Abstract
Heptahelical, or G-protein-coupled, receptors control many cellular functions and normally consist of one polypeptide chain. In contrast, heptahelical receptors that belong to the long N-terminus, group B (LNB) family are cleaved constitutively into two fragments. The N-terminal fragments (NTFs) resemble cell-adhesion proteins and the C-terminal fragments (CTFs) are typical G-protein-coupled receptors (GPCRs) with seven transmembrane regions. However, the functional roles of this cleavage and of any subsequent NTF–CTF interactions remain to be identified. Using latrophilin, a well-studied member of the LNB family, we now demonstrate that cleavage is critical for delivery of this receptor to the cell surface. On the plasma membrane, NTF and CTF behave as separate membrane proteins involved, respectively, in cell-surface reception and signalling. The two fragments can also internalise independently. However, separated NTF and CTF can re-associate on solubilisation. Agonist binding to NTF on the cell surface also induces re-association of fragments and provokes signal transduction via CTF. These findings define a novel principle of structural and functional organisation of the cleaved, two-subunit GPCRs.
Keywords: G-protein-coupled receptor, latrophilin, latrotoxin, protein processing
Introduction
Long N-terminus, group B (LNB) G-protein-coupled receptors (GPCRs) (Figure 1A) have large extracellular domains containing various cell-adhesion/recognition modules (cadherin, IgG, laminin A, lectin, etc.), and seven transmembrane regions (TMRs) resembling those of group B GPCRs (e.g. Hayflick, 2000; Stacey et al, 2000; Krasnoperov et al, 2002). Therefore, LNB receptors are thought to be natural chimeras of cell-adhesion proteins and signalling receptors, structures which could transduce cell contact cues into intracellular signals (Hamann et al, 1996; Stacey et al, 2002). Surprisingly, LNB GPCRs have been found to undergo cleavage at a ‘GPCR proteolysis site' (GPS) located within a conserved ∼60-residue domain upstream of the first TMR (Gray et al, 1996; Krasnoperov et al, 1997; Nechiporuk et al, 2001; Abe et al, 2002; Stacey et al, 2002; Obermann et al, 2003) (Figure 1A). The cleavage produces an N-terminal fragment (NTF) and a C-terminal fragment (CTF) that correspond to the cell-adhesion domain and the GPCR domain, respectively.
Figure 1.
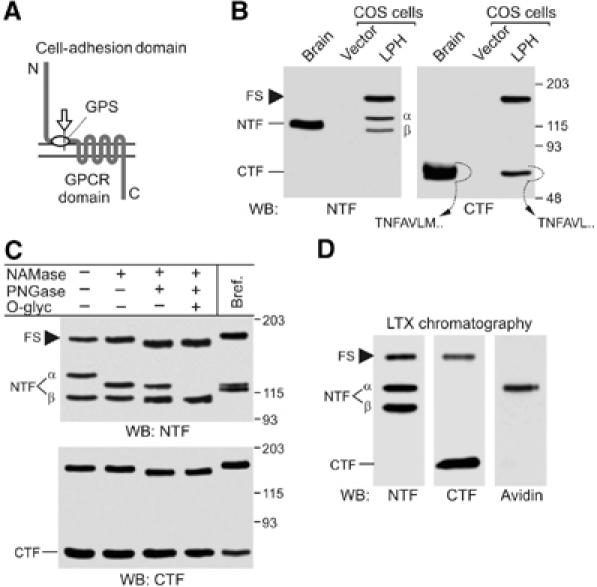
Cellular processing of latrophilin. (A) A typical LNB GPCR. Constitutive cleavage (arrow) creates two fragments corresponding to the cell-adhesion domain and GPCR domain. (B) Analysis of latrophilin expression in COS7 cells. Solubilised receptors from rat brain and COS7 cells transfected with vector or LPH were enriched by α-latrotoxin chromatography and analysed by WB. N-terminal sequences of the native and recombinant CTFs extracted from the gel are shown. (C) Analysis of post-translational modification of latrophilin. Cells were either cultured in the presence of brefeldin A or solubilised and treated with glycosidases, as indicated. For abbreviations of enzymes, see Materials and methods. (D) Identification of surface-exposed species of latrophilin. Live cells were biotinylated, solubilised, enriched on α-latrotoxin column and analysed by WB. Only αNTF is labelled with biotin. Molecular masses are shown on the right (B, C). FS, full-size latrophilin.
Proteolysis of LNB receptors occurs intracellularly, during their early post-translational modification, and only cleaved forms are found in cells/tissues in vivo (e.g. Krasnoperov et al, 1997; Obermann et al, 2003). This cleavage is, therefore, constitutive and not induced by agonists as in the case of protease-activated GPCRs (Noorbakhsh et al, 2003). Although NTFs do not contain TMRs, they remain associated with the membrane. As the two fragments co-purify after solubilisation, it has been suggested that NTFs are tethered to the cell surface due to their noncovalent interaction with CTFs (Krasnoperov et al, 1997; Abe et al, 2002; Krasnoperov et al, 2002; Qian et al, 2002). Currently, the purpose of the intracellular cleavage and the hypothetical constant interaction of the fragments on the cell surface remain unclear.
To study the processing of LNB GPCRs and the functions of their fragments, we used latrophilin (LPH) (Davletov et al, 1996; Lelianova et al, 1997), also called CIRL (Krasnoperov et al, 1997), a presynaptic receptor for α-latrotoxin implicated in the regulation of transmitter exocytosis by signalling to intracellular Ca2+ stores (Lelianova et al, 1997; Davletov et al, 1998; Ashton et al, 2001; Capogna et al, 2003; Willson et al, 2004). LPH is also the only vertebrate LNB receptor for which high-affinity exogenous agonists are known (wild-type α-latrotoxin and its mutant LTXN4C), making it a convenient model for studying receptor organisation and functions.
Our results indicate that LPH is cleaved in the endoplasmic reticulum (ER) and that this cleavage is necessary for receptor delivery to the plasma membrane. Furthermore, although NTF strongly associates with CTF upon solubilisation, the two fragments behave as independent membrane proteins on the cell surface; they are often targeted to different cell-surface locations and can internalise separately. Despite this independence, binding of LTXN4C to NTF causes association of the two receptor fragments and triggers CTF-mediated intracellular signalling to phospholipase C (PLC) and Ca2+ stores. These results provide new insights into the behaviour of this unusual subfamily of GPCRs and suggest that dynamic interactions between fragments of LNB receptors may be required for transduction of signals from at least some agonists.
Results
Cellular processing and trafficking of LPH
For initial studies of the post-translational modification of LPH, we used COS7 cells. Transiently transfected cells cleaved recombinant LPH (Figure 1B), although proteolysis was not as efficient as in neurones, and some amount of full-size protein remained in the cells. In addition, two bands of NTF (termed here α and β), but only one band of CTF, were detected by Western blotting (WB). The origin of the two NTF forms was unclear: they could be due to proteolysis at two distinct sites or due to differential glycosylation of NTF. However, the site of receptor cleavage in these cells was the same as in neurones because CTFs from transfected COS7 cells and from the brain had the same N-terminal sequence: NH2-TNFAVL (Figure 1B), consistent with that reported previously (Krasnoperov et al, 1997). Furthermore, exhaustive deglycosylation reduced both types of NTF to one band (Figure 1C), proving that αNTF and βNTF represented differently glycosylated forms of the same receptor fragment.
During the deglycosylation experiments, we noticed that neuraminidase and O-glycosidase greatly reduced the size of αNTF, but not of βNTF or the uncleaved receptor. As these enzymes remove carbohydrates attached in the Golgi complex (GC), this result meant that the full-size LPH and βNTF had not been modified in the GC. Thus, cleavage of LPH could occur in the ER. We tested this possibility by disrupting the GC with brefeldin A. This treatment did not block receptor cleavage (Figure 1C), indicating that LPH was in fact proteolysed in the ER. The efficacy of the drug was demonstrated by its blockade of surface delivery of LPH (not shown).
As uncleaved LPH was not modified in the GC, proteolysis could be necessary for its trafficking through this compartment. This was proposed previously (Krasnoperov et al, 2002) because mutations impairing cleavage of LPH prevented its delivery to the plasma membrane. However, mutations could also cause protein misfolding and directly inhibit its trafficking. To identify which forms of LPH appear on the cell surface, we labelled cells with a membrane-impermeable biotinylation reagent. All LPH species were then solubilised and isolated by α-latrotoxin affinity chromatography. Using WB with streptavidin, the biotin label was detected only in αNTF (Figure 1D). This experiment demonstrated that neither the full-size LPH nor βNTF were delivered to the plasma membrane in detectable amounts. Although some CTF was also present on the cell surface (see below), it was not labelled with biotin, probably due to the inaccessibility of its two surface lysines.
NTF anchoring in the membrane
It has been proposed that NTFs of LNB receptors are attached to the plasma membrane via their CTFs (Krasnoperov et al, 1997; Qian et al, 2002). To determine which part of CTF might be responsible for this binding, we produced a series of LPH constructs in which different numbers of original LPH residues remained downstream of the cleavage site, while the rest of CTF was replaced with sequences from an unrelated receptor (Figure 2A). The resulting constructs possessed either one TMR (LPH-B to -D) or no TMRs (LPH-E); constructs LPH-D and -E had only seven LPH amino acids (7AA; T833NFAVLM839) remaining at the N-termini of their CTFs. The C-termini of all hybrids and the wild-type LPH (LPH-A) were tagged with two c-myc epitopes (Figure 2A).
Figure 2.
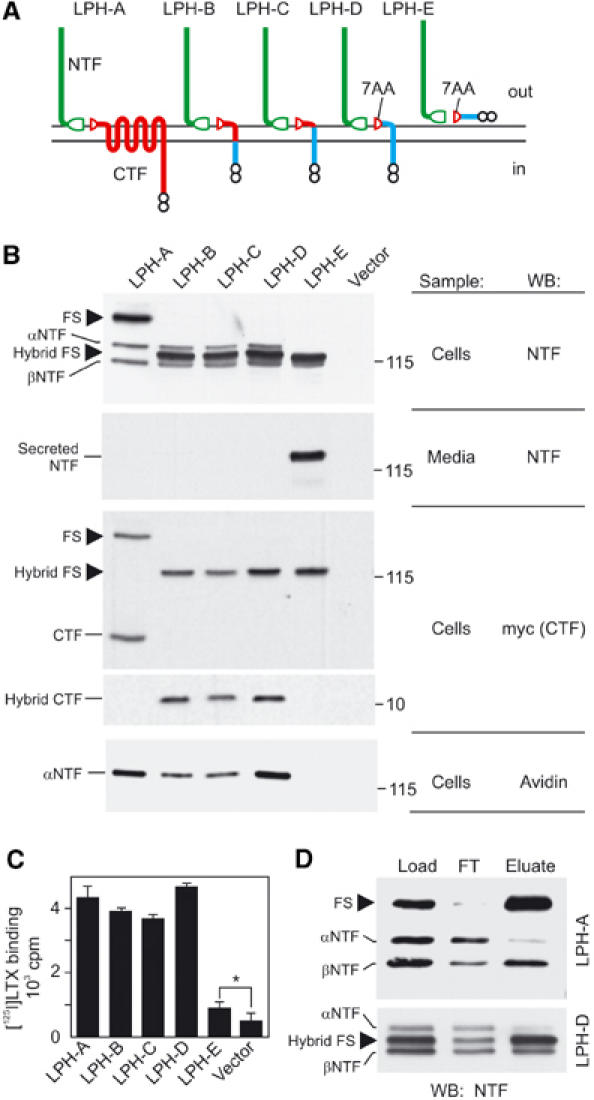
Structural requirements for latrophilin cleavage and membrane anchoring. (A) Latrophilin constructs used herein. Green, NTF of latrophilin; red, remaining original CTF sequences; blue, foreign sequence; split ellipse, cleaved GPS domain; small circles, myc epitopes. (B) Analysis of expression, cleavage, secretion and surface exposure of the LPH constructs. Transfected COS7 cells were biotinylated, solubilised, enriched on α-latrotoxin columns and analysed by WB, as indicated, in parallel with conditioned medium. Molecular masses are shown on the right. (C) Binding of radiolabelled α-latrotoxin to cells expressing LPH constructs; *P<1%, t-test. (D) Immunoprecipitation of solubilised recombinant receptors with anti-myc mAb. FT, flow-through; FS, full-size latrophilin.
All the constructs were efficiently expressed in COS7 cells and were cleaved, albeit partially (Figure 2B). The full-size receptors, stainable with both anti-NTF and anti-myc Abs, had corresponding molecular masses (Figure 2B). NTFs were processed in the same way in all the TMR-containing constructs (LPH-A to -D), with both αNTF and βNTF present (due to their extensive glycosylation, αNTFs of the hybrid constructs had larger molecular masses than the respective full-size proteins; Figure 2B). Cells expressing LPH-A to -D did not secrete any NTF, as shown by WB of the conditioned media enriched by α-latrotoxin chromatography (Figure 2B). In contrast, NTF was secreted by cells expressing TMR-less LPH-E. Biotinylation of surface proteins (as in Figure 1D) showed that only αNTFs of constructs LPH-A to -D appeared on the plasma membrane. Binding of α-latrotoxin to live cells also correlated with the presence of αNTFs (Figure 2C). Interestingly, we noticed a low but specific toxin binding to cells expressing the soluble LPH-E (Figure 2C), although NTF was not detectable on these cells by streptavidin (Figure 2B; discussed below).
The results with LPH-D indicated that the 7AA at the N-terminus of CTF were sufficient for proteolysis and NTF anchoring in the membrane. This was unexpected, and we assessed the strength of NTF–CTF interaction by co-immunoprecipitation of NTF with CTF. For both LPH-A and LPH-D, full-size proteins were essentially exhausted from solution by anti-myc mAb (Figure 2D), demonstrating the efficiency of immunoprecipitation. βNTFs were also largely pulled down, probably due to their interaction with CTF. Surprisingly, however, only a small proportion of αNTFs was immunoprecipitated (Figure 2D). Thus, the cell-surface αNTF, although not secreted, was not irreversibly bound to CTF, suggesting that it could be anchored in the plasma membrane by itself in addition to, or instead of, being tethered via CTF.
This hypothesis was evidently corroborated by α-latrotoxin binding to cells expressing LPH-E that had no TMR (Figure 2C). Two different mechanisms could explain this binding: (a) some NTF was directly anchored in the membrane; or (b) NTF was secreted and then partially adsorbed on the cell surface, creating apparent α-latrotoxin-binding sites. Direct detection of NTF on the surface of LPH-E-expressing cells could distinguish between these possibilities; if NTF were membrane-anchored (a), only cells expressing the protein would be labelled, but if NTF were secreted and re-adsorbed (b), all cells in culture would be stained indiscriminately. To increase the sensitivity of detection, we introduced V5-epitopes at the N-termini of LPH-A and LPH-E (Figure 3A). The new constructs specifically bound α-latrotoxin (Figure 3B); again, the binding was lower in cells expressing V5-LPH-E. For specific identification of LPH-expressing cells, these constructs were co-transfected with green fluorescent protein (GFP). When cells expressing GFP/V5-LPH-A or GFP/V5-LPH-E were stained with an anti-V5 mAb (Figure 3C), NTF was detected on the surface of transfected cells only, providing clear evidence for the anchoring mechanism (a).
Figure 3.
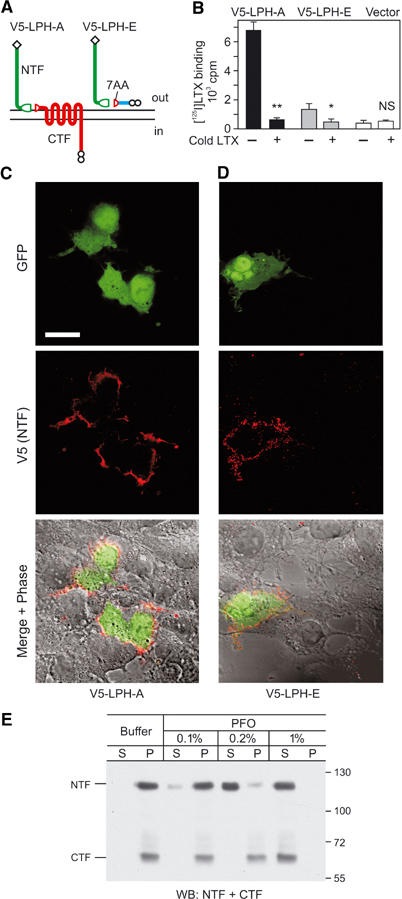
NTF can be anchored in the plasma membrane independently of CTF. (A) Modification of LPH-A and -E for improved immunodetection. Diamonds, N-terminal V5 epitopes. (B) Binding of [125I]-α-latrotoxin to COS7 cells transfected with V5-tagged constructs in the absence or presence of a 100-fold excess of cold toxin; **P<0.1%; *P<1%; NS, nonsignificant. (C, D) Immunostaining of V5-tagged constructs on the cell surface. COS7 cells co-transfected with GFP and V5-LPH-A (C) or V5-LPH-E (D) were stained with anti-V5 mAb. Detector gain for the red channel was increased in (D). Scale bar, 20 μm. (E) WB analysis of LPH-A-expressing neuroblastoma cells (see Figure 6 and respective text) treated with buffer or PFO at the indicated concentrations and centrifuged; S, supernatants; P, pellets. Note that 0.2% PFO solubilises only NTF but not CTF.
These data suggested, for the first time, that NTF could have a membrane anchor of it own. NTF apparently was not anchored via other proteins because various reagents that disrupt protein–protein interactions (dithiothreitol, high-molarity chaotropic salts, extreme acidic and alkaline conditions, urea, etc.) failed to remove NTF off the membrane (MA Rahman, YA Ushkaryov, unpublished observations). In contrast, nondenaturing detergents solubilised NTF and CTF (e.g. Figures 1 and 2). While testing different detergents, we discovered that NTF could be solubilised separately from CTF by perfluorooctanoic acid (PFO), a surfactant that does not perturb weak associations between membrane proteins and is used for nondenaturing electrophoresis of multi-subunit proteins (Ramjeesingh et al, 1999; Kedei et al, 2001). At high concentrations (1–2%), PFO solubilised both LPH fragments; however, 0.1–0.2% PFO only removed NTF from the cell membrane, leaving all CTF in the cell pellet (Figure 3E). As other receptors, which span the membrane at least once (e.g. neurexin, not shown), were not dissolved either, low concentrations of PFO probably acted by solubilising the plasma membrane only partially. Thus, NTF must be anchored in the membrane directly and independently of CTF.
Independent behaviour of LPH fragments in the cell membrane
The presence of an additional anchor on NTF meant that the LPH fragments could be independent on the cell surface. We tested this hypothesis by confocal immunofluorescent microscopy of permeabilised COS7 cells expressing LPH-A (Figure 4A) or LPH-D (not shown), and discovered an only partial overlap of the NTF and CTF staining patterns. Most cells displayed intracellular areas where the two proteins co-localised, and this could be due to the presence of either the two fragments or the uncleaved receptor. However, some immunostaining had a clearly distinct distribution: The cell surface and adjacent areas contained more CTF, while intracellular organelles stained largely for NTF. In addition, there were cells lacking NTF and those that displayed more NTF than CTF (Figure 4A).
Figure 4.
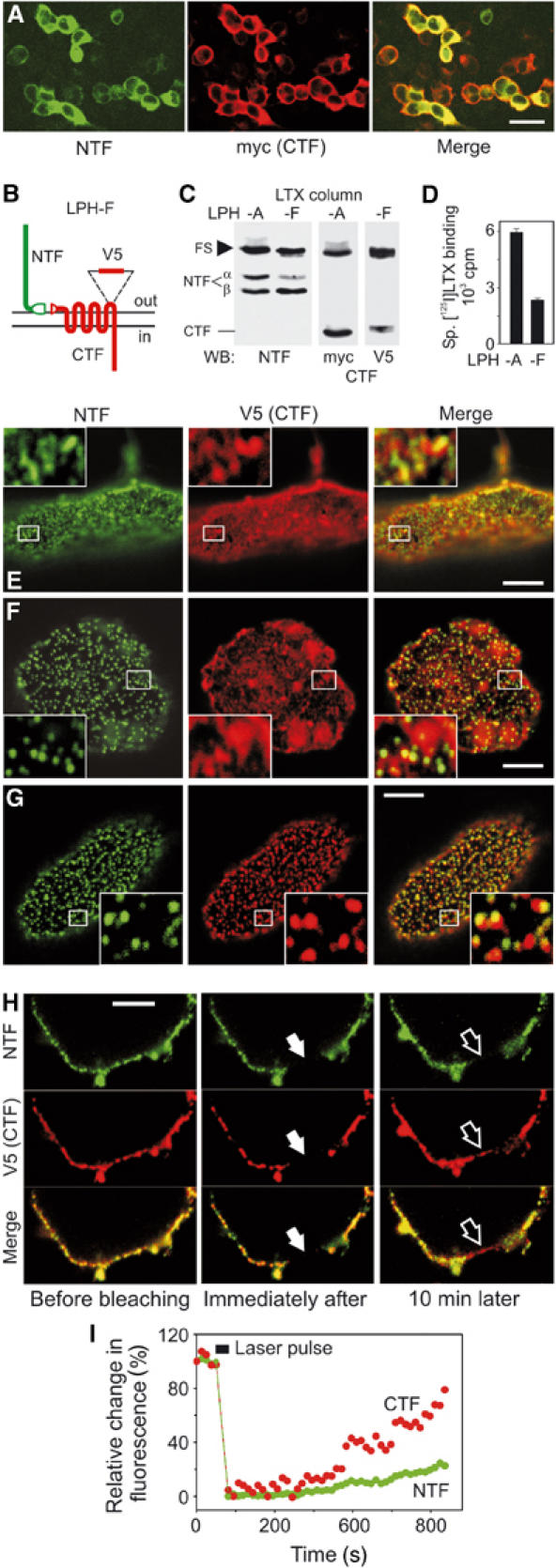
Latrophilin fragments behave as independent membrane proteins. (A) Indirect immunofluorescence of COS7 cells expressing LPH-A. (B) LPH-F with a surface-tagged CTF. (C) WB analysis of COS7 cells expressing LPH-A or -F. (D) Binding of radiolabelled α-latrotoxin to transfected cells. (E–G) Confocal images of apical membranes of nonpermeabilised cells stained for latrophilin fragments. Cells expressing LPH-F were: fixed and stained for NTF and CTF (E); or crosslinked with the primary and fluorescent secondary Abs against NTF, then stained for CTF (F); or crosslinked with primary and fluorescent secondary Abs against both fragments (G). Insets, enlarged boxed areas. NTF and CTF are independently re-arranged by crosslinking with respective Abs. (H) FRAP analysis of the lateral mobility of NTF and CTF. Cells expressing LPH-F were decorated with fluorescent primary Abs and imaged before and after photobleaching. Solid arrows, bleached area; open arrows, same area after recovery. (I) Quantification of NTF and CTF diffusion into the bleached area. Scale bars, 50 (A) and 5 (E–H) μm.
To study the behaviour of LPH fragments specifically on the cell surface, a new construct (LPH-F) was created that carried an epitope in the third extracellular loop of CTF (Figure 4B). This epitope did not block LPH-F cleavage or α-latrotoxin binding, but permitted staining of CTF with the anti-V5 mAb (Figure 4C). Note that α-latrotoxin binding correlated with the amount of αNTF (Figure 4D). Surface immunostaining of nonpermeabilised LPH-F-expressing cells revealed an incomplete co-localisation of LPH fragments (Figure 4E). However, this experiment could not determine whether NTF and CTF interacted with each other in the areas where they were both present.
We then used the method of antibody (Ab) patching (e.g. Oliferenko et al, 1999), which is based on the ability of secondary Abs to crosslink and pool together surface antigens immunodecorated with primary Abs. If two proteins interact, Ab-clumping of one of them must cause a similar change in the staining pattern of the other protein. However, it appeared that NTF and CTF were separate on the cell surface because crosslinking of NTF into well-defined patches did not change the distribution of CTF (Figure 4E and F). Similarly, when both fragments were simultaneously crosslinked with their respective Abs, NTF and CTF formed distinct clumps that overlapped only occasionally (Figure 4G).
Independent lateral mobility of NTF and CTF in the membrane was further demonstrated by fluorescence recovery after photobleaching (FRAP). Live cells expressing LPH-F were stained with fluorescent primary Abs against NTF and CTF (Figure 4H, left). Both fluorophores were then photobleached in narrow zones of the lateral plasma membrane, and re-entry of the fragments into these areas from adjacent membrane regions was monitored (Figure 4H). Quantification in Figure 4I demonstrates that the rates of lateral diffusion of CTF and NTF were different.
The use of LPH-F that could be stained extracellularly also allowed us to follow the fate of the surface-exposed LPH fragments. Incubation at 37°C of LPH-F-expressing cells decorated with primary Abs eventually led to endocytosis of the antigen–Ab complexes. Consistent with their behaviour as independent surface proteins, NTF and CTF internalised mostly separately and followed different time courses (Figure 5C and D). Quantitative assessment (Figure 5E) showed only partial (∼20%) co-localisation of the fragments in vesicles. Although the relative abundance of NTF- and CTF-containing compartments clearly changed with time, the number of vesicles containing both fragments remained constant. Each LPH fragment was often found co-localising with transferrin receptor (stained by fluorescent transferrin; not shown). These data suggested that NTF and CTF could travel through similar endocytic compartments, but their pathways did not separate or converge, and the two fragments were most probably segregated on the cell surface before internalisation.
Figure 5.
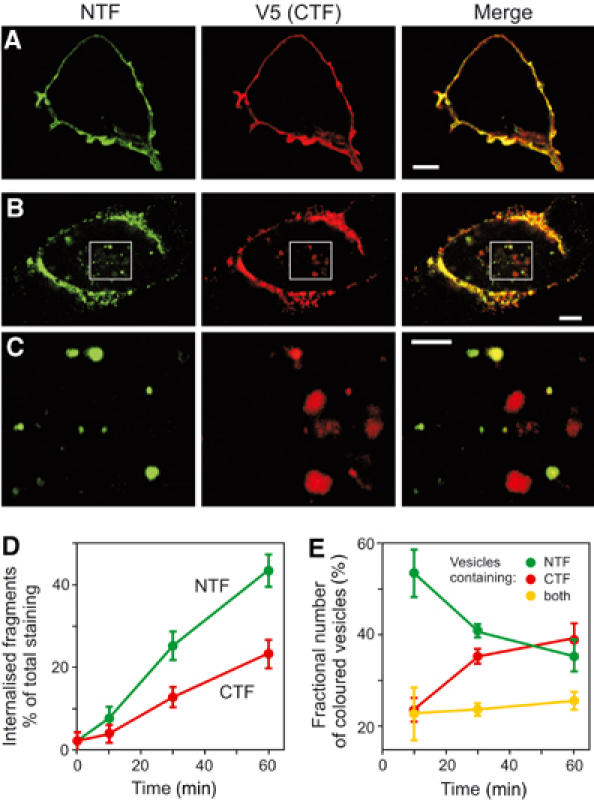
NTF and CTF can be separately internalised. (A, B) COS7 cells expressing LPH-F were decorated with both primary Abs and fixed before (A) or 30 min after (B) induction of endocytosis, then permeabilised and stained with secondary Abs. (C) Higher-magnification images of boxed areas in (B). NTF and CTF are found mostly in different vesicles. Scale bars, 5 (A, B) and 2 (C) μm. (D) Time courses of NTF and CTF endocytosis. LPH-F cells were stained as in (B), and the intensity of internalised fluorescence was measured relative to total fluorescence for each Ab. (E) Number of endocytosed vesicles containing NTF, CTF or both fragments. The data in (D, E) are the means±s.e.m., n=5–8.
NTF of LPH associates with cell-adhesion structures
To study the functional specialisation of LPH fragments, we chose neuroblastoma cells (NB2a), whose machinery for protein modification and intracellular signalling was similar to that of neurones, but which allowed the generation of stable lines expressing recombinant receptors.
In stably transfected NB2a cells, LPH-A and -D were fully cleaved and their NTFs were modified as in brain (Figure 6A). Immunostaining of permeabilised cells demonstrated that in each case all NTF was delivered to the membrane (Figure 6B). Interestingly, CTF was found both on the surface and inside the cell, suggesting that the two fragments were separated en route to, or from, the cell surface. Both NTF and CTF were present in the lateral cell membrane (Figure 6B); in contrast, the narrow protrusions of the basal membrane, contacting the substrate, contained mostly NTF (Figure 6C and D). These NTF-containing microspikes represented authentic cell-surface specialisations involved in cell adhesion, because actin cytoskeleton was attached to the base of all such structures (Figure 6E). The cell membrane surrounding the microspikes contained mostly CTF but little NTF. Thus, NTF can be targeted to structures mediating cell adhesion and/or cell contacts.
Figure 6.
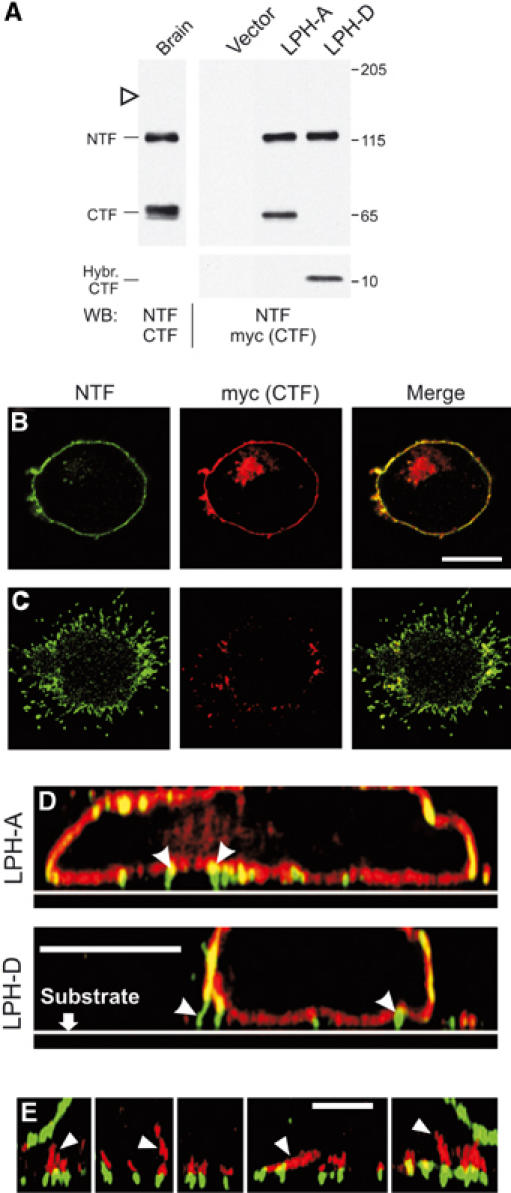
NTF associates with cell-adhesion structures. (A) WB analysis of LPH-A and -D expression in stably transfected NB2a cells, compared to brain latrophilin. Open arrowhead, expected position of full-size LPH-A. (B, C) Distribution of NTF and CTF in NB2a cells expressing LPH-A. Horizontal confocal sections were imaged near the cell's middle (B) or near the substrate (C). (D) Vertical cross-sections reconstructed from a set of overlapping horizontal confocal sections, as in (B), for cells expressing LPH-A or -D. Arrowheads, cell-adhesion structures. (E) Association of actin fibres (red; arrowheads) with NTF-containing membrane protrusions (green) in vertical cross sections reconstructed as in (D) for several individual loci of two cells. Scale bars, 10 (B–D) and 4 (E) μm.
CTF mediates intracellular signalling
CTF of LPH has seven TMRs and is probably a GPCR (Lelianova et al, 1997; Rahman et al, 1999; Capogna et al, 2003; Volynski et al, 2003). LPH can be stimulated by its agonist, α-latrotoxin from the black widow spider venom, which causes massive release of neurotransmitters (see Henkel and Sankaranarayanan, 1999). However, it is difficult to explore intracellular signalling using wild-type α-latrotoxin, because it forms large pores (reviewed in Ushkaryov, 2002). Therefore, to stimulate recombinant receptors, we used mutant latrotoxin (LTXN4C) that neither inserts into membranes nor forms pores (Volynski et al, 2003), but still triggers secretion in neurones via activation of PLC and release of stored Ca2+ (Ashton et al, 2001; Capogna et al, 2003).
We then determined the effect of mutant toxin on intracellular [Ca2+] in NB2a cells expressing LPH-A. As a negative control, we used LPH-D that could not mediate signalling via G-proteins. Cells were loaded with a fluorescent Ca2+ dye and stimulated by LTXN4C, which bound similarly to each construct (Figure 7A). As expected, intracellular Ca2+ spikes were induced in all the cells expressing LPH-A, but not in those that expressed LPH-D (Figure 7B and C). Thus, when CTF of LPH possesses seven TMRs, it is capable of mediating intracellular signals. To confirm the presence of receptors on the recorded cells, we applied native α-latrotoxin, and it induced Ca2+-permeable pores and Ca2+ influx (Figure 7B) independently of the type of receptor expressed.
Figure 7.
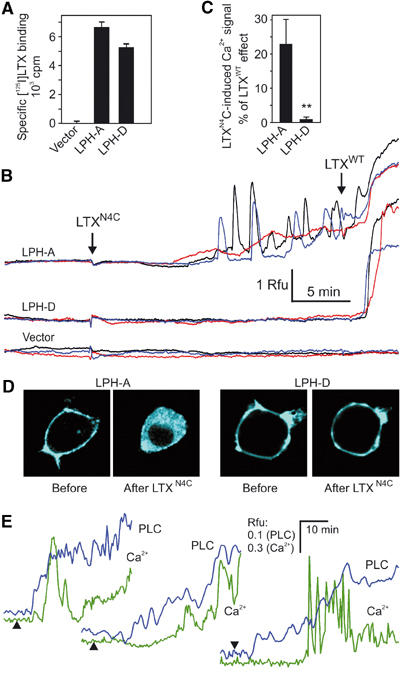
Latrotoxin induces CTF-mediated signalling. (A) Binding of [125I]-LTXN4C to NB2a cells expressing LPH-A or -D. (B) Time course of intracellular Ca2+ fluorescence in individual NB2a cells transfected with LPH-A, -D or vector and stimulated by 2.5 nM LTXN4C and native α-latrotoxin (LTXWT) (arrows). Rfu, relative fluorescence units. (C) Normalised maximal effect of LTXN4C; data are the means±s.e.m.; **P<0.1% (n=23–27 cells for each condition). (D) PLC activity visualised by translocation of CFP-PH into the cytosol after a 30-min application of 2.5 nM LTXN4C to cells expressing LPH-A or -D. (E) Time course of intracellular Ca2+ fluorescence and PLC activity in three individual LPH-A cells stimulated with LTXN4C at indicated times (arrowheads).
Although intracellular Ca2+ signals are generally believed to reflect increases in inositol-1,4,5-trisphosphate (IP3) and, therefore, PLC activation, we endeavoured to demonstrate directly that LTXN4C caused activation of this enzyme. This could be accomplished in real time by using the pleckstrin homology domain (PH) of PLCδ1 fused to cyan fluorescent protein (CFP) (van der Wal et al, 2001). CFP-tagged PH binds to inositol phospholipids on the inner side of the plasma membrane, but is released into the cytosol upon agonist-induced hydrolysis of phosphoinositides. This change can be monitored by confocal fluorescent microscopy (Figure 7D). LTXN4C induced translocation of CFP-PH only in cells expressing LPH-A, but not LPH-D, as the latter receptor was unable to transduce signals. Furthermore, PLC was activated soon after the addition of toxin, while Ca2+ spikes were delayed and followed only after a certain level of IP3 had been achieved (Figure 7E).
Direct interaction between NTF and CTF
The data reported above were intriguing. On the one hand, the fragments of LPH behaved as independent membrane proteins and could be solubilised separately by PFO (Figures 4, 5, 6 and 3E). On the other hand, NTF and CTF co-purified from Triton X-100 extracts (Figures 1B, D and 2D). To characterise any possible interaction of the solubilised fragments, we carried out exhaustive precipitation of V5-LPH-A and V5-LPH-D using immunoaffinity columns that bound either CTF (anti-myc column) or NTF (anti-V5 column). Figure 8A shows that the Ab columns completely removed their cognate fragments from the extract. The respective counterpart fragments were also largely retained by the columns, contrasting with separate behaviour of NTF and CTF on the cell surface.
Figure 8.
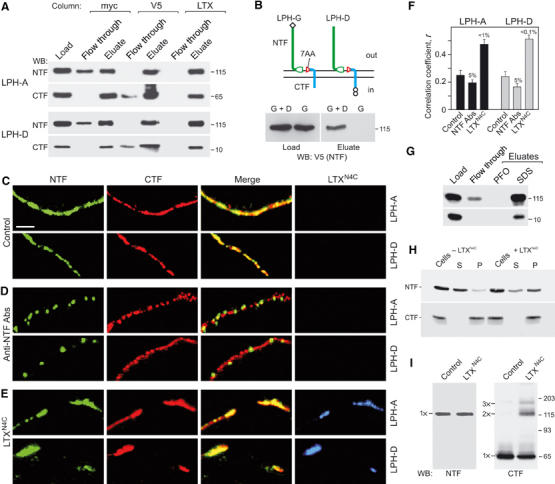
Molecular interaction between latrophilin fragments and role of LTXN4C. (A) Differential co-purification on different affinity columns of LPH fragments solubilised from NB2a cells transfected with LPH-A or LPH-D. (B) NTF from LPH-G (top) is pulled down with CTF of LPH-D by anti-myc column (bottom) upon solubilisation of NB2a cells co-expressing both constructs. (C–E) High-magnification confocal images of plasma membranes near the equator of NB2a cells expressing LPH-A or -D. Cells were fixed and stained without any treatments (C) or crosslinked with Abs against NTF (D), or treated with 2.5 nM fluorescent LTXN4C (E) before fixation and immunostaining. Scale bar, 2 μm. LTXN4C stimulates association of latrophilin fragments, while Abs crosslink NTF only. (F) Quantification of NTF and CTF co-localisation on the cell membranes. The data are the means±s.e.m. (n=25–30 cells for each condition). (G) 0.6% PFO does not break NTF–CTF complexes of LPH-D immobilised on anti-myc column, as in (A). (H) Pre-treatment of LPH-D-expressing cells with 5 nM LTXN4C inhibits solubilisation of NTF with 0.2% PFO. S, supernatant; P, pellet. (I) WB analysis of CTF oligomerisation in LTXN4C-stimulated NB2a cells expressing LPH-A.
We hypothesised, therefore, that solubilisation itself triggered an interaction between the LPH fragments. To test this idea, we co-expressed two constructs (LPH-D and -G; Figure 8B, top) endowed with different tags on opposite termini. If the fragments of each construct were truly independent in the membrane but began interacting upon solubilisation, then each CTF could bind any NTF. This proved to be the case (Figure 8B, bottom), as a large proportion of NTF from LPH-G was pulled down with CTF of LPH-D. This cross-precipitation did not result from a nonspecific interaction of V5-NTF with the anti-myc column, as demonstrated by control immunoprecipitation of LPH-G (Figure 8B).
We concluded from these findings that NTF and CTF could bind each other upon solubilisation. In addition, because there was no quantitative difference in the immunoprecipitation of LPH-A and -D, this interaction only required the 7AA from the CTF side.
α-Latrotoxin promotes NTF–CTF interaction
Despite binding only to NTF (Krasnoperov et al, 1999), α-latrotoxin requires CTF for signalling (Figure 7). Could α-latrotoxin (similar to detergent) induce NTF–CTF interaction? We noticed that while co-immunoprecipitation of the fragments was incomplete (∼70%), the α-latrotoxin column exhausted both NTF and CTF from the detergent solution (Figure 8A). This indicated that the toxin either facilitated the NTF–CTF interaction induced by detergent or simply bound to both NTF and CTF. The latter was unlikely because α-latrotoxin removed from solution not only CTF of LPH-A but also CTF of LPH-D, which contained only the 7AA of the original CTF, and this peptide was already engaged in the contact with NTF (see above).
The ability of LTXN4C to induce formation of NTF–CTF complexes on the cell surface was tested by immunostaining of NB2a cells expressing LPH-A or -D. In control experiments, LTXN4C was replaced with Abs known to induce NTF clumping without redistributing CTF (see Figure 4F). Relative changes in the NTF and CTF staining on the plasma membrane were quantified by calculating Pearson's correlation coefficient (r) between the immunofluorescence patterns of the two fragments. In untreated cells, r was low (about 0.25; Figure 8C and F), consistent with the independence of NTF and CTF. Crosslinking of NTF by Abs, not accompanied by analogous changes in CTF distribution, further decreased the r-value (Figure 8D and F). In contrast, the toxin caused a visible shift of both NTF and CTF into the same patches on the cell surface, leading to a significant increase in r (Figure 8E and F). Importantly, this clustering of the LPH fragments was observed only where the toxin was bound (Figure 8E, right).
To demonstrate that LTXN4C triggered a true molecular interaction between NTF and CTF in the membrane, we employed differential solubilisation of NTF with PFO (see Figure 3E). Molecular complexes of the two fragments are resistant to PFO (as shown by chromatography on an anti-myc column; Figure 8G); if such complexes form on the membrane after toxin binding, treatment with PFO should liberate proportionately less NTF. Indeed, when cells expressing LPH-D were exposed to LTXN4C, the amount of NTF solubilised by 0.2% PFO was decreased by 2.05±0.05-fold (n=2; Figure 8H), strongly suggesting that toxin caused formation of PFO-resistant molecular complexes of NTF and CTF.
Interestingly, treatment of LPH-A-expressing cells with LTXN4C caused formation of denaturation-resistant molecular oligomers of CTF (mainly dimers; Figure 8I). Thus, toxin binding to NTF translated into a conformational change in CTF, further supporting the idea of direct interaction between the two fragments. As in many GPCRs signal transduction is associated with formation of stable oligomers (Bouvier, 2001; Rios et al, 2001), these data also indicate that LPH signalling may be provoked by LTXN4C-induced oligomerisation of CTF.
Discussion
Based on our studies, we propose the following new scheme of organisation and functioning of LPH, which may be applicable to other LNB GPCR (as illustrated in Figure 9).
Figure 9.
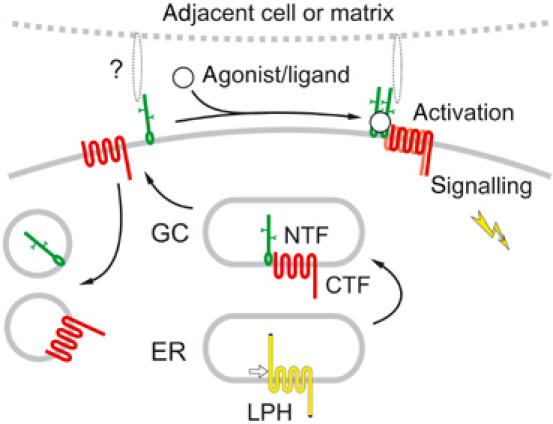
Proposed scheme of LPH processing and activation (see text).
LPH is cleaved in the ER (Figure 1). This proteolysis is required for the delivery of the mature, two-part protein to the cell surface (probably because NTF and CTF possess conflicting trafficking signals). In addition, post-translational processing endows NTF with a membrane anchor. While the precise structure of this anchor is currently unknown, our data suggest that it may be a short hydrophobic residue. CTF is not necessary for this modification (Figure 3D), but the presence of any TMR downstream of the cleavage site makes the anchoring reaction more efficient (Figure 2C and 3C), perhaps because NTF is held close to the membrane prior to cleavage.
On the cell surface, the receptor fragments behave as independent membrane proteins. They are often targeted to distinct locations (Figure 6), may be pulled into separate patches by Abs (Figure 4F and G), move in the plane of membrane at different rates (Figure 4H and I) and internalise independently (Figure 5).
Accordingly, NTF and CTF may have distinct functions. NTF contains modules implicated in surface interactions: the lectin-like domain may bind carbohydrate moieties of extracellular molecules, and the olfactomedin domain resembles a major protein of the extracellular matrix of olfactory epithelium. In our experiments, NTF showed clear association with cell-adhesion elements (Figure 6). Similarly, NTFs of other LNB receptors contain various cell-adhesion modules and mediate cell–cell and cell–matrix interactions (reviewed by Kwakkenbos et al, 2004). This may be a universal function of the diverse ectodomains of LNB receptors. On the other hand, CTF of LPH has a typical GPCR structure. LPH co-purifies with Gαq (Rahman et al, 1999) and may trigger a G-protein-coupled cascade (activation of PLC, production of IP3 and mobilisation of intracellular Ca2+; Lelianova et al, 1997; Ashton et al, 2001; Capogna et al, 2003). Stimulation of the LPH homologue in Caenorhabditis elegans by its exogenous ligand, emodepside, also activates this signalling pathway (Willson et al, 2004). Now, our data (Figure 7) demonstrate that this signalling is transduced by CTF.
As NTF binds α-latrotoxin (Krasnoperov et al, 1999), but the intracellular signal is generated via CTF, the two fragments should be able to interact. Indeed, NTF and CTF form complexes upon solubilisation, as demonstrated by their co-immunoprecipitation (Figure 8A). In contrast, on the cell surface, the fragments behave as independent membrane proteins (Figure 4). This may be due to (a) their ‘protected' conformation, (b) interaction with other proteins or (c) membrane microenvironment. Toxin binding to NTF, possibly by removing such interaction clamps, facilitates the recruitment of CTF and formation of the ternary molecular complexes (Figure 8).
The NTF–CTF association can only involve the seven N-terminal amino acids of CTF because LPH-D is indistinguishable from LPH-A in immunoprecipitation and toxin-induced clumping (Figures 2D and 8A, E). The 7AA are the most conserved residues between the cleavage sites and the TMRs in CTFs of all LNB receptors. Even conservative substitutions within this region abolish both cleavage and surface delivery of LPH (K Volynski, V Lelyanova and Y Ushkaryov, unpublished); similar mutations also inhibit proteolysis of EMR2 (Chang et al, 2003). Consequently, there must be a conserved structure in NTF that is responsible for CTF binding, and this is likely to be the GPS and the adjacent ‘stalk' domains (Chang et al, 2003), the main invariant regions in NTFs of all LNB receptors. In addition, the GPS/stalk domains could be involved in NTF anchoring in the membrane because the other NTF modules are too variable to participate in this universal function.
Currently, we can only speculate about the biological significance of this unusual receptor architecture. The synthesis of two functionally different proteins as fragments of one molecule assures their perfect stoichiometry. This organisation might be an adaptation enabling autonomous evolution of the molecule's functional domains that experience different evolutionary pressures. As the two fragments are physically separated, they can mutate independently, as long as the GPS/stalk domain is preserved. As a result, NTFs have evolved into several nonhomologous archetypes of cell-surface receptors, while CTFs acquired either seven TMRs (as in all LNB GPCRs) or one/11 TMRs (as in suREJ and polycystin-1; Mengerink et al, 2002; Qian et al, 2002). On the other hand, the association/dissociation of the fragments is likely to underpin a flexible and complex regulation of signalling and turnover. As the large NTFs contain multiple protein motifs, they could bind various ligands. Subsequent NTF–CTF interaction would allow CTFs to react to such cues. Another function of NTFs may be to bind adjacent cells or extracellular matrix (Hamann et al, 1996; Lin et al, 2001; Stacey et al, 2001; 2002) and provide temporally/spatially restricted docking sites for CTF recruitment and signalling (Figure 9). In this case, some other ligand(s) might induce the NTF–CTF interaction with subsequent signal transduction. Alternatively, CTF could be stimulated directly by its own agonists after complex formation. A further level of complexity might involve CTF interaction with NTFs from homologous receptors (see Figure 8B). Finally, the dissociation of the two receptor fragments would allow separate recycling and recovery of CTF, without disrupting NTF contacts with its extracellular partners.
While some of our conclusions are based on the use of LTXN4C, the latter is not a physiological ligand of LPH. However, LTXN4C does not induce any toxic effects typical of the wild-type toxin (see Ushkaryov et al, 2004) and stimulates LPH-mediated signalling in a manner similar to that of the small cyclical depsipeptide (Willson et al, 2004). It is possible that LTXN4C mimics the functions of two (or more) endogenous ligands of LPH; it is equally likely that only a small part of LTXN4C is involved in receptor activation. Signal transduction may be triggered by the NTF–CTF association itself, by subsequent formation of CTF oligomers (Figure 8E), or by a transient toxin contact with CTF. Importantly, the use of LTXN4C does not undermine our theory about the architecture and functions of LNB receptors because the ability to interact with each other is an intrinsic feature of their fragments.
In conclusion, our results reveal a novel principle of functional and structural organisation of LNB receptors and open new approaches to their in-depth characterisation and search for their endogenous ligands. Further study of these ‘split personality' receptors will provide new insights into the mechanisms of cell signalling.
Materials and methods
Biochemical experiments
Rabbit Abs against LPH fragments were described in Volynski et al (2000); anti-myc and anti-V5 mAbs were from Invitrogen. Iodination of α-latrotoxin, binding assays, α-latrotoxin affinity chromatography of Triton X-100-solubilised cells, preparation of LTXN4C and WB analysis was performed as outlined elsewhere (Volynski et al, 2000; 2003; Ashton et al, 2001; Capogna et al, 2003). For WB of full-size LPH or CTF with seven TMRs, samples were prepared in a conventional SDS buffer and heated to 50°C for 1 min. In some experiments, COS7 cells were biotinylated on ice for 1 h, using 0.5 mg/ml biotinamidocaptoic acid 3-sulpho-N-hydroxy-succinimide ester (Sigma-Aldrich) in PBS. Immunoprecipitation was carried out by chromatography of solubilised cells on immobilised anti-V5 and anti-myc mAbs; SDS buffer was used for elution. Where necessary, solubilised cells were deglycosylated with acyl-neuraminyl hydrolase (NAMase), peptide N-glycosidase (PNGase), or endo-α-N-acetylgalactosaminidase (O-glyc) (Sigma-Aldrich). For separate solubilisation of NTF, cells were incubated for 10 min on ice with PFO and then pelleted in a microcentrifuge. HRP-avidin was from Perbio Science.
Construction of expression plasmids
LPH constructs (LPH) were produced in pcDNA3.1 (Invitrogen). LPH-A was made by inserting two consecutive myc epitopes (EQKLISEEDL) in place of the C-terminal L1466 of the previously described full-size LPH-FS (Volynski et al, 2000). To create hybrid constructs LPH-B–E: C-terminal residues G881–S1465, E851–S1465, A840–S1465 and A840–S1465 of LPH-A were replaced, respectively, with C-terminal sequences of bovine neurexin Iα, R1479–N1523, V1457–N1523, I1447–N1523 and R1479–N1523. LPH-F was produced by inserting the V5 epitope (GKPIPNPLLGLDST) flanked by 12–13-residue spacers into the third extracellular loop of LPH-FS (between E1068 and S1069). CFP-PH was kindly provided by K Jalink (van der Wal et al, 2001).
Cell culture and transfection
Cells were cultured as described in Volynski et al (2000). COS7 cells were transiently transfected using SuperFect (Qiagen) and analysed 24 h later. Where required, 10 μM brefeldin A (Sigma-Aldrich) was added to the medium 4 h after transfection. Stable lines were generated in NB2a cells (a gift from P Salinas) by geneticin (Invitrogen) selection after FuGene6-aided transfection (Roche Diagnostics) and cell sorting (Becton Dickinson).
Confocal immunofluorescent microscopy
All images were captured and processed using a laser-scanning module (LSM510, Zeiss) mounted on an upright microscope Axioplan 2 (Zeiss). The following configurations were used in double-staining experiments: laser excitation, 488 and 543 nm; emission filters, 505–530 and >560 nm. For triple-staining experiments, the settings were: laser excitation, 488, 543 and 633 nm; emission filters, 505–530, 560–615 and >650 nm. Abs used were: primary, anti-NTF rabbit Abs, anti-myc or anti-V5 mouse mAbs; secondary, goat anti-rabbit IgG labelled with Alexa 488 and anti-mouse IgG labelled with Alexa 594 (Molecular Probes). Actin fibres were stained with TRITC-phalloidin (10 μg/ml; Sigma-Aldrich).
Transfected cells grown on poly-D-lysine-coated glass coverslips were treated by one of the following methods. Method 1: cells were fixed with 4% paraformaldehyde for 15 min at room temperature and then stained with primary and secondary Abs using conventional procedures (Figures 3C, D and 4E). Method 2: cells were processed as in Method 1, but were permeabilised with 0.1% Triton X-100 after fixation (Figures 4A, 6B–E and 8C). Method 3: live cells were processed at 0°C by (a) incubating with the primary (30 min) and secondary (30 min) Abs against NTF, fixing and subsequent staining for CTF without permeabilisation (Figure 4F), or (b) incubating with both primary Abs (30 min), then with both secondary Abs (30 min) and fixing (Figure 4G). Method 4: cells were decorated at 0°C with both primary Abs, washed and fixed either immediately (Figure 5A) or after incubation at 37°C (Figures 5B–E); finally, the cells were permeabilised and stained with secondary Abs. Method 5: cells were crosslinked with anti-NTF Abs (as in Method 3a), permeabilised and stained for CTF (Figure 8D). Method 6: cells were treated with fluorescent LTXN4C for 20 min at 0°C and stained by Method 2 (Figure 8E). Fluorescent LTXN4C was produced using an Alexa 647 labelling kit (Molecular Probes). To quantify NTF/CTF co-localisation, Pearson's correlation coefficient r was calculated for the plasma membrane (in 0.7-μm-thick confocal sections imaged near the cell's equator, where the membrane was perpendicular to the focal plane) using the LSM510 software.
FRAP experiments Primary Abs were fluorescently labelled, as appropriate, with Alexa Fluor-488 anti-rabbit IgG or Alexa Fluor-594 anti-mouse IgG2α Zenon labelling kits (Molecular Probes). Live cells expressing LPH-F were stained with fluorescent Abs at 0°C and monitored at room temperature by taking images at ∼12-s intervals with a water-immersion objective (Achroplan, 100 × , Zeiss). Fluorophores were then bleached near the cell's equator, and monitoring continued. To minimise endocytosis, each recording was completed within 15 min; the use of pre-labelled primary Abs prevented receptor patching. The acquired images were quantified using the LSM510 software and normalised to the initial value of bleaching (∼ 90% for both fluorophores).
To quantify NTF and CTF co-localisation upon endocytosis, vesicles were counted if their fluorescence in at least one channel exceeded the background (20% of maximal value), and were subdivided into three groups: ‘NTF' (red below threshold), ‘CTF' (green below threshold) and ‘both fragments' (all remaining vesicles).
Measurements of intracellular Ca2+ and PLC activation
Stably transfected NB2a cells, starved of serum for 2 days, were loaded with Fluo-3AM (Molecular Probes) using the manufacturer's protocol and equilibrated in buffer (in mM: 145 NaCl, 5.6 KCl, 5.6 glucose, 1 MgCl2, 1 CaCl2, 15 HEPES; 0.5 mg/ml BSA; pH 7.4). Images were acquired every 5 s with a water-immersion objective (Achroplan, 40 × , Zeiss), using 488 nm excitation and 505–550 nm emission filters. After a 10-min baseline recording, LTXN4C and native α-latrotoxin were added as indicated. Ca2+ fluorescence in individual cells was quantified using the LSM510 software and normalised to the baseline.
To record PLC activity, NB2a cells were transiently co-transfected with CFP-PH and LPH-A or -D and starved of serum for 20 h. Cells were imaged under the confocal microscope before and after the addition of LTXN4C (excitation, 458 nm; emission, >475 nm filter). CFP fluorescence in the cytosol was quantified using the LSM510 software. When Ca2+ and CFP signals were recorded simultaneously, crosstalk between channels was negligible.
Acknowledgments
We thank GD Kemp for protein sequencing, P Salinas for the gift of NB2a cells and K Jalink for the gift of CFP-PH construct. This work was supported by The Wellcome Trust.
References
- Abe J, Fukuzawa T, Hirose S (2002) Cleavage of Ig-Hepta at a ‘SEA' module and at a conserved G protein-coupled receptor proteolytic site. J Biol Chem 277: 23391–23398 [DOI] [PubMed] [Google Scholar]
- Ashton AC, Volynski KE, Lelianova VG, Orlova EV, Van Renterghem C, Canepari M, Seagar M, Ushkaryov YA (2001) Latrotoxin, acting via two Ca2+-dependent pathways, triggers exocytosis of two pools of synaptic vesicles. J Biol Chem 276: 44695–44703 [DOI] [PubMed] [Google Scholar]
- Bouvier M (2001) Oligomerization of G-protein-coupled transmitter receptors. Nat Rev Neurosci 2: 274–286 [DOI] [PubMed] [Google Scholar]
- Capogna M, Volynski KE, Emptage NJ, Ushkaryov YA (2003) The α-latrotoxin mutant LTXN4C enhances spontaneous and evoked transmitter release in CA3 pyramidal neurons. J Neurosci 23: 4044–4053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GW, Stacey M, Kwakkenbos MJ, Hamann J, Gordon S, Lin HH (2003) Proteolytic cleavage of the EMR2 receptor requires both the extracellular stalk and the GPS motif. FEBS Lett 547: 145–150 [DOI] [PubMed] [Google Scholar]
- Davletov BA, Meunier FA, Ashton AC, Matsushita H, Hirst WD, Lelianova VG, Wilkin GP, Dolly JO, Ushkaryov YA (1998) Vesicle exocytosis stimulated by α-latrotoxin is mediated by latrophilin and requires both external and stored Ca2+. EMBO J 17: 3909–3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletov BA, Shamotienko OG, Lelianova VG, Grishin EV, Ushkaryov YA (1996) Isolation and biochemical characterization of a Ca2+-independent α-latrotoxin-binding protein. J Biol Chem 271: 23239–23245 [DOI] [PubMed] [Google Scholar]
- Gray JX, Haino M, Roth MJ, Maguire JE, Jensen PN, Yarme A, Stetler-Stevenson MA, Siebenlist U, Kelly K (1996) CD97 is a processed, seven-transmembrane, heterodimeric receptor associated with inflammation. J Immunol 157: 5438–5447 [PubMed] [Google Scholar]
- Hamann J, Vogel B, van Schijndel GM, van Lier RA (1996) The seven-span transmembrane receptor CD97 has a cellular ligand (CD55, DAF). J Exp Med 184: 1185–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick JS (2000) A family of heptahelical receptors with adhesion-like domains: a marriage between two super families. J Recept Signal Transduct Res 20: 119–131 [DOI] [PubMed] [Google Scholar]
- Henkel AW, Sankaranarayanan S (1999) Mechanisms of α-latrotoxin action. Cell Tissue Res 296: 229–233 [DOI] [PubMed] [Google Scholar]
- Kedei N, Szabo T, Lile JD, Treanor JJ, Olah Z, Iadarola MJ, Blumberg PM (2001) Analysis of the native quaternary structure of vanilloid receptor 1. J Biol Chem 276: 28613–28619 [DOI] [PubMed] [Google Scholar]
- Krasnoperov V, Bittner MA, Holz RW, Chepurny O, Petrenko AG (1999) Structural requirements for α-latrotoxin binding and α-latrotoxin-stimulated secretion. A study with calcium-independent receptor of α-latrotoxin (CIRL) deletion mutants. J Biol Chem 274: 3590–3596 [DOI] [PubMed] [Google Scholar]
- Krasnoperov VG, Bittner MA, Beavis R, Kuang Y, Salnikow KV, Chepurny OG, Little AR, Plotnikov AN, Wu D, Holz RW, Petrenko AG (1997) Latrotoxin stimulates exocytosis by the interaction with a neuronal G-protein-coupled receptor. Neuron 18: 925–937 [DOI] [PubMed] [Google Scholar]
- Krasnoperov V, Lu Y, Buryanovsky L, Neubert TA, Ichtchenko K, Petrenko AG (2002) Post-translational proteolytic processing of the calcium-independent receptor of α-latrotoxin (CIRL), a natural chimera of the cell adhesion protein and the G protein-coupled receptor. Role of the G protein-coupled receptor proteolysis site (GPS) motif. J Biol Chem 277: 46518–46526 [DOI] [PubMed] [Google Scholar]
- Kwakkenbos MJ, Kop EN, Stacey M, Matmati M, Gordon S, Lin HH, Hamann J (2004) The EGF-TM7 family: a postgenomic view. Immunogenetics 55: 655–666 [DOI] [PubMed] [Google Scholar]
- Lelianova VG, Davletov BA, Sterling A, Rahman MA, Grishin EV, Totty NF, Ushkaryov YA (1997) Latrotoxin receptor, latrophilin, is a novel member of the secretin family of G protein-coupled receptors. J Biol Chem 272: 21504–21508 [DOI] [PubMed] [Google Scholar]
- Lin HH, Stacey M, Saxby C, Knott V, Chaudhry Y, Evans D, Gordon S, McKnight AJ, Handford P, Lea S (2001) Molecular analysis of the epidermal growth factor-like short consensus repeat domain-mediated protein–protein interactions: dissection of the CD97–CD55 complex. J Biol Chem 276: 24160–24169 [DOI] [PubMed] [Google Scholar]
- Mengerink KJ, Moy GW, Vacquier VD (2002) suREJ3, a polycystin-1 protein, is cleaved at the GPS domain and localizes to the acrosomal region of sea urchin sperm. J Biol Chem 277: 943–948 [DOI] [PubMed] [Google Scholar]
- Nechiporuk T, Urness LD, Keating MT (2001) ETL, a novel seven-transmembrane receptor that is developmentally regulated in the heart. ETL is a member of the secretin family and belongs to the epidermal growth factor-seven-transmembrane subfamily. J Biol Chem 276: 4150–4157 [DOI] [PubMed] [Google Scholar]
- Noorbakhsh F, Vergnolle N, Hollenberg MD, Power C (2003) Proteinase-activated receptors in the nervous system. Nat Rev Neurosci 4: 981–990 [DOI] [PubMed] [Google Scholar]
- Obermann H, Samalecos A, Osterhoff C, Schroder B, Heller R, Kirchhoff C (2003) HE6, a two-subunit heptahelical receptor associated with apical membranes of efferent and epididymal duct epithelia. Mol Reprod Dev 64: 13–26 [DOI] [PubMed] [Google Scholar]
- Oliferenko S, Paiha K, Harder T, Gerke V, Schwarzler C, Schwarz H, Beug H, Gunthert U, Huber LA (1999) Analysis of CD44-containing lipid rafts: recruitment of annexin II and stabilization by the actin cytoskeleton. J Cell Biol 146: 843–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian F, Boletta A, Bhunia AK, Xu H, Liu L, Ahrabi AK, Watnick TJ, Zhou F, Germino GG (2002) Cleavage of polycystin-1 requires the receptor for egg jelly domain and is disrupted by human autosomal-dominant polycystic kidney disease 1-associated mutations. Proc Natl Acad Sci USA 99: 16981–16986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MA, Ashton AC, Meunier FA, Davletov BA, Dolly JO, Ushkaryov YA (1999) Norepinephrine exocytosis stimulated by α-latrotoxin requires both external and stored Ca2+ and is mediated by latrophilin, G proteins and phospholipase C. Phil Trans R Soc Lond B 354: 379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramjeesingh M, Huan LJ, Garami E, Bear CE (1999) Novel method for evaluation of the oligomeric structure of membrane proteins. Biochem J 342: 119–123 [PMC free article] [PubMed] [Google Scholar]
- Rios CD, Jordan BA, Gomes I, Devi LA (2001) G-protein-coupled receptor dimerization: modulation of receptor function. Pharmacol Ther 92: 71–87 [DOI] [PubMed] [Google Scholar]
- Stacey M, Chang GW, Sanos SL, Chittenden LR, Stubbs L, Gordon S, Lin HH (2002) EMR4, a novel epidermal growth factor (EGF)-TM7 molecule up-regulated in activated mouse macrophages, binds to a putative cellular ligand on B lymphoma cell line A20. J Biol Chem 277: 29283–29293 [DOI] [PubMed] [Google Scholar]
- Stacey M, Lin HH, Gordon S, McKnight AJ (2000) LNB-TM7, a group of seven-transmembrane proteins related to family-B G-protein-coupled receptors. Trends Biochem Sci 25: 284–289 [DOI] [PubMed] [Google Scholar]
- Stacey M, Lin HH, Hilyard KL, Gordon S, McKnight AJ (2001) Human epidermal growth factor (EGF) module-containing mucin-like hormone receptor 3 is a new member of the EGF-TM7 family that recognizes a ligand on human macrophages and activated neutrophils. J Biol Chem 276: 18863–18870 [DOI] [PubMed] [Google Scholar]
- Ushkaryov Y (2002) Latrotoxin: from structure to some functions. Toxicon 40: 1–5 [DOI] [PubMed] [Google Scholar]
- Ushkaryov YA, Volynski KE, Ashton AC (2004) The multiple actions of black widow spider toxins and their selective use in neurosecretion studies. Toxicon 43: 527–542 [DOI] [PubMed] [Google Scholar]
- van der Wal J, Habets R, Várnai P, Balla T, Jalink K (2001) Monitoring agonist-induced phospholipase C activation in live cells by fluorescence resonance energy transfer. J Biol Chem 276: 15337–15344 [DOI] [PubMed] [Google Scholar]
- Volynski KE, Capogna M, Ashton AC, Thomson D, Orlova EV, Manser CF, Ribchester RR, Ushkaryov YA (2003) Mutant α-latrotoxin (LTXN4C) does not form pores and causes secretion by receptor stimulation. This action does not require neurexins. J Biol Chem 278: 31058–31066 [DOI] [PubMed] [Google Scholar]
- Volynski KE, Meunier FA, Lelianova VG, Dudina EE, Volkova TM, Rahman MA, Manser C, Grishin EV, Dolly JO, Ashley RH, Ushkaryov YA (2000) Latrophilin, neurexin, and their signaling-deficient mutants facilitate α-latrotoxin insertion into membranes but are not involved in pore formation. J Biol Chem 275: 41175–41183 [DOI] [PubMed] [Google Scholar]
- Willson J, Amliwala K, Davis A, Cook A, Cuttle MF, Kriek N, Hopper NA, O'Connor V, Harder A, Walker RJ, Holden-Dye L (2004) Latrotoxin receptor signaling engages the UNC-13-dependent vesicle-priming pathway in C. elegans. Curr Biol 14: 1374–1379 [DOI] [PubMed] [Google Scholar]


