Abstract
The relationship between clinical phenotypes and desmosomal gene mutations in patients with arrhythmogenic right ventricular cardiomyopathy (ARVC) is poorly characterized. Therefore, we performed a meta-analysis to explore the genotype-phenotype relationship in patients with ARVC. Any studies reporting this genotype-phenotype relationship were included. In total, 11 studies involving 1,113 patients were included. The presence of desmosomal gene mutations was associated with a younger onset age of ARVC (32.7 ± 15.2 versus 43.2 ± 13.3 years; P = 0.001), a higher incidence of T wave inversion in V1–3 leads (78.5% versus 51.6%; P = 0.0002) or a family history of ARVC (39.5% versus 27.1%; P = 0.03). There was no difference in the proportion of males between desmosomal-positive and desmosomal-negative patients (68.3% versus 68.9%; P = 0.60). The presence of desmosomal gene mutations was not associated with global or regional structural and functional alterations (63.5% versus 60.5%; P = 0.37), epsilon wave (29.4% versus 26.2%; P = 0.51) or ventricular tachycardia of left bundle-branch morphology (62.6% versus 57.2%; P = 0.30). Overall, patients with desmosomal gene mutations are characterized by an earlier onset age, a higher incidence of T wave inversion in V1–3 leads and a strong family history of ARVC.
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an uncommon inherited myocardial disease with incomplete penetrance1, characterized by ventricular arrhythmias, progressive fibrofatty replacement and even sudden cardiac death (SCD)2. To date, 13 genes have been reported in patients with ARVC, and desmosomal genes account for more than 50% of all cases, including plakoglobin (JUP), plakophilin-2 (PKP2), desmoplakin (DSP), desmoglein-2 (DSG2), and desmocollin-2 (DSC2)3. Meanwhile, non-desmosomal genes are also implicated in ARVC, such as transmembrane protein 43, desmin, titin, phospholamban, and ryanodine receptor 24,5,6. Recently, studies in patients with ARVC have shed light on the molecular mechanisms involved, and considerable progress has been made in the diagnosis and management of ARVC patients, but numerous challenges still exist due to the genetic and clinical heterogeneity in these patients7. Desmosomal gene mutations can lead to dysfunction of intercellular gap junction and electrical activity, conditions that are more vulnerable to mechanical stress8. As the most common variants, desmosomal gene mutations are closely related to several clinical characteristics, including T wave inversion, epsilon wave, onset age of ARVC and ventricular arrhythmias9,10,11. However, the definite genotype-phenotype relationship in patients with ARVC caused by desmosomal gene mutations is still poorly characterized. Moreover, data from previous studies on the clinical phenotypes of desmosomal-positive and desmosomal-negative patients are contradictory and limited due to the small population size. Therefore, we performed a meta-analysis of current studies on selected clinical features to explore the genotype-phenotype relationship in patients with ARVC caused by desmosomal gene mutations.
Methods
The protocol for the analysis and reporting of the results of this systematic review and meta-analysis was based on the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) statement12.
Literature search and study selection
We systematically searched the Cochrane Library, PubMed, and Elsevier databases through June 2016 for studies reporting the relationship between the presence of desmosomal protein gene mutations and the clinical features of ARVC. Two types of search terms were combined using the Boolean operator “and”: (1) “arrhythmogenic right ventricular dysplasia” OR “arrhythmogenic right ventricular cardiomyopathy” OR “ventricular dysplasia, right, arrhythmogenic” OR “right ventricular dysplasia, arrhythmogenic” OR “ARVC” OR “ARVD”; and (2) “plakophilin-2” OR “desmoplakin” OR “desmoglein-2” OR “desmocollin-2” OR “plakoglobin” OR “desmosome” OR “desmosomal”. We did not apply any language restrictions. In addition, we reviewed the reference lists, relevant journals, and conference abstracts for further searches.
Three independent authors (X-ZY, Z-WG and W-C) conducted the study selection and data extraction. The studies were first screened by reading the titles or abstracts to exclude the reviews, case reports, or irrelevant articles, and studies were then selected by reading the full text. Studies were considered eligible if they were: (1) cohorts of unrelated and consecutive ARVC patients where at least 2 desmosomal genes were sequenced, data on relatives were excluded from the pooled analysis; and (2) studies comparing the clinical features in ARVC patients with and without desmosomal gene mutations. If there were multiple publications, we included the study with the longest follow-up or the largest populations.
Data extraction
For each study, the following data were recorded: the first author, publication year, country or region, study population, number of cases, number of genes sequenced, positive rate of desmosomal gene mutations, and incidence rate of each desmosomal gene. We also extracted the predefined clinical features that were most frequently reported in the final selected studies: onset age of ARVC, gender, and phenotypic features on the basis of international task force criteria (TFC): global or regional dysfunction and structural alterations, T wave inversion in right precordial leads (V1–3), epsilon wave in right precordial leads (V1–3), ventricular tachycardia of left bundle-branch morphology, and family history of ARVC. Any disagreements were resolved through discussion or by a fourth researcher (H–K).
Statistical analyses
All of the statistical analyses were performed using Review Manager version 5.30 software (the Nordic Cochrane Center, Rigshospitalet, Denmark). Consistency was evaluated using the Cochrane Q test complemented with the I2 statistic, where 25% or less, 50%, and 75% or more indicated low, moderate, and high heterogeneity, respectively. The continuous variables were summarized as the mean ± standard deviation (SD), while the categorical variables were summarized as risk differences (RDs) and 95% confidence intervals (CIs). The data were pooled by the random-effects model, which was more conservative to evaluate risk estimates than the fixed-effects model. We pooled the predefined clinical features that were most frequently reported in the final selected studies (onset age of ARVC, gender, and phenotypic features on the basis of TFC). A meta-analysis of other clinical features including interventions and prognosis of ARVC (e.g., implantable cardioverter-defibrillator implantation (ICD), cardiac transplant, heart failure, and arrhythmic outcomes) was not possible because the features were inconsistently reported or not rigorously defined in the majority of the selected studies. A P value < 0.05 was considered statistically significant.
Results
Study selection
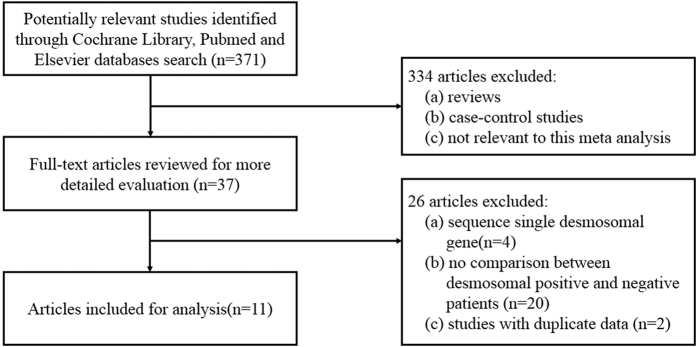
As shown in Fig. 1, we initially retrieved a total of 371 studies through the electronic retrieval and the manual search after removing duplicates. After the first screening based on abstracts or titles, 334 studies were excluded because they were reviews, case-control studies, or studies that were not relevant to our analysis. The full texts of the 37 remaining studies were reviewed, and 26 of the 37 studies were excluded because they were (a) studies that sequenced only one of the desmosomal genes (n = 4); (b) studies that did not compare the clinical features in ARVC patients with and without desmosomal mutations (n = 20); and (c) studies that had duplicate data (n = 3), with 2 of the 3 excluded due to a smaller sample size13,14.
Figure 1. Flow chart of the study selection process.
Finally, 11 studies comprising 1,113 participants were included in this meta-analysis15,16,17,18,19,20,21,22,23,24,25. The detailed characteristics of these studies are presented in Table 1. The diagnosis of patients with ARVC was made based on the original 1994 TFC26 and the revised 2010 TFC27. Four studies used the 1994 TFC16,19,20,23 and the 7 remaining studies were diagnosed according to the 2010 TFC15,17,18,21,22,24,25. In the pooled analysis, the percentage of individuals who were desmosomal-positive was 49.3% (95% CI: 39.6–59.0%).
Table 1. Characteristics of the 11 studies included in the pooled analysis.
| First Author, Year | Region | Patients (n) | Genes (n) | Desmosomal positive (%) | PKP2 (%) | JUP (%) | DSG2 (%) | DSC2 (%) | DSP (%) |
|---|---|---|---|---|---|---|---|---|---|
| Bhuiyan, 2009 | Netherlands | 84 | 3 | 32 (38.1) | 29.8 | — | 4.8 | 1.2 | — |
| Den Haan, 2009 | United States | 82 | 5 | 43 (52.4) | — | — | — | — | — |
| Fressart, 2010 | France | 135 | 5 | 62 (45.9) | 29.6 | 0 | 10.4 | 1.5 | 4.4 |
| Klauke, 2010 | Germany | 23 | 5 | 13 (56.5) | 26.1 | 0 | 8.7 | 8.7 | 8.7 |
| Cox, 2011 | Netherlands | 147 | 5 | 89 (60.5) | 53.1 | 0 | 3.4 | 1.4 | 0.7 |
| Ohno, 2013 | Japan | 35 | 4 | 20 (57.1) | 25.7 | — | 5.7 | 2.9 | 14.3 |
| Alcalde, 2014 | Spain | 30 | 6 | 19 (63.3) | 43.3 | 0 | 10.0 | 3.3 | 6.7 |
| Brun, 2014 | United States; Italy | 67 | 5 | 8 (11.9) | 7.5 | 0 | 3.0 | 0 | 1.5 |
| Groeneweg, 2015 | United States; Netherlands | 439 | 7 | 237 (54.0) | 46.0 | 0.4 | 3.9 | 1.1 | 2.5 |
| Kato, 2015 | Japan | 57 | 8 | 26 (45.6) | 19.3 | — | 12.3 | 1.8 | 5.3 |
| Medeiros-Domingo, 2016 | Switzerland | 14 | 96 | 8 (57.1) | 7.1 | 7.1 | 35.7 | 7.1 | 7.1 |
Abbreviations: JUP = plakoglobin; PKP2 = plakophilin-2; DSP = desmoplakin; DSG2 = desmoglein-2; DSC2 = desmocollin-2.
Demographic characteristics
Onset age of ARVC
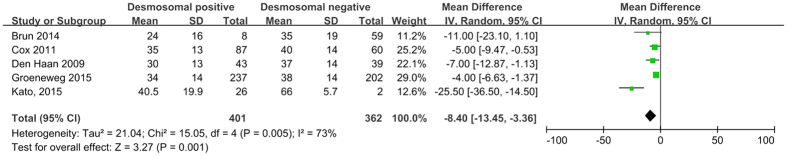
Several studies have reported a younger onset age in ARVC patients with desmosomal gene mutations19,25. In contrast, in the study by Cox et al.18, the presence of desmosomal gene mutations was not associated with an earlier onset age of ARVC. In addition, carriers with nonsense PKP2 mutations had onset at a later age than those with missense mutations15. In the pooled analysis, the average onset age for desmosomal-positive individuals was 32.7 ± 15.2 years, and the average onset age was 43.2 ± 13.3 years for desmosomal-negative individuals. There was a significant difference between these 2 groups (P = 0.001; Fig. 2), indicating that the presence of desmosomal gene mutations was associated with a younger onset age of ARVC.
Figure 2. Forest plot of the relationship between the presence of desmosomal protein gene mutations and the onset age of ARVC.
Abbreviations: ARVC = arrhythmogenic right ventricular cardiomyopathy; SD = standard deviation.
Gender
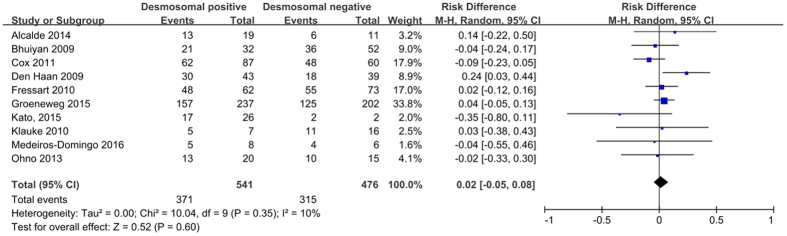
A higher prevalence of male patients is reported in the majority of ARVC cohorts11, and the male gender may be an independent predictor of appropriate ICD therapy28. In a study by Kato et al.22, the absence of desmosomal gene mutations was associated with a higher proportion of males, but other studies obtained contradictory results15,16,18,19,21,23,24,25. Overall, the pooled analysis showed no significant difference in the proportion of males between desmosomal-positive and desmosomal-negative patients (68.3% versus 68.9%; P = 0.60; Fig. 3).
Figure 3. Forest plot of the proportion of male desmosomal-positive patients with ARVC compared with desmosomal-negative patients.
Abbreviations: ARVC = arrhythmogenic right ventricular cardiomyopathy; CI = confidence interval; M-H = Mantel-Haenszel.
Phenotypic features on the basis of TFC
Structural and functional alterations
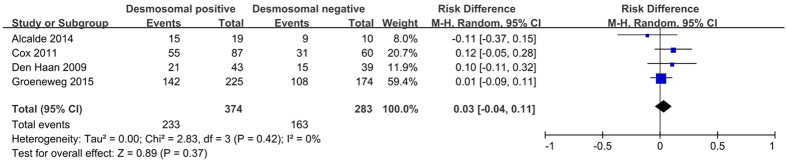
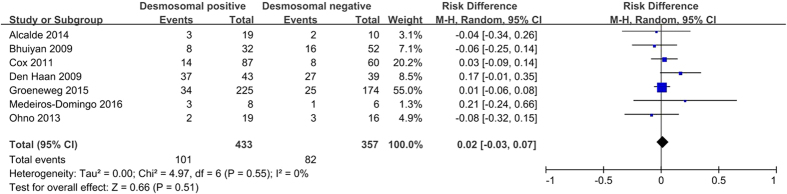
Global or regional structural and functional alterations are mainly assessed by imaging examinations with various indexes, especially in the revised 2010 TFC27,29. Despite its low prevalence, the evaluation of ARVC accounts for a disproportionately high percentage of referrals for imaging examinations29. The structural and functional alterations presented in four included studies15,18,19,21 were evaluated by both echocardiography and cardiac magnetic resonance (CMR). Moreover, only one study performed a right ventricular angiography during phenotypic evaluation21. Given that each index is inconsistently reported across studies, we regarded all the major indexes together as a category. As shown in Fig. 4, there was no difference in the proportion of structural and functional alterations in patients with desmosomal gene mutations compared to those without mutations (63.5% versus 60.5%; P = 0.37).
Figure 4. Forest plot of the proportion of structural and functional changes in desmosomal-positive patients compared with desmosomal-negative patients with ARVC.
Abbreviations: ARVC = arrhythmogenic right ventricular cardiomyopathy; CI = confidence interval; M-H = Mantel-Haenszel.
Epsilon waves in right precordial leads
The epsilon waves is one of the characteristic clinical features of ARVC. It represents a delay in depolarization of the right ventricular (RV) free wall and outflow tract in patients with ARVC30. The pooled analysis showed no difference in the presence of epsilon waves between desmosomal-positive and desmosomal-negative individuals (29.4% versus 26.2%; P = 0.51; Fig. 5).
Figure 5. Forest plot of the proportion of subjects positive for epsilon waves in desmosomal-positive compared with desmosomal-negative patients with ARVC.
Abbreviations: ARVC = arrhythmogenic right ventricular cardiomyopathy; CI = confidence interval; M-H = Mantel-Haenszel.
T wave inversion in right precordial leads
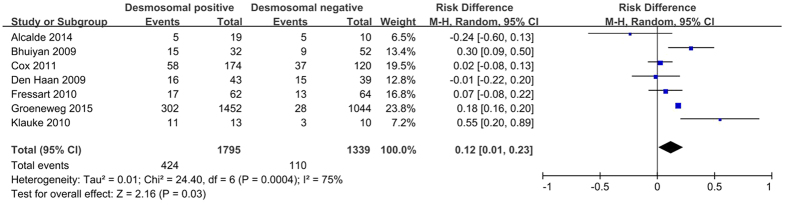
Electrocardiograms (ECGs) and signal-averaged ECGs are analyzed for depolarization and repolarization abnormalities, including T-wave inversions as the most common ECG alteration10. T wave inversion in V1–3 leads is demonstrated as one of the most sensitive and specific markers for the identification of desmosomal mutation carriers16,20. As shown in Fig. 6, the pooled analysis showed a higher incidence of T wave inversion in V1–3 leads in desmosomal-positive patients than in desmosomal-negative patients (78.5% versus 51.6%; P = 0.0002).
Figure 6. Forest plot of the proportion of negative T waves V1–3 in desmosomal-positive and desmosomal-negative patients with ARVC.
Abbreviations: ARVC = arrhythmogenic right ventricular cardiomyopathy; CI = confidence interval; M-H = Mantel-Haenszel.
Ventricular tachycardia of left bundle-branch morphology
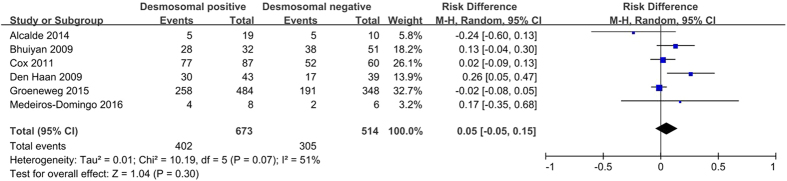
Pathologically progressive fibrofatty replacement is thought to be responsible for ventricular arrhythmias. Electrophysiological studies are not included in the diagnostic criteria, but may be important for differential diagnosis, including RV outflow tract tachycardia10. Ventricular tachycardia of left bundle-branch morphology is the major index of ventricular arrhythmia in TFC. The meta-analysis showed no statistically significant difference in the presence of ventricular tachycardia of left bundle-branch morphology between desmosomal-positive and desmosomal-negative individuals (62.6% versus 57.2%; P = 0.30; Fig. 7).
Figure 7. Forest plot of the proportion of ventricular arrhythmias of left bundle-branch morphology in desmosomal-positive compared with desmosomal-negative patients with ARVC.
Abbreviations: ARVC = arrhythmogenic right ventricular cardiomyopathy; CI = confidence interval; M-H = Mantel-Haenszel.
Family history of ARVC
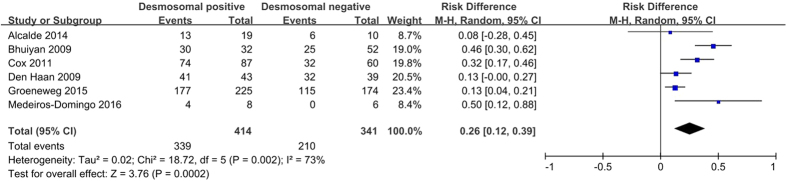
According to the study by Te Riele et al.31, one-third of first-degree relatives of ARVC probands could develop ARVC, especially siblings. Therefore, the fulfillment of TFC independent of family history is superior to conventional TFC for arrhythmic risk stratification of relatives31. Several studies have reported on the association of family history with the presence of desmosomal gene mutations, but the results remain contradictory. The pooled analysis showed a higher incidence of family history of ARVC in desmosomal-positive patients than in desmosomal-negative patients (39.5% versus 27.1%; P = 0.03; Fig. 8).
Figure 8. Forest plot of the proportion of a family history of ARVC in desmosomal-positive compared with desmosomal-negative patients with ARVC.
Abbreviations: ARVC = arrhythmogenic right ventricular cardiomyopathy; CI = confidence interval; M-H = Mantel-Haenszel.
Interventions and prognosis of ARVC
A meta-analysis of the interventions and prognosis of ARVC was not possible due to the limited phenotype data and high genetic heterogeneity. Therefore, we performed a descriptive analysis of the selected clinical features. Data on the frequency of ICD implantation in mutation carriers were contradictory. An increased prevalence of implanted ICDs was reported in the desmosomal-positive patients with ARVC32, but not in the desmosomal mutation carriers. In addition, several studies have reported the specific associations between individual mutations in the desmosomal genes and outcomes in patients with ARVC. In the study by van Tintelen et al.33, the presence of a PKP2 mutation was not associated with higher risks of endpoint events (documented sustained ventricular tachycardia episodes, ventricular fibrillation, appropriate ICD therapy, successful resuscitation and SCD). DSP mutation carriers were considerably more likely to develop heart failure, signs of left ventricular involvement and SCD14,34, but these results are contradictory for DSG2 mutation carriers14,20.
Discussion
Studies on the genotype-phenotype relationship in ARVC have improved the understanding of the molecular mechanisms leading to ARVC and the value of genetic counseling for ARVC. However, the genetic substrate, pathogenesis and clinical diagnosis of ARVC patients (especially for asymptomatic gene mutation carriers) are still poorly understood. Previous studies on the genotype-phenotype relationship in patients with ARVC are largely limited because of the small population size as well as the genetic and clinical heterogeneity. Therefore, the clinical significance of the genotype is obscure and the value of genetic analysis in developing a clinical strategy is still controversial. Our meta-analysis first expanded the sample size to explore the relationship between the presence of desmosomal gene mutations and selected clinical features.
The impact of sex on clinical course in ARVC patients is still unclear. Our analysis showed that there was no difference in the proportion of males between desmosomal-positive and desmosomal-negative patients, which is consistent with the majority of studies20,21,23,25. In addition, the incidence of desmosomal gene mutations was associated with a younger onset age of ARVC, and the initial symptoms in some young probands were more severe19,25. The presence of a PKP2 mutation is also associated with an earlier onset of symptoms and arrhythmias9. In addition, many previous studies have indicated that the presence of both single and multiple desmosomal gene mutations is associated with a younger onset age of ARVC symptoms and ventricular tachycardia14,19,33,35. Multiple desmosomal gene mutations (compound heterozygosity, digenic or trigenic heterozygosity or homozygosity) were identified in 16% of ARVC-causing desmosomal gene mutation carriers and have a close association with a younger onset age and arrhythmia35. Therefore, compound or digenic desmosomal mutations are listed as independent risk factors for ARVC according to the 2015 European Society of Cardiology (ESC) Guidelines for the management of patients with ventricular arrhythmia and the prevention of SCD36.
For the phenotypic features based on TFC, the presence of desmosomal gene mutations was associated with a higher proportion of T wave inversion in V1–3 leads (which occurred more often in PKP2 mutation carriers)33 and a family history of ARVC. ARVC is characterized by a propensity towards arrhythmia exceeding the degree of ventricular dysfunction, and T wave inversion in the V1–3 leads is a major index of repolarization according to the revised 2010 TFC27. Desmosomal proteins are involved in cell-cell adhesion and help maintain myocyte integrity3. It has been reported that the expression of connexin 43 is significantly decreased by desmosomal genes (PKP2 and JUP) mutations and is associated with the development of arrhythmia in ARVC patients37,38. The extent of negative T-waves can help estimate the amount of RV electroanatomic scar (EAS) and predict EAS-related arrhythmic risk39. Thus, desmosomal mutation may lead to abnormal repolarization of cardiomyocytes, resulting in T wave inversion of the right precordial lead on 12-leads ECG.
However, the relevant incidence of structural or functional alterations, epsilon waves, and ventricular tachycardia of left bundle-branch morphology had no associations with the presence of desmosomal gene mutations. This may because ARVC phenotypic expression is a prerequisite for the occurrence of life-threatening arrhythmias in desmosomal gene mutation carriers40 or the 3 aforementioned phenotypes may be more severe in those patients with non-desmosomal mutations such as transmembrane protein 43 and phospholamban mutations.
Due to the widely clinical application of next-generation sequencing, massive candidate gene screening can identify various mutations that may have not been identified by Sanger technology, and the genetic basis of ARVC is now better understood. Although guidelines and expert consensus suggested that comprehensive or targeted genetic screening can be useful for patients satisfying TFC41,42, genetic testing is presently handicapped by low yields, unclear clinical impact, and high background noise rates that make ARVC genetic test interpretations still challenging43. Thus, we must emphasize the necessity of a specialist in clinical practice to explain the implications of the genetic abnormality. Meanwhile, precise genotype-phenotype studies are also very important. Consistent differences between desmosomal-positive and desmosomal-negative patients suggest that, in spite of the inconsistency of each gene, desmosomal genes might be considered as a clinical entity. In accordance with our meta-analysis, desmosomal gene mutations were associated with a younger onset age, a higher prevalence of negative T waves V1–3 and a family history of ARVC. This information may help improve our understanding of the different clinical manifestations of ARVC and differential diagnosis, prognosis and treatment of patients with ARVC.
Our study also had several potential limitations. First, there were substantial differences regarding study design, case ascertainment and the assessment of clinical manifestations. The heterogeneous nature of ARVC and the inconsistency of study designs precluded the establishment of more precise genotype–phenotype relationships. For example, in this meta-analysis, the structural and functional alterations presented in four included studies are evaluated by both echocardiography and CMR, without further comparison in each index. This may cause bias and confusion, thus further studies should compare indicators of structural and functional alterations based on TFC in detail. Second, several factors might influence disease expression. For example, some mutations were unique to each family, ethnic background, and environmental factor and had low penetrance. Nevertheless, these results were stable in the sensitivity analysis. Third, because of the limited data, the diagnosis criteria of ARVC was inconsistent across studies, thus more studies with consistent diagnosis criteria will be needed in the future. Finally, a meta-analysis of specific associations between individual desmosomal gene mutations and clinical phenotypes was not possible due to limited data. Large cohort studies with a reasonable design and grouping that examine the significance among genotype, risk stratification and prognosis are also needed. The underlying mechanisms of the desmosomal genes that affects the clinical features of ARVC also deserve further investigation.
Conclusions
In summary, our meta-analysis first demonstrated that the presence of desmosomal gene mutations was associated with several clinical features, including a younger onset age of ARVC, a higher prevalence of negative T waves V1–3 and a family history of ARVC. The clinical application of genetic testing and the interpretation of genetic variations for ARVC is still challenging. Owing to the heterogeneity of ARVC, large-scale studies on the genotype-phenotype relationship with more detailed indicators in patients with ARVC are required to define the value of genetic screening for clinical practice.
Additional Information
How to cite this article: Xu, Z. et al. Genotype-phenotype relationship in patients with arrhythmogenic right ventricular cardiomyopathy caused by desmosomal gene mutations: A systematic review and meta-analysis. Sci. Rep. 7, 41387; doi: 10.1038/srep41387 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
The authors wish to acknowledge support from the National Natural Science Foundation of China [81370288, 8153000545 and 81530013] and the National Basic Research Program of China [973 Program: 2013CB531103].
Footnotes
Author Contributions H.-K. managed the project and revised the draft; X.-Z.Y., Z.-W.G. and W.-C. performed the systematic literature review, constructed the database and analyzed the data with the help of H.-L., Z.-Q.Q. and C.-X.S. All authors participated in the interpretation of the results and prepared the final version.
References
- Meurs K. M. et al. Genome-wide association identifies a deletion in the 3′ untranslated region of striatin in a canine model of arrhythmogenic right ventricular cardiomyopathy. Hum Genet 128, 315–324, doi: 10.1007/s00439-010-0855-y (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrado D. et al. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA 296, 1593–1601, doi: 10.1001/jama.296.13.1593 (2006). [DOI] [PubMed] [Google Scholar]
- Marcus F. I., Edson S. & Towbin J. A. Genetics of arrhythmogenic right ventricular cardiomyopathy: a practical guide for physicians. J Am Coll Cardiol 61, 1945–1948, doi: 10.1016/j.jacc.2013.01.073 (2013). [DOI] [PubMed] [Google Scholar]
- Roux-Buisson N. et al. Prevalence and significance of rare RYR2 variants in arrhythmogenic right ventricular cardiomyopathy/dysplasia: results of a systematic screening. Heart Rhythm 11, 1999–2009, doi: 10.1016/j.hrthm.2014.07.020 (2014). [DOI] [PubMed] [Google Scholar]
- van der Zwaag P. A. et al. Phospholamban R14del mutation in patients diagnosed with dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy: evidence supporting the concept of arrhythmogenic cardiomyopathy. Eur J Heart Fail 14, 1199–1207, doi: 10.1093/eurjhf/hfs119 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzon A. et al. Desmin mutations and arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol 111, 400–405, doi: 10.1016/j.amjcard.2012.10.017 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauce B. et al. Clinical phenotype and diagnosis of arrhythmogenic right ventricular cardiomyopathy in pediatric patients carrying desmosomal gene mutations. Heart Rhythm 8, 1686–1695, doi: 10.1016/j.hrthm.2011.06.026 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso C. et al. Ultrastructural evidence of intercalated disc remodelling in arrhythmogenic right ventricular cardiomyopathy: an electron microscopy investigation on endomyocardial biopsies. Eur Heart J 27, 1847–1854, doi: 10.1093/eurheartj/ehl095 (2006). [DOI] [PubMed] [Google Scholar]
- Dalal D. et al. Clinical features of arrhythmogenic right ventricular dysplasia/cardiomyopathy associated with mutations in plakophilin-2. Circulation 113, 1641–1649, doi: 10.1161/circulationaha.105.568642 (2006). [DOI] [PubMed] [Google Scholar]
- Haugaa K. H., Haland T. F., Leren I. S., Saberniak J. & Edvardsen T. Arrhythmogenic right ventricular cardiomyopathy, clinical manifestations, and diagnosis. Europace 18, 965–972, doi: 10.1093/europace/euv340 (2016). [DOI] [PubMed] [Google Scholar]
- Bauce B. et al. Comparison of clinical features of arrhythmogenic right ventricular cardiomyopathy in men versus women. Am J Cardiol 102, 1252–1257, doi: 10.1016/j.amjcard.2008.06.054 (2008). [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G. & Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62, 1006–1012, doi: 10.1016/j.jclinepi.2009.06.005 (2009). [DOI] [PubMed] [Google Scholar]
- Groeneweg J. A. et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy according to revised 2010 task force criteria with inclusion of non-desmosomal phospholamban mutation carriers. Am J Cardiol 112, 1197–1206, doi: 10.1016/j.amjcard.2013.06.017 (2013). [DOI] [PubMed] [Google Scholar]
- Bhonsale A. et al. Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated mutation carriers. Eur Heart J 36, 847–855, doi: 10.1093/eurheartj/ehu509 (2015). [DOI] [PubMed] [Google Scholar]
- Alcalde M. et al. Stop-gain mutations in PKP2 are associated with a later age of onset of arrhythmogenic right ventricular cardiomyopathy. PLoS One 9, e100560, doi: 10.1371/journal.pone.0100560 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuiyan Z. A. et al. Desmoglein-2 and desmocollin-2 mutations in dutch arrhythmogenic right ventricular dysplasia/cardiomypathy patients: results from a multicenter study. Circ Cardiovasc Genet 2, 418–427, doi: 10.1161/circgenetics.108.839829 (2009). [DOI] [PubMed] [Google Scholar]
- Brun F. et al. Titin and desmosomal genes in the natural history of arrhythmogenic right ventricular cardiomyopathy. J Med Genet 51, 669–676, doi: 10.1136/jmedgenet-2014-102591 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M. G. et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: pathogenic desmosome mutations in index-patients predict outcome of family screening: Dutch arrhythmogenic right ventricular dysplasia/cardiomyopathy genotype-phenotype follow-up study. Circulation 123, 2690–2700, doi: 10.1161/circulationaha.110.988287 (2011). [DOI] [PubMed] [Google Scholar]
- den Haan A. D. et al. Comprehensive desmosome mutation analysis in north americans with arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Cardiovasc Genet 2, 428–435, doi: 10.1161/circgenetics.109.858217 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fressart V. et al. Desmosomal gene analysis in arrhythmogenic right ventricular dysplasia/cardiomyopathy: spectrum of mutations and clinical impact in practice. Europace 12, 861–868, doi: 10.1093/europace/euq104 (2010). [DOI] [PubMed] [Google Scholar]
- Groeneweg J. A. et al. Clinical Presentation, Long-Term Follow-Up, and Outcomes of 1001 Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy Patients and Family Members. Circ Cardiovasc Genet 8, 437–446, doi: 10.1161/CIRCGENETICS.114.001003 (2015). [DOI] [PubMed] [Google Scholar]
- Kato K. et al. LMNA cardiomyopathy detected in Japanese arrhythmogenic right ventricular cardiomyopathy cohort. J Cardiol, doi: 10.1016/j.jjcc.2015.10.013 (2015). [DOI] [PubMed] [Google Scholar]
- Klauke B. et al. De novo desmin-mutation N116S is associated with arrhythmogenic right ventricular cardiomyopathy. Hum Mol Genet 19, 4595–4607, doi: 10.1093/hmg/ddq387 (2010). [DOI] [PubMed] [Google Scholar]
- Medeiros-Domingo A. et al. Arrhythmogenic right ventricular cardiomyopathy: implications of next-generation sequencing in appropriate diagnosis. Europace, doi: 10.1093/europace/euw098 (2016). [DOI] [PubMed] [Google Scholar]
- Ohno S. et al. Age-dependent clinical and genetic characteristics in Japanese patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ J 77, 1534–1542 (2013). [DOI] [PubMed] [Google Scholar]
- McKenna W. J. et al. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J 71, 215–218 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus F. I. et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J 31, 806–814, doi: 10.1093/eurheartj/ehq025 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A., Crawford J., Skinner J. R., Smith W. & Cardiac Inherited Diseases G. High Arrhythmic Burden but Low Mortality during Long-term Follow-up in Arrhythmogenic Right Ventricular Cardiomyopathy. Heart Lung Circ 25, 275–281, doi: 10.1016/j.hlc.2015.08.019 (2016). [DOI] [PubMed] [Google Scholar]
- Rastegar N. et al. Cardiac MR findings and potential diagnostic pitfalls in patients evaluated for arrhythmogenic right ventricular cardiomyopathy. Radiographics 34, 1553–1570, doi: 10.1148/rg.346140194 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon S. M., Healey J. S. & Morillo C. A. Epsilon waves during ventricular tachycardia in a case of arrhythmogenic right ventricular dysplasia. Circulation 122, 1752–1755, doi: 10.1161/CIRCULATIONAHA.110.959080 (2010). [DOI] [PubMed] [Google Scholar]
- Te Riele A. S. et al. Approach to family screening in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Eur Heart J 37, 755–763, doi: 10.1093/eurheartj/ehv387 (2016). [DOI] [PubMed] [Google Scholar]
- Mast T. P. et al. Prolonged Electromechanical Interval Unmasks Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy in the Subclinical Stage. J Cardiovasc Electrophysiol 27, 303–314, doi: 10.1111/jce.12882 (2016). [DOI] [PubMed] [Google Scholar]
- van Tintelen J. P. et al. Plakophilin-2 mutations are the major determinant of familial arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation 113, 1650–1658, doi: 10.1161/circulationaha.105.609719 (2006). [DOI] [PubMed] [Google Scholar]
- Sen-Chowdhry S. et al. Clinical and genetic characterization of families with arrhythmogenic right ventricular dysplasia/cardiomyopathy provides novel insights into patterns of disease expression. Circulation 115, 1710–1720, doi: 10.1161/circulationaha.106.660241 (2007). [DOI] [PubMed] [Google Scholar]
- Rigato I. et al. Compound and digenic heterozygosity predicts lifetime arrhythmic outcome and sudden cardiac death in desmosomal gene-related arrhythmogenic right ventricular cardiomyopathy. Circ Cardiovasc Genet 6, 533–542, doi: 10.1161/circgenetics.113.000288 (2013). [DOI] [PubMed] [Google Scholar]
- Priori S. G. et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC)Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 36, 2793–2867, doi: 10.1093/eurheartj/ehv316 (2015). [DOI] [PubMed] [Google Scholar]
- Yoshida T. et al. Relationships Between Clinical Characteristics and Decreased Plakoglobin and Connexin 43 Expressions in Myocardial Biopsies From Patients With Arrhythmogenic Right Ventricular Cardiomyopathy. Int Heart J 56, 626–631, doi: 10.1536/ihj.15-144 (2015). [DOI] [PubMed] [Google Scholar]
- Wang P. N., Wu S. L., Zhang B., Lin Q. X. & Shan Z. X. Function of a novel plakophilin-2 mutation in the abnormal expression of connexin43 in a patient with arrhythmogenic right ventricular cardiomyopathy. Exp Ther Med 9, 967–971, doi: 10.3892/etm.2014.2145 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzi A. et al. Electrocardiographic predictors of electroanatomic scar size in arrhythmogenic right ventricular cardiomyopathy: implications for arrhythmic risk stratification. J Cardiovasc Electrophysiol 24, 1321–1327, doi: 10.1111/jce.12246 (2013). [DOI] [PubMed] [Google Scholar]
- Zorzi A. et al. Phenotypic expression is a prerequisite for malignant arrhythmic events and sudden cardiac death in arrhythmogenic right ventricular cardiomyopathy. Europace 18, 1086–1094, doi: 10.1093/europace/euv205 (2016). [DOI] [PubMed] [Google Scholar]
- Hershberger R. E. et al. Genetic evaluation of cardiomyopathy–a Heart Failure Society of America practice guideline. J Card Fail 15, 83–97, doi: 10.1016/j.cardfail.2009.01.006 (2009). [DOI] [PubMed] [Google Scholar]
- Ackerman M. J. et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm 8, 1308–1339, doi: 10.1016/j.hrthm.2011.05.020 (2011). [DOI] [PubMed] [Google Scholar]
- Tester D. J. & Ackerman M. J. Genetic testing for potentially lethal, highly treatable inherited cardiomyopathies/channelopathies in clinical practice. Circulation 123, 1021–1037, doi: 10.1161/CIRCULATIONAHA.109.914838 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]