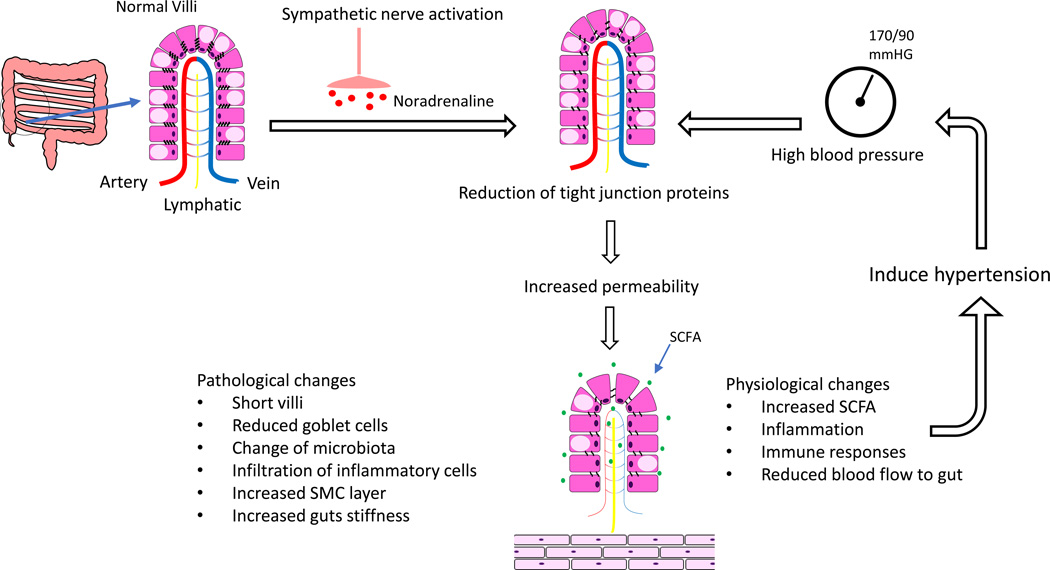
There continues to be a rapidly evolving interest in the role of the gut microbiome in cardiovascular disease. It has long been known that the gut microbiome has a fundamentally mutualistic, symbiotic relationship with the human host. However, from earlier observations using mice grown in germ-free environments to more recent advances in identifying unique metabolic products of the gut microbiome,1 the data have led to a compelling story linking cardiovascular disease to the trillions of prokaryotic organisms that live in the human gut.
In this issue of Circulation Research, Santisteban et al2 have provided provocative data demonstrating very definitively that, in two different animal models of hypertension, there is decreased expression of several tight junction proteins in the gut and a concomitant increase in intestinal permeability. Furthermore, their data show that in the SHR model, the increase in permeability is a result of increased sympathetic nerve activity prior to the development of hypertension. They therefore conclude that there is a direct, causal link between the sympathetic nerve activity derived from the central nervous system and increased gut permeability. They further hypothesize that the changes in gut permeability result in hypertension and cause a shift in the types of bacteria that are present in the gut. Finally, they have shown that the changes in sympathetic activity resulting in increased gut permeability are also associated with an increase in inflammatory cells within the intestinal wall thus potentially bringing the contributions of the immune system to hypertension into this pathophysiological mechanism.
One question raised by this manuscript is the potential relationship between increased gut permeability and changes in the gut microbiome. In the most simplistic mechanism, the increase in gut permeability would allow products of the organisms in the gut access to the tissues in the gastrointestinal tract as well as the general circulation. However, Santisteban et al2 have suggested a more complicated relationship in that they reported that there was a clear shift in the microbiome after the changes in gut permeability developed. Others have reported differences in the microbiome in hypertension3 so it is clearly not just a matter of allowing access but of also creating a dysbiosis, or an imbalance negatively impacting the beneficial flora of the intestinal tract. How this could occur is not at all clear but, in the current paper, the authors noted that mesenteric blood flow was decreased, a phenomenon that has been long been associated with increased intestinal permeability.4 A similar relationship between changes in gut permeability and dysbiosis has been reported with alcohol abuse.5 However, it was hypothesized that phenolic compounds from tyrosine breakdown by the “bad bacteria” resulted in disruption of the gut epithelial barrier, not vice versa. Whatever the initial sequence, there is a clear case for multiple, synergistic factors functioning in a positive feedback loop involving increased permeability of the gut intestinal epithelium and dysbiosis which ultimately affords the products of an imbalanced gut microbiome access to human host.
The nature of the products of the microbiome that are potentially released into the host is potentially vast and includes short chain fatty acids (SCFAs), polyamines, and activators of the aryl hydrocarbon receptor among a myriad of other potential candidates. In the case of hypertension, SCFAs are a particularly intriguing possibility as they could potentially modulate the active immune environment of the gut. Santisteban et al2 reported that there was a significant increase in T cells and monocytes/macrophages in the intestinal epithelium which suggests a potential relationship with the immunological responses that exist in the intestinal mucosa. Among their many actions, SCFAs can act as inhibitors of histone deacetylases which can in turn modify Treg cell populations as well as other immune responses.6 A mechanism such as this could conceivably begin to help to converge the roles of gut microbiome with that of the immune system in hypetesnion.7
Finally, the role of the sympathetic nervous system in inducing changes in gut permeability may have even greater impact than that proposed by the authors of this manuscript. Many factors ranging from cigarette smoking8 to mental stress9 alter the balance of the sympathetic nervous system. The potential involvement of mental stress in altered gut permeability through activation of the sympathetic nervous system is particularly intriguing. Given the extensive body of evidence linking mental stress to adverse cardiovascular outcomes10 and data indicating that chronic stress induces changes in intestinal epithelial permiability11 as well as in the gut microbiome, could changes in the intestinal epithelium play a mechanistic role in the transducing mental stress into increased cardiovascular risk? More expansively, could changes in gut permeability be another dimension to the mechanisms of action of multiple additional risk factors for hypertension that alter sympathetic nerve activity?
One of the implications of the current work and that of others in this field is that there are now new opportunities to develop novel therapeutic strategies targeting the intestinal epithelium to treat or even prevent hypertension. Probiotics have been proposed by many as a method to restore a ‘helthy” intestinal microbiome. Probiotics have been studied as potential therapeutics for hypertension by several investigators but the efficacy is modest at best in a very limited number of studies of highly variable quality12 leaving it unclear if probiotics will ever prove to be sufficiently potent therapeutic agents. However, if Santisteban et al. are correct in that sympathetic nervous system-mediated gut increased permeability precedes the development of hypertension, then perhaps our therapeutic focus should also be on these early steps in the pathological cascade. Most simplistically, could sympatholytic agents have a positive impact on the integrity of the intestinal mucosa? If we had a better understanding of the mechanism responsible for the reduction in tight junction proteins, could we employ an even more targeted approach by searching for agents that protect the integrity of the intestinal epithelium? These types of approaches could have broad impact on the ultimate impact of risk factors that alter intestinal permeability and could extend well beyond cardiovascular disease to the growing list of human conditions associated with alterations in the gut microbiome.
This manuscript by Santisteban et al. adds important new data in support of the of the overall paradigm implicating the gut microbiome in cardiovascular disease. Indeed, the data suggest that as a result of increased gut permeability, a variety of substance produced by the intestinal microbiota will gain access to their human host and ultimately lead to hypertension and potentially some of the pathological consequences of the disease. A better understating of the pathological metabolites produced by the gut microbiota, defining the mechanisms responsible for the development of dysbiosis and the molecular mechanisms linking increased sympathetic nerve activity to increased intestinal permeability are important next steps in gain a better understanding of the fundamental causes of hypertension and insights into potential novel therapeutic strategies.
Figure 1.
Overview of the impact of the impact of sympathetic nerve stimulation on gut permeability and hypertension.
Acknowledgments
W. Robert Taylor has developed intellectual property related to devices used for fecal transplantation.
Support
This work was supported by NIH PO1 HL095070
Footnotes
Conflict of Interest Statement
Kiyoko Takemiya has no relevant conflicts of interest to disclose.
References
- 1.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, Cole-Jeffrey CT, Lobaton GO, Stewart DC, Rubiano A, Simmons CS, Garcia-Pereira F, Johnson RD, Pepine CJ, Raizada MK. Hypertension-Linked Pathophysiological Alterations in the Gut. Circ Res. 2016 doi: 10.1161/CIRCRESAHA.116.309006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afsar B, Vaziri ND, Aslan G, Tarim K, Kanbay M. Gut hormones and gut microbiota: implications for kidney function and hypertension. J Am Soc Hypertens. 2016;10:954–961. doi: 10.1016/j.jash.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Ohri SK, Somasundaram S, Koak Y, Macpherson A, Keogh BE, Taylor KM, Menzies IS, Bjarnason I. The effect of intestinal hypoperfusion on intestinal absorption and permeability during cardiopulmonary bypass. Gastroenterology. 1994;106:318–323. doi: 10.1016/0016-5085(94)90588-6. [DOI] [PubMed] [Google Scholar]
- 5.Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Starkel P, Windey K, Tremaroli V, Backhed F, Verbeke K, de Timary P, Delzenne NM. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A. 2014;111:E4485–E4493. doi: 10.1073/pnas.1415174111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wenzel U, Turner JE, Krebs C, Kurts C, Harrison DG, Ehmke H. Immune Mechanisms in Arterial Hypertension. J Am Soc Nephrol. 2016;27:677–686. doi: 10.1681/ASN.2015050562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niedermaier ON, Smith ML, Beightol LA, Zukowska-Grojec Z, Goldstein DS, Eckberg DL. Influence of cigarette smoking on human autonomic function. Circulation. 1993;88:562–571. doi: 10.1161/01.cir.88.2.562. [DOI] [PubMed] [Google Scholar]
- 9.Kvetnansky R, Lu X, Ziegler MG. Stress-triggered changes in peripheral catecholaminergic systems. Adv Pharmacol. 2013;68:359–397. doi: 10.1016/B978-0-12-411512-5.00017-8. [DOI] [PubMed] [Google Scholar]
- 10.Wei J, Rooks C, Ramadan R, Shah AJ, Bremner JD, Quyyumi AA, Kutner M, Vaccarino V. Meta-analysis of mental stress-induced myocardial ischemia and subsequent cardiac events in patients with coronary artery disease. Am J Cardiol. 2014;114:187–192. doi: 10.1016/j.amjcard.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang PC, Jury J, Soderholm JD, Sherman PM, McKay DM, Perdue MH. Chronic psychological stress in rats induces intestinal sensitization to luminal antigens. Am J Pathol. 2006;168:104–114. doi: 10.2353/ajpath.2006.050575. quiz 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64:897–903. doi: 10.1161/HYPERTENSIONAHA.114.03469. [DOI] [PubMed] [Google Scholar]