Abstract
In this article, we contrast evolutionary and psychobiological models of individual development to address the idea that individual development occurring in prototypically risky and unsupportive environments can be understood as adaptation. We question traditional evolutionary explanations of individual development, calling on the principle of probabilistic epigenesis to suggest that individual development resulting from the combined activity of genes and environments is best understood to precede rather than follow from evolutionary change. Specifically, we focus on the ways in which experience shapes the development of stress response physiology, with implications for individual development and intergenerational transmission of reactive, as opposed to reflective, phenotypes. In doing so, we describe results from several analyses conducted with a longitudinal data set of 1,292 children and their primary caregivers followed from birth. Our results indicate that the effects of poverty on stress response physiology and on the development of the self-regulation of behavior represent instances of the experiential canalization of development with implications for understanding the genesis and “adaptiveness” of risk behavior.
Keywords: self-regulation, stress physiology, childhood
What is the relation between individual development and evolution? Answers to this timeless question can serve as a bellwether for causal thinking in developmental psychology (see Richards, 1987). At the evolutionary end of the continuum, individual psychological development can be seen as a relatively passive process through which ecological pressures have favored specific behaviors in distinct segments of the human population, yielding differences that can be understood as adaptive for distinct niches (e.g., Penke, Dennisen, & Miller, 2007). In contrast, at the developmental end of the continuum, individual psychological development can be seen as an active process, emphasizing malleability and the role of the individual in shaping development (e.g., Cicchetti & Tucker, 1994).
This article focuses on the relation between individual development and evolution in the study of self-regulation in children. It uses research on self-regulation behavior to illustrate and support an emerging synthesis in the relation between developmental and evolutionary theory. In the following sections, we first consider how the evolutionary origins of self-regulation in our species can help developmental psychologists think about the process of development. Many developmentalists might tend to think that the study of individual differences from an evolutionary perspective requires endorsement of the transmission of fixed traits across generations. However, emergent and innovative work in evolutionary psychological theory is starting to converge with developmental thinking in ways that suggest that neural, physiological, and behavioral plasticity itself is evolutionarily adaptive and intergenerationally transmitted. In addressing this convergence of thinking, we contrast two approaches to the question of the ontogeny of individual differences in self-regulation. We then present an evolutionarily informed developmental model in which individual differences in self-regulation arise within a generation and can be expected to vary within persons over short periods of time. We then describe findings from several analyses that highlight the malleability of development in the context of rapidly changing environmental conditions. In conclusion, we consider ways that processes of individual development resulting from natural selection enable individuals to flexibly adapt behavior to ecological conditions. In so doing, we address the central theme of this special issue, namely, to understand the adaptive benefits of behavior occurring for good and for ill in response to variation in environmental quality.
Self-Regulation as a Product of Evolution and Development
What Is Self-Regulation?
We define self-regulation as a biobehavioral system that enables the primarily volitional control of attention and emotional arousal for the purposes of reflective, goal-directed action (Blair, 2010; Blair & Ursache, 2011). As such, self-regulation is composed of behavioral, cognitive, and physiological aspects of functioning that are hierarchically organized and reciprocally integrated. By this we mean that in our theory self-regulation is understood to be composed of interrelated top-down and bottom-up components. The top-down components are referred to as executive functions (Zelazo, Muller, Frye, & Marcovitch, 2003). Executive functions include working memory, inhibitory control, and the flexible shifting of the focus of attention. Executive functions enable reasoning, planning, problem solving, and future-oriented thinking and can assist in the regulation of emotion and the modulation of behavior in response to environmental contingencies. The higher order top-down executive components of self-regulation are understood to both influence and be influenced by bottom-up, less volitional aspects of self-regulation (Blair & Dennis, 2010; Lewis & Todd, 2007). These bottom-up aspects of self-regulation include more automatic, less effortful processes associated with stress physiology, emotional arousal, and attention focusing.
The bidirectional theory of self-regulation is suggested both by the underlying neurobiology of self-regulation abilities and by the now over 100-year-old Yerkes-Dodson law in which arousal and performance on complex learning tasks are related in an inverted ∪-shape curve. Neurobiologically speaking, the prefrontal cortex (PFC), the seat of executive functions, is reciprocally interconnected with limbic and brainstem structures associated with the stress response and with emotional arousal (Barbas & Zikopoulos, 2007). Here, changes in levels of neurotransmitters (dopamine, norepinephrine, glutamate, glucocorticoids) that are in part determined by stress response physiology drive synaptic activity in PFC in the familiar inverted ∪-shape curve. At very high or very low levels of stimulation and stress arousal, synaptic activity in PFC and, consequently, executive function abilities are reduced. At moderate levels of arousal, however, synaptic activity leading to long-term potentiation in the PFC is increased, and executive function abilities are optimized (Ramos & Arnsten, 2007; Robbins & Arnsten, 2009). Notably, the curvilinear relation between physiological arousal and ability is specific to complex learning. In the instance of relatively simple learning and conditioning, such as fear conditioning and the formation of robust memories of emotionally arousing events, the relation between arousal level and learning is positive and linear (Diamond, Campbell, Park, Halonen, & Zoladz, 2007).
How Evolutionary Theory Explains the Human Capacity for Self-Regulation
One of the contributions that evolutionary psychology offers developmental theory is the insight that evolution has produced the complex, integrated, and highly flexible self-regulation system outlined above. Porges (1995, 1998) described the evolution of stress response physiology from relatively simple regulation via circulatory systems to more complex reactivity and regulation under the control of increasingly elaborated neural structures that function in a coordinated bottom-up and top-down manner. Species-typical self-regulation abilities allow for reactive, highly automatic, and phylogenetically older styles of response when needed but also enable higher order, reflective cognitive processing of past, present, and future experience. In short, the evolutionary modification of the ability to modulate approach and withdrawal tendencies through the elaboration of connections between PFC and limbic structures has undeniable benefits to reproductive fitness. Such evolutionary advantages set the stage for the progressive development of humans’ cognitive control skills that allow for reasoned planning before action, dealing with increasing environmental complexity and with emotional as well as instrumental forms of threat (Luu, Tucker, & Derryberry, 1998).
Individual Differences in Self-Regulation: Two Approaches
Evolutionary Perspectives on Individual Differences
The logic of self-regulation as a product of evolutionary pressures that enhance fitness is seemingly incontestable. Implications of this evolutionary modification and shaping of the neurobiology of self-regulation for the development of self-regulation in individuals, however, are less clear. A central question is whether evolutionary modification can be understood to have maintained adaptive individual differences within species through natural selection. That is, did nature cast a relatively wide net when crafting self-regulation abilities and, in doing so, array individuals along a continuum, from more to less reactive and regulated? And if so, could processes of evolution and individual development, including active and passive processes, help to explain within species variation and intergenerational continuity in self-regulation behavior? Such reasoning could explain individual differences in constructs such as temperament, personality, preference for challenge, delay aversion and a host of related behaviors and mental states (Bjorklund, Ellis, & Rosenberg, 2007; Buss, 2009; Confer et al., 2010). Variation, the driving force of evolution, would produce alternative profiles of self-regulation within a given species that although in general similar, would vary in the extent of reactivity and regulation. Some individuals might be argued to exhibit greater propensity for withdrawal and avoidance in response to aversive stimulation, while others would exhibit greater approach and propensity for drive in response to appetitive stimulation. In this theoretical framework, individuals would also differ in the capacity for the regulation of this reactivity (see for example Figueredo et al., 2005 for review).
Such evolutionary implications for individual development, generally, can be discerned in what can be referred to as standard evolutionary models of personality and intelligence (Buss, 2009; Penke, Denissen, & Miller, 2007). Importantly, in these models, as in the primarily developmental models described below, differences among individuals are attributable to complex intergenerationally transmitted combinations of genes, physiology (or endophenotype), and phenotypic behaviors. Notably, for individual differences in personality, the process of evolution by natural selection resulting from differential fitness associated with distinct personality profiles is what is referred to as balancing-selection, that is, that different traits are favored in distinct environments and that genotype–phenotype combinations are sorted accordingly. For example, Penke et al. (2007) argued that different stable personality styles, encompassing individuals’ styles of appraisal, expectancies and goals, as well as broader brush styles of approach and avoidance, offer different types of advantages in environments that vary on dimensions of ecological threat, harshness, and the like. In contrast, selection for intelligence is presumed to reflect the likelihood that higher cognitive ability will be associated with increased fitness across most environments, and variation will therefore be attributable to mutations that are eventually selected against, referred to as mutation-selection balance (Penke et al., 2007).
From a primarily developmental perspective on individual differences, evolutionary models of individual development are overly determined. That is, such models do not adequately acknowledge the role of context in shaping the phenotype, nor do they adequately address malleability and the process of development (Lickliter, 2007; Spencer et al., 2009). From a developmental perspective, standard evolutionary explanations of individual differences do not appear to incorporate the idea of plasticity inherent in development that is acknowledged as the “leading edge” of evolution, in the first place (West-Eberhard, 2003). In these models, development can be relegated to a subsidiary or epiphenomenal role, programmed by natural selection in a process referred to as predetermined epigenesis (Gottlieb, 2002). In these models, the assumption of stability in individual development as a hallmark of reproductive fitness would seem to work well as explanation. From a probabilistic epigenetic framework, however, high levels of intraindividual variability in self-regulation development in humans suggest a high degree of continuity (as opposed to stability; see Gottlieb, 1983) and, consequently, of malleability. That is, in a probabilistic approach, development is actively and continuously shaped by the ongoing interaction of biology and environment rather than shaped by an essentially independent and stable contribution of each. In short, although individual differences in self-regulation are undoubtedly intergenerationally transmitted, leading to continuity in profiles of behavior, the linked assumption of an intergenerationally programmed stability in individual differences does not align well with recent findings in the study of self-regulation, as we illustrate below.
Toward a Developmental Model
A more complexly specified evolutionary model that incorporates the role of individual development in the process of evolutionary modification is represented by life history theory. Life history theory describes evolution and individual development in terms of adaptive trade-offs that can be arrayed on a continuum from slow to fast (e.g., maturation and reproduction). Notably, life history theory as applied by Ellis and collaborators (Del Giudice, Ellis, & Shirtcliff, 2011; Ellis, Figueredo, Brumbach, & Schlomer, 2009) is an important advance in evolutionary thinking about individual development. Its strength is that it allows for phenotypic plasticity itself as the object of natural selection. In common with the developmental psychobiological model (Gottlieb, 1992, 1998, 2002) and the model of developmental plasticity and evolution (West-Eberhard, 2003), life history theory allows for phenotypic change in advance of any change or accommodation that might occur at the genetic level. Phenotypic modifications occur in response to environmental conditions. These modifications are then transmitted intergenerationally through a variety of mechanisms that can ultimately include genes. Phenotypic changes and changes in behavior in response to environmental conditions and, secondarily, any genetic change or accommodation are then the targets of natural selection. That is, “natural selection involves a selection for the entire developmental manifold” (Gottlieb, 1997, p .76). In the developmental and life history theory models, what is being selected for is the process through which individual behavior develops in response to experience.
Both developmental psychobiological and life history theories provide the basis for understanding the tuning of self-regulation systems to more reactive or more reflective modes of response under conditions of higher versus lower adversity (e.g., Blair, 2010; Del Giudice et al., 2011). The developmental and life history approaches appear to diverge, however, in their assumptions about malleability and the potential for within-person change as a function of changing environments. For example, Belsky and others expanded many developmentalists’ ways of thinking about time in proposing that individual differences in a given behavioral outcome in response to environmental adversity may be adaptive not only across the lifespan but across multiple generations (Belsky, Steinberg, & Draper, 1991). Life history theory, as with other evolutionary approaches, however, is unclear as to the extent to which intraindividual change is possible within the life course rather than across generations. The life history approach is also unclear as to the extent to which associations between profiles of responsiveness demonstrated by parents and life history strategies demonstrated by offspring may both be due to direct effects of chronic exposure to adversity rather than linked in a causal chain. In Belsky, Steinberg, and Draper’s (1991) framework of biological sensitivity to adversity, for example, the ecology of poverty leads to mothers’ single-partner status and insensitive caregiving, which in turn leads to daughters’ higher internalizing symptoms and earlier menarche, consequently fostering daughters’ higher likelihood of “quantity-oriented” reproductive strategy (i.e., earlier fertility and higher number of births; see also Belsky, Steinberg, Houts, Halpern-Felscher, & the NICHD Early Child Care Research Network, 2010). An alternative causal structure to explain this apparent chain of effects is that chronic disadvantage manifested across multiple institutional contexts (e.g., residential and educational segregation in unsafe neighborhoods and failing schools) directly causes both the predictors and the outcomes (see Marini & Singer, 1988 and Rubin, 1986). That is, environmental adversity may directly drive the low availability of stable, marriageable partners for mothers and daughters, as well as the high levels of psychological distress and physical weathering, such as early maturation, for both generations (see Foster, Hagan, & Brooks-Gunn, 2008).
Probabilistic Epigenesis and Experiential Canalization of Self-Regulation Development
In the following sections, we describe a probabilistic epigenetic model of self-regulation development. Clearly, flexible self-regulation can be understood to enhance evolutionary fitness, has a well-substantiated neurobiological basis, and is associated with gene variants in our species. Although a product of natural selection, however, we suggest that individual differences in self-regulation are shaped by individual experience and are malleable. We outline a model in which individual differences in self-regulation are shaped by the development of stress response physiology occurring in specific types of environments. This is an instance of a process referred to as experiential canalization (Gottlieb, 1991). In doing so, we speculate on the possible nongenomic intergenerational transmission of styles of reactivity and regulation and then provide evidence to support the experiential canalization of self-regulation development in four sets of analyses that we have conducted with data from the Family Life Project, a population-based sample of 1,292 families and children in two geographically distinct nonurban regions of poverty in the United States.
The probabilistic epigenetic view suggests that evolution enables the environment to interact with species typical characteristics to shape development in specific directions, to high or low levels of functioning on a given psychological construct or behavior. A foundational example of this is found in Gottlieb’s research on maternal imprinting in mallard duck hatchlings (see Gottlieb, 1997). Gottlieb’s embryological experiments indicated that the seemingly innate and instinctual tendency of hatchlings to identify and maintain proximity to conspecifics (mallard duck mothers) is dependent on prenatal experience. Specifically, in order to exhibit the prototypical imprinting behavior, it is necessary that the mallard experience its own hypersonic vocalizations. In the absence of this experience, imprinting can occur to a variety of species. That is, the system (genes, physiology, environmental input timed pre-, peri-, and postnatally) is plastic and open to change or canalization.
Similar findings from the work of Meaney, Champagne and others (Champagne, 2010; Meaney, 2001) also serve as clear examples of the probabilistic epigenesis of development. As shown in the rat, expression of a gene associated with glucocorticoid receptor density in the hippocampus is determined by specific types of maternal behaviors (licking and grooming/arched back nursing) that are facilitated in resource rich environments but are reduced in resource poor environments (Meaney, 2001). As a consequence, offspring manifest variation in behavioral and cognitive regulation of approach and avoidance in the face of novel and/or threatening stimuli (Champagne, 2010). As such, findings from the rat model of caregiving behavior indicate a high level of inter- and intragenerational continuity. That is, parenting style received in infancy can be determining of parenting style exhibited in adulthood. The model, however, also indicates substantial potential for malleability within a single generation; cross fostering of the biological offspring of low licking and grooming mothers by high licking and grooming mothers leads to high licking and grooming behavior (Francis, Diorio, Liu, & Meaney, 1999). Central to the interpretation of these effects is the point that variation in self-regulatory profiles occurs as a function of complex, dynamic, and rapidly unfolding processes (Jirtle & Skinner, 2007; Lickliter & Honeycutt, 2003; Markham, Toth, & Lickliter, 2006; Meaney, 2010).
Self-regulation is open to experiential influence and as such can be considered adaptive. Changes in stress physiology occurring in response to the environment lead to morphological, physiological, and psychological development that produces variation in styles of self-regulation. The central question distinguishing evolutionary and developmental models, however, concerns the limits of malleability and plasticity. That is, once set in motion, how changeable are trajectories of individual differences in self-regulation development? Research in animal models would seem to suggest that interactions between aspects of the organism and the environment in which the organism is situated occur early in development and, consequently, determine the trajectory of development. This would seem to be the case for the processes examined to date, including gene expression leading to variation in hypothalamic-pituitary-adrenal (HPA) axis reactivity (Meaney & Szyf, 2005) as well as the example of maternal imprinting (Gottlieb, 1997). Notably, however, animal models (Bredy, Grant, Champagne, & Meaney, 2003; Francis, Diorio, Plotsky, & Meaney, 2002) as well as numerous human studies (Dozier, Peloso, Lewis, Laurenceau, & Levine, 2008; Ramey & Campbell, 1984; Raver, Jones, et al., 2011) indicate considerable plasticity in development and the alteration of early trajectories. It may be that for psychological and behavioral characteristics closely linked to ongoing experience, such as self-regulation development, plasticity is inherent. Openness of the system to experience and to change would be consistent with the relatively protracted time course for cortical development (Toga, Thompson, & Sowell, 2006) and the rapid changes in cognitive abilities that characterize childhood (Ferrer & McArdle, 2004).
Experiential Canalization of Self-Regulation Via Stress System Physiology
If self-regulation development is plastic and open to experience, however, then what are the relevant shapers or canalizers of development that underlie this plasticity? In addressing this question, we focus primarily on stress response physiology and on influences on it, including the quality of the early home environment and of caregiving experience. Stress response systems, including the limbic hypothalamic-pituitary-adrenal (HPA) axis and the autonomic nervous system (ANS) are primary organizers of energy metabolism and associated attentional, emotional, and behavioral responses to stimulation (Ellis, Jackson, & Boyce, 2006; McEwen, 2000; Repetti, Taylor, & Seeman, 2002). As noted above, in the realm of self-regulation, stress hormones such as norepinephrine and cortisol potentiate either more generally reactive or reflective responses to challenge depending on the amount of hormonal increase. Consistent with the inverted ∪-shape Yerkes-Dodson curve described previously, under conditions of high environmental adversity, stress hormones are potentially at levels associated with reduced cortical activity in PFC and with increased neural activity in subcortical brain areas associated with more reactive forms of learning (Champagne et al., 2008; Diamond, Campbell, Park, Halonen, & Zoladz, 2007). Under conditions of environmental support, however, stress hormones are more likely to be at moderate levels associated with increased activity in PFC and with more reflective responses to stimulation (Aston-Jones & Cohen, 2005; Ramos & Arnsten, 2007; Robbins & Arnsten, 2009). A number of studies with both animals and humans have indicated that moderate neuromodulator increase (Alexander, Hillier, Smith, Tivarus, & Beversdor, 2007; Lupien, Maheu, Tu, Fiocco, & Schramek, 2007; Vijayraghavan, Wang, Birnbaum, Williams, & Arnsten, 2007) as well as experientially driven neural sensitivity to neuromodulator levels (McNab et al., 2009) are associated with higher levels of executive cognitive functions such as working memory and the ability to flexibly regulate the focus of attention. As such, environmental quality is hypothesized to flip the self-regulation system into either a more reflective or more reactive mode and to do so flexibly in response to experience over time.
Notably, the inverted ∪-shape relation between stress hormone levels and reflective as opposed to reactive responses to stimulation maps directly onto the hypothesized ∪-shape relation between the environmental quality and the development of stress reactivity posed by Boyce and Ellis (2005). In that model, both high and low quality environments are hypothesized to lead to higher levels of stress reactivity (Ellis, Essex, & Boyce, 2005). As extended by Blair (2010) to differential susceptibility models, the reason why highly reactive individuals in more supportive contexts develop high levels of prototypically well-regulated behavior is that supportive contexts are understood to allow for the maintenance of neuromodulator levels in ranges that are conducive to synaptic activity in PFC and to the development of reflective responses to stimulation. In more adverse contexts, however, stress reactivity is hypothesized to be less well regulated, leading to decreased activity in PFC and increased activity in brain areas associated with more reactive as opposed to reflective responses to stimulation.
Research on stress physiology provides findings indicating relations among environments, the stress response, and behavior. Specifically, in support of a malleable or flexible self-regulation system, the development of stress response physiology is characterized by what is referred to as adaptive phenotypic plasticity (Cameron et al., 2005) or adaptive calibration (Del Giudice et al., 2011). That is, variation in behavioral responses to stimulation (e.g., more reactive or more reflective) is shaped or calibrated by ecologically driven variation in experience, most notably, early in development by what is referred to as the maternal provision. As such, it would seem that variation in context can perhaps be understood to influence variation in caregiving behavior, leading to variation in self-regulation that would be expected to enhance functioning within high or low resource contexts, respectively. For example, when food availability is low and predation high, reactive rather than reflective tendencies would be potentiated and would likely be more optimal. In contrast, in resource rich environments, reflective tendencies and associated benefits would be expected to enhance reproductive fitness (Ellis et al., 2009). A central question here, however, concerns the extent to which the environment actively shapes variation in self-regulation profiles (Blair, 2010) or fine-tunes existing differences among individuals (Del Giudice et al., 2011).
Important from the developmental and life history theory perspectives is the idea that stress response systems are allostatic systems, meaning that unlike homeostatic systems such as body temperature and blood pressure, which must be maintained within a relatively narrow band of functioning to maintain physical viability, stress response systems can take on a relatively broad range of plausible values (McEwen, 2000). Under conditions of chronic adversity, stress response systems adapt or are tuned to relatively higher or lower resting levels that limit the flexible regulation of stress physiology. Under these conditions, stress hormone levels are understood to be at set points that potentiate more reactive and less reflective emotional and attentional responses to stimulation (Luu & Tucker, 2004). As a consequence, although an organism may be tuned to increase its chances of survival in a hostile environment, when conditions become increasingly supportive, it is presumed that stress system physiology readjusts to set points that facilitate executive functions and top-down, reflective self-regulation. Notably, the allostatic understanding of self-regulation development provides a clear neurobiological basis for the idea that changes in environmental context, as in the provision of high quality care and education for children facing moderate to severe psychosocial disadvantage, will result in short-term and possible long-term beneficial outcomes, potentially through the mechanism of benefits to self-regulation (Blair, Berry, & Friedman, in press; Fisher, Stoolmiller, Gunnar, & Burraston, 2007; Heckman, Malofeeva, Pinto, & Savelyev, 2009).
Empirical Examples From Our Longitudinal Data
Models of allostasis and the experiential canalization of self-regulation development provide the basis for understanding how the developing organism exhibits more reflective or more reactive modes of self-regulation over time in response to experience. To empirically test our model of self-regulation development, we have focused on executive functioning and emotion regulation. As noted previously, these represent interrelated constructs that play central roles in supporting humans’ ability to appraise and respond to challenging environmental conditions (e.g., conflict or threat) in which survival may have historically been at stake. These include in humans the tendency to use higher order, top-down reflective cognitive abilities as well as more bottom-up, automatized reactions.
Findings from our research provide evidence both for the early shaping of self-regulation profiles as well as for the malleability of self-regulation development within persons over time. Specifically, in recent work we have examined the idea that the conditions of poverty have deleterious consequences for children’s stress hormone levels as indicated by salivary cortisol. We have pursued the question of whether caregiving by adults mediates the processes by which environmental stressors “get under the skin” to affect self-regulation development. As well, we present here preliminary analyses indicating that changes in the quality of the environment in which children are developing are associated with changes in measures of executive function and, possibly, with measures of stress physiology.
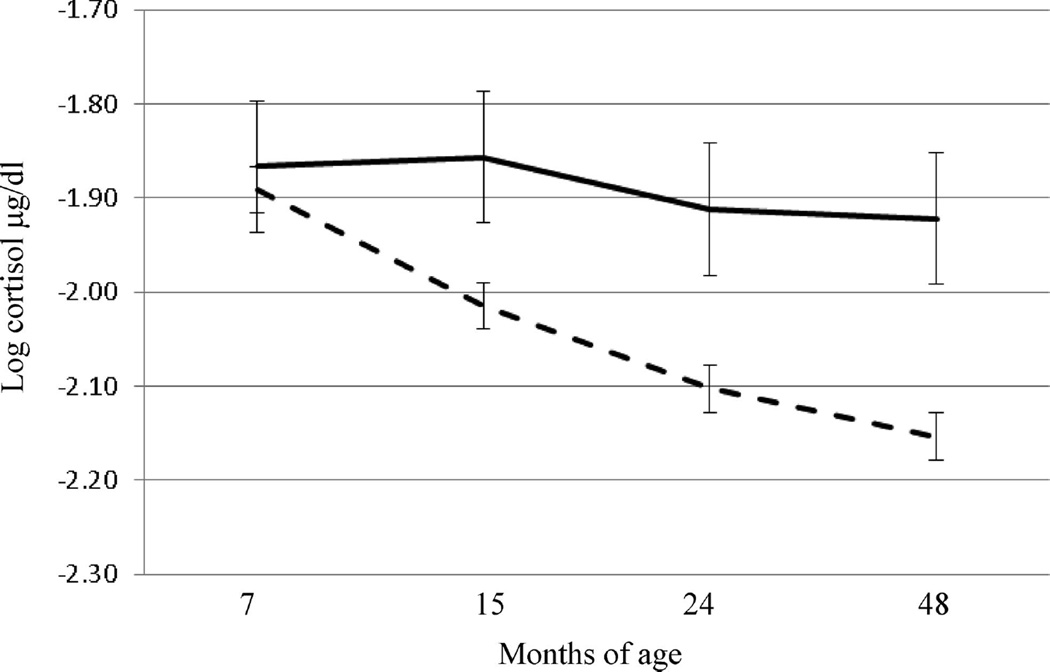
In the first of these analyses with the 1,292 families participating in the longitudinal study known as the Family Life Project, we examined the possibility that experience over the child’s first 4 years influences resting levels of salivary cortisol, an indicator of the set point of the HPA system. Here, we found both material and psychosocial aspects of poverty were relevant: Chronic exposure to inadequate housing quality, chronic exposure to perceived economic insufficiency, and turbulence in household composition (marked by adult exits from the home) were each uniquely associated with higher resting levels of cortisol over the first 4 years. Furthermore, we found that adult exits from the home interacted with cortisol levels over the first 4 years of life in a time dependent manner indicative of a process of increasing allostatic load. As seen in Figure 1, while the sample of children as a whole exhibited an expected general decrease in resting cortisol levels from 7 months to 48 months of age, those in homes characterized by a greater number of adult moves exhibited no such decline, maintaining higher resting levels across the first 4 years (Blair, Raver, Granger, Mills-Koonce, & Hibel, 2011).
Figure 1.
Relation between resting cortisol at 7, 15, 24, and 48 months of age and number of adult exits from the home; solid line = 2 or more exits, dashed line = 0 or 1 exits.
We also examined the extent to which the effects of adversity in early childhood can be understood to shape self-regulation development as indicated by executive functioning, as measured by an innovative new test battery appropriate for longitudinal use (Willoughby, Blair, Wirth, Greenberg, & the FLP Investigators, in press; Willoughby, Wirth, & Blair, 2011). We examined this possibility by testing the hypothesis that observed relations between psychosocial adversity and child executive functioning at age 3 years would be accounted for in part by relations between measures of adversity and early caregiving behavior and between early caregiving behavior and child resting cortisol levels. We found that the paths leading from adversity in the home to a latent variable representing child executive function ability were substantially reduced with the addition of paths from environmental adversity to caregiving behavior and to resting cortisol. Importantly, we found that the path from adversity to parenting to executive function was significantly mediated by a latent variable indicating children’s overall resting cortisol level in early childhood, at 7, 15, and 24 months of age (Blair, Granger, et al., 2011). Here, findings were consistent with models suggesting the tuning of generally reactive or reflective modes of responding to stimulation through information about environmental quality transmitted largely by caregiving behavior.
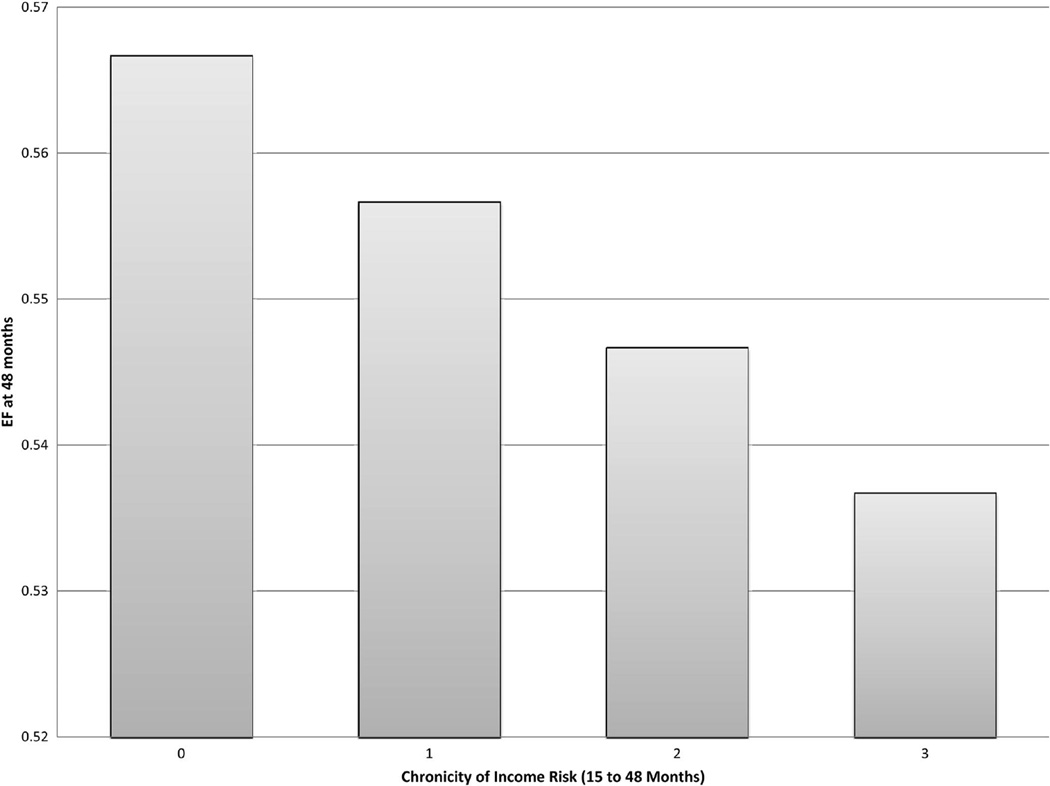
While the above findings provide evidence for canalization of development and, as such, potential for continuity and perhaps even stability, we interpret these findings primarily as evidence for plasticity (i.e., that change in the characteristics of the caregiving environment will lead to change in self-regulation.) It is of course possible, however, to interpret our findings as indicating a more fixed process (i.e., caregivers and children share background characteristics including genes that in part determine the environment, stress physiology, and associated self-regulation profiles—and that nontrivially might serve as the object of natural selection.) Consequently, in ongoing analyses, we have highlighted the importance of considering exposure to environmental conditions of adversity over time. That is, past research has often relied on measurement of environmental adversity at a single time point or cumulatively across multiple time points, with the assumption of the role of stable versus unstable environmental conditions relatively untested. In a first approach to the question of change over time in environmental adversity, we considered chronicity of exposure to low income and psychosocial strains from infancy through early childhood as predictors of children’s executive function at 48 months of age, using the test battery developed by Willoughby and Blair (Willoughby, Wirth, & Blair, 2011). As shown in Figure 2, findings indicated that chronic poverty (characterized by family income at or below the U.S. poverty threshold for 0, 1, 2, or 3 years) was more important than exposure at any one time point in predicting the cost of economic disadvantage to child executive function (Raver, Blair, Willoughby, & the FLP Investigators, 2011). Importantly, in this analysis we were careful not to discount the role of child temperament and found that cumulative exposure to environmental adversity was more strongly associated with lower executive functioning at 48 months for children exhibiting high temperamental negativity at age 7 months. Our findings are in keeping with theories of differential susceptibility offered in a number of recent studies (reviewed by Belsky & Pluess, 2009).
Figure 2.
Executive function (EF) at 48 months predicted by chronicity of income risk where chronicity (i.e., a value of 0, 1, 2, or 3) is defined as the number of approximately 12-month time periods in which family income falls at or below the U.S. poverty threshold.
In recent sets of analyses, we are further modeling changes in the conditions under which children are developing in an attempt to determine whether direct measurement of changes in environmental quality and the quality of caregiving are associated with related changes in self-regulation. In these analyses, we are examining changes from infancy to the preschool years (7–36 months) in income-to-need, maternal education, parent-child interaction quality as assessed by an observational measure (Cox, Paley, Burchinal, & Payne, 1999; National Institute of Child Health and Human Development (NICHD) Early Childcare Network, 1999), and quality of the home environment as measured by the Home Observation for Measurement of the Environment Scale (HOME; Caldwell & Bradley, 2001). Notably, across time correlations for each of these measures indicate meaningful variation from one time point to another. Correlation over time in reliable and valid measurements of the home environment and of parenting behavior by independent observers revealed only moderate stability. Correlations between adjacent time points for the measurement of the home environment were approximately r = .45. Correlations between more distal time points (e.g., 7 months and 36 months) were only approximately r = .36. Similar but slightly higher correlations were observed for the measure of parenting sensitivity.
Although much work remains to be done to identify the determinants this variation, ordinary least squares regression analyses demonstrated that changes in the quality of the home environment and changes in parenting sensitivity for each 12 month increment (e.g., from 7 to 15 months, etc.) were uniquely and meaningfully predictive of executive function ability at the child age of 48 months. For example, controlling for the initial effect of the quality of the home environment at the child age of 7 months on later executive function ability, a standard deviation change in home quality between 7 and 15 months was associated with a .14 standard deviation increment in executive function at age 48 months. Change between 15 and 24 months and between 24 and 36 months in home quality were also uniquely associated with later executive function with effects of similar magnitude. Similarly, controlling for an initial effect of parenting sensitivity at 7 months on later executive function ability, standard deviation unit changes in parenting sensitivity between measurement time points (7 to 15 months, etc.) were uniquely and incrementally related to executive function ability at age 48 months by magnitudes of .23, .18, and .10, respectively. Furthermore, these relations remained, albeit moderately reduced, with the addition of a measure of executive function ability at age 36 months. That is, change in the home environment and change in parenting sensitivity from infancy through preschool uniquely predicted executive function ability at age 48 months, even when controlling for prior executive function ability.
Examination of the relation of variation over time in the quality of the home environment and in parenting sensitivity to individual measures of child stress physiology, including salivary cortisol, salivary alpha amylase, and resting respiratory sinus arrhythmia measured at the child age of 48 months yielded no clear associations. This is likely due to the complex interrelationships among these indicators of stress physiology, leading to an absence of relations between change in the early environment and individual measures. As such, ongoing analyses will determine the best ways in which to combine the indicators in order to determine whether early stress physiology is related to changing conditions of home and parenting quality and, if so, whether it might mediate effects of environmental quality on later executive function abilities.
Conclusion and Implications
In this article we contrasted evolutionary and developmental approaches to the understanding of individual differences in self-regulation. Although there is an emerging synthesis of the two approaches, there remains a fundamental distinction between them concerning the extent to which individual differences in biobehavioral systems of self-regulation are primarily shaped by evolution, as opposed to being primarily shaped by experience. From a predominantly evolutionary framework, individual differences in self-regulation can be considered to have been selected for and to differentially interact with the environment to determine individual trajectories of psychological functioning. From a predominantly developmental perspective, however, individual differences in reactivity are understood to be shaped from conception by characteristics of the environment in which development is occurring. In presenting our research, we have attempted to highlight this distinction between the approaches and to emphasize that the distinction is subtle, important, and difficult to resolve. On the one hand, our findings, particularly on the development of cortisol levels in early childhood and on the relation of cortisol levels to executive function abilities, can be interpreted in terms of an evolutionarily determined epigenesis. That is, they can be seen to potentially illustrate the ways in which development unfolds along complex but determined lines. On the other hand, consistent with a developmental approach, we interpret our findings as indicative of an experiential shaping of development through a process of probabilistic epigenesis. In support of this probabilistic interpretation are our findings relating the chronicity of poverty to executive function abilities and relating changes in the quality of the home environment and change in the quality of parenting to later executive functioning.
Most importantly, we believe that findings from our analyses illustrate central themes in the relation between individual development and evolution and, in so doing, point to the intellectual common ground between the approaches in understanding how aspects of the environment influence individual behavior through stress system physiology. In combination, evolutionary and developmental theories provide a fertile epistemological stance against which numerous empirical questions can be posed and future biobehavioral models can be drawn and tested. Chief among the common interests of evolutionary and developmental approaches is the central theme of this special section, namely the adaptive nature of biobehavioral development occurring in adverse as opposed to supportive environments. Importantly, our findings align well with distinctions between harshness and unpredictability of environments in recent evolutionary psychology and life history theory models (Ellis et al., 2012; Belsky, Schlomer, & Ellis, 2012). In particular, we believe that in combination, these findings strongly emphasize the need for careful consideration of the ways that exposure to poverty-related hazards may remain stable for some children and may change dramatically for others over time. Central here is the conceptual value of framing individual differences not in a deficit model (which has tended to characterize much of the developmental literature) but in an experiential canalization framework. This latter framework can be productively applied to understanding behavior within context and the prospects and potential “trade-offs” of large-scale systematic efforts to bring about substantial change in individual developmental trajectories (Blair & Raver, in press). Data from “natural experiments” including economic recessions, natural disasters, and random exposures to neighborhood violence highlight the costs of exogenous shocks to children’s cognitive and emotional regulatory repertoires (Marsee, 2008; Samuelson, Krueger, Burnett, & Wilson, 2010; Sharkey, 2010; Solantaus, Leinonen, & Punamaki, 2004). Evidence from a number of randomized experiments in the fields of prevention science and educational intervention suggests that environments can be altered in positive ways with substantial discernable means to tune or shape the systems undergirding self-regulation to yield neural and physiological benefits as well as behavioral benefits (Dozier et al., 2008; O’Neal et al., 2010).
It remains to be seen whether experiments targeting infants’ cognitive and emotional self-regulation may actively shape gene expression and environmental responsiveness from caregivers in ways that serve to promote and sustain behaviors that are advantageous in those contexts. In short, we simply don’t know the limits on the malleability of development until we test the impact of multiple, sequentially staged (and randomly assigned) contrasting environmental conditions or interventions, across multiple stage-salient developmental transitions. From a developmental perspective, the limits or constraints on malleability of self-regulation profiles are few; from the standard evolutionary psychological perspective reviewed earlier, the limits on malleability would seem to be more substantial. The developmental focus on fine-grained understanding of the volatility of exposure to environmental hazard within individuals’ lifetimes and resulting discontinuities in self-regulation, however, is parallel to the focus in the evolutionary approach on phenotypic stability versus plasticity over time. The big question for both approaches concerns the sources of and modes of transmission of information needed to direct the psychological development of the individual across these shorter versus longer periods of time and across stable versus changing environmental conditions. This central question has been raised multiple times in the field of evolutionary biology (Oyama, 2000). In the field of developmental psychology, this central question has given rise to organizational (Sroufe, 1996) and dynamic systems approaches (Fogel & Thelen, 1987) that identify the combined actions of multiple elements that have rate-limiting constraints and affordances as the best approach to understanding individual development. In the search for information in systems, we believe that with evolutionary and developmental approaches one can productively pursue the idea that stress hormones fill a central role. Stress hormones act as a source of information not only from the environment to the organism but also from the organism back to the environment. Quite literally, the exogeneous stimulation of the environment can lead to changes in the stress physiology and morphology of the organism that change the behavior of the organism in ways that feedback on and maintain the conditions of the environment. The extent to which this is passed on intergenerationally or is crafted anew in individuals as development unfolds within distinct environments remains to be determined.
Acknowledgments
Support for this research was provided by National Institute of Child Health and Human Development Grants R01 HD51502 and P01 HD39667, with cofunding from the National Institute on Drug Abuse.
References
- Alexander JK, Hillier A, Smith RM, Tivarus ME, Beversdorf DQ. Beta-adrenergic modulation of cognitive flexibility during stress. Journal of Cognitive Neuroscience. 2007;19:468–478. doi: 10.1162/jocn.2007.19.3.468. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annual Review of Neuroscience. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Barbas H, Zikopoulos B. The prefrontal cortex and flexible behavior. The Neuroscientist. 2007;13:532–545. doi: 10.1177/1073858407301369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Belsky J, Schlomer GL, Ellis BJ. Beyond cumulative risk: Distinguishing harshness and unpredictability as determinants of parenting and early life history strategy. Developmental Psychology. 2012;48:662–673. doi: 10.1037/a0024454. [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy: An evolutionary theory of socialization. Child Development. 1991;62:647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, Houts R, Halpern-Felsher B the NICHD Early Child Care Research Network. The development of reproductive strategy in females: Early maternal harshness → earlier men-arche → increased sexual risk taking. Developmental Psychology. 2010;46:120–128. doi: 10.1037/a0015549. [DOI] [PubMed] [Google Scholar]
- Bjorklund DF, Ellis BJ, Rosenberg JS. Evolved probabilistic cognitive mechanisms: An evolutionary approach to gene environment development. In: Kail RV, editor. Advances in child development and behavior. Vol. 35. Oxford, England: Elsevier; 2007. pp. 1–39. [DOI] [PubMed] [Google Scholar]
- Blair C. Stress and the development of self-regulation in context. Child Development Perspectives. 2010;4:181–188. doi: 10.1111/j.1750-8606.2010.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Berry DJ, Friedman AH. The development of self-regulation in infancy and early childhood: An organizing framework for the design and evaluation of early care and education programs for children in poverty. In: Pungello E, Odom S, Gardner-Neblett N, editors. Revisioning the beginning: The implications of developmental and health science for infant/toddler care and poverty. New York, NY: Guilford; in press. [Google Scholar]
- Blair C, Dennis T. An optimal balance: Emotion-cognition integration in context. In: Calkins S, Bell M, editors. Child development at the intersection of cognition and emotion. Washington, DC: American Psychological Association; 2010. pp. 17–36. [Google Scholar]
- Blair C, Granger D, Willoughby M, Mills-Koonce R, Cox M, Greenberg MT the FLP Investigators. Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Development. 2011;82:1970–1984. doi: 10.1111/j.1467-8624.2011.01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Raver CC. Child development in the context of adversity: Experiential canalization of brain and behavior. American Psychologist. doi: 10.1037/a0027493. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Raver CC, Granger D, Mills-Koonce R, Hibel L. Allostasis and allostatic load in the context of poverty in early childhood. Development and Psychopathology. 2011;23:845–857. doi: 10.1017/S0954579411000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Ursache A. A bidirectional model of executive functions and self-regulation. In: Vohs KD, Baumeister RF, editors. Handbook of self-regulation: Research, theory, and applications. 2nd. New York, NY: Guilford; 2010. pp. 300–320. [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Grant RJ, Champagne DL, Meaney MJ. Maternal care influences neuronal survival in the hippocampus of the rat. European Journal of Neuroscience. 2003;18:2903–2909. doi: 10.1111/j.1460-9568.2003.02965.x. [DOI] [PubMed] [Google Scholar]
- Buss DM. How can evolutionary psychology successfully explain personality and individual differences? Perspectives on Psychological Science. 2009;4:359–366. doi: 10.1111/j.1745-6924.2009.01138.x. [DOI] [PubMed] [Google Scholar]
- Caldwell BM, Bradley RH. HOME Inventory Administration manual. Little Rock, AR: University of Arkansas for Medical Sciences and University of Arkansas at Little Rock; 2001. [Google Scholar]
- Cameron NM, Champagne FA, Parent C, Fish EW, Ozaki-Kuroda K, Meaney MJ. The programming of individual differences in defensive responses and reproductive strategies in the rat through variations in maternal care. Neuroscience and Biobehavioral Reviews. 2005;29:843–865. doi: 10.1016/j.neubiorev.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Champagne FA. Epigenetic perspectives on development: Evolving insights on the origins of variation. Developmental Psychobiology. 2010;52:e1–e3. [Google Scholar]
- Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, Krugers H. Maternal care and hippocampal plasticity: Evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. Journal of Neuroscience. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Tucker D. Development and self-regulatory structures of the mind. Development and Psychopathology. 1994;6:533–549. [Google Scholar]
- Confer JC, Easton JA, Fleischman DS, Goetz CD, Lewis DMG, Perilloux C, Buss DM. Evolutionary psychology: Controversies, questions, prospects and limitations. American Psychologist. 2010;65(2):110–126. doi: 10.1037/a0018413. [DOI] [PubMed] [Google Scholar]
- Cox M, Paley B, Burchinal M, Payne C. Marital perceptions and interactions across the transition to parenthood. Journal of Marriage and the Family. 1999;61:611–625. [Google Scholar]
- Del Guidice M, Ellis BJ, Shirtcliff EA. The adaptive calibration model of stress responsivity. Neuroscience and Biobehavioral Reviews. 2011;35:1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: A synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plasticity. 2007;60803:1–30. doi: 10.1155/2007/60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier M, Peloso E, Lewis E, Laurenceau J, Levine S. Effects of an attachment-based intervention on the cortisol production of infants and toddlers in foster care. Development and Psychopathology. 2008;20:845–859. doi: 10.1017/S0954579408000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Del Giudice M, Dishion TJ, Figueredo AJ, Gray P, Griskevicius V, Wilson DS. The evolutionary basis of risky adolescent behavior: Implications for science, policy, and practice. Developmental Psychology. 2012;48:598–623. doi: 10.1037/a0026220. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Essex MJ, Boyce WT. Biological sensitivity to context: II. Empirical explorations of an evolutionary-developmental theory. Development and Psychopathology. 2005;17:303–328. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Figueredo AJ, Brumbach BH, Schlomer GL. Fundamental dimensions of environmental risk: The impact of harsh versus unpredictable environments on the evolution and development of life history strategies. Human Nature. 2009;20:204–268. doi: 10.1007/s12110-009-9063-7. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Jackson JJ, Boyce WT. The stress response systems: Universality and adaptive individual differences. Developmental Review. 2006;26:175–212. [Google Scholar]
- Ferrer E, McArdle J. An experimental analysis of dynamic hypotheses about cognitive abilities and achievement from childhood to early adulthood. Developmental Psychology. 2004;40:935–952. doi: 10.1037/0012-1649.40.6.935. [DOI] [PubMed] [Google Scholar]
- Figueredo AJ, Sefcek JA, Vásquez G, Brumbach BH, King JE, Jacobs WJ. Evolutionary personality psychology. In: Buss DM, editor. Handbook of evolutionary psychology. Hoboken, NJ: Wiley; 2005. pp. 851–877. [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32:892–905. doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel A, Thelen E. Development of early expressive and communicative action: Reinterpreting the evidence from a dynamic systems perspective. Developmental Psychology. 1987;23:747–761. [Google Scholar]
- Foster H, Hagan J, Brooks-Gunn J. Growing up fast: Stress exposure and subjective weathering in emerging adulthood. Journal of Health and Social Behavior. 2008;49:162–177. doi: 10.1177/002214650804900204. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. The Journal of Neuroscience. 2002;22:7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb G. The psychobiological approach to developmental issues. In: Haith M, Campos J, editors. Handbook of child psychology. 4th. Vol. 2. New York, NY: Wiley; 1983. pp. 1–26. [Google Scholar]
- Gottlieb G. Experiential canalization of behavioral development: Theory. Developmental Psychology. 1991;27:4–13. [Google Scholar]
- Gottlieb G. Individual development and evolution: The genesis of novel behavior. New York, NY: Oxford University Press; 1992. [Google Scholar]
- Gottlieb G. Synthesizing nature-nurture: Prenatal roots of instinctive behavior. Mahwah, NJ: Erlbaum; 1997. [Google Scholar]
- Gottlieb G. Normally occurring environmental and behavioral influences on gene activity: From central dogma to probabilistic epigenesis. Psychological Review. 1998;105:792–802. doi: 10.1037/0033-295x.105.4.792-802. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Developmental-behavioral initiation of evolutionary change. Psychological Review. 2002;109:211–218. doi: 10.1037/0033-295x.109.2.211. [DOI] [PubMed] [Google Scholar]
- Heckman JJ, Malofeeva L, Pinto R, Savelyev P. The effect of the Perry Preschool program on cognitive and noncognitive skills: Beyond treatment effects. University of Chicago, IL: Department of Economics; 2009. Unpublished manuscript. [Google Scholar]
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nature Reviews Genetics. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M, Todd R. The self-regulating brain: Cortical-subcortical feedback and the development of intelligent action. Cognitive Development. 2007;22:406–430. [Google Scholar]
- Lickliter R. The dynamics of development and evolution: Insights from behavioral embryology. Developmental Psychobiology. 2007;49:749–757. doi: 10.1002/dev.20270. [DOI] [PubMed] [Google Scholar]
- Lickliter R, Honeycutt H. Developmental dynamics: Toward a biologically plausible evolutionary psychology. Psychological Bulletin. 2003;129:819–835. doi: 10.1037/0033-2909.129.6.819. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain and Cognition. 2007;65:209–237. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM. Self-regulation by the medial prefrontal cortex: Limbic representation of motive set points. In: Beauregard M, editor. Consciousness, emotional self-regulation, and the brain. Amsterdam, the Netherlands: Benjamins; 2004. pp. 123–162. [Google Scholar]
- Luu P, Tucker DM, Derryberry D. Anxiety and the motivational basis of working memory. Cognitive Therapy and Research. 1998;22:577–594. [Google Scholar]
- Marini MM, Singer B. Causality in the social sciences. Sociological Methodology. 1988;18:347–409. [Google Scholar]
- Markham R, Toth G, Lickliter R. Prenatally elevated physiological arousal interferes with perceptual learning in bobwhite quail (colinusvirginianus) embryos. Behavioral Neuroscience. 2006;120:1315–1325. doi: 10.1037/0735-7044.120.6.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsee MA. Reactive aggression and posttraumatic stress in adolescents affected by Hurricane Katrina. Journal of Clinical Child and Adolescent Psychology. 2008;37:519–529. doi: 10.1080/15374410802148152. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: From serendipity to clinical relevance. Brain Research. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, Klingberg T. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323:800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the biological definition of gene X environment interactions. Child Development. 2010;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: Life at the interface between a dynamic environment and a fixed genome. Dialogues in Clinical Neuroscience. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Child Health and Human Development (NICHD) Early Childcare Network. The effects of infant childcare on infant-mother attachment security: Results of the NICHD study of early childcare. Child Development. 1999;68:860–879. doi: 10.1111/j.1467-8624.1997.tb01967.x. [DOI] [PubMed] [Google Scholar]
- O’Neal CR, Brotman LM, Huang K, Gouley KK, Kamboukos D, Calzada EJ, Pine DS. Understanding relations among early family environment, cortisol response, and child aggression via a prevention experiment. Child Development. 2010;81:290–305. doi: 10.1111/j.1467-8624.2009.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama S. The ontogeny of information. 2nd. New York, NY: Duke University Press; 2000. [Google Scholar]
- Penke L, Dennisen JJ, Miller GF. The evolutionary genetics of personality. European Journal of Personality. 2007;21:549–587. [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology. 1995;32:301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. Love: An emergent property of the mammalian autonomic nervous system. Psychoneuroendocrinology. 1998;23:837–861. doi: 10.1016/s0306-4530(98)00057-2. [DOI] [PubMed] [Google Scholar]
- Ramey CT, Campbell FA. Preventive education for at-risk children: Cognitive consequences of the Carolina Abecedarian Project. American Journal of Mental Deficiency. 1984;88:515–523. [PubMed] [Google Scholar]
- Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: Focus on the prefrontal cortex. Pharmacology and Therapeutics. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver CC, Blair C, Willoughby M the FLP Investigators. Poverty as a predictor of 4-year-olds’ executive function: New perspectives on models of differential susceptibility. 2011 doi: 10.1037/a0028343. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver CC, Jones S, Li-Grining C, Zhai F, Bub K, Pressler E. The Chicago School Readiness Project’s impact on low-income preschoolers’ pre-academic skills: Self-regulation as a mediating mechanism. Child Development. 2011;82:362–378. doi: 10.1111/j.1467-8624.2010.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–366. [PubMed] [Google Scholar]
- Richards RJ. Darwin and the emergence of evolutionary theories of mind and behavior. Chicago IL: University of Chicago Press; 1987. [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: Monoaminergic modulation. Annual Review of Neuroscience. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB. Statistics and causal inference: Comment: Which ifs have causal answers. Journal of the American Statistical Association. 1986;81:961–962. [Google Scholar]
- Samuelson KW, Krueger CE, Burnett C, Wilson CK. Neuropsychological functioning in children with posttraumatic stress disorder. Child Neuropsychology. 2010;16:119–133. doi: 10.1080/09297040903190782. [DOI] [PubMed] [Google Scholar]
- Sharkey P. The acute effects of local homicides on children’s cognitive performance. Proceedings of the National Academy of Sciences of the USA. 2010;107:11733–11738. doi: 10.1073/pnas.1000690107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solantaus T, Leinonen J, Punamaki R. Children’s mental health in times of economic recession: Replication and extension of the family economic stress model in Finland. Developmental Psychology. 2004;40:412–429. doi: 10.1037/0012-1649.40.3.412. [DOI] [PubMed] [Google Scholar]
- Spencer JP, Blumberg MS, McMurray B, Robinson SR, Samuelson LK, Tomblin JB. Short arms and talking eggs: Why we should no longer abide the nativist-empiricist debate. Child Development Perspectives. 2009;3:79–87. doi: 10.1111/j.1750-8606.2009.00081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroufe LA. The organization of emotional life in the early years. New York, NY: Cambridge University Press; 1996. [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends in Neurosciences. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nature Neuroscience. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- West-Eberhard M. Developmental plasticity and evolution. New York, NY: Oxford University Press; 2003. [Google Scholar]
- Willoughby MW, Blair C, Wirth RJ, Greenberg MT the FLP Investigators. Executive function in early childhood: Longitudinal measurement invariance and developmental change. Psychological Assessment. doi: 10.1037/a0025779. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby M, Wirth RJ, Blair C. Contributions of modern measurement theory to measuring executive function in early childhood: An empirical demonstration. Journal of Experimental Child Psychology. 2011;108:414–435. doi: 10.1016/j.jecp.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD, Muller U, Frye D, Marcovitch S. The development of executive function in early childhood. Monographs of the Society for Research in Child Development. 2003;68 doi: 10.1111/j.0037-976x.2003.00260.x. (3, Serial No. 274) [DOI] [PubMed] [Google Scholar]