Abstract
Abnormal neurogenesis occurs during embryonic development in human diabetic pregnancies and in animal models of diabetic embryopathy. Our previous studies in a mouse model of diabetic embryopathy have implicated that high glucose of maternal diabetes delays neurogenesis in the developing neuroepithelium leading to neural tube defects. However, the underlying process in high glucose-impaired neurogenesis is uncharacterized. Neurogenesis from embryonic stem (ES) cells provides a valuable model for understanding the abnormal neural lineage development under high glucose conditions. ES cells are commonly generated and maintained in high glucose (approximately 25 mM glucose). Here, the mouse ES cell line, E14, was gradually adapted to and maintained in low glucose (5 mM), and became a glucose responsive E14 (GR-E14) line. High glucose induced the endoplasmic reticulum stress marker, CHOP, in GR-E14 cells. Under low glucose conditions, the GR-E14 cells retained their pluripotency and capability to differentiate into neural lineage cells. GR-E14 cell differentiation into neural stem cells (Sox1 and nestin positive cells) was inhibited by high glucose. Neuron (Tuj1 positive cells) and glia (GFAP positive cells) differentiation from GR-E14 cells was also suppressed by high glucose. In addition, high glucose delayed GR-E14 differentiation into neural crest cells by decreasing neural crest markers, paired box 3 (Pax3) and paired box 7 (Pax7). Thus, high glucose impairs ES cell differentiation into neural lineage cells. The low glucose adapted and high glucose responsive GR-E14 cell line is a useful in vitro model for assessing the adverse effect of high glucose on the development of the central nervous system.
Keywords: Neurogenesis, High glucose, Embryonic stem cell, Differentiation, Neural lineage cells
1. Introduction
Neurogenesis, a process of neural stem cell differentiation into neuron, in early embryo development is impaired by high glucose of maternal diabetes [1]. Impaired neurogenesis contributes to failed neurulation leading to neural tube defects (NTDs) in diabetic pregnancies [1]. Although our group and others have found some genes that critically involved in NTD formation in diabetic pregnancy [2–6], high glucose-altered gene expression responsible for impaired neurogenesis has not been characterized. Additionally, whether high glucose also adversely impacts embryonic stem cells (ESCs) differentiation into neural stem cells is unknown. Previous studies essentially focus on middle gestation stage embryos, from embryonic day 8.5 (E8.5) to E10, and have revealed the pro-apoptotic and anti-differentiation effects of maternal diabetes in the developing neuroepithelium [1,4–7]. However, it is quite a challenge to investigate the effect of high glucose of maternal diabetes on very early stage embryos, especially from zygote to E5.5 embryos, due to their extremely small sizes. ESCs, derived from pre-implantation embryos, have the capability to differentiate into all three germ layer cells [8]. It is well documented that ESCs can differentiate into neural progenitors and subsequently into neurons or glia [9–11]. ESCs differentiation into neural lineage cells provides a useful model in assessing the effect of high glucose on early neural lineage specification in mimicking embryonic development.
ESCs are generated and routinely cultured in media containing high levels of glucose (25 mM) [12]. However, physiological glucose levels have been reported to be 5.0–7.8 mM in the blood, and 0.8–2.4 mM in the human and rodent brain [14]. The high glucose level of 25 mM in regular ESC media is several-fold greater than the physiological levels of glucose (approximately 5 mM), and resembles the levels of glucose found in the bloodstream of people with diabetes [13]. In order to examine the effect of high glucose on ESCs differentiation, it is necessary to establish a stable ESC line that grows at physiological (low) glucose medium and still retains its stem cell properties. We hypothesize that ESCs maintained in high glucose can be adapted to low glucose and retain their neural differentiation potential under low glucose conditions.
Several key genes are essential for ESC differentiation into neural lineage cells. The Sox1 gene is a transcription factor expressed in the ectodermal cells committing to the neural fate [12]. Sox1 promotes neurogenesis through multiple pathways [13], and Sox1 gene deficiency in mice results in abnormal ventral forebrain development [14]. It has been reported that during embryonic development, Sox1 and Pax6 are expressed sequentially, and Sox1 expression starts upon the formation of neuroectoderm followed by Pax6 expression subsequently in radial glial cells [15]. Nestin, an intermediate filament protein, is a neural stem cell marker which is critical for neurogenesis and brain development [16–18]. The transition of neural progenitor cells to neurons or glial cells is called neurogenesis or gliogenesis, respectively [19,20]. Therefore, expression of the mature neuron and glial cell markers, TuJ1 and GFAP, is a characteristic of neural specification [21,22]. Any dysfunction of these genes during early embryonic development will alter neural lineage specification during embryonic development. High glucose may affect these key gene expression during ESC differentiation into neural lineage cells.
Here, we established a low-glucose adapted murine ESC line (GR-E14) by culturing ESCs in culture media with gradually decreasing glucose levels, starting with 11.1 mM (high glucose) and ending with 5 mM (low glucose), a level similar to that in nondiabetic [23,24]. We examined the effects of high glucose (25 mM) on the low-glucose adapted GR-E14 ESCs by determining the their ability to differentiate into neural progenitor cells, neurons, glial and neural crest cells. Our findings demonstrated that high glucose impaired ESC differentiation into neural lineage cells by inhibiting gene expression.
2. Materials and methods
2.1. mESCs maintenance and adaptation to low glucose
E14 mouse ESC line was purchased from American Type Culture Collection (ATCC). E14 cells were seeded on 6-well culture plate coated with 0.1% gelatin (Millipore, Darmstadt, Germany) at 2 × 104 cells/well in DMEM medium (Life technologies, NY, USA) containing 15% ES quality FBS (Millipore, Darmstadt, Germany), 1% NEAA (Life technologies, NY, USA), 1% glutamine (Life technologies, NY, USA), 1% β-mercaptolethanol (Millipore, Darmstadt, Germany), 1000 U/ml LIF (Millipore, Darmstadt, Germany), 1% Penicillin–Streptomycin (Life technologies, NY, USA). E14 mESC line was initially transferred to a medium containing 11.1 mM glucose for 8 passages and subsequently 8.3 mM glucose for additional 8 passages. The resultant E14 cells were then transferred to medium with 5 mM glucose for 4 passages. The E14 ESC line adapted to and maintained under low glucose (5 mM) was designated as glucose responsive E14 (GR-E14) cells.
2.2. The mESCs differentiation into neural cell lineage
GR-E14 cell differentiation into neural cell lineage cells was performed as described previously [25,26]. Cells were dissociated with accutase. After culture medium added to the cells, the cells were centrifuged at 200 g for 5 min and cell pellets were re-suspended in N2B27 media containing 25%DMEM, 25% F12 medium, 50% neurobasal medium, 1% N2 supplement, 2% B27 supplement, 1% NEAA, 1% glutamine, 1% β-mercaptolethanol,1% Penicillin–Streptomycin (all medium and supplements are from Life Technologies, NY, USA). Ten microliters of cells at density of 2 × 106 cells/ml and 50 ul Matrigel (Corning, NY, USA) were dispensed into pre-chilled 1.5-ml tubes and mixed, then immediately transferred into one well of a 48-well culture plate. The cell-Matrigel suspension was allowed to solidify at room temperature for 10 min. Additional 50 μl of Matrigel were overlaid on each cell-Matrigel suspension. The cells were incubated in 0.5 ml of N2B27 media at 37 °C for 9 days, morphology changes were monitored daily under a microscope.
2.3. RNA isolation and real time PCR
Total RNAs were isolated from differentiating cells at different differentiation days by the Trizol reagent (Invitrogen, NY, USA). cDNA were synthesized by the Quantitect Reverse Transcription Kit (Qiagen, CA, USA) and real time qPCR were performed by the RT2 Sybr Green Rox Qpcr Mastermix (Qiagen, CA, USA) in a StepOnePlus system (Applied Biosystem, NY, USA). The following primers were used for RT-qPCR: SOX1 forward (Fw) 5′-AAGGAACACCCGGATTACAAGT-3′, SOX1 reverse (Rev) 5′-GTTAGCCCAGCCGTTGACAT-3′, NESTIN forward (Fw) 5′-CCCTGAAGTCGAGGAGCTG-3′, NESTIN reverse (Rev) 5′-CCCTGAAGTCGAGGAGCTG-3′, PAX6 forward (Fw) 5′-AAGGAGGGGGAGAGAACACC-3′, PAX6 reverse (Rev) 5′-TCTGAGCTTCATCCGAGT CTT-3′, TUJ1 forward (Fw) 5′-TAGACCCCAGCGGCAACTAT-3′, TUJ1 reverse (Rev) 5′-GTTCCAGGTTCCAAGTCCACC-3′, GFAP forward (Fw) 5′-GGGGCAAAAGCACCAAAGAAG-3′, GFAP reverse (Rev) 5′-GGGA-CAACTTGTATTGTGAGCC-3′, PAX3 forward (Fw) 5′-CCGGGGCA-GAATTACCCAC-3′, PAX3 reverse (Rev) 5′-GCCGTTGATAAATACTCCT CCG-3′, PAX7 forward (Fw) 5′-TCTCCAAGATTCTGTGCCGAT-3′, PAX7 reverse (Rev) 5′-CGGGGTTCTCTCTCTTATACTCC-3′.
2.4. Immunofluorescent staining
Differentiated cysts at different time points in matrigel were extracted by the cell recovery solution (Corning, NY, USA) and dissociated into single cells with Accutase for 20 min at 37 °C and fixed with 4% paraformaldehyde (pH7.4) for 20 min at room temperature followed by permeabilization with 0.25% Triton-X100 (Sigma, St. Louis, MO, USA) for 10 min. Samples were blocked for 1 h in PBS + 10% donkey serum and incubated with the following primary antibodies overnight at 4 °C: MAP2 (1:200), TUJ1 (1:500), SOX1 (1:200), NESTIN (1:200), PAX3 (1:200). All antibodies were from Cell Signaling Technology, USA. After washing with PBS, cells were incubated with a Cy3-conjugated secondary antibody (1:1000) for 4 h followed by DAPI cell nuclear counterstaining for 10 min and mounted with aqueous mounting medium. Fluorescence images were taken on a Nikon H600L microscope with the IPLab imaging system (Scientific Instrument Company, CA, USA). For confocal florescent images, the cells were recorded by a laser scanning microscope (LSM 510 META, ZEISS, Germany).
2.5. Statistical analysis
Data was analyzed by the Sigmaplot 12.5 software (Systat software Inc, CA, USA). Experiments were repeated independently three times. Data were presented as mean ± standard deviation (SD). Statistical difference was evaluated using one-way analysis of variance (ANOVA) followed with the Tukey test.
3. Results
3.1. GR-E14 cells respond to high glucose
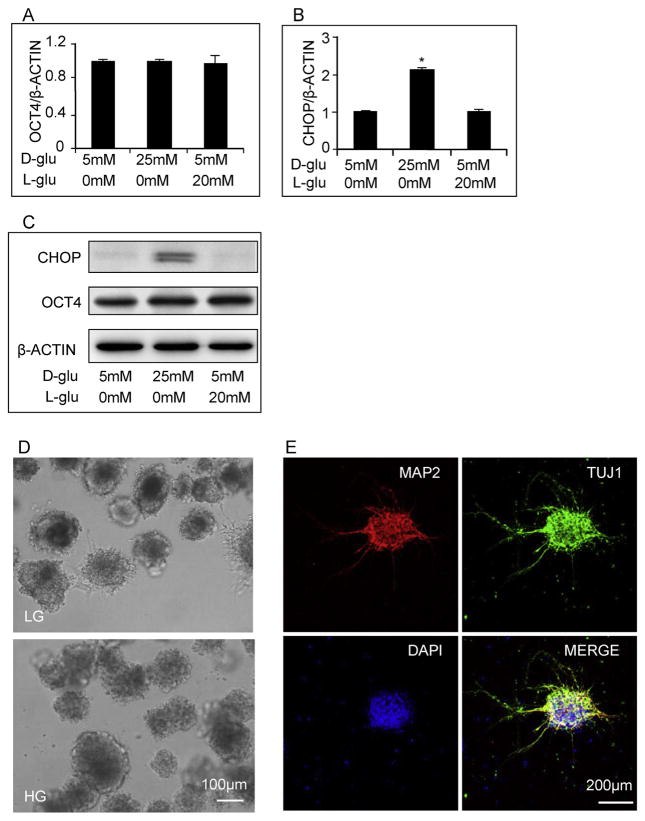
To examine whether high glucose causes ER stress, we compared the response of GR-E14 cells at high glucose (25 mM) to that at low glucose (5 mM, control). Expression of the pluripotent marker, OCT4, remained at the same levels, regardless of the concentrations of glucose in the media (Fig. 1A and C), suggesting that GR-E14 cells at various glucose levels retain their stem cell pluripotency. However, high glucose significantly up-regulated the expression of the ER stress marker CHOP (Fig. 1B, C). The glucose osmotic control group, 5 mM D-glucose + 20 mM L-glucose, did not affect CHOP expression (Fig. 1B, C).
Fig. 1. High glucose induces ER stress and delays GR-E14 cell differentiation.
levels of OCT4 mRNA (A), CHOP mRNA (B) and CHOP protein (C) from GR-E14 cells treated with low glucose (5 mM D-glucose), high glucose (25 mM D-glucose) and high L-glucose (5 mM D-glucose + 20 mM L-glucose) for 24 h. D. Morphological alteration of GR-E14 cells during neurogenesis under low glucose (5 mM) and high glucose (25 mM) at day 9. E. Immunofluorescent staining of neuron markers (MAP2 and TUJ1) during neurogenesis of GR-E14 cells under low glucose (5 mM) at day 9 of differentiation. All experiments were repeated three times (n = 3). * indicate significant difference compared with the other groups.
Under low glucose conditions, GR-E14 cells formed neurospheres at day 9 of differentiation, with outgrowth cells spreading out from the neurospheres (Fig. 1D). Immunostaining with the neuronal specific markers, MAP2 and Tuj1, showed that GR-E14 cells were differentiated into neurons under low glucose concentrations (Fig. 1E). However, under high glucose conditions, the neurospheres at differentiation day 9 were tightly impacted and outgrowth cells were hardly observed (Fig. 1D).
3.2. High glucose inhibits neural progenitor cells generation from GR-E14 cells
Sox1 and Nestin are two typical neural progenitor markers [13,17]. Under low glucose conditions, the expression of both the Sox1 and Nestin genes were dramatically increased during differentiation day 6–9 (Fig. 2A and B). Although Sox1 and Nestin gene expression in GR-E14 cells cultured under high glucose conditions increased as well, the amplitude of their expression levels were significantly lower than the levels at low glucose (Fig. 2A and B). The transcription factors Sox1 and Pax6 are expressed sequentially during early mouse embryonic neurogenesis [15]. Pax6 is involved in the progression of neuroectoderm toward radial glia [15]. Pax6 mRNA levels in GR-E14 cells cultured under low glucose conditions were increased steadily during differentiation (Fig. 2C). During the whole differentiation period we tested, Pax6 expression in cells cultured under high glucose conditions remained at the basal levels, which was almost ten times lower than that in low glucose at differentiation day 9 (Fig. 2C).
Fig. 2. High glucose inhibits neural progenitor cell generation from GR-E14 cells.
mRNA levels of SOX1 (A), NESTIN (B) and Pax6 (C) from GR-E14 cells during neurogenesis under low glucose (LG, 5 mM D-glucose) and high glucose (HG, 25 mM D-glucose) conditions. Representative images of SOX1 immunofluorescent staining (D) and SOX1 positive cell numbers (E) at differentiation day 6. Representative images of NESTIN immunofluorescent staining (F) and NESTIN positive cell numbers (G) at differentiation day 6. G. All experiments were repeated three times (n = 3). * indicate significant difference compared with the other group.
Immunostaining revealed that 37.0 ± 3.7% of Sox1 positive cells or 59.1 ± 7.0% Nestin positive cells generated under low glucose conditions, but significantly less cells generated under high glucose conditions, 12.0 ± 0.7% of Sox1 positive cells, and 18.8 ± 2.4% of Nestin positive cells (Fig. 2D–2G).
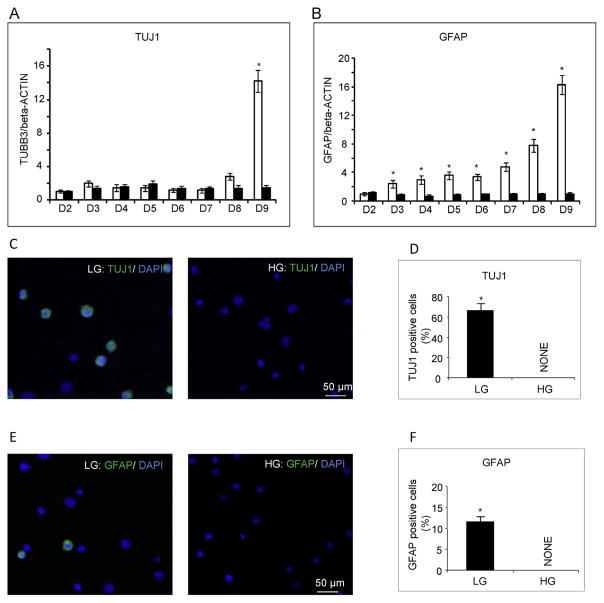
3.3. High glucose delays the differentiation of GR-E14 cells to neurons and glial cells
mRNA levels of Tuj1 (neuron marker) and GFAP (glia marker) dramatically increased from differentiation day 8–9 in GR-E14 cells cultured under low glucose conditions (Fig. 3A and B), suggesting that GR-E14 cells are capable of generating neurons or glia at this time point. However, both Tuj1 and GFAP mRNAs remained at basal levels when cells were cultured in high glucose, indicating that the formation of neurons or glia is inhibited by high glucose (Fig. 3A and B). Immunostaining demonstrated that the number of Tuj1 positive cells or GFAP positive cells was significantly higher in cells cultured under low glucose conditions than that in cells cultured under high glucose conditions (Fig. 3C–3F).
Fig. 3. High glucose impairs GR-E14 cell differentiation into neurons and glial cells.
mRNA levels of TUJ1 (A) and GFAP (B) from GR-E14 cells during neurogenesis under low glucose (LG, 5 mM D-glucose) and high glucose (HG, 25 mM D-glucose) conditions. Representative images of TUJ1 immunofluorescent staining (C) and TUJ1 positive cell numbers (D) at day 9 of differentiation. Representative images of GFAP immunofluorescent staining (E) and GFAP positive cell numbers (F) at differentiation day 9. All experiments were repeated three times (n = 3). * indicate significant difference compared with the other group.
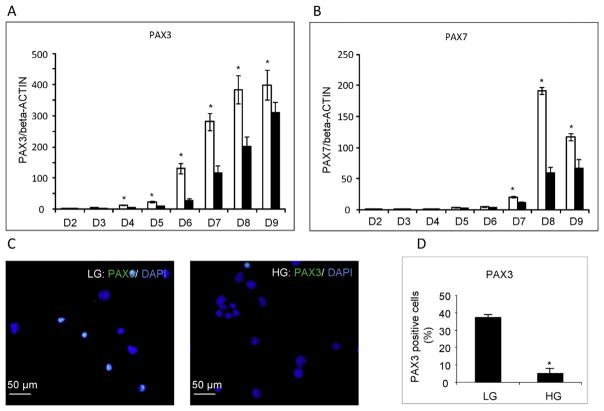
3.4. High glucose impairs GR-E14 differentiation into neural crest cells
To determine if high glucose has an inhibitory effect on neural crest cell formation, we analyzed the expression of two neural crest cell markers, Pax3 and Pax7, during GR-E14 ESCs differentiation. The expression of Pax3 and Pax7 in cells cultured under low glucose conditions started to increase at day 4 and 7, respectively, and both markers reached their highest levels at day 8 (Fig. 4A, B). Pax3 levels were significantly higher at low glucose than high glucose from differentiation day 4–9 (Fig. 4A), whereas Pax7 levels at low glucose were significantly abundant than those in high glucose from differentiation day 7–9 (Fig. 4B). Consistently, immunostaining revealed that the number of Pax3 positive cells in low glucose was significantly higher than that in high glucose (Fig. 4C, D).
Fig. 4. High glucose delays neural crest cell differentiation from GR-E14 cells.
mRNA levels of PAX3 (A) and PAX7 (B) from GR-E14 cells during neurogenesis under low glucose (LG, 5 mM D-glucose) and high glucose (HG, 25 mM D-glucose) conditions. Representative images of PAX3 immunofluorescent staining (C) and PAX3 positive cell numbers (D). All experiments were repeated three times (n = 3). * indicate significant difference compared with the other group.
4. Discussion
Maternal diabetes alters embryonic neurulation leading to NTD formation [1]. Our previous finding indicated that neurogenesis in the developing neuroepithelium was impaired by maternal diabetes [1]. However, it is unknown how high glucose affects ESCs differentiation into neural lineage cells at early development stage, such as blastocyst or zygote stages, when the tiny embryo size makes investigation difficult in humans or animal models of diabetic embryopathy. During neurulation, other neural lineage cells, such as neural crest cells, are also inhibited by maternal diabetes [27]. Therefore, it is important to create an in vitro model for investigating ESC differentiation into neural lineage cells. In the present study, the E14 mouse ESC line was gradually adapted to low glucose conditions to create a high glucose responsive ESC line, the GR-E14 line. The GR-E14 cell line was effective model in assessing the effect of high glucose on ESC differentiation into neural lineage cells. Using this model, we demonstrated for the first time that ESC differentiation into neural lineage cells was delayed and significantly impaired by high glucose.
ES cell lines are commonly established under high glucose (25 mM) conditions [28]. However this glucose concentration is significantly higher than the physiological glucose level (about 5 mM), and the ESCs generated and maintained under high glucose conditions lost their responsiveness to different glucose concentrations. Therefore, it is pivotal to obtain an ESC line which is maintained under the physiological glucose level and can respond to high glucose. In this study, an embryonic stem cell line under physiological glucose conditions was established and exhibited high glucose responsiveness. The GR-E14 cell line can be maintained more than 50 passages and still retains pluripotency. Importantly, when switched into high glucose conditions, the ER stress pathway, manifested in neurulation stage embryos from diabetic mothers [7], is activated by high glucose in GR-E14 cells. Thus, the GR-E14 cell line is a useful in vitro model for diabetic embryopathy.
Maternal diabetes impairs embryonic neurogenesis by inhibiting neural stem cell differentiation into neurons [1]. However, it is unknown whether high glucose also inhibits ESC differentiation into neural stem cells. In this study, high glucose delayed the generation of neural stem cells from GR-E14 cells. Sox1 functions as the earliest marker for neural fate decision of embryonic stem cells [29]. Nestin is expressed in immature neural progenitor cells and used as a marker for neural stem cells [30]. The expression of these two key neural stem cell markers was suppressed by high glucose during GR-E14 cell differentiation into neural stem cells. These findings support the hypothesis that high glucose inhibits embryonic stem cell differentiation into neural stem cells.
In the present study, the mature neurons appeared at differentiation day 9 in low glucose, but no neurons were present in high glucose at this time point. This finding is in agreement with our previous finding that high glucose of maternal diabetes delays neurogenesis in the developing neuroepithelium [1]. The present finding also ascertains that high glucose is a major factor in mediating the inhibitory effect of maternal diabetes on embryonic neurogenesis. The failure in neural tube closure in embryos exposed to maternal diabetes is attributed to the abnormal neural lineage specification, including neural stem cells, neurons, glia cells and neural crest cells [31]. The present study demonstrated that the differentiation of glial cells from ES cells was also suppressed by high glucose. These results collectively suggest that the ontogeny of the two major cell types, neuron and glia, in the central nervous system are adversely influenced by high glucose, which may contribute to the NTD phenotypes in diabetic mothers.
Abnormal neural crest cell development is also involved in the etiology of NTD formation in diabetic pregnancies [32]. Neural crest cells arise at the border between the neural and surface ectoderms, and migrate away once the neural tube closure occurs [33]. Abnormal expression of the neural crest markers, including Pax3 and Pax7, is commonly associated with NTD formation [34–37]. A study has reported that Pax3 expression is suppressed by high glucose during ES cell differentiation, but Pax7 expression is not affected [27]. The differentiation of neural crest cells from GR-E14 cells was suppressed by high glucose in this study. Taken together, the differentiation of the entire neural lineage cells from ES cells was inhibited by high glucose.
In summary, GR-E14 cells adapted to low glucose conditions retained their pluripotency and ability to differentiate into neural lineage cells. Both generation of neural stem cells from GR-E14 cells and neural lineage specification from neural stem cells were delayed and significantly impaired by high glucose. The GR-E14 line mimics the effect of maternal diabetes on embryonic neurulation and neurogenesis. Our study provides important insights for further understanding the effect of high glucose on neural lineage specification during embryonic development.
Acknowledgments
This work was supported by the NIH NIDDK grants R01DK083243, R01DK101972, R01DK103024, and an American Diabetes Association Basic Science Award (1-13-BS-220).
We would like to thank Dr. Julie A. Wu, Offices of the Dean and Public Affairs & Communications at the University of Maryland School of Medicine, for critical reading and editing.
Footnotes
Conflicts of interest
The authors have no competing interests to declare.
Transparency document
Transparency document related to this article can be found online at http://dx.doi.org/10.1016/j.bbrc.2016.02.117.
References
- 1.Xu C, Li X, Wang F, Weng H, Yang P. Trehalose prevents neural tube defects by correcting maternal diabetes-suppressed autophagy and neurogenesis. Am J Physiol Endocrinol Metab. 2013;305:E667–E678. doi: 10.1152/ajpendo.00185.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greene ND, Copp AJ. Neural tube defects. Annu Rev Neurosci. 2014;37:221–242. doi: 10.1146/annurev-neuro-062012-170354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu H, Yu J, Dong D, Zhou Q, Wang JY, Fang S, Yang P. High glucose-repressed CITED2 expression through miR-200b triggers the unfolded protein response and endoplasmic reticulum stress. Diabetes. 2016;65:149–163. doi: 10.2337/db15-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y, Wang F, Fu M, Wang C, Quon MJ, Yang P. Cellular stress, excessive apoptosis, and the effect of metformin in a mouse model of Type 2 diabetic embryopathy. Diabetes. 2015;64:2526–2536. doi: 10.2337/db14-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang F, Weng H, Quon MJ, Yu J, Wang JY, Hueber AO, Yang P. Dominant negative FADD dissipates the proapoptotic signalosome of the unfolded protein response in diabetic embryopathy. Am J Physiol Endocrinol Metab. 2015;309:E861–E873. doi: 10.1152/ajpendo.00215.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong D, Fu N, Yang P. MiR-17 downregulation by high glucose stabilizes thioredoxin-interacting protein and removes thioredoxin inhibition on ASK1 leading to apoptosis. Toxicol Sci. 2016 Mar;150(1):84–96. doi: 10.1093/toxsci/kfv313. http://dx.doi.org/10.1093/toxsci/kfv313. Epub 2015 Dec 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang F, Wu Y, Gu H, Reece EA, Fang S, Gabbay-Benziv R, Aberdeen G, Yang P. Ask1 gene deletion blocks maternal diabetes-induced endoplasmic reticulum stress in the developing embryo by disrupting the unfolded protein response signalosome. Diabetes. 2014;64:973–988. doi: 10.2337/db14-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamiya D, Banno S, Sasai N, Ohgushi M, Inomata H, Watanabe K, Kawada M, Yakura R, Kiyonari H, Nakao K, Jakt LM, Nishikawa S, Sasai Y. Intrinsic transition of embryonic stem-cell differentiation into neural progenitors. Nature. 2011;470:503–509. doi: 10.1038/nature09726. [DOI] [PubMed] [Google Scholar]
- 9.Germain N, Banda E, Grabel L. Embryonic stem cell neurogenesis and neural specification. J Cell Biochem. 2010;111:535–542. doi: 10.1002/jcb.22747. [DOI] [PubMed] [Google Scholar]
- 10.Kothapalli CR, Kamm RD. 3D matrix microenvironment for targeted differentiation of embryonic stem cells into neural and glial lineages. Bio-materials. 2013;34:5995–6007. doi: 10.1016/j.biomaterials.2013.04.042. [DOI] [PubMed] [Google Scholar]
- 11.Wongpaiboonwattana W, Stavridis MP. Neural differentiation of mouse embryonic stem cells in serum-free monolayer culture. J Vis Exp. 2015:e52823. doi: 10.3791/52823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pevny LH, Sockanathan S, Placzek M, Lovell-Badge R. A role for SOX1 in neural determination. Development. 1998;125:1967–1978. doi: 10.1242/dev.125.10.1967. [DOI] [PubMed] [Google Scholar]
- 13.Kan L, Israsena N, Zhang Z, Hu M, Zhao LR, Jalali A, Sahni V, Kessler JA. Sox1 acts through multiple independent pathways to promote neurogenesis. Dev Biol. 2004;269:580–594. doi: 10.1016/j.ydbio.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Malas S, Postlethwaite M, Ekonomou A, Whalley B, Nishiguchi S, Wood H, Meldrum B, Constanti A, Episkopou V. Sox1-deficient mice suffer from epilepsy associated with abnormal ventral forebrain development and olfactory cortex hyperexcitability. Neuroscience. 2003;119:421–432. doi: 10.1016/s0306-4522(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 15.Suter DM, Tirefort D, Julien S, Krause KH. A Sox1 to Pax6 switch drives neuroectoderm to radial glia progression during differentiation of mouse embryonic stem cells. Stem Cells. 2009;27:49–58. doi: 10.1634/stemcells.2008-0319. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Ji X, Li Z, Zheng H, Zheng W, Jia J, Shen H, Zhang Q, An J. Nestin overexpression promotes the embryonic development of heart and brain through the regulation of cell proliferation. Brain Res. 2015;1610:1–11. doi: 10.1016/j.brainres.2015.03.044. [DOI] [PubMed] [Google Scholar]
- 17.Lee JH, Park HS, Shin JM, Chun MH, Oh SJ. Nestin expressing progenitor cells during establishment of the neural retina and its vasculature. Anat Cell Biol. 2012;45:38–46. doi: 10.5115/acb.2012.45.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen HL, Yuh CH, Wu KK. Nestin is essential for zebrafish brain and eye development through control of progenitor cell apoptosis. PLoS One. 2010;5:e9318. doi: 10.1371/journal.pone.0009318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paridaen JT, Huttner WB. Neurogenesis during development of the vertebrate central nervous system. EMBO Rep. 2014;15:351–364. doi: 10.1002/embr.201438447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallo V, Deneen B. Glial development: the crossroads of regeneration and repair in the CNS. Neuron. 2014;83:283–308. doi: 10.1016/j.neuron.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srivastava R, Kumar M, Peineau S, Csaba Z, Mani S, Gressens P, El Ghouzzi V. Conditional induction of Math1 specifies embryonic stem cells to cerebellar granule neuron lineage and promotes differentiation into mature granule neurons. Stem Cells. 2012;31:652–665. doi: 10.1002/stem.1295. [DOI] [PubMed] [Google Scholar]
- 22.Lee HS, Han J, Lee SH, Park JA, Kim KW. Meteorin promotes the formation of GFAP-positive glia via activation of the Jak-STAT3 pathway. J Cell Sci. 2010;123:1959–1968. doi: 10.1242/jcs.063784. [DOI] [PubMed] [Google Scholar]
- 23.Abi-Saab WM, Maggs DG, Jones T, Jacob R, Srihari V, Thompson J, Kerr D, Leone P, Krystal JH, Spencer DD, During MJ, Sherwin RS. Striking differences in glucose and lactate levels between brain extracellular fluid and plasma in conscious human subjects: effects of hyperglycemia and hypoglycemia. J Cereb Blood Flow Metab. 2002;22:271–279. doi: 10.1097/00004647-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Wang F, Fisher SA, Zhong J, Wu Y, Yang P. Superoxide Dismutase 1 In Vivo Ameliorates Maternal Diabetes Mellitus†Induced Apoptosis and Heart Defects Through Restoration of Impaired Wnt Signaling. Circ Cardiovasc Genet. 2015;8:665–676. doi: 10.1161/CIRCGENETICS.115.001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Y, Carido M, Meinhardt A, Kurth T, Karl MO, Ader M, Tanaka EM. Three-dimensional neuroepithelial culture from human embryonic stem cells and its use for quantitative conversion to retinal pigment epithelium. PLoS One. 2013;8:e54552. doi: 10.1371/journal.pone.0054552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanders K, Jung JH, Loeken MR. Use of a murine embryonic stem cell line that is sensitive to high glucose environment to model neural tube development in diabetic pregnancy. Birth Defects Res A Clin Mol Teratol. 2014;100:584–591. doi: 10.1002/bdra.23281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim C, Amano T, Park J, Carter MG, Tian X, Yang X. Improvement of embryonic stem cell line derivation efficiency with novel medium, glucose concentration, and epigenetic modifications. Cloning Stem Cells. 2009;11:89–100. doi: 10.1089/clo.2008.0053. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Jiao J. Molecular biomarkers for embryonic and adult neural stem cell and neurogenesis. Biomed Res Int. 2015;2015:727542. doi: 10.1155/2015/727542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilyarov AV. Nestin in central nervous system cells. Neurosci Behav Physiol. 2008;38:165–169. doi: 10.1007/s11055-008-0025-z. [DOI] [PubMed] [Google Scholar]
- 31.Loh WT, Dheen ST, Jiang B, Kumar SD, Tay SS. Molecular and morphological characterization of neural tube defects in embryos of diabetic Swiss Albino mice. Histol Histopathol. 2011;26:965–978. doi: 10.14670/HH-26.965. [DOI] [PubMed] [Google Scholar]
- 32.Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9:557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- 33.Wilde JJ, Petersen JR, Niswander L. Genetic, epigenetic, and environmental contributions to neural tube closure. Annu Rev Genet. 2014;48:583–611. doi: 10.1146/annurev-genet-120213-092208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang D, Ighaniyan S, Stathopoulos L, Rollo B, Landman K, Hutson J, Newgreen D. The neural crest: a versatile organ system. Birth Defects Res C Embryo Today. 2014;102:275–298. doi: 10.1002/bdrc.21081. [DOI] [PubMed] [Google Scholar]
- 35.Mansouri A, Gruss P. Pax3 and Pax7 are expressed in commissural neurons and restrict ventral neuronal identity in the spinal cord. Mech Dev. 1998;78:171–178. doi: 10.1016/s0925-4773(98)00168-3. [DOI] [PubMed] [Google Scholar]
- 36.Bae CJ, Park BY, Lee YH, Tobias JW, Hong CS, Saint-Jeannet JP. Identification of Pax3 and Zic1 targets in the developing neural crest. Dev Biol. 2013;386:473–483. doi: 10.1016/j.ydbio.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murdoch B, DelConte C, Garcia-Castro MI. Pax7 lineage contributions to the mammalian neural crest. PLoS One. 2012;7:e41089. doi: 10.1371/journal.pone.0041089. [DOI] [PMC free article] [PubMed] [Google Scholar]