Abstract
There are few papers on the combination therapy of deferiprone (DFP) and deferasirox (DFX) in iron-overloaded patients with transfusion-dependent β-thalassemia major (β-TM). A total of 6 patients with β-TM (5 males and 1 female) with a mean age of 23.8±5.8 years (ranging from 17 to 31) used this treatment regimen. The mean doses of DFP and DFX were 53.9±22.2 and 29.3±6.8 mg/kg/day, respectively. The duration of treatment was 11.5±4.6 months. Their serum ferritin levels were measured to be 2800±1900 and 3400±1600 ng/mL before and after treatment, respectively (p<0.6). Their cardiac magnetic resonance imaging (MRI) T2* values were 16.69±15.35 vs 17.38±5.74 millisecond (ms) before and after treatment, respectively (p < 0.9). Although there was no significant difference between their cardiac MRI T2* values before and after treatment statistically, the values improved after combination therapy with DFP and DFX in most of the patients. Liver MRI T2 * values were changed from 2.12±0.98 to 3.03±1.51 ms after treatment (p < 0.01); Further, their liver T2* values and liver iron concentration (LIC) were improved after treatment. Our study found that cardiac MRI T2* values, liver MRI T2* values, and LIC were improved after combination therapy with DFP and DFX in β-TM patients and that DFP and DFX combination therapy could be used to alleviate cardiac and liver iron loading.
Key words: Deferiprone, deferasirox, iron overload, β-thalassemia major, MRI T2*
Competing interest statement
Conflict of interest: the authors report no conflict of interest.
Introduction
Transfusion-dependent β-thalassemia major (β-TM) is a type of inherited blood disorder resulting in severe and chronic anemia. Multi blood transfusion regimen is the first effective measure in prolonging life among these patients; however, excessive iron overload caused by repeated blood transfusions can result in multi-organ failure.1,2 For this reason, iron chelation therapy is vital for β-TM patients with iron overload. Currently, three main iron chelators are available for clinical use: deferoxamine (DFO), deferiprone (DFP), and deferasirox (DFX).1 DFO was the first iron chelator introduced in the 60s to treat iron overload; it is to be used as a continuous subcutaneous or intravenous solution, while DFP is an oral iron chelator. DFX is the most recent oral iron chelator introduced in 2003. Imported brands of DFO and DFX are available in Iran, and all three-iron chelators are made by Iranian pharmaceutical companies. In Iran, DFP is made by Avicenna Pharmaceuticals, and DFX is made by the Osvah Pharmaceutical Company as Osveral®, which has been used to successfully treat Iranian patients.3
The choice of medication is made together by the physician and the patient and depends on many factors, such as the degree of iron overload, the age of the patient, the cost of the medication, and tolerability of the medication. Some patients have a large amount of iron in their body, and one iron chelator, even in the maximum recommended dose, is not enough to induce a negative iron balance. In these cases, two iron chelators are prescribed. Combination of DFO and DFP is a classic combination now and is most recommended for severe iron overload of the heart and has been tried in our center.4,5 Combination of DFP and DFX is rather a new experience.6-8 We are sharing our experience with this combination in Thalassemia Research Center, Iran.
Materials and Methods
This study was a review of the medical records of 6 patients with β-TM using the combination treatment of DFX and DFP in 2016. Patients were included if they had high serum ferritin levels or severe iron overload, as determined by magnetic resonance imagining (MRI) T2* values, in spite of their maximum dosages of monotherapy with DFO or DFX. Patients were excluded if they were allergic to DFX or DFP or if they did not consent to the study.
The included patients’ ages, genders, histories of splenectomy, iron intakes from blood transfusions, durations and doses of iron chelation therapy, and serum ferritin levels were extracted from their medical records. As almost 250 mg of iron is contained in each PRBC (packed red blood cell) packed,9 we calculated the iron intake from blood transfusions in mg/year as follows: 250 × the number of units given during one year. The patients’ monthly CBC (complete blood count), blood urea, creatinine, aspartate aminotransferase, alanine aminotransferase, uric acid, ferritin, and 24-hour urine protein levels were measured during treatment. The patients were asked about any of their experienced clinical side effects, including skin rashes and gastrointestinal symptoms, during their monthly visits. Patients’ degrees of iron overload were assessed by serial measurements of their serum ferritin levels and their MRI T2* values in the heart and liver. The liver iron concentration (LIC) as mg/gr/dry weight of the liver was also compared between the patients. SPSS18 Software was used to conduct statistical calculations, and a paired t-test was used to calculate the difference between each before-and-after pair of measurements in order to determine the means of the changes.
Results
A total of 6 patients (5 males and 1 female) with a mean age of 23.8±5.8 years (ranging from 17 to 31) received a combination treatment of DFP and DFX. 6 patients were receiving regular blood transfusions (15 ml/kg of filtered packed cells) every 3-4 weeks. All patients had been treated with DFO, DFP, or DFX in the past for various lengths of time. The demographics, clinical states, and cardiac and liver MRI values of the patients are shown in Tables 1 and 2.
Table 1.
Demographics and clinical conditions of patients with β-thalassemia major under the combination therapy of deferiprone and deferasirox.
| Cases | Gender | Age (year) | Weight | Splenectomy | Duration of blood transfusion (years) | Transfused blood (unit/year) | Received iron via blood transfusion (Mg/year) | Combination therapy | Ferritin levels (pg/dL) | Significant complications with combination therapy | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (Mg/day) |
Duration (Month) |
Before | After | ||||||||||
| DFX | DFP | ||||||||||||
| 1 | M | 17 | 55 | No | 16 | 30 | 7500 | 1500 | 4500 | 15 | 6500 | 2150 | No |
| 2 | M | 17 | 52 | No | - | - | - | 2000 | 3000 | 15 | 1300 | 1590 | No |
| 3 | M | 24 | 40 | No | 23 | 35 | 8750 | 1500 | 3000 | 12 | 2200 | 2979 | Neutropenia |
| 4 | M | 25 | 58 | Yes | 23 | 24 | 6000 | 1500 | 1500 | 3 | 1700 | 3000 | No |
| 5 | F | 29 | 45 | Yes | 28 | 34 | 8500 | 1000 | 1500 | 10 | 3100 | 4600 | Diarrhea |
| 6 | M | 31 | 60 | No | 30 | 48 | 12,000 | 1500 | 3000 | 14 | 2100 | 5974 | No |
M, male; F, female.
Table 2.
Cardiac and liver magnetic resonance imaging T2* values of patients with β-thalassemia major under the combination therapy of deferiprone and deferasirox.
| Cases | Before combination therapy | After combination therapy | Difference between before and after | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Heart | Liver | Heart | Liver | Heart | Liver | ||||
| T2* (ms) | T2* (ms) | LIC (mg/gr/dw) | T2* (ms) | T2* (ms) | LIC (mg/gr/dw) | T2* (ms) * | T2* (ms) ** | LIC (mg/gr/dw) *** | |
| 1 | 6.95 | 1.69 | 8.20 | 7.16 | 2.06 | 6.64 | +0.21 | +0.37 | –1.56 |
| 2 | 16.46 | 1.12 | 12.72 | 22.28 | 1.78 | 7.76 | +5.82 | +0.66 | –4.96 |
| 3 | 11.16 | 3.95 | 3.31 | 14.39 | 5.65 | 2.65 | +3.23 | +1.7 | –0.66 |
| 4 | 10.87 | 1.59 | 8.76 | 18.4 | 1.81 | 7.62 | +7.53 | +0.22 | –7.62 |
| 5 | 7.46 | 2.12 | 6.44 | 21.48 | 3.81 | 3.45 | +14.02 | +1.69 | –2.99 |
| 6 | 47.22 | 2.23 | 6.1 | 20.52 | 3.08 | 4.32 | –26.7 | +0.85 | –1.78 |
LIC, liver iron concentration; Ms, millisecond.
*p<0.9,
**p<0.01,
***p<0.01.
The duration of the treatment was 11.5±4.6 months. Their serum ferritin levels were 2800±1900 and 3400±1600 ng/mL before and after treatment, respectively (p < 0.6). Their cardiac and liver MRI T2* values were 16.69±15.35 vs 17.38±5.74 and 2.12±0.98 vs 3.03±1.51 milliseconds (ms) before and after treatment, respectively (p < 0.9 and p < 0.01, respectively). There was a significant difference in LIC after combination therapy with DFP and DFX (7.59±3.16 vs 5.41±2.22 mg/g/dry, p < 0.01). The changes in the cardiac and liver T2* values, LIC, and serum ferritin levels before and after treatment are shown in Figures 1-4.
Figure 1.
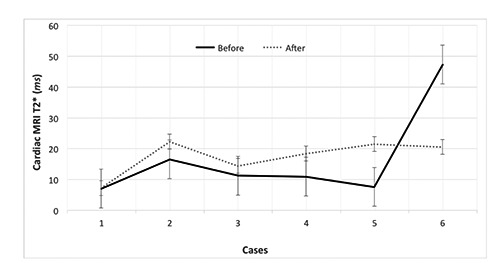
Cardiac magnetic resonance imaging (MRI) T2*changes in patients with β-thalassemia major before and after combination therapy with deferiprone and deferasirox.
Figure 2.
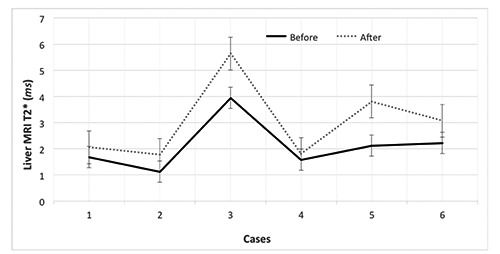
Liver magnetic resonance imaging (MRI) T2*changes in patients with β-thalassemia major before and after combination therapy with deferiprone and deferasirox.
Figure 3.
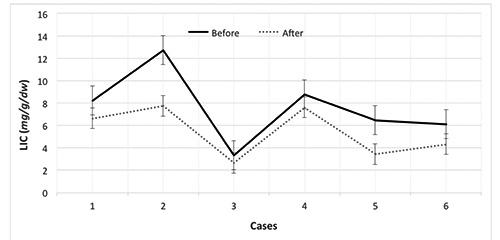
Liver iron concentration (LIC) changes in patients with β-thalassemia major before and after combination therapy with deferiprone and deferasirox.
Figure 4.
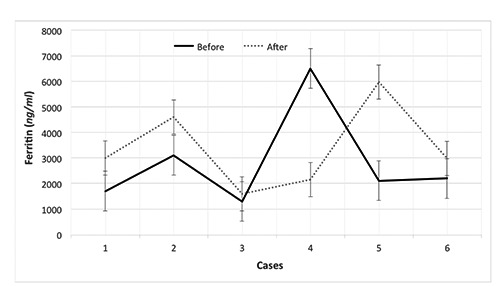
Ferritin changes in patients with β-thalassemia major before and after combination therapy with deferiprone and deferasirox.
One of the patients (case 6) experienced a drop in his cardiac MRI T2* value after combination therapy with DFP and DFX; however, the cardiac status of this patient was in the normal range both before and after therapy. Two patients (33.33%) discontinued treatment due to neutropenia and diarrhea. Currently, three patients (50%) continue treatment and have not experienced any complications.
Discussion
The main objective of this study was to demonstrate the effects of DFP and DFX combination treatment on cardiac and liver MRI values in iron-overloaded β-TM patients. The cardiac MRI T2* values increased in all cases except in patient 6. However, the change in this patient’s cardiac MRI T2* value was in the normal range, and the patient did not have cardiac siderosis according to MRI. Although there was no significant difference between the cardiac MRI T2* values before and after treatment statistically (p < 0.05), the cardiac MRI T2* values improved after combination therapy with DFX and DFP in the majority of the patients. Further, our findings showed that the liver T2* values and LIC were improved after treatment. In addition, patient 5 had a remarkable increase in their cardiac MRI T2* value after 10 months of DFX and DFP combination therapy. We believe that this considerable change was caused by more compliance with the new regimen over the course of the patients’ first year of its use.
The doses of DFP and DFX used in our study were 53.9±22.2 and 29.3±6.8 mg/kg/day, respectively. We utilized DFP and DFX combination therapy in various doses according to the patient’s clinical situation and laboratory data. The drugs’ doses used in the literature were 75 mg/kg/day for DFP and 30 mg/kg/day for DFX).7
The use of this combination (DFP and DFX) was more effective to clear the heart and the liver from iron siderosis. This is likely a result of the synergistic effects and ability of DFX10 and DFP11 to enter cells and chelate intracellular iron. Evidence on the co-administration of DFX and DFP has only been provided by a few studies.6-8 There are some case reports on the successful combined oral chelation therapy of DFX and DFP in β-TM patients.6,7 A case study reported a 25-year-old β-TM female patient who received DFX and DFP combination therapy. A dose of DFX was given at 25 mg/kg/day. Then, 3 doses of DFP at 75 mg/kg/day were added to the treatment. The patient’s serum ferritin levels decreased from 4200 to 1596 ng/mL after 1 year of treatment. Moreover, their cardiac and liver MRI-T2* values increased from 10.3 to 15 ms and 1.7 to 6.78 ms, respectively.7 A reduction of serum ferritin was observed after 8 months of combined therapy (less than 100 ng/mL before month 8).
In our study, the patients’ serum ferritin levels raised in all cases expect in patient 1. Several studies show that serum ferritin can provide information regarding the total iron store of patients; however, the association is not very strong.12 Although serum ferritin is traditionally measured in all centers for this reason, MRI is the most sensitive method for the appraisal of excess iron accumulation in the liver and heart of β-TM patients.13 We speculate that ferritin may increase following other events, such as inflammation or infection, during DFP and DFX combination therapy.14
Gomer et al. used DFX and DFP combination therapy in 15 β-TM patients for 12 months. The patients’ serum ferritin levels, urinary iron excretion, and MRI T2* values were compared before and after the intervention. Gomer et al. found the combination to be safe and effective.15 In addition, a randomized open-label trial demonstrated that DFX and DFP combination therapy improved heavily iron-overloaded β-TM patients’ cardiac T2* values after one year of treatment with 2 doses of DFP at 75 mg/kg/day and 1 dose of DFX at 30 mg/kg/day.16
A previous study evaluated the safety and efficacy of DFP and DFX combination treatment in 36 patients with β-TM with a mean age of 13±6.9 years. The patients had a mean serum ferritin level of 6768±4145 µg/L. Their mean serum ferritin level dramatically decreased to 3275±618 µg/L after 1 year (p < 0.001). The mean doses of DFP and DFX were 84.4±5.2 (61-100 mg/kg/day) and 33.4±5.2 (20-40 mg/kg/day), respectively. Of the 36 patients, 30 experienced transient gastrointestinal complications, joint problems, increased serum transaminases and creatinine, and/or transient proteinuria. Three patients discontinued treatment following persistent abdominal pain or joint problems during the study. The study found that oral combination therapy with DFP and DFX is effective for patients with a suboptimal response to monotherapy.8 In the study, patients’ serum ferritin levels decreased after combination therapy with DFX and DFP in contrast to the results of our study. However, the researchers did not evaluate the patients’ heart and liver MRI values, which is the most reliable method to estimate the intracellular iron stores of these organs.
Another previous work evaluated the efficacy of DFX in 407 patients with transfusion-dependent TM in 9 different centers.3 Their mean age was 11.5±7.4 years, and 50.5% of the patients were female. The mean dose of DFX prior to treatment was 23.5±4.9 mg/kg, and the maximum dose was 40 mg/kg. In the study, the mean baseline serum ferritin level was 2210±1175 ng/dL. A significant decrease in the mean serum ferritin level occurred after one year of DFX monotherapy. Of the participants, 148 patients (36.37%) experienced at least one side effect, such as raised serum transaminase or creatinine levels. The main causes of withdrawal included an unsatisfactory response in 24 patients (5.8%), poor compliance in 21 patients (5.1%), and adverse effects in 13 patients (3.1%). The researchers concluded that the efficacy and safety of DFX monotherapy were promising.3
Some limitations of our study included our small number of patients included and our short study period; a large-scale cohort study is required to confirm our findings. More studies are also expected to define the optimal dosing, duration, safety, and cost effectiveness of DFX and DFP combination therapy in iron-overloaded patients with β-TM.
Conclusions
Combination therapy with DFX and DFP is a new regimen for β-TM patients with a heavy iron overload. Our study showed that heart and liver MRI T2* values and LIC were improved after combination therapy with DFX and DFP in β-TM patients. Alternative therapy with DFX and DFP could be considered to alleviate cardiac and liver iron loading in these patients. The authors conclude that, while these results are encouraging, future studies should include a larger number of participants.
References
- 1.Xia S, Zhang W, Huang L, et al. Comparative efficacy and safety of deferoxamine, deferiprone and deferasirox on severe thalassemia: a meta-analysis of 16 randomized controlled trials. PLoS One 2013;8:e82662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kosaryan M, Aliasgharian A. Iron chelator medications used by patients with beta thalassemia major, Thalassemia Research Center, Iran, 2015. Sci J Iran Blood Transfus 2015. [In press]. [Google Scholar]
- 3.Eshghi R, Farahmandinia Z, Molavi M, et al. Efficacy and safety of Iranian made Deferasirox (Osveral) in Iranian major thalassemic patients with transfusional iron overload: A one year prospective multicentric open-label non-comparative study. Daru 2011;19:240-8. [PMC free article] [PubMed] [Google Scholar]
- 4.Rashidi ghader F, Kosaryan M, Karami H. Assessment of the effect of combination therapy with deferiprone and desferrioxamine on cardiac complications in major Thalassemia. JMUMS 2007;17:12-9. [Google Scholar]
- 5.Karami H, Kosaryan M, Abolghasemi H, et al. Deferiprone plus deferoxamine versus deferoxamine iron chalation in beta thalassemia major. Sci J Iran Blood Transfus Organ 2011;7:227-34. [Google Scholar]
- 6.Voskaridou E, Christoulas D, Terpos E. Successful chelation therapy with the combination of deferasirox and deferiprone in a patient with thalassaemia major and persisting severe iron overload after single-agent chelation therapies. Br J Haematol 2011;154:654-6. [DOI] [PubMed] [Google Scholar]
- 7.Alavi S, Sadeghi E, Ashenagar A. Efficacy and safety of combined oral iron chelation therapy with deferasirox and deferiprone in a patient with beta-thalassemia major and persistent iron overload. Blood Res 2014;49:72-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Totadri S, Bansal D, Bhatia P, et al. The deferiprone and deferasirox combination is efficacious in iron overloaded patients with β–thalassemia major: a prospective, single center, open–label study. Pediatr Blood Cancer 2015;62:1592-6. [DOI] [PubMed] [Google Scholar]
- 9.Ozment CP, Turi JL. Iron overload following red blood cell transfusion and its impact on disease severity. Biochim Biophys Acta 2009;1790:694-701. [DOI] [PubMed] [Google Scholar]
- 10.Cappellini M. Iron-chelating therapy with the new oral agent ICL670 (Exjade®). Best Pract Res 2005;18:289-98. [DOI] [PubMed] [Google Scholar]
- 11.Piga A, Roggero S, Salussolia I, et al. Deferiprone. Ann N Y Acad Sci 2010;1202:75-8. [DOI] [PubMed] [Google Scholar]
- 12.Beutler E, Felitti V, Ho NJ, et al. Relationship of body iron stores to levels of serum ferritin, serum iron, unsaturated iron binding capacity and transferrin saturation in patients with iron storage disease. Acta Haematol 2002;107:145-9. [DOI] [PubMed] [Google Scholar]
- 13.Taghizadeh Sarvestani R, Moradveisi B, Kompany F, et al. Correlation between heart and liver iron levels measured by MRI T2* and serum ferritin in patients with β-thalassemia major. Int J Pediatr 2016;4:1559-67. [Google Scholar]
- 14.Wang W, Knovich MA, Coffman LG, et al. Serum ferritin: past, present and future. Biochim Biophys Acta 2010;1800:760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomber S, Jain P, Sharma S, et al. Comparative efficacy and safety of oral iron chelators and their novel combination in children with thalassemia. Indian Pediatr 2016;53:207-10. [DOI] [PubMed] [Google Scholar]
- 16.Elalfy MS, Adly AM, Wali Y, et al. Efficacy and safety of a novel combination of two oral chelators deferasirox/deferiprone over deferoxamine/deferiprone in severely iron overloaded young beta thalassemia major patients. Eur J Haematol 2015; 95:411-20. [DOI] [PubMed] [Google Scholar]


