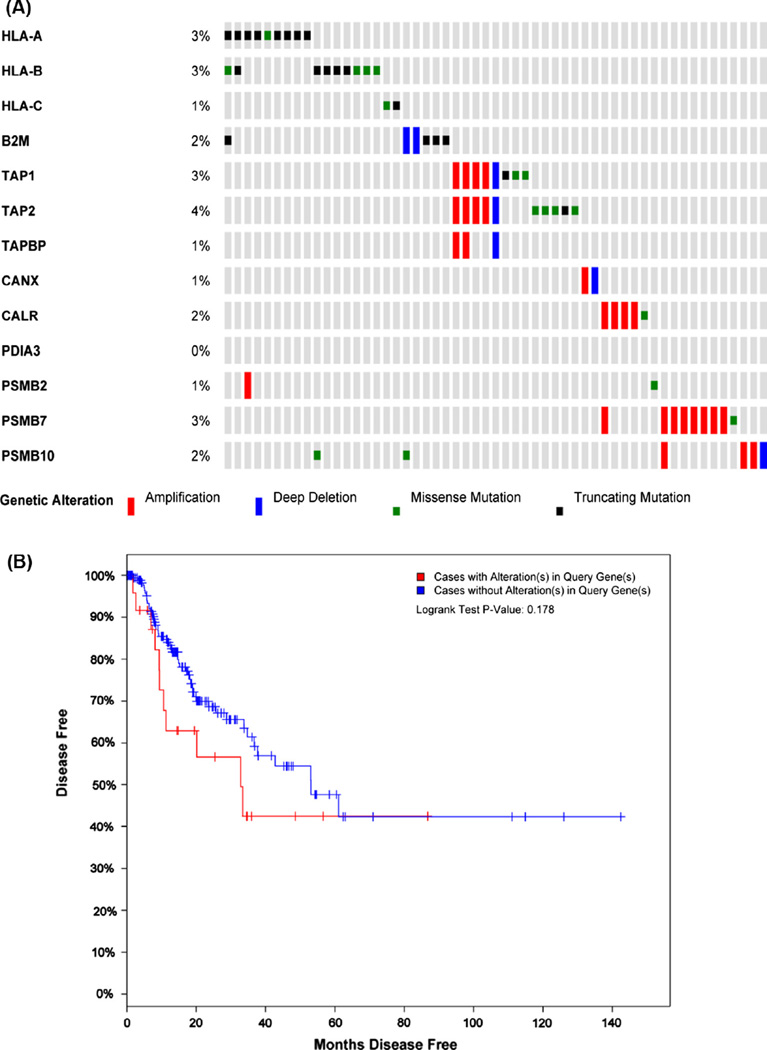
In order to further extend the in vitro findings of the abnormalities in the expression of HLA class I APM components described in the previous section herein we address the APM mutational landscape in human cancers taking advantage of the well annotated TCGA database with particular focus in squamous cell carcinomas of the lung and the head and neck. In the setting of lung carcinomas, a recent study profiled 178 tumor specimens and showed previously unreported loss-of-function mutations in the HLA class I gene locus in 3% of the specimens analyzed, with the alterations being mostly non-sense and splice site mutations [71]. Similarly, in the setting of HNC a recently published study reported that 3% of the specimens (n = 279) had mutations in the HLA-A, HLA-B and B2M loci [72], however this report did not further address if other APM components were concomitantly altered. We further analyzed TCGA database for genetic alterations in APM components (LMP2, LMP7, LMP10, TAP1, TAP2, Tapasin, Calreticulin, Calnexin, ERp57, β2-microglobulin) and HLA class I loci (HLA-A, HLA-B and HLA-C). Interestingly, we found that 20% of specimens (55/279) harbored genetic alterations in APM components and HLA class I genes (Fig. 2A), being HLA-A, HLA-B, TAP2 and LMP7 the most frequently mutated genes (4%, respectively). Importantly, the HLA-A and HLA-B loci had almost exclusively truncating mutations, while the TAP1 and TAP2 loci harbored an equivalent frequency of point mutations (missense and non-sense mutations) and gene amplification mutations (Fig. 2A). Moreover, we found that patients who did not have alterations in APM components and HLA class I loci showed longer disease free survival than those who did (53 vs. 32 months median months disease free survival, respectively. P = 0.178) (Fig. 2B), while overall survival did not show a significant difference (data not shown). Interestingly, when we analyzed the tendency of mutation co-occurrence, we found that mutations in the TAP1, TAP2 and TAPBP (Tapasin) as well as HLA-A and HLA-B loci had a statistically significant co-occurrence odds ratio (Table 1). Therefore, genetic alterations in the APM components and HLA class I are somewhat frequent in the TCGA cohort (20% out of 279 specimens) and clinically important as a potential prognostic biomarkers for survival in HNC patients, or resistance to immunotherapy.
Fig. 2.
The cancer genome atlas (TCGA) mutational landscape of APM components in head and neck cancer. (A) TCGA data from 279 HNC specimens with whole sequencing and copy number alterations (CNA) was accessed via the cBio portal (www.cbioportal.org). 20% of specimens (55/279) harbored genetic alterations in the APM gene sequences queried. An OncoPrint view was generated for each gene queried, the most altered APM genes were TAP2 (4%), TAP1 (3%), HLA-A and HLA-B (3% respectively) and PSMB7 (LMP7, 3%). Interestingly the mutations found in the HLA-A and HLA-B loci were almost exclusively truncating or missense mutations (black and green slots, respectively) while for the rest of the APM genes queried were mostly gene amplifications or deletions (red and blue slots, respectively). B2M, β2-microglobulin; TAPBP, tapasin; CANX, calnexin; CALR, calreticulin; PDIA3, ERP57; PSMB2, LMP2; PSMB7, LMP7; PSMB10, LMP10. (B) Disease free Kaplan–Meier survival curve of 276 HNC tumor specimens annotated in the TCGA with clinical data available showed that cancer patients with no genetic alterations in the APM components had better prognosis than those who had alterations (53.09 vs. 32.82 median months disease free). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Co-occurring mutations in APM components. TAP1-TAP2, TAP1-TAPBP, TAP2-TAPBP and HLA-A-HLA-B showed a highly significant tendency towards co-occurring mutations as quantified by Log Odds Ratio and Fisher’s exact test (P < 0.0001).
| Gene A | Gene A | Log odds ratio | P-value |
|---|---|---|---|
| TAP1 | TAP2 | >3 | <0.001 |
| TAP1 | TAPBP | >3 | <0.001 |
| TAP1 | TAPBP | >3 | <0.001 |
| HLA-A | HLA-B | 2.373 | 0.03 |
Immunological consequence of APM defects
Since antigen processing and presentation is a crucial event for the initiation of effector adaptive immune responses, defects in APM components and HLA class I mediated antigen presentation will most likely impair the effector phase of CTL dependent antitumor immune responses. Indeed, as we previously reported, tumor cells express tumor antigen peptides loaded onto HLA class I molecules, however, they were not sufficient to stimulate specific CTL responses, as determined by IFNγ secretion or specific target tumor cell lysis [47]. Interestingly, only when tumor targets were pulsed with exogenous antigen-derived peptide or incubated with IFNγ, they were capable of eliciting an effective CTL activation, which suggests that tumor cells may load defective or non-immunogenic peptides onto HLA class I molecules, most likely due to defects in APM components reflecting deficiencies in the generation, transport or loading of immunogenic endogenous peptides [47,54]. In fact, it has been shown that proteasome abnormalities can diminish or suppress the degradation of cytosolic proteins and generation of antigenic peptides. Moreover, alterations in the conformation of the immunoproteasome could lead to aberrant peptide generation ultimately affecting the tumor cell antigen repertoire. Likewise, TAP and chaperone defects could lead to deficient transport of peptides into the ER lumen and affect HLA class I loading and structural stabilization and complex expression on the cell surface.
In addition, as evidence of the immunological relevance of APM defects, several reports demonstrate that the expression of APM components correlates with the extent of CD8+ T cell infiltration in HNC and other malignancies [29,41,58,59,73]. Some other reports showed that impaired antigen recognition by CD8+ T lymphocytes is associated with low expression of APM components [47,74]. Importantly, the integrity of APM components is not only important for initiating an effective adaptive immune response through the generation of proper immunogenic peptides but also for maintaining normal cellular processes such as degradation of misfolded proteins, relieving ER stress and therefore inhibiting ER-stress induced apoptosis. In this scenario, tumor cells must retain these basic survival functions that the APM is responsible for, but downregulate or suppress those more specialized functions that are involved in the generation of immunogenic peptides. This exquisite regulation of expression may lead to a negative immune selection, where those tumor cells with selective non-essential APM defects survive and present HLA class I loaded with antigen peptides to CD8+ T cells and are subject to immunoediting. While those with extensive abnormalities in the proteasome-dependent protein degradation pathway and APM undergo apoptosis [75].
Noteworthy, HNC becomes a model of tumor immune evasion not only because its chemical as well viral etiology but also because it harbors immunoescape mechanisms shared by many cancer types. This is particularly true, regarding downregulation of HLA class I antigen presentation since it overexpresses the epidermal growth factor receptor (EGFR) which leads to the overactivation of multiple oncogenic downstream pathways [76]. One downstream signal is the activation of protein tyrosine phosphatase non-receptor type 11 (PTNP11), best known as src homology containing protein 2 (SHP2). EGFR-dependent SHP2 overactivation downregulates STAT1 mediated synthesis of HLA class I molecules and APM components by two mechanisms. First, by directly dephosphorylating phospho-STAT1, and second, by enhancing mitogen activated protein kinase (MAPK) pathway mediated APM component downregulation [76–78]. Indeed, experimental depletion of SHP2 induced upregulation of APM components, specific HLA class I TA presentation and generation of TA specific CTL [77].
Clinical significance and strategies to reverse APM defects
The findings presented in this review support the notion that downregulation of APM components and HLA class I antigen presentation is a common mechanism of immune evasion shared by many cancers. However, more important is to investigate whether these defects can influence the course of the disease. Several lines of evidence support the fact that abnormalities in APM components correlate with disease aggressiveness and clinicopathological outcome. In this regard, TAP1 has been shown to be an independent prognostic factor for metastases in melanoma [41]. Additionally, reduced HLA class I expression associates with advanced stage disease [40]. In the setting of HNC it has been reported that downregulation of LMP2, TAP1, TAP2 and tapasin varies from 18 to 80% in clinical specimens [11,58,79]. Moreover, Ogino et al. reported that expression level of LMP2, LMP7, TAP1, TAP2 and HLA class I predicts patient survival [58], where higher expression of APM components correlates with better survival. Additionally, the expression level of LMP7 in HNC specimens has been inversely associated with disease recurrence [11]. Therefore, these findings support the view that APM component abnormalities seen in vitro can affect the clinical outcome of disease and emerge as a possible prognostic biomarker of disease.
In addition, specific CTL-mediated tumor cell recognition plays a pivotal role initiating adaptive immune responses not only by mediating lysis of tumor targets but also expanding the antitumor immune response by secreting Th1 type cytokines, which will most likely activate antigen presenting cells in the tumor microenvironment and expand effector T cell activation. Hence, strategies to counteract tumor cell escape from T cell recognition become important. In the setting of HNC, defects in APM components are functional rather than structural in most of the cases, since IFNγ treatment could restore TAP function [6,80] and induce HLA class I dependent CTL activation and specific tumor cell lysis [54]. Therefore, strategies to enhance IFNγ signaling or alternatively STAT1 activation may be important in order to rescue immunogenic antigen presentation. As discussed previously, the EGFR becomes a readily targetable molecule to inhibit since its activation diminishes STAT1 phosphorylation via upregulation of the phosphatase SHP2. Indeed, as we recently reported, cetuximab-mediated specific EGFR blockade increased expression of APM components, TAP1 and TAP2 as well as HLA class I molecules through upregulation of IFNγ receptor I (IFNγRI) on the cell surface and subsequently STAT1 phosphorylation. Additionally, cetuximab treatment enhanced EGFR specific CTL recognition and restored tumor cell HLA class I expression in vitro. Importantly, these findings were more prominent in clinical responders than in non-responders to cetuximab therapy [81]. Moreover, we recently found that EGFR blockade could also downregulate PD-L1 surface expression in tumor cells [82], therefore diminishing the otherwise aberrant co-inhibitory signal 2 mediated by ligation of its cognate receptor programmed death 1 (PD-1) expressed in effector T cells. Importantly, we should emphasize that anti-PD-1/PD-L1 therapy would not be as successful without an effective signal 1, which involves a fully functional APM, tumor antigen processing and presentation. Consequently, reversing tumor cell downregulation of APM components by indirectly upregulating STAT1 activation and downregulating PD-L1 mediated suppression through EGFR blockade presents as a useful, clinically relevant strategy to overcome tumor immune escape.
Conclusions
The limited efficacy of conventional therapies including chemoradiotherapy and surgery in most types of cancer support the development of alternative strategies to overcome tumor proliferation. Overall, the findings presented in this article argue in favor of immunotherapy as a means to enhance adaptive immune responses in order to control tumor progression. T cell based immunotherapies have gained interest in the last decade due their success in tumor eradication in animal models, however their efficacy in the treatment of human cancers has not been as compelling [83,84]. This discrepancy may be due to a defective antigen presentation on the tumor end. In fact, as discussed here, abnormalities in APM components and HLA class I expression is the rule rather the exception in various types of cancers including HNC. Further characterization of these defects at the basic and clinical level may bring up new strategies to reverse defective antigen processing and presentation and enhance efficacy of specific tumor antigen vaccines and T cell-based immunotherapies that will ultimately rely in the onset of a strong and long-lasting adaptive effector immune response against tumors.
Acknowledgments
This work was supported by National Institute of Health grants R01 DE19727, P50 CA097190, 1R01 CA 206517-01, CA110249 and University of Pittsburgh Cancer Center Support Grant P30CA047904.
Footnotes
Conflict of interest statement
The authors disclose no potential conflicts of interest.
References
- 1.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 2.Ostrand-Rosenberg S. Animal models of tumor immunity, immunotherapy and cancer vaccines. Curr Opin Immunol. 2004;16:143–150. doi: 10.1016/j.coi.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Marsman M, Jordens I, Griekspoor A, Neefjes J. Chaperoning antigen presentation by MHC class II molecules and their role in oncogenesis. Adv Cancer Res. 2005;93:129–158. doi: 10.1016/S0065-230X(05)93004-2. [DOI] [PubMed] [Google Scholar]
- 4.Moretta L, Bottino C, Pende D, Vitale M, Mingari MC, Moretta A. Human natural killer cells: molecular mechanisms controlling NK cell activation and tumor cell lysis. Immunol Lett. 2005;100:7–13. doi: 10.1016/j.imlet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie HB, Davidson HC, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res. 2013;19:1858–1872. doi: 10.1158/1078-0432.CCR-12-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campoli M, Chang CC, Oldford SA, Edgecombe AD, Drover S, Ferrone S. HLA antigen changes in malignant tumors of mammary epithelial origin: molecular mechanisms and clinical implications. Breast Dis. 2004;20:105–125. doi: 10.3233/bd-2004-20112. [DOI] [PubMed] [Google Scholar]
- 7.Campoli M, Ferrone S, Zea AH, Rodriguez PC, Ochoa AC. Mechanisms of tumor evasion. Cancer Treat Res. 2005;123:61–88. doi: 10.1007/0-387-27545-2_3. [DOI] [PubMed] [Google Scholar]
- 8.Marincola FM, Wang E, Herlyn M, Seliger B, Ferrone S. Tumors as elusive targets of T-cell-based active immunotherapy. Trends Immunol. 2003;24:335–342. doi: 10.1016/s1471-4906(03)00116-9. [DOI] [PubMed] [Google Scholar]
- 9.Seliger B, Cabrera T, Garrido F, Ferrone S. HLA class I antigen abnormalities and immune escape by malignant cells. Semin Cancer Biol. 2002;12:3–13. doi: 10.1006/scbi.2001.0404. [DOI] [PubMed] [Google Scholar]
- 10.Whiteside TL. Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol. 2006;16:3–15. doi: 10.1016/j.semcancer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Meissner M, Reichert TE, Kunkel M, Gooding W, Whiteside TL, Ferrone S, et al. Defects in the human leukocyte antigen class I antigen processing machinery in head and neck squamous cell carcinoma: association with clinical outcome. Clin Cancer Res. 2005;11:2552–2560. doi: 10.1158/1078-0432.CCR-04-2146. [DOI] [PubMed] [Google Scholar]
- 12.Ciechanover A. Intracellular protein degradation: from a vague idea thru the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Biochim Biophys Acta. 2012;1824:3–13. doi: 10.1016/j.bbapap.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Watts C. The endosome-lysosome pathway and information generation in the immune system. Biochim Biophys Acta. 2012;1824:14–21. doi: 10.1016/j.bbapap.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yewdell JW. Not such a dismal science: the economics of protein synthesis, folding, degradation and antigen processing. Trends Cell Biol. 2001;11:294–297. doi: 10.1016/s0962-8924(01)02030-x. [DOI] [PubMed] [Google Scholar]
- 15.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 16.Koegl M, Hoppe T, Schlenker S, Ulrich HD, Mayer TU, Jentsch S. A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly. Cell. 1999;96:635–644. doi: 10.1016/s0092-8674(00)80574-7. [DOI] [PubMed] [Google Scholar]
- 17.Aki M, Shimbara N, Takashina M, Akiyama K, Kagawa S, Tamura T, et al. Interferon-gamma induces different subunit organizations and functional diversity of proteasomes. J Biochem. 1994;115:257–269. doi: 10.1093/oxfordjournals.jbchem.a124327. [DOI] [PubMed] [Google Scholar]
- 18.Shin EC, Seifert U, Kato T, Rice CM, Feinstone SM, Kloetzel PM, et al. Virus-induced type I IFN stimulates generation of immunoproteasomes at the site of infection. J Clin Invest. 2006;116:3006–3014. doi: 10.1172/JCI29832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaczynska M, Rock KL, Spies T, Goldberg AL. Peptidase activities of proteasomes are differentially regulated by the major histocompatibility complex-encoded genes for LMP2 and LMP7. Proc Natl Acad Sci USA. 1994;91:9213–9217. doi: 10.1073/pnas.91.20.9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toes RE, Nussbaum AK, Degermann S, Schirle M, Emmerich NP, Kraft M, et al. Discrete cleavage motifs of constitutive and immunoproteasomes revealed by quantitative analysis of cleavage products. J Exp Med. 2001;194:1–12. doi: 10.1084/jem.194.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arora S, Lapinski PE, Raghavan M. Use of chimeric proteins to investigate the role of transporter associated with antigen processing (TAP) structural domains in peptide binding and translocation. Proc Natl Acad Sci USA. 2001;98:7241–7246. doi: 10.1073/pnas.131132198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins CF, Linton KJ. The ATP switch model for ABC transporters. Nat Struct Mol Biol. 2004;11:918–926. doi: 10.1038/nsmb836. [DOI] [PubMed] [Google Scholar]
- 23.Parcej D, Tampe R. ABC proteins in antigen translocation and viral inhibition. Nat Chem Biol. 2010;6:572–580. doi: 10.1038/nchembio.410. [DOI] [PubMed] [Google Scholar]
- 24.Ortmann B, Androlewicz MJ, Cresswell P. MHC class I/beta 2-microglobulin complexes associate with TAP transporters before peptide binding. Nature. 1994;368:864–867. doi: 10.1038/368864a0. [DOI] [PubMed] [Google Scholar]
- 25.Grandea AG, 3rd, Lehner PJ, Cresswell P, Spies T. Regulation of MHC class I heterodimer stability and interaction with TAP by tapasin. Immunogenetics. 1997;46:477–483. doi: 10.1007/s002510050308. [DOI] [PubMed] [Google Scholar]
- 26.Hatomoto S, Ohama K, Baba E, Awai A, Tominaga M. Patients with limited life possibilities (1): support of cancer patients. Interactions with patients who suspected cancer. Kurinikaru Sutadi. 1984;5:103–108. [PubMed] [Google Scholar]
- 27.Lehner PJ, Surman MJ, Cresswell P. Soluble tapasin restores MHC class I expression and function in the tapasin-negative cell line .220. Immunity. 1998;8:221–231. doi: 10.1016/s1074-7613(00)80474-4. [DOI] [PubMed] [Google Scholar]
- 28.Sant A, Yewdell J. Antigen processing and recognition. Curr Opin Immunol. 2003;15:66–68. doi: 10.1016/s0952-7915(02)00020-1. [DOI] [PubMed] [Google Scholar]
- 29.Ogino T, Shigyo H, Ishii H, Katayama A, Miyokawa N, Harabuchi Y, et al. HLA class I antigen down-regulation in primary laryngeal squamous cell carcinoma lesions as a poor prognostic marker. Cancer Res. 2006;66:9281–9289. doi: 10.1158/0008-5472.CAN-06-0488. [DOI] [PubMed] [Google Scholar]
- 30.Liu Q, Hao C, Su P, Shi J. Down-regulation of HLA class I antigen-processing machinery components in esophageal squamous cell carcinomas: association with disease progression. Scand J Gastroenterol. 2009;44:960–969. doi: 10.1080/00365520902998679. [DOI] [PubMed] [Google Scholar]
- 31.Qifeng S, Bo C, Xingtao J, Chuanliang P, Xiaogang Z. Methylation of the promoter of human leukocyte antigen class I in human esophageal squamous cell carcinoma and its histopathological characteristics. J Thorac Cardiovasc Surg. 2011;141:808–814. doi: 10.1016/j.jtcvs.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 32.Ayshamgul H, Ma H, Ilyar S, Zhang LW, Abulizi A. Association of defective HLA-I expression with antigen processing machinery and their association with clinicopathological characteristics in Kazak patients with esophageal cancer. Chin Med J (Engl) 2011;124:341–346. [PubMed] [Google Scholar]
- 33.Hirata T, Yamamoto H, Taniguchi H, Horiuchi S, Oki M, Adachi Y, et al. Characterization of the immune escape phenotype of human gastric cancers with and without high-frequency microsatellite instability. J Pathol. 2007;211:516–523. doi: 10.1002/path.2142. [DOI] [PubMed] [Google Scholar]
- 34.Kloor M, Becker C, Benner A, Woerner SM, Gebert J, Ferrone S, et al. Immunoselective pressure and human leukocyte antigen class I antigen machinery defects in microsatellite unstable colorectal cancers. Cancer Res. 2005;65:6418–6424. doi: 10.1158/0008-5472.CAN-05-0044. [DOI] [PubMed] [Google Scholar]
- 35.Atkins D, Breuckmann A, Schmahl GE, Binner P, Ferrone S, Krummenauer F, et al. MHC class I antigen processing pathway defects, ras mutations and disease stage in colorectal carcinoma. Int J Cancer. 2004;109:265–273. doi: 10.1002/ijc.11681. [DOI] [PubMed] [Google Scholar]
- 36.Dierssen JW, de Miranda NF, Ferrone S, van Puijenbroek M, Cornelisse CJ, Fleuren GJ, et al. HNPCC versus sporadic microsatellite-unstable colon cancers follow different routes toward loss of HLA class I expression. BMC Cancer. 2007;7:33. doi: 10.1186/1471-2407-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero JM, Jimenez P, Cabrera T, Cozar JM, Pedrinaci S, Tallada M, et al. Coordinated downregulation of the antigen presentation machinery and HLA class I/beta2-microglobulin complex is responsible for HLA-ABC loss in bladder cancer. Int J Cancer. 2005;113:605–610. doi: 10.1002/ijc.20499. [DOI] [PubMed] [Google Scholar]
- 38.Cathro HP, Smolkin ME, Theodorescu D, Jo VY, Ferrone S, Frierson HF., Jr Relationship between HLA class I antigen processing machinery component expression and the clinicopathologic characteristics of bladder carcinomas. Cancer Immunol Immunother. 2010;59:465–472. doi: 10.1007/s00262-009-0765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seliger B, Stoehr R, Handke D, Mueller A, Ferrone S, Wullich B, et al. Association of HLA class I antigen abnormalities with disease progression and early recurrence in prostate cancer. Cancer Immunol Immunother. 2010;59:529–540. doi: 10.1007/s00262-009-0769-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kageshita T, Hirai S, Ono T, Hicklin DJ, Ferrone S. Down-regulation of HLA class I antigen-processing molecules in malignant melanoma: association with disease progression. Am J Pathol. 1999;154:745–754. doi: 10.1016/S0002-9440(10)65321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamarashev J, Ferrone S, Seifert B, Boni R, Nestle FO, Burg G, et al. TAP1 downregulation in primary melanoma lesions: an independent marker of poor prognosis. Int J Cancer. 2001;95:23–28. doi: 10.1002/1097-0215(20010120)95:1<23::aid-ijc1004>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 42.Hasim A, Abudula M, Aimiduo R, Ma JQ, Jiao Z, Akula G, et al. Post-transcriptional and epigenetic regulation of antigen processing machinery (APM) components and HLA-I in cervical cancers from Uighur women. PLoS ONE. 2012;7:e44952. doi: 10.1371/journal.pone.0044952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dovhey SE, Ghosh NS, Wright KL. Loss of interferon-gamma inducibility of TAP1 and LMP2 in a renal cell carcinoma cell line. Cancer Res. 2000;60:5789–5796. [PubMed] [Google Scholar]
- 44.Respa A, Bukur J, Ferrone S, Pawelec G, Zhao Y, Wang E, et al. Association of IFN-gamma signal transduction defects with impaired HLA class I antigen processing in melanoma cell lines. Clin Cancer Res. 2011;17:2668–2678. doi: 10.1158/1078-0432.CCR-10-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bandoh N, Ogino T, Katayama A, Takahara M, Katada A, Hayashi T, et al. HLA class I antigen and transporter associated with antigen processing downregulation in metastatic lesions of head and neck squamous cell carcinoma as a marker of poor prognosis. Oncol Rep. 2010;23:933–939. doi: 10.3892/or_00000717. [DOI] [PubMed] [Google Scholar]
- 46.Feenstra M, Veltkamp M, van Kuik J, Wiertsema S, Slootweg P, van den Tweel J, et al. HLA class I expression and chromosomal deletions at 6p and 15q in head and neck squamous cell carcinomas. Tissue Antigens. 1999;54:235–245. doi: 10.1034/j.1399-0039.1999.540304.x. [DOI] [PubMed] [Google Scholar]
- 47.Lopez-Albaitero A, Nayak JV, Ogino T, Machandia A, Gooding W, DeLeo AB, et al. Role of antigen-processing machinery in the in vitro resistance of squamous cell carcinoma of the head and neck cells to recognition by CTL. J Immunol. 2006;176:3402–3409. doi: 10.4049/jimmunol.176.6.3402. [DOI] [PubMed] [Google Scholar]
- 48.Pandha H, Rigg A, John J, Lemoine N. Loss of expression of antigen-presenting molecules in human pancreatic cancer and pancreatic cancer cell lines. Clin Exp Immunol. 2007;148:127–135. doi: 10.1111/j.1365-2249.2006.03289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dissemond J, Gotte P, Mors J, Lindeke A, Goos M, Ferrone S, et al. Association of TAP1 downregulation in human primary melanoma lesions with lack of spontaneous regression. Melanoma Res. 2003;13:253–258. doi: 10.1097/00008390-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Seliger B, Ritz U, Abele R, Bock M, Tampe R, Sutter G, et al. Immune escape of melanoma: first evidence of structural alterations in two distinct components of the MHC class I antigen processing pathway. Cancer Res. 2001;61:8647–8650. [PubMed] [Google Scholar]
- 51.Yang T, McNally BA, Ferrone S, Liu Y, Zheng P. A single-nucleotide deletion leads to rapid degradation of TAP-1 mRNA in a melanoma cell line. J Biol Chem. 2003;278:15291–15296. doi: 10.1074/jbc.M300954200. [DOI] [PubMed] [Google Scholar]
- 52.Ayalon O, Hughes EA, Cresswell P, Lee J, O’Donnell L, Pardi R, et al. Induction of transporter associated with antigen processing by interferon gamma confers endothelial cell cytoprotection against natural killer-mediated lysis. Proc Natl Acad Sci USA. 1998;95:2435–2440. doi: 10.1073/pnas.95.5.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma W, Lehner PJ, Cresswell P, Pober JS, Johnson DR. Interferon-gamma rapidly increases peptide transporter (TAP) subunit expression and peptide transport capacity in endothelial cells. J Biol Chem. 1997;272:16585–16590. doi: 10.1074/jbc.272.26.16585. [DOI] [PubMed] [Google Scholar]
- 54.Ferris RL, Whiteside TL, Ferrone S. Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin Cancer Res. 2006;12:3890–3895. doi: 10.1158/1078-0432.CCR-05-2750. [DOI] [PubMed] [Google Scholar]
- 55.Mehta AM, Jordanova ES, van Wezel T, Uh HW, Corver WE, Kwappenberg KM, et al. Genetic variation of antigen processing machinery components and association with cervical carcinoma. Genes Chromosom Cancer. 2007;46:577–586. doi: 10.1002/gcc.20441. [DOI] [PubMed] [Google Scholar]
- 56.Fowler NL, Frazer IH. Mutations in TAP genes are common in cervical carcinomas. Gynecol Oncol. 2004;92:914–921. doi: 10.1016/j.ygyno.2003.11.037. [DOI] [PubMed] [Google Scholar]
- 57.Chen HL, Gabrilovich D, Tampe R, Girgis KR, Nadaf S, Carbone DP. A functionally defective allele of TAP1 results in loss of MHC class I antigen presentation in a human lung cancer. Nat Genet. 1996;13:210–213. doi: 10.1038/ng0696-210. [DOI] [PubMed] [Google Scholar]
- 58.Ogino T, Bandoh N, Hayashi T, Miyokawa N, Harabuchi Y, Ferrone S. Association of tapasin and HLA class I antigen down-regulation in primary maxillary sinus squamous cell carcinoma lesions with reduced survival of patients. Clin Cancer Res. 2003;9:4043–4051. [PubMed] [Google Scholar]
- 59.Liu Y, Komohara Y, Domenick N, Ohno M, Ikeura M, Hamilton RL, et al. Expression of antigen processing and presenting molecules in brain metastasis of breast cancer. Cancer Immunol Immunother. 2012;61:789–801. doi: 10.1007/s00262-011-1137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang CC, Pirozzi G, Wen SH, Chung IH, Chiu BL, Errico S, et al. Multiple structural and epigenetic defects in the human leukocyte antigen class I antigen presentation pathway in a recurrent metastatic melanoma following immunotherapy. J Biol Chem. 2015;290:26562–26575. doi: 10.1074/jbc.M115.676130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang Q, Zhang J, Qi B, Shen C, Xie W. Downregulation of HLA class I molecules in primary oral squamous cell carcinomas and cell lines. Arch Med Res. 2009;40:256–263. doi: 10.1016/j.arcmed.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto H, Perez-Piteira J, Yoshida T, Terada M, Itoh F, Imai K, et al. Gastric cancers of the microsatellite mutator phenotype display characteristic genetic and clinical features. Gastroenterology. 1999;116:1348–1357. doi: 10.1016/s0016-5085(99)70499-3. [DOI] [PubMed] [Google Scholar]
- 63.de Miranda NF, Nielsen M, Pereira D, van Puijenbroek M, Vasen HF, Hes FJ, et al. MUTYH-associated polyposis carcinomas frequently lose HLA class I expression – a common event amongst DNA-repair-deficient colorectal cancers. J Pathol. 2009;219:69–76. doi: 10.1002/path.2569. [DOI] [PubMed] [Google Scholar]
- 64.Bicknell DC, Kaklamanis L, Hampson R, Bodmer WF, Karran P. Selection for beta 2-microglobulin mutation in mismatch repair-defective colorectal carcinomas. Curr Biol. 1996;6:1695–1697. doi: 10.1016/s0960-9822(02)70795-1. [DOI] [PubMed] [Google Scholar]
- 65.Cabrera T, Collado A, Fernandez MA, Ferron A, Sancho J, Ruiz-Cabello F, et al. High frequency of altered HLA class I phenotypes in invasive colorectal carcinomas. Tissue Antigens. 1998;52:114–123. doi: 10.1111/j.1399-0039.1998.tb02274.x. [DOI] [PubMed] [Google Scholar]
- 66.Cabrera T, Maleno I, Collado A, Lopez Nevot MA, Tait BD, Garrido F. Analysis of HLA class I alterations in tumors: choosing a strategy based on known patterns of underlying molecular mechanisms. Tissue Antigens. 2007;69(Suppl 1):264–268. doi: 10.1111/j.1399-0039.2006.00777.x. [DOI] [PubMed] [Google Scholar]
- 67.White CA, Thomson SA, Cooper L, van Endert PM, Tampe R, Coupar B, et al. Constitutive transduction of peptide transporter and HLA genes restores antigen processing function and cytotoxic T cell-mediated immune recognition of human melanoma cells. Int J Cancer. 1998;75:590–595. doi: 10.1002/(sici)1097-0215(19980209)75:4<590::aid-ijc16>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 68.Maleno I, Lopez-Nevot MA, Cabrera T, Salinero J, Garrido F. Multiple mechanisms generate HLA class I altered phenotypes in laryngeal carcinomas: high frequency of HLA haplotype loss associated with loss of heterozygosity in chromosome region 6p21. Cancer Immunol Immunother. 2002;51:389–396. doi: 10.1007/s00262-002-0296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cabrera T, Salinero J, Fernandez MA, Garrido A, Esquivias J, Garrido F. High frequency of altered HLA class I phenotypes in laryngeal carcinomas. Hum Immunol. 2000;61:499–506. doi: 10.1016/s0198-8859(00)00097-5. [DOI] [PubMed] [Google Scholar]
- 70.Seliger B. Molecular mechanisms of MHC class I abnormalities and APM components in human tumors. Cancer Immunol Immunother. 2008;57:1719–1726. doi: 10.1007/s00262-008-0515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cancer Genome Atlas Research N. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cancer Genome Atlas N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Han LY, Fletcher MS, Urbauer DL, Mueller P, Landen CN, Kamat AA, et al. HLA class I antigen processing machinery component expression and intratumoral T-Cell infiltrate as independent prognostic markers in ovarian carcinoma. Clin Cancer Res. 2008;14:3372–3379. doi: 10.1158/1078-0432.CCR-07-4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Racanelli V, Leone P, Frassanito MA, Brunetti C, Perosa F, Ferrone S, et al. Alterations in the antigen processing-presenting machinery of transformed plasma cells are associated with reduced recognition by CD8+ T cells and characterize the progression of MGUS to multiple myeloma. Blood. 2010;115:1185–1193. doi: 10.1182/blood-2009-06-228676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leone P, Shin EC, Perosa F, Vacca A, Dammacco F, Racanelli V. MHC class I antigen processing and presenting machinery: organization, function, and defects in tumor cells. J Natl Cancer Inst. 2013;105:1172–1187. doi: 10.1093/jnci/djt184. [DOI] [PubMed] [Google Scholar]
- 76.Concha-Benavente F, Srivastava RM, Ferrone S, Ferris RL. EGFR-mediated tumor immunoescape: the imbalance between phosphorylated STAT1 and phosphorylated STAT3. Oncoimmunology. 2013;2:e27215. doi: 10.4161/onci.27215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leibowitz MS, Srivastava RM, Andrade Filho PA, Egloff AM, Wang L, Seethala RR, et al. SHP2 is overexpressed and inhibits pSTAT1-mediated APM component expression, T-cell attracting chemokine secretion, and CTL recognition in head and neck cancer cells. Clin Cancer Res. 2013;19:798–808. doi: 10.1158/1078-0432.CCR-12-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Agazie YM, Hayman MJ. Molecular mechanism for a role of SHP2 in epidermal growth factor receptor signaling. Mol Cell Biol. 2003;23:7875–7886. doi: 10.1128/MCB.23.21.7875-7886.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferris RL, Hunt JL, Ferrone S. Human leukocyte antigen (HLA) class I defects in head and neck cancer: molecular mechanisms and clinical significance. Immunol Res. 2005;33:113–133. doi: 10.1385/IR:33:2:113. [DOI] [PubMed] [Google Scholar]
- 80.Chang CC, Campoli M, Ferrone S. Classical and nonclassical HLA class I antigen and NK Cell-activating ligand changes in malignant cells: current challenges and future directions. Adv Cancer Res. 2005;93:189–234. doi: 10.1016/S0065-230X(05)93006-6. [DOI] [PubMed] [Google Scholar]
- 81.Srivastava RM, Trivedi S, Concha-Benavente F, Hyun-Bae J, Wang L, Seethala RR, et al. STAT1-induced HLA class I upregulation enhances immunogenicity and clinical response to anti-EGFR mAb cetuximab therapy in HNC patients. Cancer Immunol Res. 2015;3:936–945. doi: 10.1158/2326-6066.CIR-15-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Concha-Benavente F, Srivastava RM, Trivedi S, Lei Y, Chandran U, Seethala RR, et al. Identification of the cell-intrinsic and extrinsic pathways downstream of EGFR and IFNgamma that induce PD-L1 expression in head and neck cancer. Cancer Res. 2015 doi: 10.1158/0008-5472.CAN-15-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science. 2004;305:200–205. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 84.Rosenberg SA. Development of effective immunotherapy for the treatment of patients with cancer. J Am Coll Surg. 2004;198:685–696. doi: 10.1016/j.jamcollsurg.2004.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]