Abstract
Studies of lung diseases in vitro often rely on flat, plastic-based monocultures, due to short lifespan of primary cells, complicated anatomy, lack of explants, etc. We hereby present a native 3D model with cues for repopulating epithelial cells. Abilities of mesenchymal stem cells (MSC) to modulate bacterial lipopolysaccharide (LPS) and cigarette smoke-induced injury to pulmonary epithelium were tested in our model. Post-mortem human lung tissue was sliced, cut and decellularized. Resulting matrix pads were reseeded with pulmonary epithelium (A549 line). Markers of the layer integrity and certain secreted proteins in the presence of cigarette smoke extract (CSE) and LPS were assessed via Western blot, ELISA and RT-PCR assays. In parallel, the effects of MSC paracrine factors on exposed epithelial cells were also investigated at gene and protein levels. When cultured on native 3D matrix, A549 cells obtain dual, type I- and II-like morphology. Exposure to CSE and LPS leads to downregulation of several epithelial proteins and suppressed proliferation rate. MSC medium added to the model restores proliferation rate and some of the epithelial proteins, i.e. e-cadherin and beta-catenin. CSE also increases secretion of pro-inflammatory cytokines by epithelial cells and upregulates transcription factor NFκB. Some of these effects might be counteracted by MSC in our model. We introduce repopulated decellularized lung matrix that highly resembles in vivo situation and is convenient for studies of disease pathogenesis, cytotoxicology and for exploring therapeutic strategies in the human lung context in vitro. MSC paracrine products have produced protecting effects in our model.
Keywords: Native lung matrix, Disease modeling, Decellularisation, Mesenchymal stem cells paracrine effects
Introduction
Destructive chronic lung diseases cause an increasing burden for the health of modern humans and the healthcare sector in general. Despite all efforts from the scientific community and health care providers, mortality and morbidity from chronic obstructive pulmonary disease (COPD) and similar diseases constantly grows (Lopez et al. 2006). COPD, a major cause of death and morbidity worldwide, is characterized by expiratory airflow limitation that is not fully reversible, deregulated chronic inflammation and emphysematous destruction of the lungs. Conventional COPD therapies remain palliative and regenerative approaches for disease management are not available yet. COPD pathogenetic mechanisms are constantly revisited and now include some novel aspects, e.g. shifts in lung microflora (Sze et al. 2012), autoimmune component of the disease (Cosio et al. 2002) and other than cigarette smoke environmental risk factors, that include indoor and outdoor air pollution, dust and fumes (Forbes et al. 2009; Hopkinson and Polkey 2015). Scientific community still lacks a holistic understanding of COPD development and only several mechanisms are well described and generally recognized, i.e. unregulated inflammation, proteolysis/antiproteolysis imbalance and destroyed repair mechanisms, while novel topics, like deviated microbiota, air pollutants-related damage and autoimmune process within the lung tissue, are only gaining attention. Considerable influx of new data from the clinic, in vivo and in vitro studies stimulate to search for concise understanding of COPD nowadays.
Tools to study diseases of lung parenchyma, like emphysema in COPD, in vitro are limited due to complex architecture and functional properties of the alveolar tissue. Several experimental possibilities exist, such as conventional flat cultures of lung epithelium, pulmonary cell cultures on synthetic matrices and native matrix-based models (Mahadeva and Shapiro 2002; Sakagami 2006; Krimmer and Oliver 2011; Ojo et al. 2014b). In vivo strategies for bioartificial lung creation and transplantation are explored and require engineering of viable lung architecture with ventilation, perfusion and gas exchange function. Successful attempts are reported, including perfusion of the bioartificial lung by the recipient’s blood and air, and also provision of gas exchange after the transplantation in vivo (Ott et al. 2010). In addition, suitability of decellularized lung matrix fragments for repopulation with various cells, including mesenchymal stem cells, lung fibroblasts and lung epithelial cells, was recently demonstrated (O’Neill et al. 2013). In the other study, the repopulation of the whole rat lung with pulmonary epithelial cells revealed that hierarchical cellular organization and efficient repopulation of matrix might be achieved in vitro (Petersen et al. 2010). Moreover, in the elegant work of Wagner and co-workers post-mortem human lung tissue from normal but not COPD subjects was shown to support growth of human bronchial epithelial cells, endothelial progenitor cells, mesenchymal stem cells and lung fibroblasts. Authors suggest that altered 3D environment might be the reason for the impaired cell proliferation on COPD lung matrix (Wagner et al. 2014). Several innovative lung tissue models are also proposed, i.e. “lung-on-chip” and microfluidic systems (Huh et al. 2010). These systems are particularly suited for precise detection of the interaction between the cell and inhaled particles, various drugs action studies and gas exchange-related tests. However, they are not well suited to accommodate spatial and chemical composition that is crucial for the survival and function of progenitor cells. Several attempts to replace the lung matrix with simplified collagen scaffolds, acellular matrices of lung-unrelated materials and similar experiments are reported with controversial results (Sueblinvong and Weiss 2010). Addressing all these issues comprises the major challenge for the creation of functional human lung tissue model in vitro.
The most important features of new lung model would be its flexibility and complexity, i.e. the ability to cultivate various types of lung cells and easy access and test of cellular and sub-cellular components under various experimental conditions. Additionally, an ideal in vitro model of lung alveolar tissue would allow precise, local and continuous assessment of extracellular matrix and cells, levels of inflammatory activity, proteolysis balance and intracellular signaling events. The idea of our study is to design native and relevant 3D lung model from post-mortem decellularized (DCL) lung tissue and evaluate growth and survival of human lung epithelial cells (line A549) in various COPD-related experimental designs. A549 in our model represent type II epithelial cells that are known as self renewing in culture and able to differentiate into alveolar-like structures “alveospheres” containing type II and type I epithelial cells (Barkauskas et al. 2013).
We have also investigated paracrine effects of human mesenchymal stem cells (MSC) in the protection of lung epithelial cells in our model. In the context of alveolar tissue MSC have been shown to contribute to lung regeneration in numerous experimental settings, including homing and attenuation of endotoxin-induced acute lung injury (Gupta et al. 2007; Lee et al. 2009). Promising potential of MSC has been studied in several ways and detectable therapeutic benefits were often attributed to paracrine mechanisms, since engraftment was low (van Haaften et al. 2009). Moreover, this and other studies show that MSC-secreted products reduce structural lung cells apoptosis, accelerate healing and enhance endothelial functions (van Haaften et al. 2009; Chen et al. 2010). MSC reparative function was already tested in humans in a clinical trial involving systemic administration of MSC for COPD patients (Weiss et al. 2013). However, no clinical improvement was detected and MSC-based regenerative strategies still need to be validated.
Materials and methods
Chemicals and cell lines
Post-mortem lung tissue samples (redundant from pathological studies) were obtained from the Department of Pathology, Vilnius Clinical Hospital (Lithuania) and stored at −20 °C. The procedure was approved by the Lithuanian Bioethics Committee (Approval #158200-14-740-265). Donors without the diagnosis of pulmonary pathology and obvious disease signs in the lungs were chosen.
Human MSC were isolated and expanded from bone marrow aspirates following the described protocol (approved by the Lithuanian Bioethics Committee, #158200-14-741-257, informed consent collected) with some modifications (23). Briefly, bone marrow aspirate was seeded in DMEM supplemented with 10 % fetal bovine serum (FBS) and 100 U/ml penicillin–streptomycin (PS) (all from Biochrom, Berlin, Germany) without prior gradient centrifugation. Cells employed for the experiments were derived from 28-year old female donor undergoing orthopedic surgery. Cells of 2nd–3rd passage were used. MSC were cultured to confluence and medium was collected 24 h after cell growth in fresh medium. Medium was diluted (1:1) with epithelial cell culture medium prior to application. In some experiments MSC were cultured on the membrane of inserts with pore size 0.4 µm.
Human cell line A549 was purchased from ATCC (Rockville, MD, USA) and expanded in F-12 K medium (Thermo Scientific, Waltham, MA, USA) supplemented with 10 % FBS and antibiotics.
All cell cultures were maintained in serum-free medium for 12 h prior to and during the experiments.
Reagent 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) was from AppliChem (Maryland Heights, MO, USA). Bradford protein assay, protein standards, electrophoresis gels were purchased from Bio-Rad (Hercules, CA, USA). Cigarette smoke extract (CSE) was provided by Murty Pharmaceuticals (Lexington, KY, USA). DNase I, LPS, RIPA, Laemmli buffers were from Sigma-Aldrich (St. Louis, MO, USA); RNase was from Genaxxon (Ulm, Germany); Western blot membrane—from Roche (Mannheim, Germany).
Preparation of native matrix model
A model was developed by slicing and cutting (hand driven blades) frozen post-mortem human lung tissue into discs of desired size (according to multi-dish well size). Tissue without obvious bronchial, vascular and other macro elements was chosen for slicing. First, frozen tissue was sliced into approximately 0.5 mm thick slices, then cut into round-shaped discs and thoroughly washed with deionized water and subjected to the treatment with CHAPS buffer (8 mM CHAPS, 1 M NaCl, 25 mM EDTA in PBS, 2 % PS) for 24 h, washed again and incubated for 1 h with SDS buffer (1.8 mM SDS, 1 M NaCl, and 25 mM EDTA in PBS with 2 % PS). Incubation with nucleases (DNase I and RNase A) for another hour was followed by 6 washes to completely remove the detergents and enzymes. Remaining RNA was extracted with QIAzol buffer (Qiagen, Hilden, Germany), DNA—using the PureLink Genomic DNA Mini Kit (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s protocols. DNA and RNA were quantified and qualified with the NanoPhotometer™ Pearl (Implen, Munich, Germany).
The decellularized matrix was afterwards repopulated with alveolar epithelium cells (A549 line). Matrices were equilibrated in the cell culture medium prior to cell seeding (2 h). To repopulate 1 cm2 of the matrix, 1 × 106 cells were resuspended in 300 µL of medium. Suspension was added to the matrix (in low-adherence plates, Nunc™, Roskilde, Denmark) and incubated for 30 min at 37 °C allowing the cells to settle into the scaffold. Recellularized slices were then submerged in tissue culture medium and incubated further.
Cell proliferation and viability assays
A549 cells seeded on native matrix were exposed to soluble CSE (0.1 %) and LPS (0.1 µg/ml) and compared to control cells. The CCK-8 (Dojindo Molecular Technologies, Rockville, MD, USA) assay was used to measure proliferation in our model according to the manufacturer’s instructions. Every 24 h cells were exposed to fresh CCK-8 reagent with subsequent incubation for 3 h and absorption was measured at 450 nm. Proliferation rate was measured for 4 or 5 consecutive days. In parallel, proliferation of cells cultured on 2D conventional plastic surface was tested. Proliferation rate in 2D and 3D cultures was compared in CSE-, LPS-exposed and control cells.
Histology and immunohistochemistry
Immunohistochemical assays were performed on formalin-fixed paraffin-embedded lung tissue sections. Paraffin-embedded specimens were cut into 2 µm sections and processed in DAKO autostainer Plus System (Glostrup, Danemark) further. Following primary mouse monoclonal anti-human antibodies were used: anti-surfactant protein (SP)-A, 1:100; anti-β-catenin, 1:100; anti-thyroid transcription factor-1 (TTF-1), 1:250 (all from Leica, Wetzlar, Germany); anti-E-cadherin, 1:50; anti-cytokeratin 7, 1:400; anti-pancytokeratin, 1:250 (all from DAKO) and anti-Forkhead box P3 (FOXP3), 1:100 (Abcam, Cambridge, UK). Lung tissue elastin fibers were stained with Verhoeff’s stain according to the routine histological protocol. Elastin makes complex with an iron-hematoxylin and remains stained blue-back to black, while remaining tissue elements are decolorized.
Electrophoresis and Western blotting
Reseeded native lung matrix was lysed in RIPA buffer and protein concentration was determined by Bradford assay. Lysates were subjected to the electrophoresis and blotted using a BioRad system. Primary antibodies to e-cadherin, Forkhead box protein A1 (FOXA1), beta-actin (all from Abcam) and β-catenin (Invitrogen) at 1:1000 dilutions were used. Bands were visualized by the incubation with appropriate secondary antibodies (1:2000) and chemoluminescence substrate (Thermo Scientific).
Cytokine release assay and transcription factor NFκB
Relative expression levels of 102 human soluble proteins were determined using the Proteome Profiler Human XL Cytokine Array (R&D Systems, Wiesbaden, Germany) containing capture antibodies spotted in duplicate on nitrocellulose membranes. The spot intensities were evaluated by densitometry using software GelQuantNET. Analytes tested with this array and their coordinates are listed in the Table 1.
Table 1.
Cytokine array analytes and their coordinates
Levels of NFκB p65 in whole cell lysates were detected by ELISA-type assay according to the manufacturer’s instructions (ThermoScientific/Pierce). Briefly, NFκB p65 proteins in the lysates were hybridized to the NFκB consensus sequence bound on the bottom of the wells and subsequently detected by the primary and secondary antibodies.
Gene expression studies
Tissue was flash-frozen in liquid nitrogen, homogenized, suspended in QIAzol buffer (Qiagen, Hilden, Germany) and RNA extracted according to the manufacturer’s protocol. RNA was subsequently purified with RNeasy Mini Spin columns (Qiagen) and measured by the NanoPhotometer™ Pearl (Implen).
RNA samples were treated with DNase I (Thermo Fisher Scientific) according to the manufacturer’s protocol and reverse-transcribed to cDNA with the Maxima®First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). PCRs were performed using Maxima® Probe qPCR Master Mix (2X) (Fermentas, Vilnius, Lithuania) and Stratagene MX-3005P detection instrument (Agilent Technologies, Waldbronn, Germany). The TaqMan® Gene Expression Assays (Applied Biosystems, Darmstadt, Germany) for 9 genes were used for gene expression analysis (Table 2). The PCR reactions were run in triplicate. Three reference genes were used in the experiments, i.e. beta-2-microglobulin (B2M), beta actin (ACTB) and ribosomal protein S9 (RPS9). RPS9 was selected for normalization.
Table 2.
Taqman®Gene Expression Assays used for gene expression analysis
| Gene, assay ID | Encoded protein |
|---|---|
| 1. RPS9 (reference gene) Hs02339424_g1 | 40S ribosomal protein S9 |
| 2. SFTPB Hs01090667_m1 | Surfactant protein SP-B |
| 3. OCLN Hs00170162_ml | Occludin |
| 4. CLDN1 Hs00221623_ml | Claudin-1 |
| 5. CAV1 Hs00971716_m1 | Caveolin-1 |
| 6. MMP1 Hs00899658_m1 | Matrix metallopeptidase 1 |
| 7. MMP2 Hs01548727_m1 | Matrix metallopeptidase 2 |
| 8. MMP9 Hs00234579_m1 | Matrix metallopeptidase 9 |
| 9. MMP14 Hs01037009_g1 | Matrix metallopeptidase 14 |
Statistical analysis
Statistical analysis was performed using of Sigma software (SigmaPlot, 2004, version 9.0). The differences in measured parameters between the study groups were analyzed for their statistical significance with the two-sided t test from three independent samples. Significance was determined at the 5 % level. Data are expressed as mean ± SD. The gene expression ratio was calculated by using 2−∆∆Ct equation and paired-samples t test on ∆Ct values was used to confirm statistical significance. Differences in DNA and RNA amounts were evaluated with one way ANOVA test followed by post hoc Bonferroni‘s multiple comparison test.
Results
Lung tissue model preparation
We have established a valid and simple method to reuse post-mortem or post-surgery lung tissue and employ it as a scaffold/matrix for cell cultures when the decellularisation procedure of the whole organ is complicated or impossible due to non functional vascular or airway system necessary for the perfusion with DCL solutions. Steps of preparation of native matrix model are visually presented in Fig. 1 and described in Materials and Methods section. Briefly, post mortem lung tissue (fragments) has been taken by pathologists during 12 h after the death, frozen at −20 °C and transported to the laboratory. Upon arrival frozen tissue (Fig. 1a) was cut into approximately 1 cm3 pieces and sliced into approximately 0.5 mm thick slices by hand-driven blades (Fig. 1b). Subsequently, circular disks were cut and subjected to the decellularisation procedure (Fig. 1c, arrow). The final preparation of lung tissue-derived matrix was employed for further experiments (Fig. 1d) after the equilibration in the cell culture medium for 2 h. Possibility to mount DCL lung matrix pad on CellCrown® was also tested (Fig. 1d).
Fig. 1.
Lung matrix-based model design. a Human post-mortem lung tissue lumps were frozen (−20 °C), sliced into approximately 0.5 mm thick sections b with subsequent cut of circular pieces that were subjected to decellularisation (c, arrow). Decellularisation, repopulation and further experiments were carried-out in the low-adherent wells in multi-well dishes. For certain experiments decellularized matrix pads may be mounted on a CellCrown® tissue holder (d)
Quality of decellularisation
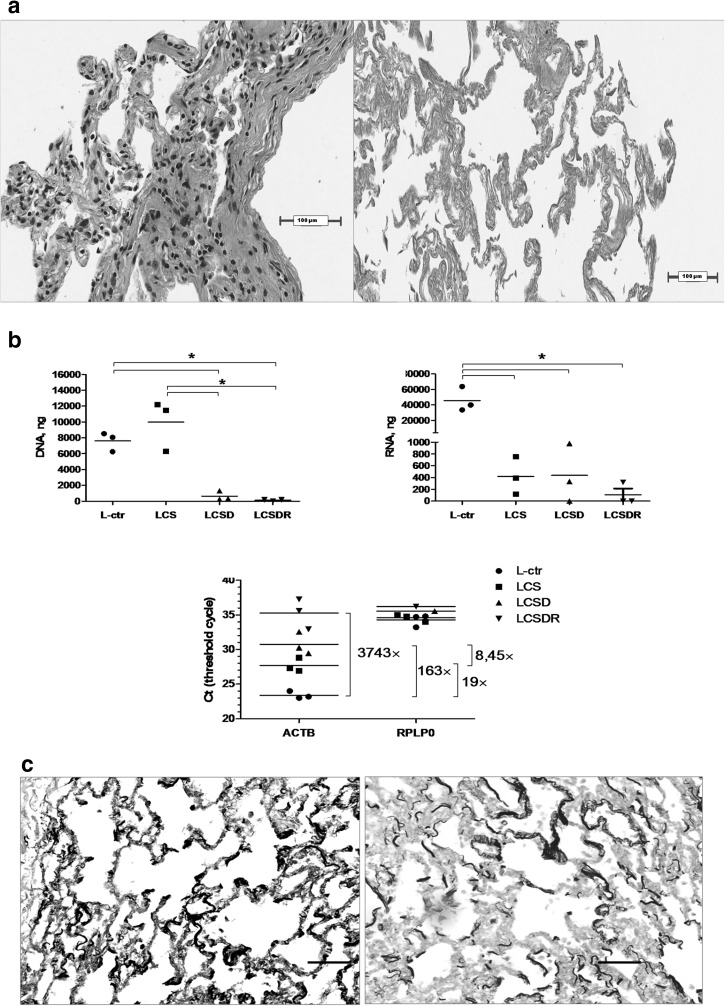
In this study we have adopted a mild decellularisation procedure that includes important step of treatment with DNA and RNA degrading enzymes, i.e. nucleases (DNase and RNase). The quality of decellularisation process was confirmed by few different methods. The absence of donor lung cells in DCL tissue matrix was demonstrated by the eosin/hematoxylin staining (Fig. 2a). In parallel, the residual amounts of DNA and RNA were investigated prior and after the decellularisation. Our data show that decellularisation of lung tissue by CHAPS and SDS has not sufficiently removed genetic material of the donor cells (LCS treatment group in Fig. 2b), whereas the addition of DNA nuclease was more efficient (LCSD treatment group). The most effective removal of nucleic acids was observed after the treatment with CHAPS, SDS, DNase and RNase (LCSDR treatment group). On the other hand, harsh decellularisation could damage lung matrix structure and content, and affect subsequent attachment of repopulating cells. Therefore we have investigated a presence of elastin before and after the decellularisation. As presented in Fig. 2c, elastin is adequately preserved after our DCL procedure.
Fig. 2.
Decellularisation quality assessment. a Cellular content in lung matrix was tested prior and after the decellularisation by eosin/hematoxylin staining, left and right panel, respectively; b DNA (left panel) and RNA (right panel) content in differently decellularized lung tissue are presented. L-ctr–untreated lung sample; LCS—lung tissue treated with CHAPS and SDS; LCSD—lung tissue treated with CHAPS, SDS and DNase; LCSDR—lung sample treated with CHAPS, SDS, DNase and RNase. Expression of housekeeping genes (lower panel): ACTB denotes β-actin, RPLP0–Ribosomal Protein, Large, P0. Both are housekeeping genes although RPLP0 is, perhaps, not suited for our model. *Significance of p < 0.05; c Estimation of elastin content in the native lung matrix detected by Verhoeff’s elastin staining before and after decellularisation. Scale bar–100 µm
Repopulation of lung tissue matrix with A549 cells
After matrix preparation and equilibration with cell culture medium, A549 cells were seeded into the matrix and their presence was assessed and confirmed by the eosin/hematoxylin staining after 4 days in culture (Fig. 3a). Dark blue nuclei and cellular structures show the presence of newly seeded A549 cells. However, proliferation rate of A549 cell on the DCL lung matrix compared to the growth in conventional 2D conditions was slightly lower (Fig. 3b). This might be explained by the additional time required for the cells to penetrate and attach to the scaffold, and/or by partial differentiation of type II cells (initial A549 cells) into the type I cells as pointed by arrow in the Fig. 3a. The data presented in Fig. 3c show that A549 cells grown on the native scaffold for 4 days express typical epithelial markers, i.e. e-cadherin, cytokeratin-7, beta-catenin, pan-cytokeratins, FOXP3, SP-A and some of them are positive for type I epithelial cell marker TTF-1 (arrow). It is known that production of TTF-1 is higher in flattened, enlarged, type I-like cells in comparison to clustered, cylindrical cells (type II-like). Our data suggest that part of A549 cells may spontaneously differentiate into type I alveolar cells upon contact with the native matrix leading to the lower total proliferation rate as compared to flat plastic-based cultures.
Fig. 3.
Growth of A549 cells on native lung matrix. a Morphology of A549 cells on native matrix 4 days after reseeding. Magnification 4× (left panel) and 10× (right panel). Arrow points to the flattened type I-like epithelial cells; b Proliferation rate of A549 cells on native 3D matrix in comparison to regular 2D culture conditions on plastic. Proliferation was monitored by metabolic CCK-8 assay by measuring absorption at 450 nm every 24 h after seeding for several days. Data are presented as mean ± SD. Each point is representative of not less than 5 independent experiments; c Expression of typical epithelial markers, i.e. e-cadherin, cytokeratin-7, beta-catenin, pan-cytokeratins, FOXP3, SP-A and type I epithelium-related TTF-1
Reactivity of pulmonary epithelium to the toxic exposures
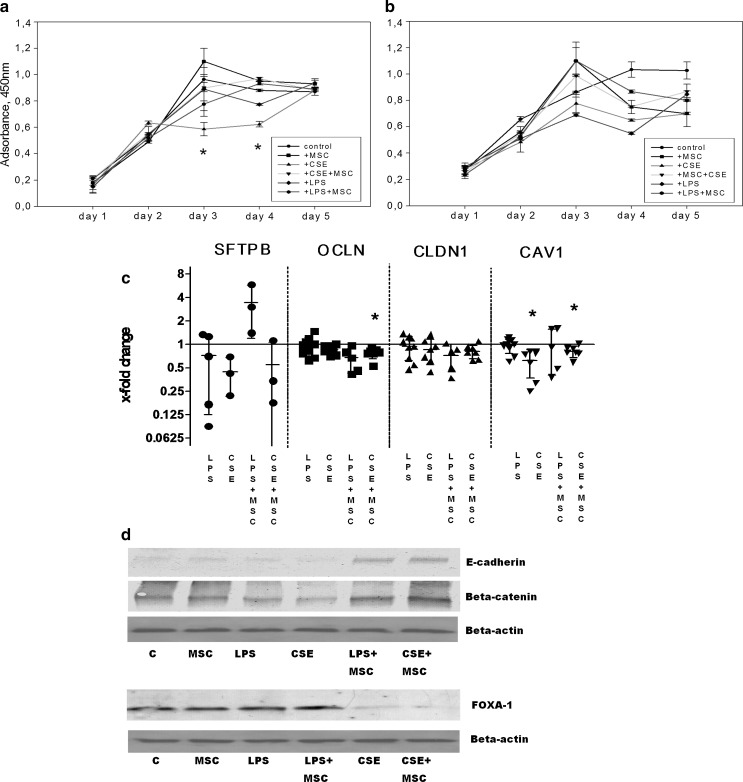
Survival of A549 cells under exposure to bacterial LPS and CSE and the possible protective role of MSC secreted products were investigated next. Data in Fig. 4a and 4b show that proliferation of A549 cells starts to decrease on the third day of exposure to CSE treatment in 3D cultures. The decrease was statistically significant when compared to control cells or to CSE and MSC medium treated cells. However, this decrease was more pronounced in 3D cultures but not in conventional flat cultures, suggesting that 3D-cultured cells have not only obtained less proliferative phenotype but also remained more susceptible to the certain damaging factors. Other treatment groups exhibited no significant shifts of cell proliferation. We have further investigated the mRNA levels of functional epithelial proteins, i.e. transportation related protein caveolin-1 and tight junction proteins occludin and claudin1 (Fig. 4c). Our findings show that expression of all three proteins mentioned above was downregulated after exposure to LPS and CSE, with most prominent suppressive effect of CSE on caveolin-1.
Fig. 4.
CSE and LPS effects on epithelium integrity-related proteins. a Proliferation rate (assessed by means of CCK-8 assay) of 3D matrix-cultured A549 cells exposed to CSE (0.1 %) and LPS (0.1 µg/ml) for 5 days. Data are presented as mean ± SD, *p < 0.05 in comparison to control and CSE + MSC exposed cells; b Proliferation rate of A549 cells (flat, plastic-based cultures) exposed to CSE (0.1 %) and LPS (0.1 µg/ml) for 5 days. Data are presented as mean ± SD. c Changes of mRNA levels of surfactant protein B (SFTPB), occludin (OCLN), claudin-1 (CLDN1) and caveolin (CAV1) as estimated by RT-PCR after the exposures to CSE (0.1 %) and LPS (0.1 µg/ml) and MSC conditioned media for 18 h. PCRs were performed using TaqMan® Gene Expression Assays (genes are listed in the Table 2). Reference gene–ribosomal protein S9 (RPS9). Paired t test assay on ∆Ct values was used to confirm statistical significance. Results are representative of at least 3 independent experiments. *p < 0.05; d Expression of e-cadherin, beta-catenin and FOXA-1 proteins detected by Western blotting. Beta-actin was tested in parallel as a loading control. CSE, LPS and MSC conditioned medium was added where indicated. Representative protein expression from 3 independent experiments is shown
On the protein level, the exposure of A549 cells to CSE and LPS has led to dowregulation of e-cadherin and beta-catenin (Fig. 4d). When MSC-secreted products were added to the model, levels of e-cadherin and beta-catenin were restored and even up-regulated (Fig. 4d). However, the expression of FOXA1, lung epithelium maturation-related transcription factor, was strongly downregulated by CSE but not LPS exposure, revealing different molecular mechanisms behind these two exposure models. Reparatory effect of MSC medium on the CSE-induced dowregulation of FOXA-1 was negligible (Fig. 4d).
Effects of MSC on pro-inflammatory activation of pulmonary epithelium
Previous experiments demonstrate that CSE-induced toxic effect might be different and stronger than that of LPS. Therefore, to examine the impact of CSE (0.1 %, 18 h) in more details, the semi-quantitative assessment of 102 soluble proteins was performed (Fig. 5a). We have found that exposure of A549 cells to CSE augments secretion of inflammation-associated factors MMP-9, chemokine GROα and LPS binding receptor CD14 (Fig. 5b). In addition, exposure of A549 cells to CSE has elevated the extracellular level of cystatin C, the main inhibitor of cysteine proteases, and chemoattractant for Th2 cells–TARC. The activation of vitamin D binding protein was very low. The indirect simultaneous co-cultivation of CSE-exposed epithelial cells with MSC has normalized levels of cystatin C and CD14. MSC medium has also downregulated these factors when applied to unexposed cells. No significant effects of MSC medium on the expression of remaining pro-inflammatory proteins were detected.
Fig. 5.
Proinflammatory cytokine secretion and levels of transcription factor NFκB. a Semi-quantitative determination of cytokines and soluble receptors was performed in a 102 protein analyte array. Analytes tested and their coordinates are listed in Table 1, shifted analytes are indicated in the picture. Cells were exposed to CSE (0.1 %) and LPS (0.1 µg/ml) for 18 h. During this experiment MSC were cultured on the cell culture inserts above the repopulated lung tissue matrix; b Densitometry graph of secreted pro-inflammatory factors; c Level of proinflammatory transcription factor NFκB p65 protein as detected by colorimetric ELISA-like assay. Protein level of the transcription factor was determined in the whole cell lysates. Data are presented as mean ± SD from 3 independent experiments. P level of significance is 0.05
Levels of NFκB p65 protein, an inflammation-related transcription factor, were significantly up-regulated by CSE and LPS (Fig. 5c). The addition of MSC medium simultaneously with LPS has normalized level of NFκB p65, while in the case of CSE it was only slightly down-regulated. Thus, the mechanism of CSE-induced inflammation and injury might be different compared to the LPS. This finding correlates with the viability shifts (Fig. 4a) and changes in the level of transcription factor FOXA1 (Fig. 4d).
Discussion
Our native matrix model enabling convenient cytotoxicity tests, screening for treatment strategies, individual disease modeling and other experimental studies in the context of real human lung is presented herein. The architecture and the microenvironment of the model highly resembles lung situation in vivo. We have investigated a suitability of thin, mild detergent treated post mortem lung sections with minimized loss of extracellular component elastin for the further repopulation and tissue modeling. Recently, a similar study reported that decellularisation of rat lung tissue can remove over 98 % of intracellular donor proteins retaining the highest amount of extracellular matrix comprising proteoglycans and glycoproteins (Hill et al. 2015). The decellularized human lung tissue matrix in our study was reseeded with human lung epithelial type II cells (A549) for the investigation of toxic COPD-related irritants, i.e. bacterial LPS and cigarette smoke extract. Protective capabilities of MSC secreted products were tested as well.
Lung tissue model presented here is indispensible when only fragments of tissue are available and the whole organ decellularisation becomes impossible. Utilization of such in vitro lung tissue spans from toxicology studies to screening and testing of novel therapeutics. When possible, whole lungs of human-, mouse- or rat-origin are processed and employed for similar applications (Ott et al. 2010; Krimmer and Oliver 2011; Mishra et al. 2012; Booth et al. 2012; Ojo et al. 2014a). Recently, it was shown that fragmented human and porcine lung tissue can be decellularized by CHAPS and SDS without perfusion and applied as biological scaffold for lung tissue engineering (O’Neill et al. 2013). In the current study we have added DNase and RNase treatment step. According to our data detergent treatment would leave matrix contaminated with detectable and transcribable donor genetic information with subsequent biologic effects. Taken together we demonstrate that human lung tissue matrix decellularized by CHAPS and SDS with the addition of both nucleases contains significantly lower amounts of donor DNA and RNA, comparable elastin content and serves well for the reseeding and variety of further assays in vitro.
Many cell types were used by different research groups for reseeding of lung matrices. Structural lung cells, induced pluripotent stem cells, MSC, transduced type I pulmonary cells and other cell types are currently listed in several publications with varying success (Swain et al. 2010; Huh et al. 2010; Ojo et al. 2014b). We have employed the most widely used alveolar epithelium cell line A549 that represents important features of type II alveolar epithelium and is used by the majority of researchers worldwide. It appears that A549 cells may actually possess natural capabilities of type II cells that are activated by matrix cues. This is in corroboration with other reports showing that type II epithelial cells serve as progenitors and are able to convert to type I respiratory cells and repair damaged alveolar epithelium in the case of injury or during lung development (Barkauskas et al. 2013). Similarly, the ability of A549 cells to spontaneously differentiate into type I cells and obtain their differential properties, like expression of TTF-1 and enlarged flat phenotype, over the time has been reported previously (Khubchandani and Snyder 2001; Swain et al. 2010; Zu et al. 2012) and further supports our results.
The effects of CSE and LPS on the A549 cells are studied for quite some time now (Leanderson and Tagesson 1992; Lannan et al. 1994; Wollmer and Evander 1994; Hoshino et al. 2001; Victoni et al. 2014). They include down-regulated proliferation, impaired cell attachment, DNA damage and suppressed levels of surfactant proteins. Similarly, LPS and CSE exposure also suppresses cell proliferation and levels of beta-catenin protein, e-cadherin protein and caveolin-1 mRNA in our native matrix model. In corroboration, recent studies also show that besides the regulation of barrier functions, tight junctions are important for cell cycle, cell migration, proliferation and regeneration (Kasper et al. 1995; Phillips et al. 2008; Flozak et al. 2010; Shiozaki et al. 2012). In addition, MSC exhibited ability to modulate tight junctions-associated proteins e-cadherin and beta-catenin when added together with CSE and LPS in our model. Recently, similar MSC effects were demonstrated on cystic fibrosis epithelium (Carbone et al. 2014).
Cytotoxic effects of LPS and CSE in our model include not only diminished cell proliferation capacity and levels of epithelial tight junction proteins, but also inflammatory activation that is partially restored by MSC. We have detected several proinflammatory proteins peeking up in the array from CSE-exposed epithelial cells with the strong up-regulation of MMP-9, cystatin C and GROα and mildly elevated vitamin D BP, TARC and CD14 levels. However, we have not detected any significant increase of commonly reported pro-inflammatory cytokines, like IL-6, IL-1β, IL-8, and TNFα suggesting that CSE-induced toxic effects might differ due to cell culture environment, dose and exposure model chosen. However, some sole analytes, e.g. MMP-9, and their up-regulation reflects similar tendencies reported by other authors (Braber et al. 2011; Overbeek et al. 2013). In general, expression of MMPs in lung tissue is described as a characteristic of tissue remodeling associated with various biological processes, such as development, inflammation, migration of cells, wound repair and other (Legrand et al. 1999; Itoh et al. 2002; Barnes et al. 2003; Wolf et al. 2003; Greenlee et al. 2007; Crosby and Waters 2010). Secretion of pro-inflammatory chemokine GROα was increased by CSE in our native matrix model in contrast to some other reports (Victoni et al. 2014) suggesting that study model and matrix origin might be important. Moreover, differential secretion of several other cytokines reported by Victoni et al. (2014) suggested that results from flat cultures and 3D native or synthetic cultures should always be weighted and compared cautiously. This is further supported by our results on cell proliferation, where 3D cultures were more susceptible to CSE toxicity than flat cultures. In corroboration with other studies, the up-regulated secretion of soluble CD14 by CSE was detected in our model (Tsutsumi-Ishii and Nagaoka 2003). CD14 is a known activator of inflammation in epithelial cells (Frey et al. 1992; Hailman et al. 1994; Ulevitch and Tobias 1995; Cario et al. 2000). In addition, up-regulation of cystatin C in CSE-exposed cells was also detected. The correlation of cystatin C expression with the smoking status and emphysema degree in patients is documented (Rokadia and Agarwal 2012). In parallel to enhanced cytokine levels we have also found increased activity of NFκB p65 in our model cells. Other authors have also shown that cytokine release from A549 cells under the stimulation with bacterial irritants is dependent on NFκB (Sachse et al. 2006). Transcriptional factor NFκB is a well recognized mediator of inflammatory processes in laboratory animals, patients and in vitro studies (Baeza-Squiban et al. 1999; Griesenbach et al. 2000; Aldonyte et al. 2004, 2008). Studies targeting NFκB for treatment purposes are ongoing (Griesenbach et al. 2000; Aikawa et al. 2002). As our data suggest, MCS-secreted products might be considered as one of the treatment options protecting epithelial cells from injuries.
In conclusion, we report that mild decellularisation of small tissue fragments preserves native structures and provides a functional lung matrix for cell repopulation. Cells might be conveniently cultured for the prolonged periods of time and subjected to the influence of various noxious and/or pro-regenerative agents. Furthermore, we show that MSC-secreted products may influence reactivity of pulmonary epithelium towards bacterial LPS and cigarette smoke. We believe that native matrix model proposed in this study represents a much-needed, relevant and convenient platform to study physiologic and pathologic processes within the alveolar compartment of the human lung.
Acknowledgments
We would like to thank Dr. A. Rimkevicius for the assistance with post-mortem lung tissue collection, colleagues at National Center of Pathology, at Vilnius University Hospital Santariskiu Clinic for the excellent immunohistochemistry work and surgeons at the Dept. of Traumatology at the Republic Hospital of Vilnius University for their help with bone marrow collection.
Funding
This work was supported by European Social Fund (ESF) under the Development Action Program of Human Resources by implementation of the project “Execution of functions assigned to Lithuanian Research Council for implementation of the Global Grant measure” (Grant # VP1-3.1-SMM-07-K-03-052).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Ieva Bruzauskaite and Jovile Raudoniute have contributed equally to this work.
References
- Aikawa Y, Yamamoto M, Yamamoto T, et al. An anti-rheumatic agent T-614 inhibits NF-kappaB activation in LPS- and TNF-alpha-stimulated THP-1 cells without interfering with IkappaBalpha degradation. Inflamm Res. 2002;51:188–194. doi: 10.1007/PL00000291. [DOI] [PubMed] [Google Scholar]
- Aldonyte R, Eriksson S, Piitulainen E, et al. Analysis of systemic biomarkers in COPD patients. COPD. 2004;1:155–164. doi: 10.1081/COPD-120030828. [DOI] [PubMed] [Google Scholar]
- Aldonyte R, Hutchinson TE, Jin B, et al. Endothelial alpha-1-antitrypsin attenuates cigarette smoke induced apoptosis in vitro. COPD. 2008;5:153–162. doi: 10.1080/15412550802092936. [DOI] [PubMed] [Google Scholar]
- Baeza-Squiban A, Bonvallot V, Boland S, Marano F. Airborne particles evoke an inflammatory response in human airway epithelium. Activation of transcription factors. Cell Biol Toxicol. 1999;15:375–380. doi: 10.1023/A:1007653900063. [DOI] [PubMed] [Google Scholar]
- Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PWHB. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22:672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- Booth AJ, Hadley R, Cornett AM, et al. Acellular Normal and Fibrotic Human Lung Matrices as a Culture System for In Vitro Investigation. Am. J. Respir. Crit. Care Med. Am J Respir Crit Care Med. 2012;186814:866–876. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braber S, Koelink PJ, Henricks PAJ, et al. Cigarette smoke-induced lung emphysema in mice is associated with prolyl endopeptidase, an enzyme involved in collagen breakdown. Am J Physiol Lung Cell Mol Physiol. 2011;300:L255–L265. doi: 10.1152/ajplung.00304.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone A, Castellani S, Favia M, et al. Correction of defective CFTR/ENaC function and tightness of cystic fibrosis airway epithelium by amniotic mesenchymal stromal (stem) cells. J Cell Mol Med. 2014;18:1631–1643. doi: 10.1111/jcmm.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cario E, Rosenberg IM, Brandwein SL, et al. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- Chen Y, Xiang LX, Shao JZ, et al. Recruitment of endogenous bone marrow mesenchymal stem cells towards injured liver. J Cell Mol Med. 2010;14:1494–1508. doi: 10.1111/j.1582-4934.2009.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosio MG, Majo J, Cosio MG (2002) Inflammation of the Airways and Lung Parenchyma in COPD * : Role of T Cells Inflammation of the Airways and Lung Parenchyma in COPD * Role of T Cells. 4–9. doi: 10.1378/chest.121.5 [DOI] [PubMed]
- Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol. 2010;298:L715–L731. doi: 10.1152/ajplung.00361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flozak AS, Lam AP, Russell S, et al. Beta-catenin/T-cell factor signaling is activated during lung injury and promotes the survival and migration of alveolar epithelial cells. J Biol Chem. 2010;285:3157–3167. doi: 10.1074/jbc.M109.070326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes LJL, Kapetanakis V, Rudnicka AR, et al. Chronic exposure to outdoor air pollution and lung function in adults. Thorax. 2009;64:657–663. doi: 10.1136/thx.2008.109389. [DOI] [PubMed] [Google Scholar]
- Frey EA, Miller DS, Jahr TG, et al. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee KJ, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev. 2007;87:69–98. doi: 10.1152/physrev.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesenbach U, Scheid P, Hillery E, et al. Anti-inflammatory gene therapy directed at the airway epithelium. Gene Ther. 2000;7:306–313. doi: 10.1038/sj.gt.3301078. [DOI] [PubMed] [Google Scholar]
- Gupta N, Su X, Popov B, et al. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- Hailman E, Lichenstein HS, Wurfel MM, et al. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med. 1994;179:269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RC, Calle EA, Dzieciatkowska M, et al. Quantification of extracellular matrix proteins from a rat lung scaffold to provide a molecular readout for tissue engineering. Mol Cell Proteomics. 2015;14:961–973. doi: 10.1074/mcp.M114.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkinson NS, Polkey MI. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2015;374:1964. doi: 10.1016/S0140-6736(09)62115-2. [DOI] [PubMed] [Google Scholar]
- Hoshino Y, Mio T, Nagai S, et al. Cytotoxic effects of cigarette smoke extract on an alveolar type II cell-derived cell line. Am J Physiol Lung Cell Mol Physiol. 2001;281:L509–L516. doi: 10.1152/ajplung.2001.281.2.L509. [DOI] [PubMed] [Google Scholar]
- Huh D, Matthews BD, Mammoto A, et al. Reconstituting Organ-Level Lung Functions on a Chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Matsuda H, Tanioka M, et al. The role of matrix metalloproteinase-2 and matrix metalloproteinase-9 in antibody-induced arthritis. J Immunol. 2002;169:2643–2647. doi: 10.4049/jimmunol.169.5.2643. [DOI] [PubMed] [Google Scholar]
- Kasper M, Huber O, Grossmann H, et al. Immunocytochemical distribution of E-cadherin in normal and injured lung tissue of the rat. Histochem Cell Biol. 1995;104:383–390. doi: 10.1007/BF01458132. [DOI] [PubMed] [Google Scholar]
- Khubchandani KR, Snyder JM. Surfactant protein A (SP-A): the alveolus and beyond. FASEB J Off Publ Fed Am Soc Exp Biol. 2001;15:59–69. doi: 10.1096/fj.00-0318rev. [DOI] [PubMed] [Google Scholar]
- Krimmer DI, Oliver BGG. What can in vitro models of COPD tell us? Pulm Pharmacol Ther. 2011;24:471–477. doi: 10.1016/j.pupt.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Lannan S, Donaldson K, Brown D, MacNee W. Effect of cigarette smoke and its condensates on alveolar epithelial cell injury in vitro. Am J Physiol. 1994;266:L92–100. doi: 10.1152/ajplung.1994.266.1.L92. [DOI] [PubMed] [Google Scholar]
- Leanderson P, Tagesson C. Cigarette smoke-induced DNA damage in cultured human lung cells: role of hydroxyl radicals and endonuclease activation. Chem Biol Interact. 1992;81:197–208. doi: 10.1016/0009-2797(92)90034-I. [DOI] [PubMed] [Google Scholar]
- Lee JW, Fang X, Gupta N, et al. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand C, Gilles C, Zahm JM, et al. Airway epithelial cell migration dynamics. MMP-9 role in cell-extracellular matrix remodeling. J Cell Biol. 1999;146:517–529. doi: 10.1083/jcb.146.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27:397–412. doi: 10.1183/09031936.06.00025805. [DOI] [PubMed] [Google Scholar]
- Mahadeva R, Shapiro SD. Chronic obstructive pulmonary disease * 3: experimental animal models of pulmonary emphysema. Thorax. 2002;57:908–914. doi: 10.1136/thorax.57.10.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra DK, Thrall MJ, Baird BN, et al. Human lung cancer cells grown on acellular rat lung matrix create perfusable tumor nodules. Ann Thorac Surg. 2012;93:1075–1081. doi: 10.1016/j.athoracsur.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill JD, Anfang R, Anandappa A, et al. Decellularization of Human and Porcine Lung Tissues for Pulmonary Tissue Engineering. Ann Thorac Surg. 2013;96:1046–1056. doi: 10.1016/j.athoracsur.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo O, Lagan AL, Rajendran V, et al. Pathological changes in the COPD lung mesenchyme–novel lessons learned from in vitro and in vivo studies. Pulm Pharmacol Ther. 2014;29:121–128. doi: 10.1016/j.pupt.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Ojo O, Lagan AL, Rajendran V, et al. Pathological changes in the COPD lung mesenchyme - Novel lessons learned from in??vitro and in??vivo studies. Pulm Pharmacol Ther. 2014 doi: 10.1016/j.pupt.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Ott HC, Clippinger B, Conrad C, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- Overbeek SA, Braber S, Koelink PJ, et al. Cigarette smoke-induced collagen destruction; key to chronic neutrophilic airway inflammation? PLoS ONE. 2013;8:e55612. doi: 10.1371/journal.pone.0055612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TH, Calle EA, Zhao L, et al. Tissue-Engineered Lungs for in Vivo Implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BE, Cancel L, Tarbell JM, Antonetti DA. Occludin independently regulates permeability under hydrostatic pressure and cell division in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2008;49:2568–2576. doi: 10.1167/iovs.07-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokadia HK, Agarwal S. Serum cystatin C and emphysema: results from the National Health and Nutrition Examination Survey (NHANES) Lung. 2012;190:283–290. doi: 10.1007/s00408-012-9374-z. [DOI] [PubMed] [Google Scholar]
- Sachse F, Von Eiff C, Stoll W, et al. Induction of CXC chemokines in A549 airway epithelial cells by trypsin and staphylococcal proteases—a possible route for neutrophilic inflammation in chronic rhinosinusitis. Clin Exp Immunol. 2006;144:534–542. doi: 10.1111/j.1365-2249.2006.03089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami M. In vivo, in vitro and ex vivo models to assess pulmonary absorption and disposition of inhaled therapeutics for systemic delivery. Adv Drug Deliv Rev. 2006;58:1030–1060. doi: 10.1016/j.addr.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Shiozaki A, Bai X, Shen-Tu G, et al. Claudin 1 mediates TNFalpha-induced gene expression and cell migration in human lung carcinoma cells. PLoS ONE. 2012;7:e38049. doi: 10.1371/journal.pone.0038049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueblinvong V, Weiss DJ. Stem Cells and Cell Therapy Approaches in Lung Biology and Diseases. Transl Res. 2010;156:188–205. doi: 10.1016/j.trsl.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain RJ, Kemp SJ, Goldstraw P, et al. Assessment of Cell Line Models of Primary Human Cells by Raman Spectral Phenotyping. Biophys J. 2010;98:1703–1711. doi: 10.1016/j.bpj.2009.12.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze MA, Dimitriu PA, Hayashi S, et al. The lung tissue microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:1073–1080. doi: 10.1164/rccm.201111-2075OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi-Ishii Y, Nagaoka I. Modulation of Human β-Defensin-2 Transcription in Pulmonary Epithelial Cells by Lipopolysaccharide-Stimulated Mononuclear Phagocytes Via Proinflammatory Cytokine Production. J Immunol. 2003;170:4226–4236. doi: 10.4049/jimmunol.170.8.4226. [DOI] [PubMed] [Google Scholar]
- Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- Van Haaften T, Byrne R, Bonnet S, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med. 2009;180:1131–1142. doi: 10.1164/rccm.200902-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoni T, Gleonnec F, Lanzetti M, et al. Roflumilast N-oxide prevents cytokine secretion induced by cigarette smoke combined with LPS through JAK/STAT and ERK1/2 inhibition in airway epithelial cells. PLoS ONE. 2014;9:e85243. doi: 10.1371/journal.pone.0085243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DE, Bonenfant NR, Parsons C, et al. Comparative Decellularization and Recellularization of Normal versus Emphysematous Human Lungs. Biomaterials. 2014;35:3281–3297. doi: 10.1016/j.biomaterials.2013.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss DJ, Casaburi R, Flannery R, et al. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013;143:1590–1598. doi: 10.1378/chest.12-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M, Clark-Lewis I, Buri C, et al. Cathepsin D Specifically Cleaves the Chemokines Macrophage Inflammatory Protein-1α, Macrophage Inflammatory Protein-1β, and SLC That Are Expressed in Human Breast Cancer. Am J Pathol. 2003;162:1183–1190. doi: 10.1016/S0002-9440(10)63914-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmer P, Evander E. Biphasic pulmonary clearance of 99mTc-DTPA in smokers. Clin Physiol. 1994;14:547–559. doi: 10.1111/j.1475-097X.1994.tb00413.x. [DOI] [PubMed] [Google Scholar]
- Zu Y-F, Wang X-C, Chen Y, et al. Thyroid transcription factor 1 represses the expression of Ki-67 and induces apoptosis in non-small cell lung cancer. Oncol Rep. 2012;28:1544–1550. doi: 10.3892/or.2012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]