Abstract
Bin1/M-amphiphysin-II is an amphiphysin-II isoform highly expressed in transverse tubules of adult striated muscle and is implicated in their biogenesis. Bin1 contains a basic unique amino-acid sequence, Exon10, which interacts with certain phosphoinositides such as phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2), to localize to membranes. Here we found that Exon10 also binds to the src homology 3 (SH3) domain of Bin1 itself, and hence blocks the binding of the SH3 domain to its canonical PxxP ligands, including dynamin. This blockage was released by addition of PI(4,5)P2 in vitro or in cells overexpressing phosphatidylinositol 4-phosphate 5-kinase. The Exon10-binding interface of the Bin1 SH3 domain largely overlapped with its PxxP-binding interface. We also show that the PLCδ pleckstrin homology domain, another PI(4,5)P2-binding module, cannot substitute for Exon10 in Bin1 function in transverse tubule formation, and suggest the importance of the dual biochemical properties of Exon10 in myogenesis. Our results exemplify a novel mechanism of SH3 domain regulation, and suggest that the SH3-mediated protein–protein interactions of Bin1 are regulated by Exon10 so that it may only occur when Bin1 localizes to certain submembrane areas.
Keywords: Bin1, phosphoinositide, protein–protein interaction, SH3 domain, transverse tubule
Introduction
The src homology 3 (SH3) domain is a noncatalytic protein–protein interaction module found in a great variety of cytoplasmic and membrane-associated proteins, such as signaling adaptors, cytoskeleton-associated proteins and proteins with enzymatic activity. The SH3 domain has a hydrophobic shallow groove defined by conserved aromatic residues on one side of its structure, which serves as the binding surface to proline-rich peptides of an all-trans left-handed polyproline type II helix with the minimal consensus PxxP (Mayer, 2001; Musacchio, 2003). PxxP ligands bind with low affinity but this may be enhanced by multiple interactions. It has been thought that SH3 domains may have general functions such as increasing local protein concentration, altering protein subcellular localization or mediating the assembly of multiprotein complexes. SH3 domains in a few proteins, on the other hand, have been shown to bind to non-PxxP peptides, such as PxxDY, RKxxYxxY, WxxxFxxLE and RxxK, in which peptides interact with areas either distinct or overlapping with the PxxP-binding site (Lock et al, 2000; Mayer 2001; Kami et al, 2002; Berry et al, 2002; Latour et al, 2003). Although isolated SH3 domains can bind constitutively to their cognate ligands in vitro, some SH3-binding reactions have been suggested to be regulatable in vivo (Mayer, 2001; Musacchio, 2003).
Amphiphysin possesses an NH2-terminal BAR domain and a COOH-terminal SH3 domain (Wigge and McMahon, 1998). Mammals possess two amphiphysin genes, amphiphysin-I and II. Amphiphysin-I is a neuronal protein enriched in nerve terminals and is likely to be involved in endocytosis, and was originally identified as a human autoantigen in paraneoplastic Stiff-man syndrome (David et al, 1996). Amphiphysin-II is widely expressed in different tissues, and is most highly expressed in the brain and striated muscle (Butler et al, 1997). Amphiphysin-II consists of greater than six isoforms generated by alternative mRNA splicing, all of which contain the common COOH-terminal SH3 domain (Ramjaun and McPherson, 1998), by which amphiphysin-II binds to canonical PxxP sequences of other proteins, such as synaptojanin and dynamin2 (Wigge and McMahon, 1998). Amphiphysin-II isoforms bearing the clathrin- and AP-2-binding sites, such as amphiphysin-IIa, have been suggested to be involved in the late stage of endocytosis in brain (Butler et al, 1997; Wigge and McMahon, 1998). In contrast, amphiphysin-II isoforms without the clathrin- and AP-2-binding sites, such as Bin1, whose expression is highly induced during C2C12 differentiation in myogenesis (Wechsler-Reya et al, 1998), are thought to be involved in the membrane remodeling of excitation–contraction coupling machinery in muscle (Butler et al, 1997; Razzaq et al, 2001).
Lee et al (2002) have shown that Bin1 is involved in transverse tubule (T-tubule) biogenesis in striated muscle. T-tubules are specialized invaginations of the plasma membrane that serve to propagate action potentials to the interior of muscle fibers (Flucher, 1992). Morphological studies have suggested that T-tubules are formed by a process similar to endocytosis, which is however not accompanied by the pinching-off of vesicles (Ishikawa, 1968). Bin1 contains a basic amino-acid sequence (RKKSKLFSRLRRKKN) encoded by exon10 (hereafter this module is called Exon10), which is unique to Bin1 among the known amphiphysin-II isoforms (Ramjaun and McPherson, 1998). Lee et al (2002) have also shown that Exon10 can associate with certain phosphoinositides, such as phosphatidylinositol-4-phosphate and phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2), but not with phosphatidylinositol-3,4-bisphosphate, phosphatidylinositol-3,5-bisphosphate (PI(3,5)P2), phosphatidylserine (PS) or phosphatidylinositol. T-tubules are rich in PI(4,5)P2, and interaction of Exon10 with PI(4,5)P2 appears to be crucial for localization of Bin1 to T-tubules (Lee et al, 2002).
In this study, we found that besides phosphoinositides, Exon10 also has affinity toward SH3 domain of Bin1 itself, and acts to mask the SH3 domain from binding to its canonical PxxP ligands. Our results indicate that binding of the Bin1 SH3 domain to PxxP ligands is regulated by Exon10 so that it only occurs when Bin1 is localized to certain membrane areas via the binding of Exon10 to phosphoinositides. We also provide evidence supporting that such dual properties of Exon10 are important for normal T-tubule formation in myogenesis.
Results
Exon10 acts to block the binding of Bin1 to dynamin2 in vitro
Bin1 is an amphiphysin-II isoform generated by alternative mRNA splicing. During our study of amphiphysin-II isoforms involved in endocytosis, we found that Bin1 does not bind to the proline-rich domain (PRD) of dynamin2 in vitro, while other isoforms, such as amphiphysin-IIm (IIm), bind strongly (data not shown; see Figure 1). Bin1 is a close isoform of amphiphysin-IIm: both have the COOH-terminal SH3 domain responsible for PxxP binding, but lack the clathrin- and AP-2-binding sites (see Figure 1A). However, Bin1 is unique in that it is the only isoform bearing Exon10 (Ramjaun and McPherson, 1998). We thus tested whether the presence of Exon10 causes the absence of binding to the PRD of dynamin2. SH3P9 is a naturally occurring Exon10-deleted isoform of Bin1 (see Figure 1A). We also made a mutant form of amphiphysin-IIm in which Exon10 was inserted (IIm+Exon10; Figure 1A). To examine the in vitro binding ability of these proteins with dynamin2, we expressed Bin1, SH3P9, amphiphysin-IIm and the IIm+Exon10 mutant in COS-7 cells, and the cell lysates were incubated with GST-tagged dynamin2, produced in COS-7 cells and purified on glutathione beads. As shown in Figure 1B, SH3P9 and amphiphysin-IIm bound to GST-dynamin2, while Bin1 and the IIm+Exon10 mutant did not. Although there is another splicing difference between Bin1 and amphiphysin-IIm (see Figure 1A), results using these four constructs indicate that the presence of Exon10 is primarily responsible for the inability of Bin1 to bind to dynamin2 in vitro.
Figure 1.
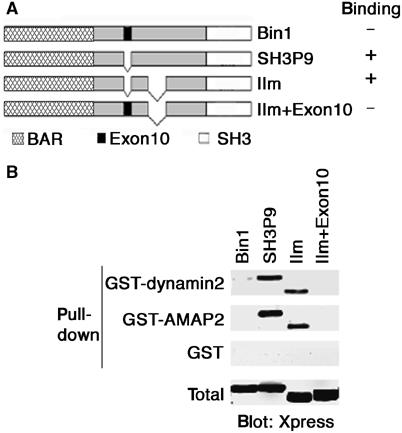
Exon10 is responsible for the inability of Bin1 to bind to dynamin2 in vitro. (A) Structures of Bin1, its related isoforms (SH3P9 and IIm) and a synthetic mutant (IIm+Exon10). (B) In vitro binding of Bin1 and its related proteins to GST-dynamin2 and GST-AMAP2. A 5 μg portion of GST-dynamin2, GST-AMAP2 or GST alone was incubated with 250 μg of COS-7 cell lysate each expressing His-Xpress-tagged Bin1, SH3P9, IIm and IIm+Exon10, and co-precipitated proteins were analyzed by immunoblotting using an anti-Xpress antibody. Total includes 20 μg of the cell lysates. Full-length GST-dynamin2 and full-length GST-AMAP2, expressed in COS-7 cells and purified on glutathione beads, were used. GST was produced in E. coli and purified on glutathione beads.
The amphiphysin-II SH3 domain not only binds to the dynamin2 PRD but also binds to PRDs of other proteins, such as synaptojanin (Wigge and McMahon, 1998) and AMAP2 (also called PAG3 or Papα; Hashimoto et al, 2004). We then examined whether the presence of Exon10 in Bin1 also acts to block its binding to AMAP2 in vitro. As shown in Figure 1B, we found that Bin1 does not bind to GST-AMAP2, while SH3P9 binds strongly. Similarly, the IIm+Exon10 mutant did not bind to GST-AMAP2, while wild-type amphiphysin-IIm bound strongly (Figure 1B). Therefore, Bin1 acts similarly with regard to its binding to AMAP2 and dynamin2 in vitro.
Possible binding of Exon10 with the Bin1 SH3 domain
We then explored the underlying mechanism as to how the presence of Exon10 acts to block the binding of Bin1 to other proteins via its SH3 domain. For this purpose, we divided Bin1 into two regions—only the SH3 domain, tagged with GST, and the rest of the protein (Bin1ΔSH3), tagged with the His-Xpress tag—and tested their binding in vitro. As a control, we also made an SH3-deleted mutant of SH3P9 (SH3P9ΔSH3) tagged with the His-Xpress tag. We found that His-Xpress-Bin1ΔSH3 binds strongly to GST-Bin1 SH3, while His-Xpress-SH3P9ΔSH3 does not (Figure 2A). Therefore, the presence of Exon10 appears to render Bin1ΔSH3 the ability to physically interact with the SH3 domain of Bin1.
Figure 2.
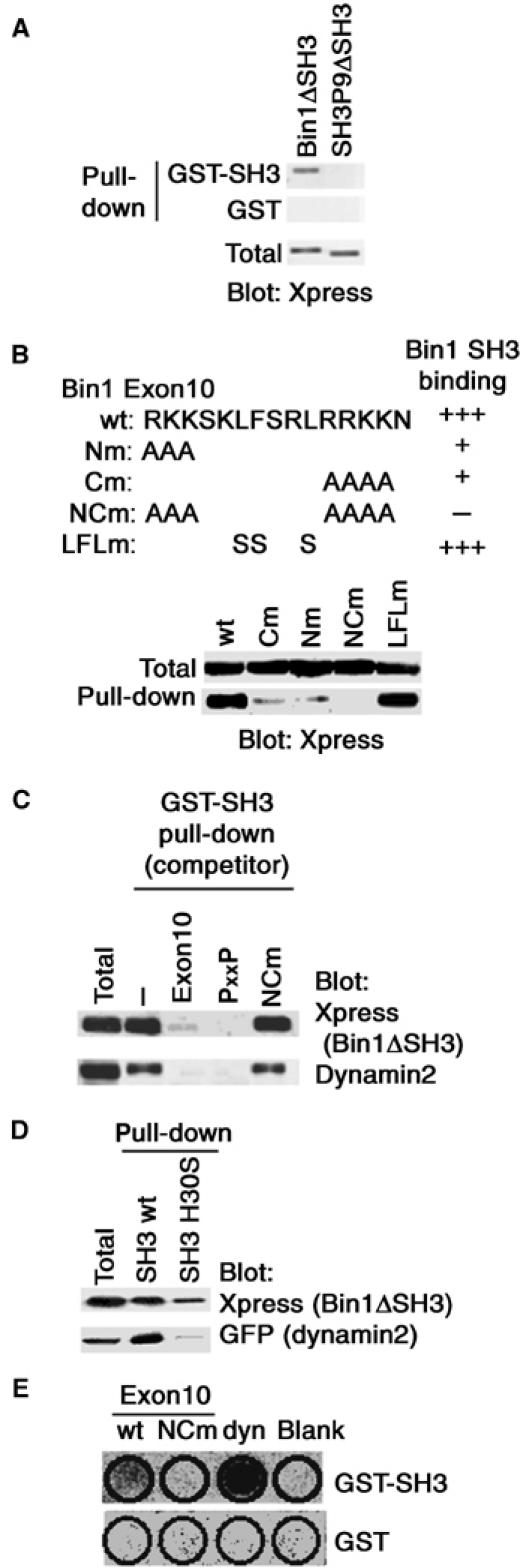
Exon10 binding to the Bin1 SH3 domain. (A) A 10 μg portion of GST-Bin1 SH3 or GST alone was incubated with 250 μg of lysate from COS-7 cells expressing His-Xpress-tagged Bin1ΔSH3 or SH3P9ΔSH3, to test the binding of Exon10 to the Bin1 SH3 domain. (B) Basic amino-acid clusters of Exon10 are necessary for binding to the Bin1 SH3 domain. The NH2- and COOH-terminal basic amino-acid clusters or the central nonpolar amino acids of Exon10 were mutated, as indicated (upper panel). His-Xpress-tagged Bin1ΔSH3 proteins, in which Exon10 was mutated, were expressed in COS-7 cells, and tested for their binding to GST-Bin1 SH3 in vitro (lower panels). (C) Competition of the Exon10 peptide with the dynamin PxxP peptide in binding to the Bin1 SH3 domain. A 10 μg portion of GST-Bin1 SH3 was incubated with 1 mM each of the Exon10 peptide, the dynamin PxxP peptide or the NCm mutant peptide, and then incubated with 250 μg of lysate from COS-7 cells expressing His-Xpress-Bin1ΔSH3, to test the binding. (D) Nonidentical binding of the Bin1 SH3 domain to Exon10 and the dynamin PxxP peptide. A measure of 10 μg of GST-Bin1 SH3 or its H30S mutant was incubated with 250 μg of lysate from COS-7 cells expressing His-Xpress-Bin1ΔSH3 or EGFP-dynamin2, to test the binding. (E) Peptides for Exon10 (wt), its mutant (NCm) and the dynamin PxxP motif (dyn) were synthesized on a membrane, and hybridized with radiolabeled GST-Bin1 SH3 or GST alone. In (A–D), proteins co-precipitated with GST fusion proteins were analyzed by immunoblotting using antibodies against each tag, or an anti-dynamin antibody, as indicated. Total includes 20 μg of the cell lysates used in each assay. In the lower panel in (C), binding of endogenous dynamin2 with GST-Bin1 SH3 was also analyzed.
To examine the specificity of Exon10 as well as the biochemical nature of this binding, we next made mutants of Exon10 in which basic amino-acid clusters at the NH2- and COOH-termini, and nonpolar amino acids located near the center, were each mutated to alanines or serines (Nm, Cm, NCm and LFLm; Figure 2B). These His-Xpress-Bin1ΔSH3 proteins with mutated Exon10 sequence were then expressed in COS-7 cells and examined for their binding to GST-Bin1 SH3 in vitro. We found that the Nm and Cm mutations both partially block the binding to GST-Bin1 SH3, and the NCm mutation blocks the binding almost completely (Figure 2B). The LFLm mutation, on the other hand, did not affect the binding (Figure 2B). Therefore, both the NH2- and COOH-terminal basic amino-acid clusters of Exon10, but not the central nonpolar residues, appear to be involved in the binding of Bin1ΔSH3 to the Bin1 SH3 domain.
Competition of the Exon10 peptide with the dynamin PxxP peptide for binding to the Bin1 SH3 domain
To further explore the nature of the Exon10-mediated binding of Bin1ΔSH3 to the Bin1 SH3 domain, we next examined whether this binding competes with the binding of the dynamin PxxP peptide to the Bin1 SH3 domain. For this purpose, GST-Bin1 SH3 was preincubated with a synthetic 15-amino-acid-long peptide of Exon10, RKKSKLFSRLRRKKN, or the dynamin PxxP peptide, PPQVPSRPNRAPPGV (Owen et al, 1998), and then incubated with lysate from COS-7 cells expressing His-Xpress-Bin1ΔSH3. Under our condition in which the Exon10 peptide blocks His-Xpress-Bin1ΔSH3 binding to GST-Bin1 SH3, we found that the dynamin PxxP peptide also completely blocks the binding (Figure 2C, upper panel). COS-7 cells express endogenous dynamin2. Both the Exon10 and the PxxP peptides also blocked GST-Bin1 SH3 binding to dynamin2 (Figure 2C, lower panel). The NCm mutant of the Exon10 peptide, used as a negative control, did not block these bindings (Figure 2C). Thus, Exon10 and the dynamin PxxP peptide appear to act competitively with each other in their binding to the Bin1 SH3 domain.
The crystal structure of the Bin1 SH3 domain has been reported, and mutation of His30 into serine (the H30S mutant) has been shown to abolish its binding to the dynamin PxxP peptide (Owen et al, 1998). Under our condition in which the H30S mutation of GST-Bin1 SH3 abolished its binding to GFP-dynamin2 almost completely, we found that this mutation only partially affects its binding to His-Xpress-Bin1ΔSH3 (Figure 2D). Thus, the binding interfaces of the Bin1 SH3 domain to Bin1ΔSH3 and to the dynamin PxxP peptide appear to be nonidentical.
Direct interaction of Exon10 with the Bin1 SH3 domain, and the large overlap in its binding site with the dynamin2 PxxP-binding site
We next tried to examine the direct interaction between Exon10 and the Bin1 SH3 domain. As mentioned earlier, several SH3 domains have been shown to bind to basic motifs with the critical sequence RxxK, and this sequence is in fact present in Exon10. We first performed a surface plasmon resonance assay using BIACORE to examine their interaction. The above-described synthetic peptides for Exon10 and dynamin2 PxxP were analyzed for their interaction with GST-Bin1 SH3, and a synthetic peptide of the Exon10 NCm was used as a negative control. In this assay, we detected significant plasmon resonance signals between the Exon10 peptide and GST-Bin1 SH3 (data not shown). However, the levels of the resonance signals were much lower than those observed between the dynamin2 PxxP peptide and GST-Bin1 SH3, and were too weak to reach a plateau to calculate rate constants of the kinetics. We then assessed their direct binding by synthesizing a similar set of peptides, corresponding to Exon10 (wt), its NCm mutant (NCm) and the dynamin PxxP peptide (dyn), on a filter membrane and incubating them with radiolabeled GST-Bin1 SH3. As shown in Figure 2E, the Exon10 peptide itself exhibited detectable binding to the Bin1 SH3 domain. However, the binding of Exon10 appeared to be much weaker than the binding of the dynamin PxxP motif to the Bin1 SH3 domain, consistent with the above-mentioned results of the surface plasmon resonance assay.
We then analyzed the interaction between Exon10 and the Bin1 SH3 domain by NMR. The simplest and most popular approach to detect such interactions is to measure chemical shift perturbations, in which the amide chemical shifts in 15N–1H-heteronuclear single quantum correlation (HSQC) spectra of an 15N-labeled protein are compared in the absence and presence of a nonlabeled ligand (Ikegami et al, 1998). The locations of resonances that undergo chemical shift perturbations are then mapped onto the tertiary structure of the protein, which represent the putative binding site. We succeeded in assigning the amide resonances in the main chain using a series of three-dimensional NMR experiments with the help of the data described by Pineda-Lucena and Arrowsmith (2001). We then measured chemical shift perturbations of 15N-labeled Bin1 SH3 domain in the presence of the Exon10 peptide or the dynamin PxxP peptide. From these analyses, we determined the binding site of the dynamin PxxP peptide on the Bin1 SH3 domain, which was almost the same as reported previously (Owen et al, 1998). We also found that Exon10 interacts directly with the Bin1 SH3 domain, and the binding site for Exon10 largely overlaps with that for the PxxP motif (Figure 3). Moreover, comparison of scales of the perturbations with and without NaCl indicated that Exon10 binds to the Bin1 SH3 domain more strongly under salt-free conditions than under 150 mM salt condition, while the opposite was seen in the case of the PxxP peptide (Figure 3). Therefore, the nature of the binding of the Exon10 peptide and the PxxP peptide to the Bin1 SH3 domain appears to be different: Exon10 mostly electrostatically, while the PxxP peptide may bind hydrophobically, although they appear to bind to very similar sites of the Bin1 SH3 domain.
Figure 3.
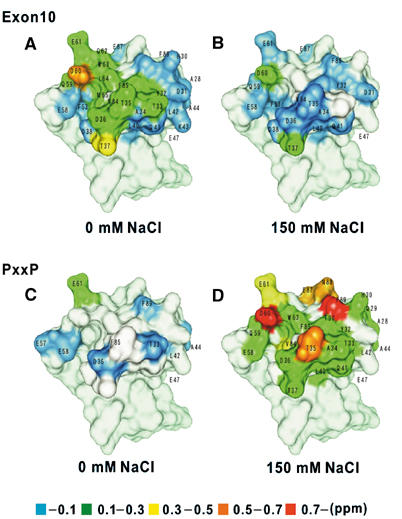
The interface of interaction of the Bin1 SH3 domain with Exon10 and the canonical PxxP ligand. Residues in the Bin1 SH3 domain in which amide resonances were perturbed in an 15N–1H-HSQC spectrum upon interaction with the Exon10 peptide (A, B) or the dynamin PxxP peptide (C, D) are shown on the crystal structure reported by Owen et al (1998). The degree of chemical shift changes is color-coded on the structures. The left figures (A, C) show the perturbation under salt-free conditions, and the right figures (B, D) show perturbation in 150 mM NaCl. The figures were created with the CHIMERA software (Huang et al, 1996).
Amphiphysin-II plays a crucial role in receptor-mediated endocytosis such as that of the transferrin (Tfn) receptor, and it has been shown that overexpression of the Bin1 SH3 domain blocks the uptake of Tfn (Wigge et al, 1997). To obtain evidence for the interaction of Exon10 with the Bin1 SH3 domain in vivo, we next microinjected the Bin1 SH3 domain and the Exon10 peptide into COS-7 cells. As shown in Figure 4, coinjection of the Exon10 peptide was able to alleviate the Bin1 SH3 domain-mediated inhibition of Tfn uptake. Microinjection of the Exon10 peptide itself also acted to block the Tfn uptake, which is likely due to the inhibition of SH3-mediated protein interactions of endogenous amphiphysin-II proteins.
Figure 4.
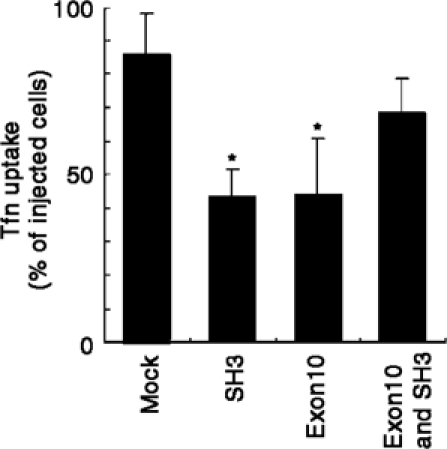
Possible interaction of Exon10 with the Bin1 SH3 domain in vivo. Whether Exon10 can restore the inhibition of Tfn uptake caused by the microinjection of the Bin1 SH3 domain was examined as described in Supplementary material. Microinjection-positive cells were identified by the autofluorescence from coinjected fluorescein isothiocyanate. Results are shown as means±s.d. of three independent experiments. More than 50 cells were examined in each experiment. *P<0.01 against values of the mock.
PI(4,5)P2 allows Bin1 to bind to dynamin
The primary structure of Exon10 is very similar to that of the PI(4,5)P2-binding motif, K/RxxxxKxK/RK/R (Martin, 1998). Lee et al (2002) have shown that the amino acids 1–282 of Bin1 (the BAR* domain) bind to phosphoinositides such as PI(4,5)P2. The BAR* domain contains Exon10, and Exon10 has been shown to be primarily responsible for this binding (Lee et al, 2002). We then examined whether the binding of phosphoinositides to Exon10 affects its binding to the Bin1 SH3 domain. Bin1ΔSH3 (amino acids 1–373 of Bin1) includes the BAR* domain, and we confirmed that Bin1ΔSH3 exhibits similar binding to phosphoinositides, as reported for the BAR* domain (data not shown). We expressed His-Xpress-Bin1ΔSH3 in COS-7 cells, and purified the protein using nickel beads. After elution from the beads, His-Xpress-Bin1ΔSH3 proteins were incubated with liposomes containing phosphoinositides, and then with GST-Bin1 SH3. Immunoblotting of proteins co-precipitating with GST-Bin1 SH3 revealed that preincubation with PI(4,5)P2-containing liposomes significantly inhibits binding of Bin1ΔSH3 to the Bin1 SH3 domain, while preincubation with PI(3,5)P2 or PS did not notably affect the binding (Figure 5A). PI(3,5)P2 and PS do not bind to the BAR* domain (Lee et al, 2002) nor to Bin1ΔSH3 (data not shown). Therefore, PI(4,5)P2 binding to Exon10 appears to be competitive with the binding of Exon10 to the Bin1 SH3 domain. On the other hand, inositol 1,4,5-trisphosphate did not affect binding of the Bin1ΔSH3 region to the Bin1 SH3 domain (data not shown).
Figure 5.
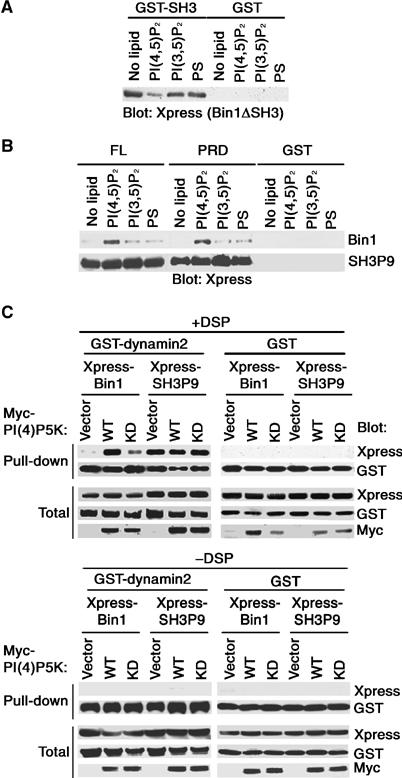
Regulation of binding of the Bin1 SH3 domain by phosphoinositides. (A) PI(4,5)P2 inhibits the binding of Exon10 to the Bin1 SH3 domain. A 3 μg portion of purified His-Xpress-Bin1ΔSH3 protein was incubated with or without 40 μg of liposomes containing different phosphoinositides or PS, as indicated. They were then incubated with 10 μg of GST-Bin1 SH3 domain or GST alone, to test their binding. (B) PI(4,5)P2 allows binding of full-length Bin1 to dynamin2. A 3 μg portion of His-Xpress-Bin1 or His-Xpress-SH3P9 was incubated with or without 40 μg of liposomes, as indicated. They were then incubated with 4 μg of GST-dynamin2 or 10 μg of GST-dynamin2 PRD or GST alone, to test the binding. (C) Regulation of binding of the Bin1 SH3 domain by phosphoinositides in COS-7 cells. COS-7 cells were expressed with wild-type myc-PIP5Kβ (WT), its kinase-dead mutant (KD) or the empty vector (vector), with GST-dynamin2 plus His-Xpress-Bin1 or His-Xpress-SH3P9, as indicated. After in situ crosslinking using DSP (upper panels) or without crosslinking (lower panels), GST-dynamin2 was pulled down with glutathione beads from 500 μg of cell lysate and co-precipitating proteins were analyzed as described in Materials and methods. A control also included GST instead of GST-dynamin2. In (A–C), proteins co-precipitated with GST fusion proteins or GST alone were analyzed by immunoblotting against each tag, as indicated. Total includes 20 μg of cell lysate.
We next examined whether phosphoinositides affect the binding of full-length Bin1 to full-length dynamin2 in vitro. As a control, full-length SH3P9 was used, which does not possess Exon10. Purified full-length His-Xpress-Bin1 or His-Xpress-SH3P9 proteins were prepared as above, and incubated with liposomes and then with full-length GST-dynamin2. We found that full-length Bin1 gains the ability to bind to full-length dynamin2 in the presence of PI(4,5)P2, while PI(3,5)P2 and PS could only weakly exert such an effect (Figure 5B, upper panel). In contrast, binding of SH3P9 to dynamin2 was not notably affected by any of these phosphoinositides or PS (Figure 5B, lower panel). Dynamin uses its pleckstrin homology (PH) domain for phosphoinositide binding. To avoid the possibility that the above interaction of dynamin2 and Bin1 was due to the liposome-mediated bridging of these two proteins, we confirmed that GST-dynamin2 PRD also binds to Bin1 in the presence of PI(4,5)P2 (Figure 5B). Therefore, PI(4,5)P2 appears to have the ability to release the Bin1 SH3 domain from Exon10, and allow the Bin1 SH3 domain to bind to the PxxP ligand of dynamin2.
We then investigated whether such phosphoinositide-mediated regulation of Bin1 SH3 binding can occur in vivo. A large fraction of intracellular PI(4,5)P2 is generated by phosphatidylinositol 4-phosphate 5-kinase (PI(4)P5K) activity (Yin and Janmey, 2003). Overexpression of PI(4)P5Ks, such as PI(4)P5Kβ, in COS-7 cells has been shown to significantly augment cellular levels of PI(4,5)P2 (Davis et al, 1997). We overexpressed myc-tagged PI(4)P5Kβ (WT) in COS-7 cells, together with GST-dynamin2 and His-Xpress-Bin1. A myc-tagged kinase-dead mutant of PI(4)P5Kβ (KD) and an empty vector (vector) were included as controls. To detect intracellular protein binding, we treated cells with membrane-permeable dithiobis-succinimidylpropionate (DSP) to crosslink cellular proteins. Cells were then first solubilized in a 1% SDS solution to avoid nonspecific protein binding. As shown in the upper panels of Figure 5C, amounts of His-Xpress-Bin1 co-precipitated with GST-dynamin2 were remarkably augmented when PI(4)P5K was overexpressed, as compared to those in control cells expressing the KD mutant or an empty vector. Co-precipitation of His-Xpress-SH3P9 with GST-dynamin2, which we used as another control, was not affected by PI(4)P5K overexpression, and the proteins constitutively bound to each other even in cells expressing the KD mutant or an empty vector (Figure 5C, upper panels). Such co-precipitation was not seen when cells were not treated with DSP (Figure 5C, lower panels). These results are consistent with the notion that intracellular PI(4,5)P2 allows the binding of Bin1 to dynamin2, in which Exon10 plays an important role.
Exon10 in myogenesis
Our results and previous literature indicate that Exon10 has dual biochemical properties: one is to bind to certain phosphoinositides, and the other is to bind to the SH3 domain of Bin1 itself to mask its binding to PxxP ligands. Thus, one can envisage that such dual properties of Exon10 may play a role in regulating the binding of the Bin1 SH3 domain so that it may occur only when Bin1 binds to certain phosphoinositides. However, one question still remained: why does Exon10 have to possess the property to mask the SH3 domain of Bin1 itself until being bound to phosphoinositides? SH3 domains of other proteins, for example those of Cool-1 and Cool-2, which possess the PH domain for phosphoinositide binding, can bind to their PxxP ligands even in the absence of phosphoinositides (Manser et al, 1998; Fuentes et al, 2003). Therefore, binding of the SH3 domains of these proteins does not appear to be regulated by phosphoinositides, unlike in the case of the Bin1 SH3 domain. We analyzed the necessity of the dual properties of Exon10, rather than simply being able to bind to PI(4,5)P2, for Bin1 function. Bin1 expression and high levels of PI(4,5)P2 production are simultaneously induced during differentiation of C2C12 cells (Wechsler-Reya et al, 1998; Lee et al, 2002). T-tubules are rich in PI(4,5)P2, and interaction of Exon10 with PI(4,5)P2 appears to be crucial for the localization of Bin1 to T-tubules (Lee et al, 2002). On the other hand, the PH domain of PLCδ also exhibits a high and specific affinity to PI(4,5)P2, and it has been demonstrated that GFP-tagged PLCδ PH domain localizes to T-tubules in differentiated C2C12 cells (Lee et al, 2002). We made a Bin1 swap mutant in which Exon10 was replaced by the PH domain of PLCδ. This swap mutant, when tagged with GST, retained a similar activity in vitro to bind to PI(4,5)P2-containing liposomes, as did GST-PLCδ PH (data not shown). Small interfering RNA (siRNA)-mediated knock-down of Bin1 protein expression in C2C12 cells has previously demonstrated the necessity of Bin1 for T-tubule biogenesis (Lee et al, 2002). We expressed wild-type Bin1, its swap mutant and SH3P9 (an Exon10-deleted form of Bin1) using the adenovirus-mediated cDNA transfer method in C2C12 cells, which had been subjected to serum starvation to induce differentiation and which were pretreated with a Bin1 siRNA duplex. We used Bin1 cDNA of human origin, which does not contain the target sequence of the mouse Bin1 siRNA duplex used to knock down endogenous expression in C2C12 cells. We found that expression of caveolin3, a marker for myogenesis and T-tubules (Tang et al, 1996; Parton et al, 1997), was still induced in the Bin1 siRNA-treated cells after differentiation (Figure 6A), unlike a previous report in which Bin1 knock-down abolished caveolin3 expression (Lee et al, 2002). This difference may be due to the different timing of Bin1 siRNA treatment between these two experiments. Bin1 siRNA-treated C2C12 cells expressing exogenous Bin1, the swap mutant or SH3P9 also expressed caveolin3 after differentiation (Figure 6A). Expression of dynamin2 was neither affected by Bin1 siRNA treatment nor by the expression of any of these cDNAs (Figure 6A). Nevertheless, T-tubule formation seemed to be severely affected by knock-down of Bin1 expression, and was not restored by expression of the swap mutant or SH3P9, while it was restored by expression of wild-type Bin1 (Figure 6B). The subcellular localization of these two proteins, the swap mutant and SH3P9, did not indicate a normal morphology of T-tubules in almost all of the differentiated cells. Staining of these cells with caveolin3 also indicated that most of the cells failed to generate normal T-tubule structures (Figure 6B). These results indicate the necessity of Exon10 for T-tubule formation, and suggest that the ability of the PLCδ PH domain to bind to PI(4,5)P2 is not enough to compensate for the role played by Exon10 in T-tubule formation. These results are consistent with our notion that the dual properties of Exon10, rather than the property of simply binding to certain phosphoinositides, are important for the cellular function of Bin1.
Figure 6.
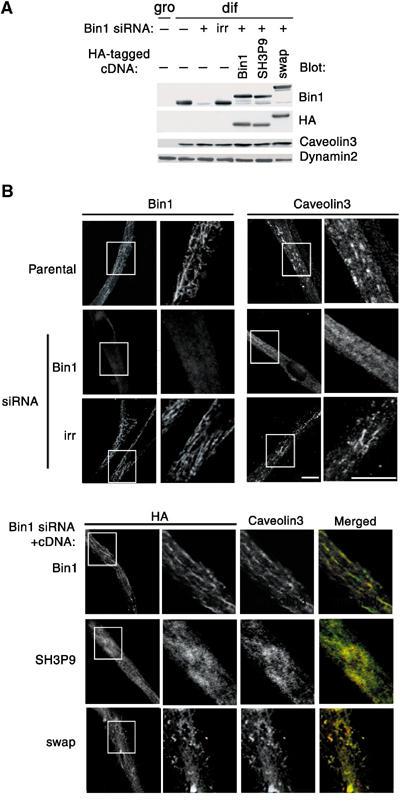
The PLCδ PH domain cannot substitute for Exon10 in the function of Bin1 during T-tubule formation. C2C12 cells, starved for serum to induce differentiation (dif), were treated with a Bin1 siRNA duplex, and then infected with adenoviruses encoding HA-tagged wild-type Bin1, the Bin1 swap mutant or SH3P9, as described in Materials and methods. An irrelevant siRNA duplex (irr) was used as a control. (A) Protein expression. A 30 μg portion of each cell lysate was subjected to immunoblotting analysis using antibodies as indicated. Cell lysates from parental C2C12 cells at the growing phase (gro) are also included. (B) Immunolabeling of cells. Cells were fixed and immunolabeled using antibodies against Bin1, the HA tag and caveolin3, as indicated. Differentiated C2C12 cells (parental) and differentiated siRNA-treated cells are shown in the upper panels. Distinct cell staining is observed in the upper panels. Bin1 siRNA-treated C2C12 cells expressing exogenous Bin1, the swap mutant or SH3P9 after differentiation are shown in the lower panels, in which merged images of each of the left two columns are also shown. The boxes in the lower magnification images indicate the areas shown in the higher magnification images. Representative images are shown from more than 50 cells examined in each experiment of the siRNA treatment and the cDNA transfection. Bar, 10 μm.
Discussion
In this report, we found a novel function of Exon10, a 15-amino-acid-long peptide rich in basic amino acids unique to Bin1. Exon10 has previously been shown to bind to certain phosphoinositides. We found here that Exon10 also possesses the ability to interact directly with the SH3 domain of Bin1 itself. We suggest that Exon10 thereby acts to mask the SH3 domain from binding to other proteins until Exon10 is occupied by phosphoinositides. Conversely, it may also be possible that binding of the Bin1 SH3 domain to PxxP ligands regulates the binding of Bin1 to phosphoinositides. We also provided evidence supporting that the dual properties of Exon10 are important for Bin1 function in T-tubule formation.
Our NMR analyses indicate that the site of binding of Exon10 to the Bin1 SH3 domain largely, although not completely, overlaps with that of the canonical PxxP-binding site of dynamin2. Our biochemical results fully support this model. We showed that the Exon10 peptide competes with the dynamin2 PxxP peptide in binding to the Bin1 SH3 domain. We also showed, on the other hand, that the H30S mutant of the Bin1 SH3 domain, which is unable to bind to dynamin2, can still bind to the Exon10-containing Bin1ΔSH3 fragment. However, the physicochemical nature of binding of these two peptides is different. By analyzing chemical shift perturbations of the NMR signals, we showed that the interaction between Exon10 and the Bin1 SH3 domain weakens upon an increase in environmental salt concentration, while in contrast the interaction between the PxxP peptide and the Bin1 SH3 domain is strengthened in the presence of salt. The latter observation is consistent with the current model that canonical PxxP peptides bind to SH3 domains mostly via hydrophobic interactions (Mayer, 2001; Musacchio, 2003). The former observation, on the other hand, suggests that the interaction between Exon10 and the Bin1 SH3 domain might be primarily mediated by a force generated by electrostatic interactions. The amphiphysin-II SH3 domain (including the Bin1 SH3 domain) has the notable feature of bearing an extensive patch of negative electrostatic potential on the surface, which largely overlaps with the dynamin PxxP-binding site (Owen et al, 1998). Other SH3 domains, such as those of Abl and Sem5, do not have such an extensive negative patch on the surface (Owen et al, 1998). Our NMR analysis indicated that most of the amino-acid residues within this acidic patch, Asp36, Asp38, Glu58, Asp60 and Glu61, are involved in binding to Exon10 (see Figure 3). Consistent with this, we also showed that basic amino acids of Exon10, rather than the nonpolar residues, are necessary for its binding to the Bin1 SH3 domain (see Figure 2B).
The PxxP motifs were originally identified as consensus SH3 ligands (Ren et al, 1993). However, in addition to the Bin1 SH3 domain, several other SH3 domains have recently been shown to bind both to basic amino acids and the canonical PxxP sequence. For example, the SH3 domain of Gads, STAM2 and Fyn possess negatively charged surfaces and can bind to the RxxK motif present in other proteins such as SLP-76, UBPY and SAP, respectively (Chan et al, 2003; Liu et al, 2003; Kaneko et al, 2004). On the other hand, it has also been shown that the binding of ligands to several of the SH3 domains is regulatable. For example, binding of the PxxP peptide to the Nck SH3(C) domain may be regulated by phosphorylation of the peptide (Zhao et al, 2000). Phosphorylation of Thr780 of dynamin1, located near the PxxP motif, also blocks its binding to amphiphysin (Tan et al, 2003; Tomizawa et al, 2003). The competitive binding of different types of PxxP ligands to the same SH3 domain may also be considered as a type of regulation of SH3 binding, as occurs in the binding of the myosin1 SH3 domain to the PxxP ligands of WIP/verprolin and WASP (Mayer, 2001), and binding of the Cool/Pix SH3 domain to the PxxP ligands of Pak and Cbl (Flanders et al, 2003). Moreover, regulation of binding of the p47phox SH3(N) domain to its target p22phox appears to be analogous to that of the Bin1 SH3 domain: it has been proposed that binding of the p47phox SH3(N) domain is blocked by an intramolecular interaction within the p47phox protein, and this blockage is released by serine phosphorylation of p47phox (Ago et al, 2003; Groemping et al, 2003). However, the underlying molecular mechanisms of the regulation of SH3 binding between p47phox and Bin1 are different. Hence, there is no precedented demonstration of the regulation of binding of an SH3 domain by a peptide rich in basic amino acids in the same protein, as well as by phospholipids, as we have shown for the Bin1 SH3 domain.
We were interested in investigating whether this type of regulation of binding of the SH3 domain is only seen with Bin1. A search of a protein family database (Pfam, http://www.sanger.ac.uk/Software/Pfam/) indicated that the human genome encodes several proteins, which possess both an SH3 domain and a short sequence rich in basic amino acids. Such proteins include endophilin, ZO-1, DLG, HS1, Abl and SLAP. Since the SH3 domain of endophilin also binds to dynamin (Ringstad et al, 1997), we examined whether this SH3 domain can bind to the basic amino-acid sequence of the same protein, KKLEGRRLDFDYKKKRQ. As was done with Bin1, we divided endophilin2 into two fragments: only the SH3 domain, and the rest of the entire protein, endophilin2ΔSH3. We, however, found that these two fragments do not bind to each other (C Kojima and H Sabe, unpublished data, 2002). We also found that the basic amino-acid sequence of endophilin2 does not bind to the Bin1 SH3 domain (C Kojima and H Sabe, unpublished data, 2002), indicating the specificity of the Bin1 SH3 domain.
Having found that Exon10 has dual biochemical properties, our next concern was why Exon10 must simultaneously regulate the binding of the SH3 domain of the same protein, in addition to its role in binding to phosphoinositides to be recruited to membranes. To explore this issue, we made a swap mutant of Bin1, in which Exon10 was replaced by the PLCδ PH domain. Our results suggest that the property possessed by the PLCδ PH domain of simply being able to be recruited to membranes by binding to PI(4,5)P2 may not be enough to fully substitute for Exon10 function in Bin1 with regard to its role in T-tubule formation. Both Bin1 expression and the high levels of production of PI(4,5)P2 are simultaneously induced during the differentiation of C2C12 cells, as mentioned earlier. Our results altogether indicate that the simultaneity of the binding of the SH3 domain to other proteins with its membrane localization might be important for the function of Bin1 in T-tubule formation, although we do not know the precise stepwise process as to how the complicated structures of T-tubules are built during myogenesis. Such simultaneity has also been reported with amphiphysin-I with regard to its binding to dynamin and AP-2/clathrin in endocytosis (Farsad et al, 2003).
In conclusion, we showed in this paper that PI(4,5)P2 plays a crucial role in regulating SH3-mediated protein–protein interactions of Bin1, a scaffold protein involved in membrane remodeling. PI(4,5)P2 has been shown to act as a major player in regulating protein–protein interactions of several scaffold proteins for cytoskeletal remodeling, such as vinculin, talin and ERM (Ezrin/Radixin/Moesin) (Yin and Janmey, 2003). Production of PI(4,5)P2 and the related phosphoinositides is tightly coupled with various intracellular processes, such as cytoskeletal remodeling and membrane remodeling. Many other types of protein–protein interactions may in fact be under the control of PI(4,5)P2 or the related phosphoinositides, some of which are perhaps also important for the achievement of the simultaneous occurrence of different events during intracellular dynamic processes.
Materials and methods
Cells
COS-7 cells were maintained at 37°C in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal calf serum (FCS; Hyclone). C2C12 cells were maintained in DMEM with 10% FCS, supplemented with additional 2 mM glutamine. Differentiation of C2C12 cells was induced by culturing cells with DMEM containing 0.1% FCS, 5 μg/ml Tfn, 5 μg/ml insulin and an additional 2 mM glutamine for 4 days, as described previously (Hu et al, 1999).
Protein binding and immunoblotting
Preparation of COS-7 cell lysates using 1% NP-40 buffer, and protein binding assays using GST fusion proteins and glutathione beads (Amersham Pharmacia) were performed as described previously (Kondo et al, 2000).
For PIP(4)P5K overexpression, 2 × 106 COS-7 cells in a 100-mm culture dish were transfected with 3 μg of pcDNA3 myc-PI(4)5Kβ WT or pcDNA3 myc-PI(4)5Kβ KD, together with 5 μg each of pEBG-dynamin2 and pcDNA3.1/HisC-Bin1 or 5 μg each of pEBG-dynamin2 and pcDNA3.1/HisC-SH3P9, using lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Control empty vectors were also used. At 24 h after the transfection, in situ crosslinking using DSP (Pierce) was performed according to a method described previously (Hüttlmaier et al, 1998). In brief, cells were washed twice with phosphate-buffered saline (PBS), and then incubated with 0.5 mM DSP in PBS for 30 min at room temperature. After washing once with PBS and incubating with 0.2 M glycine in PBS for 5 min, cells were lysed in 100 μl of RIPA buffer containing 1% SDS (1% Nonidet P-40, 1% sodium deoxycholate, 1% sodium dodecyl sulfate, 150 mM NaCl, 20 mM Tris–HCl (pH 7.4), 5 mM EDTA, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 5 μg/ml aprotinin, 2 μg/ml leupeptin and 3 μg/ml pepstatin A) supplemented with 20 mM glycine and 15 U/ml DNase (Wako). After 5 min incubation on ice, the suspension was diluted with 900 μl of RIPA buffer without SDS and passed through a 23-G needle 10 times to shear DNA, and lysates were obtained by centrifugation. DSP was cleaved during boiling of the protein samples with Laemmli's SDS sample buffer.
Liposomes were made according to a method described previously (Lee et al, 2002). His-Xpress-Bin1 proteins were expressed in COS-7 cells, and purified on Ni-NTA agarose beads (QIAGEN) according to the manufacturer's instructions by lysing cells with 1% Triton X-100 solution (1% Triton X-100, 500 mM NaCl, 20 mM Tris–HCl (pH 7.4), 0.5 mM EGTA, 2 mM MgCl2, 0.1% β-mercaptoethanol and 1 mM phenylmethylsulfonyl fluoride). Recombinant proteins were then eluted with the same solution containing 100 mM imidazol, and dialyzed against a buffer (20 mM Tris–HCl (pH 7.4), 200 mM NaCl, 100 mM KCl and 1 mM EGTA). The proteins were then incubated with liposomes consisting of 70% phosphatidylcholine, 20% PS and 10% phosphoinositide or PS for 15 min at 37°C in the same dialysis buffer, and then incubated with the GST-SH3 protein or a control GST protein for 2 h at 4°C, both of which were produced in bacteria and purified on glutathione beads.
NMR
NMR analysis was performed according to a method described previously (Ikegami et al, 1998). In brief, the Bin1 SH3 domain (amino acids 374–454) was cloned into pGEX-6P-1, to be fused in-frame to the COOH-terminus of GST, and expressed in Escherichia coli in M9 medium containing 15N-labeled ammonium chloride or 15N-labeled ammonium chloride plus 13C-labeled glucose, as described previously. After purification using glutathione beads, proteins were incubated with PreScission Protease (Amersham Biosciences) at 4°C for 6 h to be separated from the GST tag. The isolated Bin1 SH3 protein contained an extra GPKGSSGA sequence at its NH2-terminus. The 1H, 15N and 13C chemical shifts of the main chain of Bin1-SH3 were assigned by the multidimensional and multiresonance NMR methods at 303 K with a Bruker DRX-500 spectrometer using 1.0 mM 15N-, 13C-labeled Bin1-SH3 dissolved in a 20 mM NaH2PO4–Na2HPO4 (pH 6.8) buffer containing 10 mM NaCl and 10% D2O. For analyses of the interactions of the Bin1 SH3 domain with the ligand peptides (Exon10: RKKSKLFSRLRRKKN; dynamin: PPQVPSRPNRAPPGV), an equimolar amount of each ligand was added to 0.2 mM of 15N-labeled Bin1-SH3 dissolved in a 20 mM KH2PO4–K2HPO4 (pH 7.4) buffer containing 150 mM (or 0 mM) NaCl and 10% D2O. The WATERGATE and Water-flip-back versions of 15N–1H-HSQC spectra were obtained at 303 K with a Bruker AV-400M spectrometer at the proton base frequency of 400.13 MHz.
siRNA-mediated interference and rescue experiments
Oligonucleotides used for the mouse Bin1 siRNA were generated as described previously (the pair 1 sequence; Lee et al, 2002). An oligonucleotide duplex with an irrelevant sequence was purchased from Dharmacon. A total of 2 × 105 C2C12 cells in a 35-mm dish were induced to differentiate by serum starvation. After 24 h, cells were transfected with 25 nM siRNA duplexes using the HVJ Envelope VECTOR KIT (Ishihara Sangyo, Osaka, Japan) according to the manufacturer's instructions, and incubated for 4 h followed by medium change. After further incubation for 20 h, cells were infected with adenoviruses bearing human Bin1 cDNA or its mutants at a concentration of 5 × 109 PFU/ml, and incubated for 4 h before medium change. After a further 48 h of incubation, cells were subjected to immunoblotting analysis and to immunofluorescent analysis.
The following information is described in Supplementary material: antibodies and chemicals, cDNAs and their expression, peptide binding, immunofluorescence microscopy, supplemental information of NMR analysis and Tfn uptake.
Supplementary Material
Supplementary material
Acknowledgments
We are grateful to Alan Aderem and Masato Hirata for their generous gifts of plasmid DNAs, Yoshiaki Ito for C2C12 cells and Helena Akiko Popiel for critical reading of the manuscript. We thank Atsuko Yamada, Manami Hiraishi and Yumiko Shibata for their technical assistance, and Mayumi Yoneda for her secretarial work. This work was supported in part by Grants-in-aid from the Ministry of Education, Science, Sports and Culture of Japan (MESSC), and Grants from Takeda Pharmaceutical Co. and the Uehara Memorial Life Science Foundation. CK is a recipient of the MESSC Studentship of the 21th Century Center of Excellence Program.
References
- Ago T, Kuribayashi F, Hiroaki H, Takeya R, Ito T, Kohda D, Sumimoto H (2003) Phosphorylation of p47phox directs phox homology domain from SH3 domain toward phosphoinositides, leading to phagocyte NADPH oxidase activation. Proc Natl Acad Sci USA 100: 4474–4479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DM, Nash P, Liu S K-W, Pawson T, McGlade CJ (2002) A high-affinity Arg-X-X-Lys SH3 binding motif confers specificity for the interaction between Gads and SLP-76 in T cell signaling. Curr Biol 12: 1336–1341 [DOI] [PubMed] [Google Scholar]
- Butler MH, David C, Ochoa G-C, Freyberg Z, Daniell L, Grabs D, Cremona O, De Camilli P (1997) Amphiphysin II (SH3P9; BIN1), a member of the amphiphysin/Rvs family, is concentrated in the cortical cytomatrix of axon initial segments and nodes of ranvier in brain and around T tubules in skeletal muscle. J Cell Biol 136: 1355–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan B, Lanyi A, Song HK, Griesbach J, Simarro-Grande M, Poy F, Howie D, Sumegi J, Terhirst C, Eck MJ (2003) SAP couples Fyn to SLAM immune receptors. Nat Cell Biol 5: 155–160 [DOI] [PubMed] [Google Scholar]
- David C, McPherson PS, Mundigl O, De Camilli P (1996) A role of amphiphysin in synaptic vesicle endocytosis suggested by its binding to dynamin in nerve terminals. Proc Natl Acad Sci USA 93: 331–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JN, Rock CO, Cheng M, Watson JB, Ashmun RA, Kirk H, Kay RJ, Roussel MF (1997) Complementation of growth factor receptor-dependent mitogenic signaling by a truncated type I phosphatidylinositol 4-phosphate 5-kinase. Mol Cell Biol 17: 7398–7406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsad K, Slepnev V, Ochoa G, Daniell L, Hauke V, De Camilli P (2003) A putative role for intramolecular regulatory mechanisms in the adaptor function of amphiphysin in endocytosis. Neuropharmacology 45: 787–796 [DOI] [PubMed] [Google Scholar]
- Flanders JA, Feng Q, Bagrodia S, Laux MT, Singavarapu A, Cerione RA (2003) The Cbl proteins are binding partners for the Cool/Pix family of p21-activated kinase-binding proteins. FEBS Lett 550: 119–123 [DOI] [PubMed] [Google Scholar]
- Flucher BE (1992) Structural analysis of muscle development: transverse tubules, sarcoplasmic reticulum, and the triad. Dev Biol 154: 245–260 [DOI] [PubMed] [Google Scholar]
- Fuentes EJ, Karnoub AE, Booden MA, Der CJ, Campbell SL (2003) Critical role of the pleckstrin homology domain in Dbs signaling and growth regulation. J Biol Chem 278: 21188–21196 [DOI] [PubMed] [Google Scholar]
- Groemping Y, Lapouge K, Smerdon SJ, Rittinger K (2003) Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell 113: 343–355 [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Hashimoto A, Yamada A, Kojima C, Yamamoto H, Tsutsumi T, Higashi M, Mizoguchi A, Yagi R, Sabe H (2004) A novel mode of action of an ArfGAP, AMAP2/PAG3/PAPα, in Arf6 function. J Biol Chem 279: 37677–37684 [DOI] [PubMed] [Google Scholar]
- Hu Y, Cascone PJ, Cheng L, Sun D, Nambu JR, Schwartz LM (1999) Lepidopteran DALP, and its mammalian ortholog HIC-5, function as negative regulators of muscle differentiation. Proc Natl Acad Sci USA 96: 10218–10223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Couch GS, Pettersen EF, Ferrin TE (1996) Chimera: an extensible molecular modeling application constructed using standard components. Pacific Symp Biocomput 1: 724 [Google Scholar]
- Hüttlmaier S, Mayboroda O, Harbeck B, Jarchau T, Jockusch BM, Rüdiger M (1998) The interaction of the cell-contact proteins VASP and vinculin is regulated by phosphatidylinositol-4,5-bisphosphate. Curr Biol 8: 479–488 [DOI] [PubMed] [Google Scholar]
- Ikegami T, Kuraoka I, Saijo M, Kodo N, Kyogoku Y, Morikawa K, Tanaka K, Shirakawa M (1998) Solution structure of the DNA- and RPA-binding domain of the human repair factor XPA. Nat Struct Biol 5: 701–706 [DOI] [PubMed] [Google Scholar]
- Ishikawa H (1968) Formation of elaborate networks of T-system tubules in cutured skeletal muscle with special reference to the T-system formation. J Cell Biol 38: 51–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kami K, Takeya R, Sumimoto H, Kohda D (2002) Diverse recognition of non-PxxP peptide ligands by the SH3 domains from p67phox, Grb2 and Pex13p. EMBO J 21: 4268–4276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Kumasaka T, Ganbe T, Sato T, Miyazawa K, Kitamura N, Tanaka N (2004) Structural insight into modest binding of a non-PXXP ligand to the signal transducing adaptor molecule-2 src homology 3 domain. J Biol Chem 279: 48162–48168 [DOI] [PubMed] [Google Scholar]
- Kondo A, Hashimoto S, Yano H, Nagayama K, Mazaki Y, Sabe H (2000) A new paxillin-binding protein, PAG3/Papα/KIAA0400, bearing an ADP-ribosylation factor GTPase-activating protein activity, is involved in paxillin recruitment to focal adhesions and cell migration. Mol Biol Cell 11: 1315–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latour S, Roncagalli R, Chen R, Bakinowski M, Shi X, Schwartzberg PL, Davidson D, Veillette A (2003) Binding of SAP SH2 domain to FynT SH3 domain reveals a novel mechanism of receptor signaling in immune regulation. Mol Cell 11: 471–481 [DOI] [PubMed] [Google Scholar]
- Lee E, Marcucci M, Daniell L, Pypaert M, Weisz OA, Ochoa G-C, Farsad K, Wenk MR, De Camilli P (2002) Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science 297: 1193–1196 [DOI] [PubMed] [Google Scholar]
- Liu Q, Berry D, Nash P, Pawson T, McGlade CJ, Li SS-C (2003) Structural basis for specific binding of the Gads SH3 domain to an RxxK motif-containing SLP-76 peptide: a novel mode of peptide recognition. Nat Cell Biol 8: 149–154 [DOI] [PubMed] [Google Scholar]
- Lock LS, Royal I, Naujokas MA, Park M (2000) Identification of an atypical Grb2 carboxyl-terminal SH3 domain binding site in Gab docking proteins reveals Grb2-dependent and -independent recruitment of Gab1 to receptor tyrosine kinases. J Biol Chem 275: 31536–31545 [DOI] [PubMed] [Google Scholar]
- Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L (1998) PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell 1: 183–192 [DOI] [PubMed] [Google Scholar]
- Martin TFJ (1998) Phosphoinositide lipids as signaling molecules: common themes for signal transduction, cytoskeletal regulation, and membrane trafficking. Annu Rev Cell Dev Biol 14: 231–264 [DOI] [PubMed] [Google Scholar]
- Mayer BJ (2001) SH3 domains: complexity in moderation. J Cell Sci 114: 1253–1263 [DOI] [PubMed] [Google Scholar]
- Musacchio A (2003) How SH3 domains recognize proline. Adv protein chem 61: 211–268 [DOI] [PubMed] [Google Scholar]
- Owen DJ, Wigge P, Vallis Y, Moore JDA, Evans PR, McMahon HT (1998) Crystal structure of the amphiphysin-2 SH3 domain and its role in the prevention of dynamin ring formation. EMBO J 17: 5273–5285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, Way M, Zorzi N, Stang E (1997) Caveolin-3 associates with developing T-tubules during muscle differentiation. J Cell Biol 136: 137–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda-Lucena A, Arrowsmith CH (2001) 1H, 13C and 15N resonance assignments and secondary structure of the c-Myc binding domain (MBD) and the SH3 domain of the tumor suppressor Bin1. J Biomol NMR 19: 191–192 [DOI] [PubMed] [Google Scholar]
- Ramjaun AR, McPherson PS (1998) Multiple amphiphysin II splice variants display differential clathrin binding: identification of two distinct clathrin-binding sites. J Neurochem 70: 2369–2376 [DOI] [PubMed] [Google Scholar]
- Razzaq A, Robinson IM, McMahon HT, Skepper JN, Su Y, Zelhof AC, Jackson AP, Gay NJ, O'Kane CJ (2001) Amphiphysin is necessary for organization of the excitation–contraction coupling machinery of muscles, but not for synaptic vesicle endocytosis in Drosophila. Genes Dev 15: 2967–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R, Mayer BJ, Cicchetti P, Baltimore D (1993) Identification of a 10-amino acid proline-rich SH3 binding site. Science 259: 1157–1161 [DOI] [PubMed] [Google Scholar]
- Ringstad N, Nemoto Y, De Camilli P (1997) The SH3p4/SH3p8/SH3p13 protein family: binding partners for synaptojanin and dynamin via a Grb2-like Src homology 3 domain. Proc Natl Acad Sci USA 94: 8569–8574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan TC, Valova VA, Malladi CS, Graham ME, Berven LA, Jupp OJ, Hansra G, McClure SJ, Sarcevic B, Boadle RA, Larsen MR, Cousin MA, Robinson PJ (2003) Cdk5 is essential for synaptic vesicle endocytosis. Nat Cell Biol 8: 701–710 [DOI] [PubMed] [Google Scholar]
- Tang Z, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS, Nishimoto I, Lodish HF, Lisanti MP (1996) Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem 271: 2255–2261 [DOI] [PubMed] [Google Scholar]
- Tomizawa K, Suneda S, Lu Y-F, Oda Y, Kinuta M, Ohshima T, Saito T, Wei F-Y, Matsushita M, Li S-T, Tsutsui K, Hisanaga S-I, Mikoshiba K, Takei K, Matsui H (2003) Cophosphorylation of amphiphysin I and dynamin I by Cdk5 regulates clathrin-mediated endocytosis of synaptic vesicles. J Cell Biol 163: 813–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler-Reya R, Elliott KJ, Prendergast GC (1998) A role for the putative tumor suppressor Bin1 in muscle cell differentiation. Mol Cell Biol 18: 566–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigge P, McMahon HT (1998) The amphiphysin family of proteins and their role in endocytosis at synapse. Trends Neurosci 21: 339–344 [DOI] [PubMed] [Google Scholar]
- Wigge P, Vallis Y, Mcmahon HT (1997) Inhibition of receptor-mediated endocytosis by the amphiphysin SH3 domain. Curr Biol 7: 554–560 [DOI] [PubMed] [Google Scholar]
- Yin HL, Janmey PA (2003) Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol 65: 761–789 [DOI] [PubMed] [Google Scholar]
- Zhao ZS, Manser E, Lim L (2000) Interaction between PAK and Nck: a template for Nck targets and role of PAK autophosphorylation. Mol Cell Biol 20: 3906–3917 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material


