Abstract
The authors examine the effects of poverty-related adversity on child development, drawing upon psychobiological principles of experiential canalization and the biological embedding of experience. They integrate findings from research on stress physiology, neurocognitive function, and self-regulation to consider adaptive processes in response to adversity as an aspect of children’s development. Recent research on early caregiving is paired with research in prevention science to provide a reorientation of thinking about the ways in which psychosocial and economic adversity are related to continuity in human development.
Keywords: stress, self-regulation, preventive intervention, poverty, development
Perceptual and cognitive development in infancy and early childhood, perhaps throughout the life span, are to a considerable extent characterized by the shaping and constraining of abilities by experience, by gain through loss. A widely cited illustrative example of such environmentally induced specificity in development is a decline in the ability to discriminate phonetic contrasts in infancy. Between approximately 6 and 10 months of age, infants lose the ability to discriminate phonemes in nonnative languages while maintaining and strengthening the ability to discriminate phonemes in the native language (Kuhl, Williams, Lacerda, Stevens, & Lindblom, 1992; Werker & Tees, 1986). With such a trade-off or narrowing of development in infancy, however, come gains in perceptual ability that enable the coordination of multiple sensory attributes (Pons, Lewkowicz, Soto-Faraco, & Sebastián-Galleés, 2009). The tuning of perceptual networks to attend primarily to regularly occurring contrasts precedes the development of the ability to integrate across perceptual networks, facilitating the emergence of more elaborate and complex types of perceptual experience (Lewkowicz & Ghazanfar, 2009).
The progressive selection and shaping of abilities has also been proposed as a chief characteristic of life span development (Baltes, Staudinger, & Lindenberger, 1999). In life span theory, development in later adulthood has been described as a process of selective optimization with compensation, in which ability within a given domain is maintained by a narrowing of the focus and scope of activities within that domain in order to compensate for a gradual decline in ability. Although only one of several competing theories concerning development in middle and later adulthood, the theory of selective optimization with compensation embodies the idea that the shaping of behavior by biology and experience represents a general developmental process involving trade-offs. As development takes away with one hand, it gives with the other.
The idea that development is shaped by biology and experience coactively to promote specific abilities over others is known as experiential canalization (Gottlieb, 1991, 1997). Experiential canalization describes a general developmental process through which biology and typically occurring experience combine, often in ways that go largely unnoticed, to influence behavior. A foundational demonstration of the process of the experiential canalization of development is provided by Gilbert Gottlieb’s research on the development of the recognition of the maternal call in mallard and wood duck hatchlings. Recognition of the maternal call, in which the hatchling orients to the vocalizations of its own species and not to those of another, appears to be a classic example of instinctual behavior, meaning that it is hardwired and innate. Gottlieb demonstrated, however, that the wiring that underlies this behavior is malleable and that this seemingly instinctual behavior is driven as much by experience as by genes. A central idea in the canalization model is that experience induces functional activity from the behavioral level to the cellular level to shape development to maximize functioning within a specific expected environment. As such, the environment in combination with genetic background directs the process of development; this combination functions as the source of information in a developmental system. In other words, directions for development are not simply encoded in DNA or present in the environment in a predetermined sense (Oyama, 2000); rather, genetic information and environmental information coactively and probabilistically determine behavioral and psychological development.
Without reference to experiential canalization, little in development makes sense. That is, without taking into account functional relations across levels of analysis, explanations for processes of development become overly determined, and the individual contributions of either biology or experience, of nature or nurture, are overemphasized (Lickliter & Honeycutt, 2003). Accordingly, experiential canalization, or the selective optimization of behavior in response to experience, is a central aspect of what is known as the developmental psychobiological model. This model offers a framework for understanding the implications of developmental trade-offs, of opportunities taken or foreclosed, that are inherent in distinct developmental pathways. Such a perspective on development provides for greater complexity and specificity as well as for greater probability of change or reversibility than is implied by an additive or simple interactive model of biological and environmental inputs leading to child outcomes.
Poverty, Parenting, and the Psychobiology of Self-Regulation
In this article we apply the developmental psychobiological model of experiential canalization to research on children’s development in the context of poverty-related adversity in an effort to break new ground for interpreting recent research findings and for designing future research and preventive and therapeutic interventions. Consideration of psychobiological processes may be particularly important for understanding the ways in which variation in typical experience associated with socioeconomic status (SES) affects child development. It is well established that the material and psychosocial contexts of poverty adversely affect multiple aspects of development in children (Bradley & Corwin, 2002; Duncan & Brooks-Gunn, 2000; Noble, McCandliss, & Farah, 2007). Poverty affects where and how family members live, limiting housing options to those that are often characterized by higher levels of crowding, violence, and lack of safety (Evans, 2004; Kohen, Leventhal, Dahinten, & McIntosh, 2008). Economic hardship exacerbates conflict between adults, with children in poor households facing a higher probability of disrupted social relationships with key adults in their lives (Watson & McLanahan, 2011). As parents struggle with a range of stressors, the probability of parents’ depressive symptoms, emotional distress, and expressions of anger and aggression in the household also rises, with cascading effects on children’s psychological development (Ackerman & Brown, 2010; Foster & Brooks-Gunn, 2009; Molnar, Buka, Brennan, Holton, & Earls, 2003). Children in conditions of economic hardship face a wide array of dangers (e.g., higher probability of exposure to environmental teratogens such as lead, higher levels of noise and crowding, and lower levels of household and neighborhood safety) and simultaneously lower access to supportive environments, such as high-quality child care (Brooks-Gunn & Duncan, 1997).
The material and psychosocial hardships of poverty are very real, and their effects on development are often severe. As such, these effects, generally speaking, have tended to be characterized within a deficit model in which children are seen as lacking specific inputs, whether environmental or genetic or both, that are needed to avoid compromised development. For example, a gradient between the amount of input, such as maternal language, and output, such as vocabulary development in children, is well established and has been shown to covary with income (Hart & Risley, 1985). Consideration of the context of poverty only from a deficit-oriented, input– output perspective on child development, however, is of less theoretical and empirical value than one might hope. An important feature of the experiential canalization model is that it indicates the relevance of focusing not only on the absence of particular types of stimulation but also on the presence of alternative types of stimulation that actively shape development to meet a specific set of contingencies. Although cognition and behavior in children from low-income homes are often clearly differentiated from those of their middle-income counterparts, there is little to suggest that the mechanisms underlying the observed differences, whether defined in terms of environmental factors or in terms of genetic similarity, are best explained in an additive, input– output fashion. The principle of experiential canalization indicates the need to focus on the ways in which variables across levels of analysis, from the genetic to the social, combine to shape development in favor of one trajectory over another and to promote continuity for good and for ill.
Experiential Canalization of Development in Low- Versus High-Resource Environments
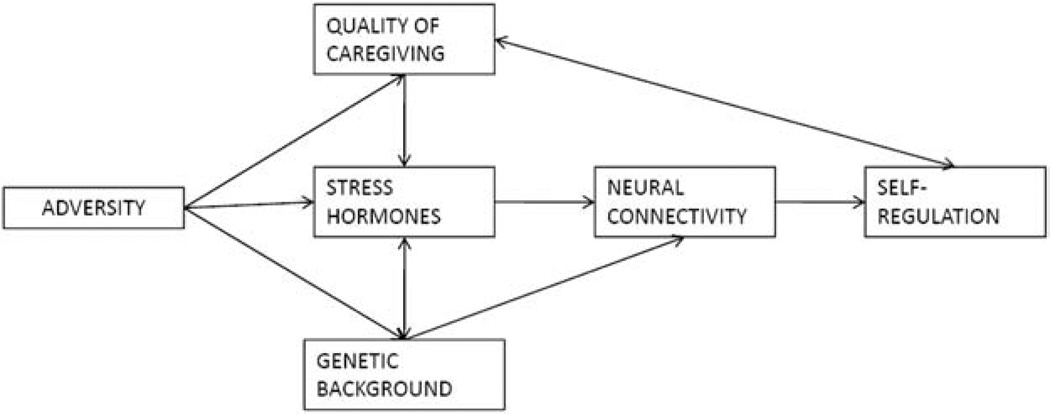
Recent advances in neuroscience and neuropsychology illustrate the developmental psychobiological model and experiential canalization of self-regulation development in children. In the model shown in Figure 1, characteristics of the environment influence parents’ psychological functioning and in turn the quality of caregiving they provide. Quality of caregiving is then in turn hypothesized to act as a key mediator of the linkage between children’s exposure to poverty-related hazard and subsequent physiological, neurobiological, and cognitive development. In this process, the development of stress physiology is a central component of the model, one in which stress hormone levels act as a primary canalizer or mechanism through which cognitive and social-emotional development in early childhood is shaped by experience—most specifically, the development of neural systems important for self-regulation, defined here as the primarily volitional regulation of attention, emotion, and executive functions for the purposes of goal-directed actions (Blair & Ursache, 2011).
Figure 1.
Model of the Experiential Canalization of Self-Regulation Development
The basis for the experiential canalization model of self-regulation development is found in a number of animal models that examined the effects of early experience on development. This research (primarily with rats) has demonstrated that chronic stress in the prenatal and/or very early neonatal periods has multiple negative sequelae. These studies demonstrate that early stress alters gene expression and induces structural changes as well as changes in connectivity in brain areas that underlie stress response physiology (Karssen et al., 2007; Liston et al., 2006; Patel, Katz, Karssen, & Lyons, 2008; Radley, Arias, & Sawchenko, 2006). In turn, alteration of stress response physiology influences activity in neural systems that underlie self-regulation abilities, including executive functions (Cerqueira, Mailliet, Almeida, Jay, & Sousa, 2007; Holmes & Wellman, 2009), or what can be considered tendencies to a more reflective or more reactive response to experience. This is because stress hormones are modulators of neural activity in the brain (Arnsten, 2000; Yuen et al., 2009) and at moderate levels of increase lead to long-term synaptic potentiation in corticolimbic circuitry associated with prefrontal cortex (PFC), the seat of executive function abilities. At very high or very low levels of neuroendocrine increase, however, synaptic activity in PFC circuitry is decreased, and activity in brain systems associated with more reactive forms of learning and behavior is increased (Ramos & Arnsten, 2007; Segal, Richter-Levin, & Maggio, 2010).
Given the relations among the constructs in Figure 1, it appears that one way in which early experience may shape or program the development of the organism is by altering neural connectivity and sensitivity to neuroendocrine levels in pathways that underlie tendencies to more reactive as opposed to more reflective responses to experience. With an increasingly established relation between stress hormone levels and self-regulation, a central research question concerns the extent to which effects of environmental quality on the neural substrate that supports self-regulation are mediated by and can be remediated through early experience, particularly early caregiving. The idea that caregiving acts as a key mediator of the effect of poverty on child development has been shown in a number of studies (McLoyd, 1998). Research on early experience in animal models indicates the psychobiological shaping of behavior and cognition through early caregiving effects on stress physiology. In one widely cited example, Meaney and collaborators (Meaney & Szyf, 2005) have shown that variation in naturally occurring maternal behavior in the rat during the offspring’s first eight postnatal days is associated with the expression of a gene that codes for the density of neural receptors for glucocorticoids in the hippocampus. This effect is a highly meaningful one in that glucocorticoid receptor density is central to the regulation of neuroendocrine levels, which, as noted above, are highly relevant to the activity in brain areas associated with more volitional and proactive responses to stimulation (Robbins & Arnsten, 2009) and with complex learning and memory (Liu, Diorio, Day, Francis, & Meaney, 2000).
Although there are many interesting aspects of the developmental process linking early caregiving experience to later behavior in the animal models described above, one of the most important from the standpoint of developmental psychobiology is that variation in the maternal behaviors in the rat that initiate the cascade from the behavioral to the genetic level and back again is in part driven by the quality of the environment in which development is occurring. The idea here is that environmental quality leads to particular types of caregiving behaviors that initiate a physiological cascade leading to patterns of development that are appropriate or beneficial for that environment (Cameron et al., 2005; Meaney, 2001, 2010). Such coactions among genes, behavior, and environments provide new insight into processes of development. These and other studies provide growing evidence to support the idea that early caregiving and stress physiology serve as primary conduits or sources of information in a developmental system. As such, early caregiving can be understood to shape the development of child behavior in ways that are appropriate for the context in which development is occurring.
Adaptation and Change in Development
The notion of developmental trade-offs in the experiential canalization approach is consistent with a rationale for future research that might profitably investigate the idea that adversity in the context of poverty shapes neural development and perhaps also molecular genetic processes and leads to adaptations in behavior and mental states that are relevant to that environment. Adaptive shaping of behavior in low-resource environments, however, should not be taken to imply the development of necessarily optimal or desirable states of functioning. On the contrary, adaptation to low-resource environments involves short-term “benefits” as well as long-term “costs” to the organism, both psychologically as well as physically, that are due to increased stress on organ systems resulting from alterations to stress and immune system functioning. Recent epidemiological findings suggest that low SES is consistently related to poorer health in later life (Jackson et al., 2004; Miller et al., 2009). One mechanistic interpretation of these findings is that alterations to stress and immune system functioning in children in low-SES homes represent an adaptive trade-off. For example, low-SES background has been associated with up-regulation of genes associated with adrenergic function and down-regulation of genes associated with the regulation of the hypothalamic–pituitary– adrenal (HPA) axis (Miller et al., 2009). Increased adrenergic and glucocorticoid responses to stimulation would enable a more reactive and faster response to threats, both physical and psychosocial, and as such would confer an advantage in unsafe environments. Such a trade-off, however, would come with short- and long-term costs to health and well-being that would preferentially shape physical and psychological development along particular trajectories while limiting the likelihood of development along others.
The experiential canalization approach offers a sharper lens through which to reexamine models of poverty and child development. Although research on the canalization of development through caregiving and stress physiology is in an early stage, a growing number of studies provide a neurobiological basis for well-documented associations between poverty and child physical and psychological health and development. For example, a longitudinal study with pre- and early adolescent children has demonstrated that a cumulative risk index composed of psychosocial and physical characteristics of the home environment differentiated high- from low-SES homes and was positively and linearly associated with an index of stress physiology biomarkers in children that included cardiovascular function, body mass index, and overnight levels of catecholamines and cortisol. Furthermore, the risk index was associated with reduced delay of gratification, increased learned helplessness, greater psychological distress, and reduced working memory in children, indicating links among poverty, stress physiology, and self-regulation (Evans, 2003; Evans & English, 2002; Evans & Schamberg, 2009).
A second longitudinal study beginning at birth that we have been conducting with a large group of collaborators has followed a sample of 1,292 children from predominantly low-income and nonurban communities. This study, known as the Family Life Project, has demonstrated that poverty is associated with elevated cortisol in infancy and early childhood and that this association is mediated through characteristics of the household (Blair, Raver, et al., 2011; Hibel, Granger, Blair, Cox, & the Family Life Project Investigators, 2011). Furthermore, this study has shown that, as outlined above, parenting sensitivity mediates the relation between poverty and stress physiology (Blair et al., 2008; Mills-Koonce et al., 2011) and that, in combination, parenting sensitivity and elevated cortisol mediate the association between poverty and low levels of executive function abilities in children (Blair, Granger, et al., 2011).
Studies of young children in conditions of more extreme forms of compromised caregiving involving neglect and maltreatment in infancy and early childhood also provide evidence of experiential canalization in the context of adversity. Children who have experienced exceptionally harsh treatment from caregivers demonstrate alterations to HPA axis function including hyperactivity followed by later hyporeactivity (Gunnar, Fisher, & the Early Experience, Stress, and Prevention Network, 2006). An important aspect of this model is that it can be extended to include exposure to aggression from peers as well as adults. In a sample of monozygotic twins discordant for the experience of bullying, the bullied members of the twin pairs showed blunted, or hyporesponsive, cortisol secretion in response to a lab stressor compared with their nonbullied but genetically identical counterparts (Ouellet-Morin et al., 2011). In contrast, the nonbullied twins showed an expected increase in cortisol secretion in response to the moderately stressful lab task. These findings highlight the neurophysiological and behavioral trade-offs that accompany the process of experiential canalization in the face of environmental adversity. In discussing their findings, the investigators squarely considered these trade-offs and addressed the question of whether the blunted, hyporesponsive HPA axis profile demonstrated by the bullied children’s response to psychosocial stress was “adaptive or detrimental” (Ouellet-Morin et al., 2011, p. 580).
Here development may be giving with one hand—conferring developmental advantage through blunting of the HPA axis to protect the brain from iatrogenic effects of prolonged cortisol elevations—while taking away with the other—the blunting of the cortisol response leading to longer term health costs. This set of trade-offs is manifested at the behavioral level as well in that chronic exposure to others’ anger and aggression tunes children’s attention and responsiveness in favor of heightened vigilance to emotionally negative stimuli (Cicchetti & Rogosch, 2009; Pollak, Messner, Kistler, & Cohn, 2009; Pollak, Vardi, Putzer Bechner, & Curtin, 2005). Such heightened vigilance may be beneficial in the short run, keeping children alert and ready to respond when facing potentially threatening situations or interactions at home or at school. That same level of behavioral vigilance may translate to a level of wariness or reticence with unfamiliar teachers and peers that carries longer term social costs. These findings provide support for the idea that chronic exposure to adversity, such as can occur more frequently in the context of poverty, actively shapes physiological and behavioral development in ways that are adaptive for that context.
Implications of Experiential Canalization for Reversibility, Reoptimization, and Intervention
As noted above, physiological, cognitive, and behavioral adaptations to the context of adversity and compromised caregiving can result in objectively worse chances of positive life course outcomes. Applied to children in poverty, such a psychobiological process in development might understandably be associated with short-term “beneficial” adaptations but potentially harmful long-term sequelae. In the context of maltreatment specifically, or in low-resource, unpredictable caregiving environments more generally, altered HPA axis responsivity, biased attributional style, and hypervigilance to environmental cues allow for more rapid learning and response to conditions of threat (Champagne et al., 2008; Pollak, 2008). These processes, however, also increase the chances of negative interpersonal interactions and high levels of difficulty in social contexts such as school (Cicchetti & Rogosch, 2009).
Attention to potential exchanges or trade-offs that make given behaviors rewarded and rewarding versus problematic provides insight into ways that poverty-related adversity may profoundly shape children’s development. Attention to these trade-offs, however, also underscores ways in which neurocognitive and behavioral profiles of self-regulation can be altered. A central implication of the experiential canalization approach is that the shaping of development by experience offers an opportunity for repair and reversal. Just as the system is open to shaping and selective optimization in the face of high levels of disadvantage, so too might the system be reoptimized to meet changing environmental demands and conditions. A sanguine implication of models of experiential canalization, however, is that there are few if any opportunities for an “easy fix”: In setting aside input– output models of development, seemingly straightforward solutions for altering children’s self-regulation and executive function will have a lower probability of success than will interventions that take canalizing processes across multiple levels into account.
Repair Through Mediating Mechanisms of Caregiving
In the developmental psychobiological framework, experiential and biological influences on development are highly intertwined. For this reason, supporting adults to maintain high levels of responsiveness, consistency, and warmth can be expected to lead to more flexible regulation of stress physiology with cascading influences on child self-regulation. A considerable body of research in prevention science demonstrates that parenting intervention can be successful in altering the quality of caregiving that adults provide to young children while those adults navigate a large number of stressful poverty-related hazards. Recent parent training programs have shown significant success in helping adults to acquire new caregiving goals and schema, alter their use of negative forms of discipline, and engage in more sensitive and responsive and less coercive and inept forms of caregiving, with short-term reductions in young children’s behavioral dysregulation (Brotman, Gouley, Klein, Castellanos, & Pine, 2003; Dishion et al., 2008; Dozier et al., 2009; Izard, Sann, Spelke, & Streri, 2009; Landry, Smith, Swank, & Guttentag, 2008; Mendelsohn et al., 2005; Webster-Stratton, 1998).
For example, implementation of multiple years of the SAFEChildren intervention supporting parenting practices among low-income families facing high levels of violence led to significant increases in parents’ use of more stable, consistent forms of caregiving and limit setting, with concomitant improvements in children’s regulation of attention, impulsivity, and behavior (Tolan, Gorman-Smith, Henry, & Schoeny, 2009). Effect sizes of these interventions range from small impacts with “dilute” forms of intervention (see Dishion et al., 2008) to larger effect sizes for more intensive intervention efforts (e.g., d = .5 for improvement in responsive caregiving and child attention deployment; Landry et al., 2008). An essential point from the perspective of the experiential canalization of development is that these models of parent skills training often engage parents through attention to parents’ own regulatory profiles of affect, behavior, and cognition in conditions of high environmental stress (Dishion et al., 2008; Izard et al., 2009; Fisher & Stoolmiller, 2008). Equally important is that careful experimental designs in “real-world” contexts have dramatically increased our ability to make clear causal inferences regarding the role of caregiving provided by adults as a critical mediating mechanism in canalization models of human development.
Studies of parenting training also provide some evidence of effects on aspects of child self-regulation, including attention and emotion regulation (Landry, Smith, & Swank, 2006). As well, evaluations of outcomes for children experiencing extreme caregiving disruption that results in foster care placement provide initial support for a process by which changes in caregiving behavior are associated with changes in stress physiology that should be conducive to executive function abilities and more reflective self-regulation of behavior. Children of foster care parents receiving training in emotionally supportive and contingent behavior demonstrated a more typical pattern of diurnal cortisol change (higher morning levels and a consistent decline through the day) as well as lower overall cortisol levels (Dozier, Peloso, Lewis, Laurenceau, & Levine, 2008; Fisher, Stoolmiller, Gunnar, & Burraston, 2007). Similarly, preschool children at risk for conduct disorder in families receiving an intervention to promote responsive parenting demonstrated an appropriate increase in cortisol in anticipation of a moderate social challenge relative to children in a randomly assigned control group (Brotman et al., 2007).
Evidence from the obstacles and successes encountered within parenting interventions also highlights the extent to which canalizing processes are bidirectional and reciprocal and the extent to which attention to this reciprocity, as a process of the active maintenance of patterns of behavior, is needed to promote intervention efficacy. Children’s behaviors and regulatory profiles that may have been adaptive in the context of past environmental contingencies may also shape future environmental contingencies, eliciting continued styles of suboptimal care from adults in ways that are sometimes difficult to disrupt in intervention contexts that focus only on adult behavior change. For example, in the preventive intervention for children in foster care noted above, family members needed interventionists’ support in order to avoid becoming caught in an escalating cycle of rising negative arousal, biased cognitive attributions, and behavioral responses of dismissiveness, disengagement, and withdrawal (Dozier, 2005; Dozier et al., 2009). In light of this cycle of escalating dysregulation and withdrawal, Dozier and colleagues (2009) designed their intervention to support foster parents’ ability to structure a set of environmental contingencies that might canalize children’s more optimal trajectories of regulation over time. In so doing, the interventionists considered that there are also significant trade-offs in shifting into new patterns of emotional regulation and that caregivers need to be supported in making greater investments in approaching rather than withdrawing during emotionally negative bouts of interaction with their foster children. The implication from such an approach is that caregivers would need to concomitantly shift the expectations and responses of others in additional environmental contexts, including, for example, teachers in their children’s preschools, in order for their children’s altered forms of self-regulation to be sustained rather than transitory. In short, canalizing models indicate that change in multiple, rather than single, environmental contexts is necessary if newly canalized trajectories of responding are to be supported over time.
While promising, the above findings provide limited but suggestive evidence of canalizing processes in development and the potential malleability of development through intervention and support. Within this framework, a key question concerns whether intervening in cycles of maladaptive caregiving and dysregulation in parent– child interactions yields linked improvements both in child stress physiology and self-regulation abilities. Although no intervention studies to date have fully tested such a model, the findings described above strongly indicate the need for direct empirical examinations of this model. As well, two important points related to the foregoing concern prenatal experience as well as the possibility that some children may be more or less sensitive or susceptible to alterations to experience. A substantial literature on the relation of prenatal experience to postnatal development indicates that canalizing processes begin early and can have meaningful implications for later self-regulation (Davis & Sandman, 2010; Markham, Toth, & Lickliter, 2006). Similarly, the growing literature on differential susceptibility or biological sensitivity to context suggests that temperamental and physiological differences among children are central to the processes by which biology and experience coactively shape development (Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van Ijzendoorn, 2011).
Alternative Pathways to Repair Through Mediating Mechanisms: Classroom-Based Intervention
Although findings emerging from the recent parenting interventions discussed above are promising, it is important to underscore that behavioral change may be easier to engineer among some parents than among others (e.g., among full-time working parents who are not able to attend extensive trainings and workshops). Further, the estimated size of the effect or impact of those interventions on child outcomes is generally small and may not be sustained unless the “dose” of intervention is high and continues across several developmental periods (see Landry et al., 2008). A clear implication from both theoretical and policy perspectives is that for many children, interventions targeting the quality of caregiving represent only partial rather than full solutions to children’s self-regulatory difficulty.
One benefit of an experiential canalization approach is that multiple ecological contexts can be viewed as positive canalizers of self-regulation development. Those new, enriched environmental contexts can be viewed as mediators of the impact of poverty-related hazard on long-term trajectories of development (Blair, 2002). For example, results from recent preschool intervention trials suggest that exposure to cognitively stimulating and behaviorally well-managed classrooms benefits low-income children’s executive functioning (Bierman, Nix, Greenberg, Blair, & Domitrovich, 2008; Diamond, Barnett, Thomas, & Munro 2007; Morris, Raver, Lloyd, & Millenky, 2009; Raver, Jones, et al., 2011). An important qualification to these findings is that it remains to be seen whether interventions would benefit children to an even greater extent if both home and preschool contexts were targeted. However, these intervention trials offer compelling evidence that out-of-home environments may serve as additional mediating influences for young children’s developmental trajectories. These findings also provide powerful empirical support for the claim that new experiences of environmental enrichment can be structured to capitalize on those biobehavioral and neurocognitive processes, such as the development of executive functioning, that may be “late breaking” in developmental time.
This theoretically motivated recognition of stage salience in considering experiential canalization might be profitably applied to a next generation of interventions: How can neuroendocrine and neurocognitive reorganizations that coincide with key developmental transitions in early childhood, middle childhood, and early adolescence be targeted through intervention, and in what settings would such interventions have maximal impact? We are only just beginning to understand processes through which stress response physiology, corticolimbic neural circuitry, and self-regulation behavior may be shaped in developmental periods extending past infancy. Pressing questions regarding normative patterns of change in stress physiology as well as specific biobehavioral mechanisms through which change occurs need to be tested within experimental contexts offered by randomized controlled trials.
Experimental changes in the environmental conditions of poverty itself offer an additional, powerful way to test theoretical propositions laid out by the experiential canalization approach. Do such interventions lead to reductions in psychological stress, or lower wear and tear on the part of adults and children, and are hypothesized reductions in allostatic load in low-income families associated with measurable changes in children’s neuroendocrine and neurocognitive functioning? Although evidence is sparse, a quasi-experimental study from the Opportunidades poverty-alleviation program in Mexico suggests that among children at high risk (with caregivers experiencing high depressive symptoms at baseline), community-level efforts to reduce poverty are associated with lower average cortisol levels in children (Fernald & Gunnar, 2009). To our knowledge, studies of antipoverty programs (such as evaluations of the benefits of the Earned Income Tax Credit and of conditional cash transfers) have not yet included assessments of whether children’s self-regulatory trajectories are affected. However, we view this potential line of inquiry as promising, with the caveat that the induction and facilitation of new patterns of stress reactivity and behavior may be limited unless we are committed to substantial and sustained efforts to intervene across ecological settings and across time.
Conclusion
The evidence reviewed in the foregoing sections helps us to recognize that exposure to environmental adversity is a primary shaper of development from the cellular to the behavioral and social levels. Environmental exposure, as a primary constituent of development, is like other contributors to development, malleable. Poverty presents a remediable rather than a static set of environmental conditions that must be borne by families and children. The environmental conditions of poverty, however, work to maintain continuity by constraining change across levels of analysis. Conceiving of development as a process of continuity through adaptation provides us with large, new empirical territory in which to test models of experiential canalization and the limits of developmental change. To do that, we can deploy hybrid models of experimental design at the policy level combined with careful measurement at the biobehavioral and neurocognitive levels to detect developmental benefits across a broad array of pathways. Such hybrid models of scientific inquiry also allow us to ask whether the timing of intervention is central to limiting the ultimate developmental cost of the hazard and maximizing the opportunity for remedy.
Throughout this article we have argued that development is shaped by biology and experience coactively to promote specific abilities over others, in processes of gain through loss (Gottlieb, 1997). We have argued that advances in developmental science may be powerfully fueled by recognition of the potential trade-offs posed by a given behavior and the environmental contingencies that make such a behavior rewarded and rewarding versus problematic or costly to the individual. We have also outlined new ways to conceptualize developmental continuity as a result of both socially and biobehaviorally mediated processes rather than as a result of faulty or inadequate environmental input or genetic vulnerability. By considering the processes linking early experience, stress physiology, and gene expression as canalizing forces that shape the development of brain and behavior, we offer a model of development that is fundamentally plastic and remarkably complex and that veers markedly away from simple input–output, deficit-compensation models. In so doing, we hope to shed light on the new directions that the fields of developmental science, prevention science, and public policy may take in the years ahead.
Acknowledgments
Clancy Blair acknowledges support from Eunice Kennedy Shriver National Institute of Child Health and Human Development Grants R01 HD51502 and P01 HD39667 (with co-funding from the National Institute on Drug Abuse) and from Institute of Education Sciences Grant R305A100058. C. Cybele Raver acknowledges support from Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant R01 HD046160.
Biographies

Clancy Blair

C. Cybele Raver
REFERENCES
- Ackerman BP, Brown ED. Physical and psychosocial turmoil in the home and cognitive development. In: Evans G, Wachs TD, editors. Chaos and its influence on children’s development: An ecological perspective. Washington, DC: American Psychological Association; 2010. pp. 35–47. [Google Scholar]
- Arnsten AFT. Through the looking glass: Differential noradrenergic modulation of prefrontal cortical function. Neural Plasticity. 2000;7:133–146. doi: 10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes PB, Staudinger UM, Lindenberger U. Lifespan psychology: Theory and application to intellectual functioning. Annual Review of Psychology. 1999;50:471–507. doi: 10.1146/annurev.psych.50.1.471. [DOI] [PubMed] [Google Scholar]
- Bierman KL, Nix RL, Greenberg MT, Blair C, Domitrovich CE. Executive functions and school readiness intervention: Impact, moderation, and mediation in the Head Start REDI program. Development and Psychopathology. 2008;20:821–843. doi: 10.1017/S0954579408000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C. School readiness: Integrating cognition and emotion in a neurobiological conceptualization of children’s functioning at school entry. American Psychologist. 2002;57:111–127. doi: 10.1037//0003-066x.57.2.111. [DOI] [PubMed] [Google Scholar]
- Blair C, Granger DA, Kivlighan KT, Mills-Koonce R, Willoughby M, Greenberg MT the Family Life Project Investigators. Maternal and child contributions to cortisol response to emotional arousal in young children from low-income, rural communities. Developmental Psychology. 2008;44:1095–1109. doi: 10.1037/0012-1649.44.4.1095. [DOI] [PubMed] [Google Scholar]
- Blair C, Granger D, Willoughby M, Mills-Koonce R, Cox M, Greenberg MT the FLP Investigators. Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Development. 2011;82:1970–1984. doi: 10.1111/j.1467-8624.2011.01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Raver CC, Granger D, Mills-Koonce R, Hibel L the Family Life Project Key Investigators. Allostasis and allostatic load in the context of poverty in early childhood. Development and Psychopathology. 2011;23:845–857. doi: 10.1017/S0954579411000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Ursache A. A bidirectional model of executive functions and self-regulation. In: Baumeister RF, Vohs KD, editors. Handbook of self-regulation. 2nd. New York, NY: Guilford Press; 2011. pp. 300–320. [Google Scholar]
- Bradley RH, Corwyn RF. Socioeconomic status and child development. Annual Review of Psychology. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, Duncan GJ. The effects of poverty on children. The Future of Children. 1997;7:55–71. [PubMed] [Google Scholar]
- Brotman LM, Gouley K, Huang K, Kamboukos D, Fratto C, Pine DS. Effects of a psychosocial family-based preventive intervention on cortisol response to a social challenge in preschoolers at high risk for antisocial behavior. Archives of General Psychiatry. 2007;64:1172–1179. doi: 10.1001/archpsyc.64.10.1172. [DOI] [PubMed] [Google Scholar]
- Brotman LM, Gouley KK, Klein RG, Castellanos FX, Pine DS. Children, stress, and context: Integrating basic, clinical, and experimental prevention research. Child Development. 2003;74:1053–1057. doi: 10.1111/1467-8624.00589. [DOI] [PubMed] [Google Scholar]
- Cameron NM, Champagne FA, Parent C, Fish EW, Ozaki-Kuroda K, Meaney MJ. The programming of individual differences in defensive responses and reproductive strategies in the rat through variations in maternal care. Neuroscience and Biobehavioral Reviews. 2005;29:843–865. doi: 10.1016/j.neubiorev.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. Journal of Neuroscience. 2007;27:2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, Krugers H. Maternal care and hippocampal plasticity: Evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. Journal of Neuroscience. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Adaptive coping under conditions of extreme stress: Multilevel influences on the determinants of resilience in maltreated children. New Directions for Child and Adolescent Development. 2009;124:47–59. doi: 10.1002/cd.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Development. 2010;81:131–148. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Barnett WS, Thomas J, Munro S. Preschool program improves cognitive control. Science. 2007 Nov 30;318:1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishion TJ, Shaw D, Connell A, Gardner F, Weaver C, Wilson M. The family check-up with high-risk indigent families: Preventing problem behavior by increasing parents’ positive behavior support in early childhood. Child Development. 2008;79:1395–1414. doi: 10.1111/j.1467-8624.2008.01195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier M. Challenges of foster care. Attachment & Human Development. 2005;7(1):27–30. doi: 10.1080/14616730500039747. [DOI] [PubMed] [Google Scholar]
- Dozier M, Lindheim O, Lewis E, Bick J, Bernard K, Peloso E. Effects of a foster parent training program on young children’s attachment behaviors: Preliminary evidence from a randomized clinical trial. Child & Adolescent Social Work Journal. 2009;26:321–332. doi: 10.1007/s10560-009-0165-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier M, Peloso E, Lewis E, Laurenceau JP, Levine S. Effects of an attachment-based intervention on the cortisol production of infants and toddlers in foster care. Developmental Psychopathology. 2008;20:845–859. doi: 10.1017/S0954579408000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GJ, Brooks-Gunn J. Family poverty, welfare reform, and child development. Child Development. 2000;71:188–196. doi: 10.1111/1467-8624.00133. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to the environment: An evolutionary-neurodevelopmental theory. Development and Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychology. 2003;39:924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- Evans GW. The environment of childhood poverty. American Psychologist. 2004;59:77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Evans GW, English K. The environment of poverty: Multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Development. 2002;73:1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences, USA. 2009;106:6545–6549. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald LC, Gunnar MR. Poverty-alleviation program participation and salivary cortisol in very low-income children. Social Science & Medicine. 2009;68:2180–2189. doi: 10.1016/j.socscimed.2009.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M. Intervention effects on foster parent stress: Associations with child cortisol levels. Development and Psychopathology. 2008;20:1003–1021. doi: 10.1017/S0954579408000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32:892–905. doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster H, Brooks-Gunn J. Toward a stress process model of children’s exposure to physical family and community violence. Clinical Child and Family Psychology Review. 2009;12:71–94. doi: 10.1007/s10567-009-0049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb G. Experiential canalization of behavioral development: Theory. Developmental Psychology. 1991;27:4–13. [Google Scholar]
- Gottlieb G. Synthesizing nature–nurture: Prenatal roots of instinctive behavior. Mahwah, NJ: Erlbaum; 1997. [Google Scholar]
- Gunnar MR, Fisher PA the Early Experience, Stress, and Prevention Network. Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Development and Psychopathology. 2006;18:651–677. [PubMed] [Google Scholar]
- Hart B, Risley T. Meaningful differences in the everyday experience of young American children. Baltimore, MD: Paul H. Brookes; 1985. [Google Scholar]
- Hibel LC, Granger DA, Blair C, Cox M the Family Life Project Investigators. Maternal sensitivity buffers the adrenocortical implications of intimate partner violence exposure during early childhood. Development and Psychopathology. 2011;23:689–701. doi: 10.1017/S0954579411000010. [DOI] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neuroscience and Biobehavioral Reviews. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izard V, Sann C, Spelke ES, Streri A. Newborn infants perceive abstract numbers. Proceedings of the National Academy of Sciences, USA. 2009;106:10382–10385. doi: 10.1073/pnas.0812142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson B, Kubzansky LD, Cohen S, Weiss S, Wright RJ CARDIA Study. A matter of life and breath: Childhood socioeconomic status is related to young adult pulmonary function in the CARDIA study. International Journal of Epidemiology. 2004;33:271–278. doi: 10.1093/ije/dyh003. [DOI] [PubMed] [Google Scholar]
- Karssen AM, Her S, Li JZ, Patel PD, Meng F, Bunney WE, Lyons DM. Stress-induced changes in primate prefrontal profiles of gene expression. Molecular Psychiatry. 2007;12:1089–1102. doi: 10.1038/sj.mp.4002095. [DOI] [PubMed] [Google Scholar]
- Kohen DE, Leventhal T, Dahinten VS, McIntosh CN. Neighborhood disadvantage: Pathways of effects for young children. Child Development. 2008;79:156–169. doi: 10.1111/j.1467-8624.2007.01117.x. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Williams KA, Lacerda F, Stevens KN, Lindblom B. Linguistic experience alters phonetic perception in infants by 6 months of age. Science. 1992 Jan 31;255:606–608. doi: 10.1126/science.1736364. [DOI] [PubMed] [Google Scholar]
- Landry SH, Smith KE, Swank PR. Responsive parenting: Establishing early foundations for social, communication, and independent problem-solving skills. Developmental Psychology. 2006;42:627–642. doi: 10.1037/0012-1649.42.4.627. [DOI] [PubMed] [Google Scholar]
- Landry SH, Smith KE, Swank PR, Guttentag C. A responsive parenting intervention: The optimal timing across early childhood for impacting maternal behaviors and child outcomes. Dvelopmental Psychology. 2008;44:1335–1353. doi: 10.1037/a0013030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowicz DJ, Ghazanfar AA. The emergence of multisensory systems through perceptual narrowing. Trends in Cognitive Sciences. 2009;13:470–478. doi: 10.1016/j.tics.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Lickliter R, Honeycutt H. Developmental dynamics: Toward a biologically plausible evolutionary psychology. Psychological Bulletin. 2003;129:819–835. doi: 10.1037/0033-2909.129.6.819. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. Journal of Neuroscience. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal neurogenesis, and cognitive development in rats. Nature Neuroscience. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Markham RG, Toth G, Lickliter R. Prenatally elevated physiological arousal interferes with perceptual learning in bobwhite quail (Colinus virginianus) embryos. Behavioral Neuroscience. 2006;120:1315–1325. doi: 10.1037/0735-7044.120.6.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoyd VC. Socioeconomic disadvantage and child development. American Psychologist. 1998;53:185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the biological definition of gene × environment interactions. Child Development. 2010;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: Life at the interface between a dynamic environment and a fixed genome. Dialogues in Clinical Neuroscience. 2005;7(2):103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn AL, Dreyer BP, Flynn V, Tomopoulos S, Rovira I, Tineo W, Nixon AF. Use of videotaped interactions during pediatric well-child care to promote child development: A randomized, controlled trial. Journal of Developmental and Behavioral Pediatrics. 2005;26:34–41. [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Sciences, USA. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills-Koonce WR, Garrett-Peters P, Barnett M, Granger D, Blair C, Cox MJ Family Life Project Key Investigators. Father contributions to cortisol responses in infancy and early childhood. Developmental Psychology. 2011;47:388–395. doi: 10.1037/a0021066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar BE, Buka SL, Brennan RT, Holton JK, Earls F. A multilevel study of neighborhoods and parent-to-child physical aggression: Results from the Project on Human Development in Chicago neighborhoods. Child Maltreatment. 2003;8:84–97. doi: 10.1177/1077559502250822. [DOI] [PubMed] [Google Scholar]
- Morris P, Raver CC, Lloyd CM, Millenky M. Can teacher training in classroom management make a difference for children’s experiences in preschool? A preview of findings from the Foundations of Learning Demonstration (MDRC Working Paper) 2009 Retrieved from http://www.mdrc.org/publications/527/full.pdf. [Google Scholar]
- Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Developmental Science. 2007;10:464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I, Danese A, Bowes L, Shakoor S, Ambler A, Pariante CM, Arseneault L. A discordant monozygotic twin design shows blunted cortisol reactivity among bullied children. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:574–582. doi: 10.1016/j.jaac.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama S. The ontogeny of information: Developmental systems and evolution. 2nd. Durham, NC: Duke University Press; 2000. [Google Scholar]
- Patel PD, Katz M, Karssen AM, Lyons DM. Stress-induced changes in corticosteroid receptor expression in primate hippocampus and prefrontal cortex. Psychoneuroendocrinology. 2008;33:360–367. doi: 10.1016/j.psyneuen.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD. Mechanisms linking early experience and the emergence of emotions: Illustrations from the study of maltreated children. Current Directions in Psychological Science. 2008;17:370–375. doi: 10.1111/j.1467-8721.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Messner M, Kistler DJ, Cohn JF. Development of perceptual expertise in emotion recognition. Cognition. 2009;110:242–247. doi: 10.1016/j.cognition.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Vardi S, Putzer Bechner AM, Curtin JJ. Physically abused children’s regulation of attention in response to hostility. Child Development. 2005;76:968–977. doi: 10.1111/j.1467-8624.2005.00890.x. [DOI] [PubMed] [Google Scholar]
- Pons F, Lewkowicz DJ, Soto-Faraco S, Sebastián-Gallés N. Narrowing of intersensory speech perception in infancy. Proceedings of the National Academy of Sciences, USA. 2009;106:10598–10602. doi: 10.1073/pnas.0904134106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. Journal of Neuroscience. 2006;26:12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos BP, Arnsten AFT. Adrenergic pharmacology and cognition: Focus on the prefrontal cortex. Pharmacology & Therapeutics. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver CC, Jones SM, Li-Grining C, Zhai F, Bub K, Pressler E. The Chicago School Readiness Project’s impact on low-income preschoolers’ preacademic skills: Self-regulation as a mediating mechanism. Child Development. 2011;82:362–378. doi: 10.1111/j.1467-8624.2010.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: Monoaminergic modulation. Annual Review of Neuroscience. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M, Richter-Levin G, Maggio N. Commentary: Stress induced routing of hippocampal connectivity: A hypothesis. Hippocampus. 2010;20:1332–1338. doi: 10.1002/hipo.20751. [DOI] [PubMed] [Google Scholar]
- Tolan PH, Gorman-Smith D, Henry D, Schoeny M. The benefits of booster interventions: Evidence from a family-focused prevention program. Prevention Science. 2009;10:287–297. doi: 10.1007/s11121-009-0139-8. [DOI] [PubMed] [Google Scholar]
- Watson T, McLanahan S. Marriage meets the Joneses: Relative income, identity, and marital status. Journal of Human Resources. 2011;46:482–517. doi: 10.3368/jhr.46.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster-Stratton C. Preventing conduct problems in Head Start children: Strengthening parent competencies. Journal of Consulting and Clinical Psychology. 1998;66:715–730. doi: 10.1037//0022-006x.66.5.715. [DOI] [PubMed] [Google Scholar]
- Werker JF, Tees RC. The effect of multilingualism on phonetic perceptual flexibility. Applied Psycholinguistics. 1986;7:141–155. [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proceedings of the National Academy of Sciences, USA. 2009;106:14075–14079. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]