Abstract
The present study examined the apoptosis inducing effects of Amaranthus spinosus L. aqueous extract in Allium cepa root meristematic cells and human erythrocytes. Cytogenetic assay revealed many apoptosis inducing cytogenetic aberrations viz., cytoplasmic breakage, cytoplasmic disintegration, cytoplasmic shrinkage, receding of cytoplasm, cytoplasmic vacuolation, enucleated cell, ghost cell, nuclear vacuolation, nuclear fragmentation and nuclear disintegration. A remarkable modification of red blood cell surface morphology was observed in the result of RBC assay. The treated RBCs show membrane blebbing and shrinkage, features typical for apoptosis in nucleated cells. Significant induction of cell death was observed in treated Allium root tip cells after Evans blue staining, disclosing the membrane damage potential of the plant extract. TTC assay results in reduced mitochondrial/metabolic activity in Allium root tip cells after treatment, designating the adverse effect of plant extract on mitochondrial respiratory chain. These results confirm the apoptosis inducing potential of A. spinosus extract. Confirming the present results by further in vitro studies, it can be effectively targeted against cell proliferation during cancer treatment by inducing apoptosis. Thus from the present investigation it can be concluded that the aqueous extract of A. spinosus exhibited apoptosis induction and cytotoxic activities.
Keywords: Amaranthus spinosus, Apoptosis, Blebbing, Fragmentation, Shrinkage, Vacuolation
Introduction
Studying the biological activities of plants is an unending process since many species of plants contain substances of medicinal value, which are yet to be explored. The specific phytochemicals for many plant based bioactivities are not yet fully understood. A large number of plants are constantly being screened for their possible medicinal value, since plants serve as an important therapeutic agent as well as important raw material for the manufacture of traditional and modern medicines. It has also been observed that ethnomedicinal plants frequently serve as a source of new drugs with little or no side effects. Epidemiologic studies have suggested that fruit and vegetable consumption reduces the risk of certain types of human cancers (Meyskens and Szabo 2005). Recently, many natural foods were found to exhibit pharmacological effects, and may have potential uses in cancer chemotherapy (Hwang et al. 2007). Therefore, plants have been examined to identify new and effective anticancer compounds, as well as to elucidate mechanisms of cancer prevention via apoptosis. Many previous studies had revealed that plants induced anticancer activity by inhibiting the multiplication and growth of cancer cells by inducing apoptosis in them.
Amaranthus spinosus L. is commonly known as Mullencheera in Malayalam, is a spinous and noxious weed commonly seen in cultivated as well as fallow lands. It is a monoecious erect spiny herb, traditionally used to treat various diseases. In Ayurveda, the plant is used as a digestive, laxative, diuretic, stomachic, antipyretic, and improves the appetite and biliousness. It cures blood diseases, leprosy, bronchitis, piles and leucorrhoea (Kirtikar and Basu 2001). The plant extract possesses severe effects in hematology (Olufemi et al. 2003). It shows immunomodulatory (Tatiya et al. 2007), anthelmintic (Assiak et al. 2002), analgesic (Krishnamurthi et al. 2010), antidiabetic, antihyperlipidemic, spermatogenic (Girija and Lakshman 2011; Sangameswaran and Jayakar 2008), hepatoprotective (Zeashan et al. 2008, 2009,), anticancer (Cristine et al. 2013), antioxidant (Odhavo et al. 2007; Zeashan et al. 2009) and chemoprotective activities (Kumar et al. 2010). A. spinosus contains several active constituents like alkaloids, flavonoids, glycosides, phenolic acids, steroids, terpenoids, saponins, betalains, β-sitosterol, stigmasterol, rutin, catechuic tannins, etc. Betalains in the stem bark of A. spinosus were identified as amaranthine, isoamaranthine, hydroxycinnamates, quercetin and kaempferol glycosides (Srinivasan et al. 2005; Ibewuike et al. 1997; Rastogi and Mehrotra 1999; Stintzing and Carle 2004; Hilou et al. 2006). It also contains amaranthoside, a lignin glycoside, amaricin, a coumaroyl adenosine along with stigmasterol glycoside, betaine such as glycinebetaine and trigonelline (Azhar-ul-Haq et al. 2006; Blunden et al. 1999). Betalains are well-known for their antioxidant, anticancer, antiviral and antiparasitosis activities (Kapadia et al. 1995, 1996; Patkai et al. 1997).
Apoptosis induction in HT-29 colon cancer cells by crude saponin from the roots of Platycodon grandiflorum (SPR) via both caspase- dependent and independent pathways was observed by Kim et al. (2008). In Caspase-dependent pathway, SPR increased the expression of the pro-apoptotic protein, Bax and decreased the expression of anti-apoptotic protein, Bcl-2, and subsequently, activated caspase-9 in the HT-29 cells, which in turn activated caspase-3. In caspase-independent pathway SPR elevated AIF protein expression and AIF translocation into the nucleus. AIF is a mitochondrial apoptosis inducing factor implicated in apoptosis as well as a mitochondrial flavoprotein that translocates to the nucleus following apoptotic stimuli. In the nucleus, AIF induces partial DNA fragmentation and chromatin condensation. AIF appears to promote apoptosis independent of caspase, although it likely acts in a cooperative manner with other factors to promote nuclear apoptosis.
A remarkable modification in RBCs surface morphology was observed in the result of RBC assay. Similar to other cell types, erythrocytes have to be eliminated after their physiological life span (Daugas et al. 2001). Beyond this, mechanisms are required for the removal of defective erythrocytes. In other cell types, the primary mechanism of clearance is apoptosis (Gulbins et al. 2000; Green and Reed 1998). Erythrocytes are devoid of mitochondria and nuclei (key organelles in the apoptotic machinery of other cells) and were considered unable to undergo apoptosis, until a study revealed Ca2+-ionophore ionomycin triggers breakdown of phosphatidylserine asymmetry (leading to annexin binding), membrane blebbing and shrinkage of erythrocytes, features typical for apoptosis in nucleated cells (Gulbins et al. 2000; Berg et al. 2001). Reports pertaining the effect of plant extracts on erythrocyte apoptosis are in their infancy. For instance, an altered RBC profile was observed after treatment with A. spinosus extract (Olufemi et al. 2003). Furthermore, Saponin the major anti-nutritional compound present in most of the Amaranthus species was reported to induce 100% hemolysis at concentrations of 10 and 15 μg/ml (Arias et al. 2010). Thus in addition to the confirmation of the apoptosis inducing potential via A. cepa root tip cells, the present study was performed to assess whether erythrocyte apoptosis could be induced by the crude extract of A. spinosus, a known trigger of genotoxic damage in other cell types (Joshua et al. 2010; Harsha 2011).
Materials and methods
Collection of assay systems
Fresh plant materials of A. spinosus L. was collected from Calicut University campus in Malappuram district of Kerala, India (10°7′34″N; 75°53′25″E; 45–50 m). The plant was identified taxonomically and voucher specimen was herbarized (CALI 123743). For cytotoxic assay certified bulbs of Allium cepa were purchased from agricultural vendor. For RBC assay human blood was purchased from blood bank. 2, 3, 5-triphenyl tetrazolium chloride (TTC) and Evans blue were purchased from HiMedia chemical laboratory (Mumbai, India).
Preparation of test solutions
Fresh aqueous leaf extracts of A. spinosus were prepared with the help of mortar and pestle. To make the stock solution, 1 g of leaf was weighed and ground in 100 ml distilled water. Low concentrations of the extract viz., 0.005, 0.01, 0.05 and 0.1% were prepared for toxicity analysis by diluting the stock solution with distilled water.
Cytotoxic assay on Allium cepa root meristematic cells
Uniform sized healthy bulbs of A. cepa were sorted and planted in sterilized sandy soil without manure to prevent cellular alterations. Germinated bulbs with healthy roots (1–2 cm) were collected at peak mitotic period (9–10 am) and washed in distilled water. Bases of onion bulbs bearing roots were suspended in extract solutions. Onion bulbs treated with distilled water and 0.1% methyl parathion were taken as negative and positive control, respectively. After the treatment for various durations (1/2, 1, 2 and 3 h), a few healthy root tips excised from each bulb were washed thoroughly with distilled water and immediately fixed in ethanol/glacial acetic acid (2:1) fixative (modified Carnoy’s fluid) for 1 h. After hydrolysis in 1 N HCl for 15 min at room temperature, mitotic squash preparations were made with improved techniques (Sharma and Sharma 1980) using 2% acetocarmine.
After the preparation of micro slides, the numbers of damaged cells and total cells were counted from five different fields of view using 40× of light microscope (Olympus CX21FSI, Tokyo, Japan). The percentage of each aberrations observed were tabulated and photomicrographs were taken by using AmScope MU Series digital camera.
Evaluation of membrane damage using RBCs
The apoptosis inducing capacity of the plant extract on human erythrocytes was tested by treating the blood samples with 0.1% of A. spinosus extract for 1 h at room temperature, and with saline solution as control group. Blood smears were prepared, dried, fixed and stained by May-Grunwald-Giemsa method (Junqueira and Carneiro 2004; Maiworm et al. 2008). After that, the blood smears were scanned under 100× of light microscope (Olympus CX21FSI, Japan) and images of red blood cells were taken by using an AmScope MU Series digital camera.
Evaluation of cell death using Evans blue staining
The loss of cell viability was studied using the Evans/Trypan blue staining method as reported earlier (Achary et al. 2008) with slight modification. The germinated bulbs were initially treated with four concentrations viz., 0.1, 0.2, 0.4 and 0.8% of A. spinosus extract for 24 h. Root tips treated with distilled water and 0.1% methyl parathion for 24 h were used as negative and positive control, respectively. Control and treated roots were then stained with 0.25% (w/v) aqueous solution of Evans blue for 15 min and subsequently washed with distilled water for 30 min. The roots were then macro-imaged for a qualitative estimation of cell death. For a quantitative estimation, 10 root tips of equal length were excised and soaked with 3 ml of N, N-dimethylformamide for 1 h at room temperature. The absorbance of Evans blue released was measured at 600 nm.
Evaluation of metabolic activity using TTC staining
After exposure to treatment and control as mentioned above, the control and treated roots were immersed in 0.5% 2, 3, 5-triphenyl tetrazolium chloride (TTC) and kept at 35 ± 1 °C for 15 min in the dark. Subsequently, the root tips were rinsed with distilled water and imaged (Shaymurat et al. 2012). In addition, 10 root tips were excised from each bulb and the colored complex triphenyl formazan was extracted in 95% ethanol. The absorbance was read at 490 nm.
Statistical analysis
Data obtained were subjected to statistical analysis, DMRT and one-way ANOVA to determine mean separation and significance of treatments using SPSS 20 (SPSS Inc., Chicago, IL, USA). All results were expressed as mean ± SE and differences between corresponding controls and exposure treatments were considered statistically significant at p < 0.05.
Results
RBC assay
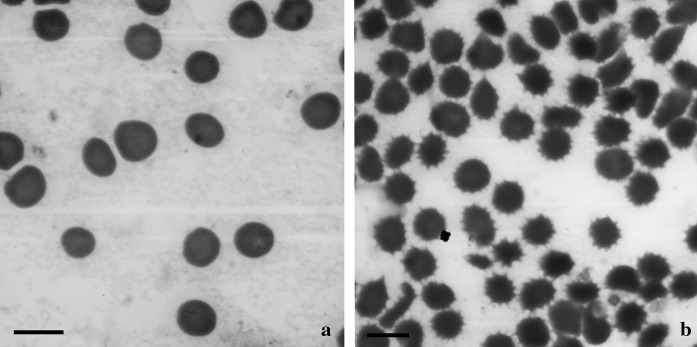
The RBC assay revealed significant alterations in the cell shape and surface features. Human erythrocytes incubated with A. spinosus aqueous extract shows significant morphological changes from a normal discoid to an echinocytic form (Fig. 1b), while the general discoid feature was maintained in the control group (Fig. 1a). The appearance of several protuberances (blebbing) on the cell surface, shrinkage and marked loss of the central concavity, typical of echinocytes was also observed. The modifications in the RBC membrane and/or cytoskeletal structural assembly may be the possible reason for this abnormal condition, which indicates the possible effect of A. spinosus extract on RBC membrane and cytoskeletal structure.
Fig. 1.
Effect of aqueous extract of A. spinosus on surface morphology of human Red Blood Cells. a Control showing normal discoid erythrocyte b Treated RBCs showing membrane blebs. Bar 10 µm
Cytotoxic assay on Allium cepa root meristematic cells
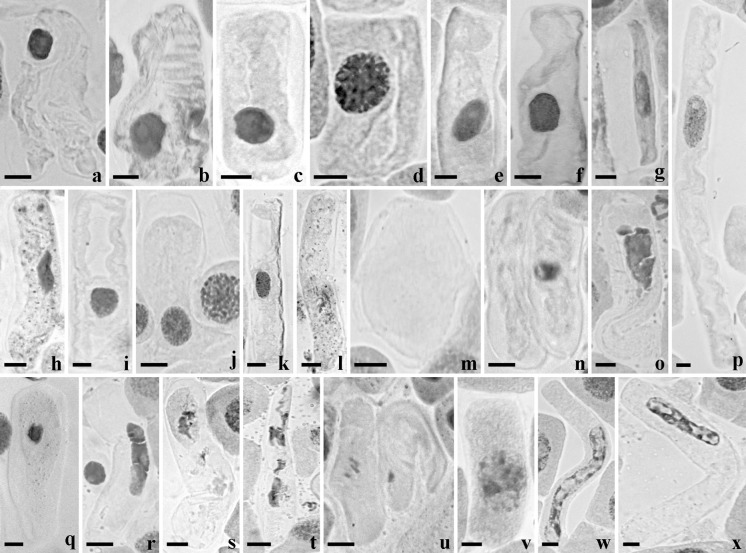
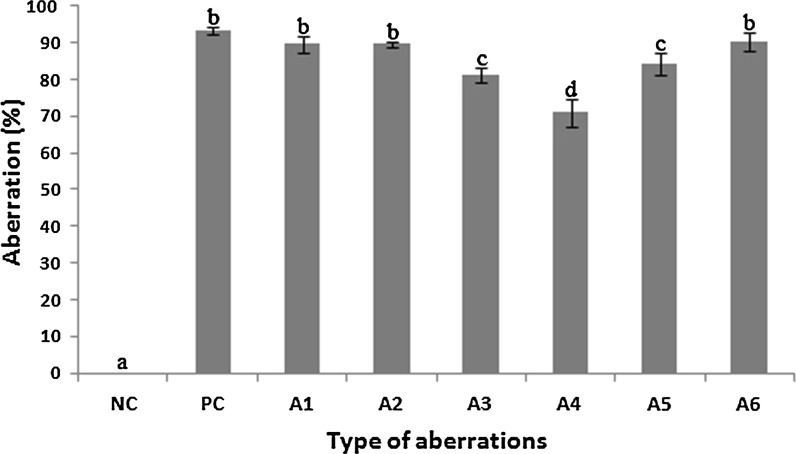
Cytotoxic assay resulted in dose-dependent apoptosis inducing cytogenetic toxicity via cytoplasmic breakage (Fig. 2a–d), cytoplasmic disintegration (Fig. 2a, b, s, t), cytoplasmic shrinkage (Fig. 2c, e–g, p), receding of cytoplasm (Fig. 2e–g, h, l, q), cytoplasmic vacuolation (Fig. 2i–k), enucleated cell (Fig. 2m), ghost cell (Fig. 2l), nuclear vacuolation (Fig. 2w, x), nuclear fragmentation and nuclear disintegration (Fig. 2n, o, q, r–v). All concentrations tested were found to be significant, but highest toxicity was observed at 0.1% during 3 h treatment (Fig. 3). The percentage of toxicity observed for each aberration at 0.1% during 3 h treatment include cytoplasmic breakage and disintegration (89.66 ± 2.18), shrinkage and receding of cytoplasm (89.66 ± 0.88), cytoplasmic vacuolation (81.33 ± 2.02), enucleated and ghost cell (71 ± 3.78), nuclear vacuolation (84.33 ± 2.90), nuclear fragmentation and disintegration (90.33 ± 2.40) (Fig. 3). There was no toxic effect observed in root tip cells in the negative control (NC-Distilled water) but the positive control (PC-0.1% methyl parathion) exhibited maximum toxicity (93.33 ± 1.20) after 3 h treatment.
Fig. 2.
Apoptosis inducing cytogenetic aberrations persuaded by aqueous extract of A. spinosus on root meristematic cells of A. cepa. a, b Cytoplasmic breakage and disintegration; c cytoplasmic breakage and shrinkage; d cytoplasmic breakage; e–g cytoplasmic shrinkage and receding of content; h receding cytoplasm and nuclear peak; i–k cytoplasmic vacuolation; l ghost cell showing receding cytoplasm; m enucleated cell; n two cells showing stages of nuclear disintegration and disappearance; o, r nuclear fragmentation; p giant cell showing cytoplasmic shrinkage and nuclear lesion; q nuclear disintegration and receding cytoplasm; s, t cytoplasmic and nuclear disintegration; u two cells showing stages of nuclear fragmentation and disappearance; v nuclear disintegration; w, x nuclear vacuolation in giant cells. Bar 20 µm
Fig. 3.
Induction of various cytogenetic aberrations by 0.1% A. spinosus aqueous extract after 3 h treatment in A. cepa root tip cells. (NC) Negative control—Distilled water; (PC) Positive control—0.1% Methyl parathion; A1—Cytoplasmic breakage and disintegration, A2—Shrinkage and receding of cytoplasm, A3—Cytoplasmic vacuolation, A4—Enucleated and ghost cell, A5—Nuclear vacuolation, A6—Nuclear fragmentation and disintegration; (0%)—Minimum aberration as in NC (Distilled water), (100%)—Maximum aberration beyond PC (0.1% Methyl parathion). Mean values within a graph followed by the same letters are not significantly different at P < 0.05 as determined by Duncan’s multiple range tests
Evans blue staining assay
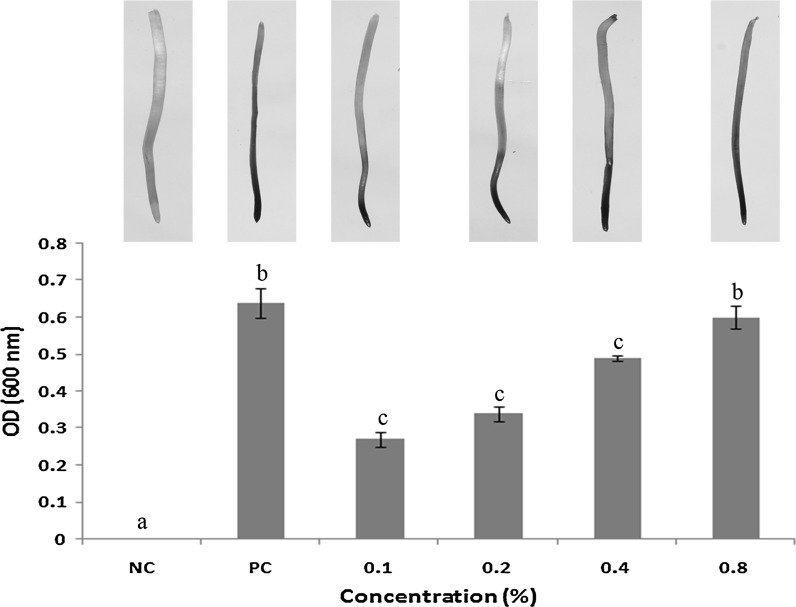
Evaluation of apoptotic activity of A. spinosus aqueous extract using Evans blue staining method revealed significant induction of membrane damage and subsequent cell death in treated A. cepa root meristematic cells. Severe apoptotic activity was observed after treatment with positive control (0.64 ± 0.04), whereas no cell death was observed in negative control (Distilled water). In treatment, apoptotic potential of plant extract was found to be dose-dependent, since maximum cell death (0.60 ± 0.03) was observed after 0.8% treatment with plant extract. Evans blue dye was used as a marker of membrane integrity and thereby cell death. Living cells have the ability to exclude the dye at the plasma membrane, while cells with a damaged membrane are unable to exclude the dye and are stained blue (Vargas et al. 2006). The roots exposed to different concentrations of plant extract and positive control (0.1% Methyl parathion) showed a dose-dependent uptake of the blue dye when compared to negative control (Distilled water) roots (Fig. 4), indicating the presence of certain apoptosis inducing phytochemicals in the aqueous extract of A. spinosus.
Fig. 4.
Effect of A. spinosus aqueous extract on cell viability- Evans blue staining. Graph showing a significant dose-dependent increase in cell death, the root tips of A. cepa showing dead cells (blue), living cells (white); (NC) Negative control—Distilled water; (PC) Positive control—0.1% Methyl parathion. Mean values within a graph followed by the same letters are not significantly different at P < 0.05 as determined by Duncan’s multiple range tests. (Colour figure online)
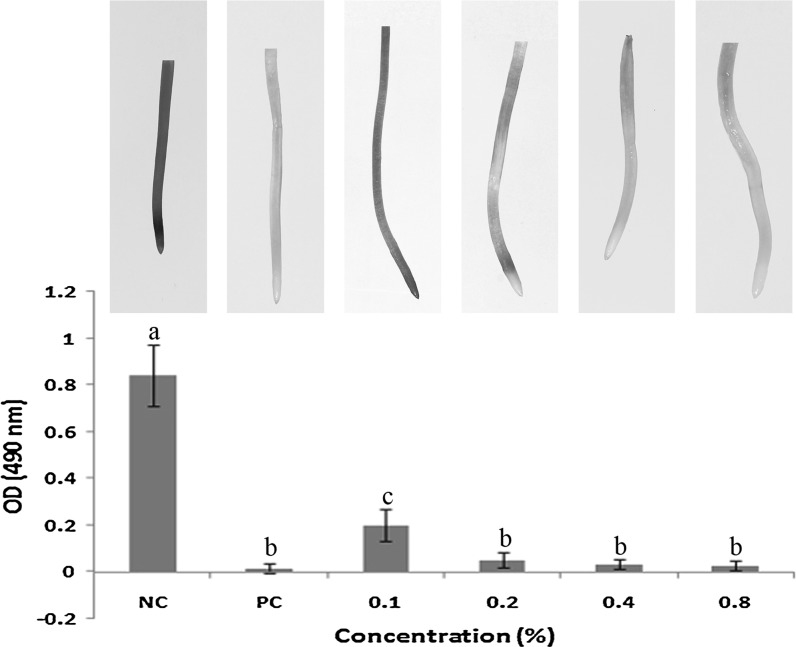
TTC staining assay
The effect of A. spinosus aqueous extract on mitochondrial function was tested using TTC staining technique and it resulted in significant decrease in mitochondrial activity in treated A. cepa root meristematic cells. The inhibition of mitochondrial/metabolic activity was found to be maximum in positive control (0.01 ± 0.02), hence it remained unstained. A dose-dependent inhibition was observed in the treatment group, since it shows a dose-dependent decrease in stainability (Fig. 5). The least mitochondrial activity (0.02 ± 0.02) was observed after 0.8% treatment with the plant extract. The negative control (Distilled water) having active mitochondria got stained maximally indicating highest mitochondrial/metabolic activity (0.84 ± 0.13). Roots exposed to plant extracts and positive control (0.1% Methyl parathion) was unable to reduce triphenyltetrazolium chloride (TTC) to insoluble red colored triphenyl formazan (TF), indicating decreased activity of the mitochondrial respiratory chain. Severe mitochondrial dysfunction observed in the present study indicates the possible cytotoxicity of the A. spinosus extract.
Fig. 5.
Effect of A. spinosus aqueous extract on mitochondrial activity- TTC staining. Graph showing dose-dependent decrease in mitochondrial activity, the root tips showing viable cells with active mitochondria stained red. (NC) Negative control—Distilled water; (PC) Positive control—0.1% Methyl parathion. Mean values within a graph followed by the same letters are not significantly different at P < 0.05 as determined by Duncan’s multiple range tests. (Colour figure online)
Discussion
Apoptosis is a physiological process characterized by chromatin condensation, membrane blebbing, cell shrinkage and DNA fragmentation (Sharma et al. 2007). Cytotoxic assay resulted several cytogenetic aberrations having features indicative of cell death. Treated cells showed cytoplasmic breakage, cytoplasmic disintegration, cytoplasmic shrinkage, receding of cytoplasm, cytoplasmic vacuolation, enucleated cell, ghost cell, nuclear vacuolation, nuclear fragmentation and nuclear disintegration. Extreme mitotic inhibition by A. spinosus aqueous extract in A. cepa root tip cells was reported by Prajitha and Thoppil (2016), indicating its cytotoxic potential. These results corroborate a previous report in which thymoquinone induced antimitotic activity and cell death associated aberrations including nuclear membrane disruption, nuclear fragmentation, shrinkage of the plasma membrane, leakage of cell lysate, degradation of cell walls, enlargement of vacuoles and condensation of nuclei in Allium root tip cells (Hassanien et al. 2013). Excessive vacuolation is an indicative of upcoming cell death. The upregulation of a variety of vacuolar hydrolytic enzymes occur during Plant PCD (Hiraiwa et al. 1999). These enzymes attack various organelles and nuclear DNA, leading to cell death.
The present study demonstrates that A. spinosus aqueous extract is a powerful stimulus for erythrocyte apoptosis. Even though erythrocytes lack nuclei and mitochondria, they showed some of the morphological features of apoptosis, such as membrane blebbing and cell shrinkage. In the present study a significant modification of RBCs membrane via blebbing was observed. The defects in cytoskeleton influence membrane blebbing because it is important in cell shape maintenance. The cytoskeleton was largely described as a subcellular structure involved in oxidative modifications leading to cell injury (Bellomo et al. 1990; Malorni et al. 1993). Erythrocytes become damaged by oxidation, which consumes endogenous reducing substances (Richards et al. 2000). This damage then leads to shape changes and increased rigidity by alteration in the erythrocyte lipid bilayer (peroxidation) and oxidation of labile groups in the proteins of the cytoskeleton. Methemoglobin, the product of haemoglobin denaturation may cause damage to red cell membranes by oxidation of membrane sulfhydryl groups and peroxidation of lipids (Chiu and Lubin 1989). This kind of suicidal death of erythrocytes is called eryptosis, characterized by cell shrinkage, nuclear condensation, mitochondrial depolarization, membrane blebbing and cell membrane phospholipid scrambling with phosphatidylserine exposure at the cell surface. Phosphatidylserine-exposing erythrocytes are recognised by macrophages, which engulf and degrade the affected cells. Reported triggers of eryptosis include osmotic shock, oxidative stress, toxic chemicals such as aluminium, mercury, lead etc., phytocomponents such as curcumin, valinomycin etc., diseases such as malaria, sickle-cell anemia, β-thalassemia, iron deficiency etc. Eryptosis allows defective erythrocytes to escape hemolysis. On the other hand, excessive eryptosis favours the development of anemia (Foller et al. 2008).
Blebs are balloon-like, quasi-spherical protrusions of the plasma membrane that are found in association with cell injury. In damaged cells, the presence of blebs correlates with impending cell death. The molecular mechanisms behind apoptotic blebbing are not yet clear, although several recent studies showed involvement of the actomyosin cytoskeleton and its regulators. Membrane blebs appear when actin attachments between plasma membrane and the underlying cytoskeleton are weakened (Cunningham 1995). For apoptotic blebs, several mechanisms have been proposed, which include cleavage and phosphorylation of cytoskeletal proteins (Mills et al. 1999). The echinocyte formation due to plasma membrane disruption induced by malathion in fish erythrocytes was observed by Sawhney and Johal (2000). The erythrocyte membrane seems to be most affected depicting increased porosity and fluidity. According to them the plasma membrane disruption occurred due to disturbed lipid microenvironment and increased lipid peroxidation after the exposure to malathion. Bonarska-Kujawa et al. (2014) reported echinocyte formation in RBCs after exposure to 0.1 and 0.01 mg/ml concentration of Ribes nigrum extracts.
In the TTC assay, a significant decrease in mitochondrial activity was observed. Here the roots exposed to the plant extract were unable to reduce triphenyltetrazolium chloride (TTC) into insoluble red colored triphenyl formazan (TF) indicating the decreased activity of the mitochondrial respiratory chain (Vargas et al. 2006). This result points out the possible cytotoxicity of the tested plant extract on the metabolism and cell viability of the experimental organism. The cleavage of triphenyltetrazolium chloride into red colored triphenyl formazan derivative by living cells is the principle of the TTC assay. The reductions of TTC only take place in metabolically active cells, the level of activity is a measure of the viability of the cells. The resulted metabolic inactivation might be due to the active phytochemicals present in A. spinosus extract.
Plant derived natural products such as flavonoids, terpenes, alkaloids etc. have received wide attention in recent years due to their diverse pharmacological activities including cytotoxic and cancer chemopreventive effects (Babu et al. 2002). Flavonoids, such as curcumin, genistein, quercetin, luteolin, kaempferol etc., are known to have cell line specific antiproliferative and apoptosis inducing activity (Ren et al. 2003). A. spinosus contains many antioxidant components such as phenols, flavanoids, tannins, betalains, terpenoids, carotenoids etc. According to Agati et al. (2012) flavonoids can interfere in the mitotic process and therefore induce apoptosis depending on their nature and concentration. The ethanol extract of A. spinosus was reported to cause significant reduction in packed cell volume, RBC and Hb (Olufemi et al. 2003). Saponins are subgroups of glycosides and are known to cause haemolysis of red blood cells (Lawrence et al. 1997). A. spinosus contains significant levels of saponins, which are potential antinutrients. Saponins are toxic compounds that protect the crop against attack by birds, pests etc. The membranolytic activity of saponins has been appreciated for some time and has been exploited in the assay of such compounds by hemolysis of red blood cells. More recently, it has been demonstrated that saponins can damage intestinal mucosal cells by altering cell membrane permeability and interfering with active transport (Gee et al. 1993). Saponin from Platycodon grandiflorum induced apoptosis in HT-29 colon cancer cells (Kim et al. 2008). The apoptosis inducing activity of plant derived polyphenols in cancer cells due to their antioxidant property was observed by Hadi et al. (2003). Natural products discovered from medicinal plants have played a vital role in the management of cancer. Plant based medication has definitely found a role in cancer healing (chemotherapy), and the mechanism of interaction between many phytochemicals and cancer cells has been studied extensively. In particular, there is growing interest in the pharmacological estimation of various plants used in Indian traditional system of medicine. Cytotoxic specificity of plant extracts is expected to be due to the occurrence of different classes of compounds, especially toxic compounds such as saponins, tannins etc. The antitumor potential of A. spinosus against EAC bearing Swiss albino mice was reported previously (Joshua et al. 2010). Decrease in tumor volume and viable cell count, while increase in mean survival time and non-viable tumor cell count, when compared to the mice of the EAC control group was observed in the result. Chemoprevention, which consists of the use of synthetic or natural agents (alone or in combination) to block the development of cancer in human beings, is an extremely promising strategy for cancer prevention. The control of cell proliferation is fundamental in maintaining cellular homeostasis and loss of this mechanism is a principle hallmark of cancer cells. Thus the inhibition of tumor cell growth without side effects is recognized as an important target for cancer therapy (Koppikar et al. 2010).
In this study, A. spinosus caused apoptosis inducing toxicity in Allium and human erythrocytes. Apoptosis is a well-known biological response expressed by cells after suffering DNA damage and is a useful marker for screening compounds for subsequent development as possible anticancer agent (Arulvasu et al. 2010). Most of the cytotoxic antitumor drugs in recent use have been shown to induce apoptosis in susceptible cells. It has been established that apoptotic cells exhibited DNA fragmentation at inter-nucleosomal sites followed by morphological changes and loss of membrane integrity (Fan et al. 2005). An earlier study confirmed that, the cytotoxic potential of Hymenodictyon excelsum is closely associated with chromatin condensation, one of the markers for apoptosis (Khairunnisa and Karthik 2014). The loss of chromatin integrity is often induced by activated caspases. Several apoptosis inducing nuclear changes were observed viz., nuclear vacuolation, fragmentation and disintegration. It could be established that nuclear changes as a part of apoptosis are followed by loss of membrane integrity thereby making the membrane Evans blue permeable. This induction of apoptosis in cancer cells make them more susceptible for host phagocytic clearance without initiating inflammation which can be attributed for the tumoricidal activity of the extract. The apoptosis inducing potential of A. spinosus can be exploited as an antiproliferative agent for cancer chemoprevention. Lectin, a carbohydrate binding protein from the seeds of Amaranthus viridis showed significant antiproliferative activity towards HB98 and P388D1 murine cancer cell lines (Kaur et al. 2006).The presence of lectin was also reported in A. spinosus. Treatment with methanolic leaf extract of A. spinosus showed significant inhibition in tumor development and decrease in viable cell count in cancers of breast, colorectal, liver and normal cell lines (Vero), indicating its antitumor activity (Rajasekaran et al. 2014). The antiproliferative activity of A. spinosus extract towards normal cell line (Vero) indicated its severe cytotoxic potential. It may require specifically targeted treatment of this extract towards cancer cells.
Cancer is a neoplastic disorder caused by excessive cellular proliferation. Apoptosis is a key regulator of tissue homeostasis and imbalances between cell death and proliferation may result in tumor formation (Fulda and Debatin 2003). The objective of using anticancer agents is to induce apoptosis related signaling, disrupt cell proliferation in cancer cells. In HT-29 and HepG2 cells the ethyl ether extract of Amaranthus viridis showed cell growth inhibition by apoptosis (Jin et al. 2013).
Conclusion
Studies and interests in cancer chemoprevention by the biological activity and pharmaceutical value of naturally occurring substances, derived from food and medicinal plants, have increased recently. The discovery of natural products with specific action on tumor cells would be helpful in cancer chemoprevention or chemotherapy (Kametani et al. 2007). A. spinosus containing some toxic phytoconstituents, was reported to cause apoptosis, thereby revealing its potential cytotoxicity. The present study confirms this fact. This cytotoxicity of A. spinosus extract can be exploited in cancer treatment to lead cancer cells for apoptosis, since medicinal plants frequently serve as a source of new drugs with little or no side effects. Findings of the present study suggesting the possible therapeutic use of A. spinosus, require additional investigation with isolated compounds in vitro systems for understanding the molecular mechanism/s.
Acknowledgements
First author kindly acknowledges Kerala State Council for Science, Technology and Environment for providing financial assistance through KSCSTE fellowship.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Achary VMM, Jena S, Panda KK, Panda BB. Aluminium induced oxidative stress and DNA damage in root cells of Allium cepa L. Ecotoxico Environ Safe. 2008;70:300–310. doi: 10.1016/j.ecoenv.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Agati G, Azzarello E, Pollastri S, Tattini M. Flavonoids as antioxidants in plants: location and functional significance. Plant Sci. 2012;196:67–76. doi: 10.1016/j.plantsci.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Arias M, Quijano JC, Haridas V, Gutterman JU, Lemeshko VV. Red blood cell permeabilization by hypotonic treatments, saponin and anticancer avicins. BBA Mol Basis Dis. 2010;1798:1189–1196. doi: 10.1016/j.bbamem.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Arulvasu C, Prabhu D, Manikandan R, Srinivasan P, Dinesh D, Babu G, Sellamuthu S. Induction of apoptosis by the aqueous and ethanolic leaf extract of Vitex negundo L. in MCF-7 human breast cancer cells. Int J Drug Discov. 2010;2:1–7. [Google Scholar]
- Assiak IE, Olufemi BE, Ayonde GO, Onigemo MA. Preliminary studies on the effects of Amaranthus spinosus leaf extract as an anthelmintic in growing pigs. Trop Vet. 2002;20:126–129. doi: 10.4314/tv.v20i2.4521. [DOI] [Google Scholar]
- Azhar-ul-Haq M, Afza N, Khan SB, Muhammad P. Coumaroyl adenosine and lignan glycoside from Amaranthus spinosus Linn. Pol J Chem. 2006;80:259–263. [Google Scholar]
- Babu BH, Shylesh BS, Padikkala J. Tumour reducing and anticarcinogenic activity of Acanthus ilicifolius in mice. J Ethno Pharmacol. 2002;79:27–33. doi: 10.1016/S0378-8741(01)00347-6. [DOI] [PubMed] [Google Scholar]
- Bellomo G, Mirabelli F, Vairetti M, Iosi F, Malorni W. Cytoskeleton as a target in menadione-induced oxidative stress in cultured mammalian cells. I. Biochemical and immunocytochemical features. J Cell Physiol. 1990;143:118–128. doi: 10.1002/jcp.1041430116. [DOI] [PubMed] [Google Scholar]
- Berg CP, Engels IH, Rothbart A, Lauber K, Renz A, Schlosser SF, Schulze-Osthoff K, Wesselborg S. Human mature red blood cells express caspase-3 and caspase 8, but are devoid of mitochondrial regulators of apoptosis. Cell Death Differ. 2001;8:1197–1206. doi: 10.1038/sj.cdd.4400905. [DOI] [PubMed] [Google Scholar]
- Blunden G, Yang M, Janicsak MI, Carabot-Cuervo A. Betaine distribution in the Amaranthaceae. Biochem Syst Ecol. 1999;27:87–92. doi: 10.1016/S0305-1978(98)00072-6. [DOI] [Google Scholar]
- Bonarska-Kujawa D, Cyboran S, Zylka R, Oszmianski J, Kleszczynska H. Biological activity of blackcurrant extracts (Ribes nigrum L.) in relation to erythrocyte membranes. BioMed Res Int. 2014;2014:1–13. doi: 10.1155/2014/783059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu D, Lubin B. Oxidative haemoglobin denaturation and RBC destruction: the effect of haem on red cell membranes. Semin Hematol. 1989;26:128–135. [PubMed] [Google Scholar]
- Cristine DO, Melinda L, Ade Z, Ajeng D, Anas S, Rizky A. Anticancer properties of daily-consumed vegetables Amaranthus spinosus, Ipomoea aquatica, Apium graveolens and Manihot utilisima to LNCaP prostate cancer cell lines. J Nat Pharm. 2013;4:67–70. doi: 10.4103/2229-5119.110366. [DOI] [Google Scholar]
- Cunningham CC. Actin polymerization and intracellular solvent flow in cell surface blebbing. J Cell Biol. 1995;129:1589–1599. doi: 10.1083/jcb.129.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugas E, Cande C, Kroemer G. Erythrocytes: death of a mummy. Cell Death Differ. 2001;8:1131–1133. doi: 10.1038/sj.cdd.4400953. [DOI] [PubMed] [Google Scholar]
- Fan TJ, Han LH, Cong RH, Liang J. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin. 2005;37:719–727. doi: 10.1111/j.1745-7270.2005.00108.x. [DOI] [PubMed] [Google Scholar]
- Foller M, Huber SM, Lang F. Erythrocyte programmed cell death, critical review. IUBMB Life. 2008;60:661–668. doi: 10.1002/iub.106. [DOI] [PubMed] [Google Scholar]
- Fulda S, Debatin KM. Apoptosis pathways in neuroblastoma therapy. Cancer Lett. 2003;197:131–135. doi: 10.1016/S0304-3835(03)00091-0. [DOI] [PubMed] [Google Scholar]
- Gee JM, Price KR, Ridout CL, Wortley GM, Hurrel RF, Johnson IT. Saponins of quinoa (Chenopodium quinoa): effects of processing on their abudance in quinoa products and their biological effects on intestinal mucosal tissue. Sci Food Agric. 1993;63:201–209. doi: 10.1002/jsfa.2740630206. [DOI] [Google Scholar]
- Girija K, Lakshman K. Anti-hyperlipidemic activity of methanol extracts of three plants of Amaranthus in triton-WR 1339 induced hyperlipidemic rats. Asian Pac J Trop Biomed. 2011;1:62–65. doi: 10.1016/S2221-1691(11)60125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Gulbins E, Jekle A, Ferlinz K, Grassme H, Lang F. Physiology of apoptosis. Am J Physiol Renal Physiol. 2000;279:F605–F615. doi: 10.1152/ajprenal.2000.279.4.F605. [DOI] [PubMed] [Google Scholar]
- Hadi SM, Asad SF, Singh S, Ahmad A, Khan NU. A putative mechanism for anticancer and apoptosis inducing properties of plant derived polyphenolic antioxidants. In: Manjumdar DK, Govil JN, Singh VK, editors. Recent progress in medicinal plants. Studium press, USA: hytochemistry and pharmacology II; 2003. [Google Scholar]
- Harsha VS. In vitro antibacterial activity of Amaranthus spinosus root extracts. Pharmacophore. 2011;2:266–270. [Google Scholar]
- Hassanien SE, Ramadan AM, Azeiz AZA, Mohammeda RA, Hassan SM, Shokry AM, Atef A, Kamal KBH, Rabah S, Sabir JSM, Abuzinadah OA, El-Domyati FM, Martin GB, Bahieldin A. Thymoquinone causes multiple effects, including cell death on dividing plant cells. C R Biol. 2013;336:546–556. doi: 10.1016/j.crvi.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Hilou A, Nacoulma OG, Guiguemde TR. In vivo antimalarial activities of extracts from Amaranthus spinosus L. and Boerhaavia erecta L. in mice. J Ethnopharmacol. 2006;103:236–240. doi: 10.1016/j.jep.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hiraiwa N, Nishimura M, Hara-Nishimura I. Vacuolar processing enzyme is self-catalytically activated by sequential removal of the C-terminal and N-terminal propeptides. FEBS Lett. 1999;447:213–216. doi: 10.1016/S0014-5793(99)00286-0. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Kim JY, Cha MR, Park HR. Effect of methanolic extract from silkworm droppings on proliferation and caspase activity in HT-29 human colon cancer cells. J Med Food. 2007;10:467–472. doi: 10.1089/jmf.2006.165. [DOI] [PubMed] [Google Scholar]
- Ibewuike JC, Ogundaini AO, Bohlin L, Ogungbamila FO. Antiin-flammatory activity of selected Nigerian medicinal plants. Niger J Nat Prod Med. 1997;1:10–14. [Google Scholar]
- Jin Y, Xuan Y, Chen M, Chen J, Jin Y, Piao Tao J. Antioxidant, antiinflammatory and anticancer activities of Amaranthus viridis L. extracts. Asian J Chem. 2013;16:8901–8904. [Google Scholar]
- Joshua LS, Pal VC, Kumar KLS, Sahu RK, Roy A. Antitumor activity of the ethanol extract of Amaranthus spinosus leaves against EAC bearing swiss albino mice. Der Pharm Lett. 2010;2:10–15. [Google Scholar]
- Junqueira LC, Carneiro J. Histologia basica. Rio de Janeiro: Guanabara Koogan; 2004. [Google Scholar]
- Kametani S, Oikawa T, KojimaYA Kennedy DR, Norikura T, Honzawa M, Matsui YI. Mechanism of growth inhibitory effect of cape Aloe extract in Ehrlich Ascites tumor cells. J Nutr Sci Vitaminol. 2007;53:540–546. doi: 10.3177/jnsv.53.540. [DOI] [PubMed] [Google Scholar]
- Kapadia G, Balasubramanian V, Tokuda H, Iwashima A, Nishino H. Inhibition of 12-O-tetradecanoylphorbol-13-acetate induced Epstein- Barr virus early antigen activation by natural colorant. Cancer Lett. 1995;115:173–178. doi: 10.1016/S0304-3835(97)04726-5. [DOI] [PubMed] [Google Scholar]
- Kapadia G, Tokuda H, Harukuni K, Takao M, Nishino H. Chemoprevention of lung and skin cancer by Beta vulgaris (beet) root extract. Cancer Lett. 1996;100:211–214. doi: 10.1016/0304-3835(95)04087-0. [DOI] [PubMed] [Google Scholar]
- Kaur N, Dhuna V, Kamboj SS, Agrewala JN, Singh J. A novel antiproliferative and antifungal lectin from Amaranthus viridis Linn seeds. Protein Pept Lett. 2006;13:897–905. doi: 10.2174/092986606778256153. [DOI] [PubMed] [Google Scholar]
- Khairunnisa K, Karthik D. Evaluation of in vitro apoptosis induction, cytotoxic activity of Hymenodictyon excelsum (Roxb) Wall in Dalton’s lymphoma ascites (DLA) and Lung fibroblast—Mouse L929 cell lines. JAPS. 2014;4:11–17. [Google Scholar]
- Kim JY, Park KW, Moon KD, Lee MK, Choi J, Yee ST, Shim KH, Seo KI. Induction of apoptosis in HT-29 colon cancer cells by crude saponin from Platycodi radix. Food Chem Toxicol. 2008;46:3753–3758. doi: 10.1016/j.fct.2008.09.067. [DOI] [PubMed] [Google Scholar]
- Kirtikar KR, Basu BD. Indian medicinal plants. 2. Dehradun, Uttaranchal: Oriental Enterprises; 2001. [Google Scholar]
- Koppikar SJ, Choudhari AS, Suryavanshi SA, Kumari S, Chattopadhyay S, Kaul-ghanekar R. Aqueous cinnamon extract (ACE-c) from the bark of Cinnamomum cassia causes apoptosis in human cervical cancer cell lines (SiHa) through loss of mitochondrial membrane potential. BMC Cancer. 2010;10:210. doi: 10.1186/1471-2407-10-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthi SKM, Venugopalan R, Suresh KS, Perumal P. Evaluation of analgesic activity of Amaranthus spinosus Linn. leaves in mice. J Pharm Res. 2010;3:3088–3089. [Google Scholar]
- Kumar BSA, Lakshman K, Jayaveera KN, Shekar DS, Nandeesh R, Velmurugan C. Chemoprotective and antioxidant activities of methanolic extract of Amaranthus spinosus leaves on paracetamol induced-liver damage in rats. Acta Med Sal. 2010;39:68–74. [Google Scholar]
- Lawrence DR, Bennett PN, Brown MS. Chemical pharmacology. 8. Singapore: Longman Publishers Ltd; 1997. pp. 465–466. [Google Scholar]
- Maiworm AI, Presta GA, Santos-Filho SD, Paoli SD, Giani TS, Fonseca AS, Bernardo-Filho M. Osmotic and morphological effects on red blood cell membrane: action of an aqueous extract of Lantana camera. Braz J Pharmacog. 2008;18:42–46. [Google Scholar]
- Malorni W, Iosi F, Donelli G, Caprari P, Salvati AM, Cianciulli P. A new, striking morphologic feature for the human erythrocyte in hereditary spherocytosis: the blebbing pattern. Blood. 1993;81:2821–2822. [PubMed] [Google Scholar]
- Meyskens FL, Szabo E. Diet and cancer: the disconnect between epidemiology and randomized clinical trials. Cancer Epidemiol Biomarkers Prev. 2005;14:1366–1369. doi: 10.1158/1055-9965.EPI-04-0666. [DOI] [PubMed] [Google Scholar]
- Mills JC, Stone NL, Pittman RN. Extranuclear apoptosis. The role of the cytoplasm in the execution phase. J Cell Biol. 1999;146:703–708. doi: 10.1083/jcb.146.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odhavo B, Beekrum S, Akula US, Baijnath H. Preliminary assess-ment of nutritional value of traditional leafy vegetables in Kwazulu-Natal. South Africa. J Food Compos Anal. 2007;20:430–435. doi: 10.1016/j.jfca.2006.04.015. [DOI] [Google Scholar]
- Olufemi BE, Assiak IE, Ayoade GO, Onigemo MA. Studies on the effects of Amaranthus spinosus leaf extract on the hematology of growing pigs. Afr J Biomed Res. 2003;6:149–150. [Google Scholar]
- Patkai G, Barta J, Varsanyi I. Decomposition of anticarcinogen factors of the beet root during juice and nectar production. Cancer Lett. 1997;114:105–106. doi: 10.1016/S0304-3835(97)04636-3. [DOI] [PubMed] [Google Scholar]
- Prajitha V, Thoppil JE. Genotoxic and antigenotoxic potential of the aqueous leaf extracts of Amaranthus spinosus Linn. using Allium cepa assay. S Afr J Bot. 2016;102:18–25. doi: 10.1016/j.sajb.2015.06.018. [DOI] [Google Scholar]
- Rajasekaran S, Dinesh MG, Kansrajh C, Baig FHA. Amaranthus spinosus leaf extracts and its anti-inflamatory effects on cancer. IJRPB. 2014;2:1058–1064. [Google Scholar]
- Rastogi RP, Mehrotra BN. Compendium of Indian medicinal plants. 1. New Delhi: Central Drug Research Institute Lucknow & NISCAIR; 1999. [Google Scholar]
- Ren W, Qiao Z, Wang H, Zhu L, Zhang L. Flavonoids: promising anticancer agents. Med Res Rev. 2003;23:519–534. doi: 10.1002/med.10033. [DOI] [PubMed] [Google Scholar]
- Richards RS, Roberts TK, Mathers D, Dunstan RH, McGregor NR, Butt HL. Investigation of erythrocyte oxidative damage in rheumatoid arthritis and chronic fatigue syndrome. J Chronic Fatigue Syndr. 2000;6:37–46. doi: 10.1300/J092v06n01_04. [DOI] [Google Scholar]
- Sangameswaran B, Jayakar B. Anti-diabetic, anti-hyperlipidemic and spermatogenic effects of Amaranthus spinosus Linn. on streptozotocin-induced diabetic rats. J Nat Med. 2008;62:79–82. doi: 10.1007/s11418-007-0189-9. [DOI] [PubMed] [Google Scholar]
- Sawhney AK, Johal MS. Erythrocyte alterations induced by Malathion in Channa punctatus (Bloch) Bull Environ Contam Toxicol. 2000;64:398–405. doi: 10.1007/s001280000014. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Sharma A. Chromosome techniques—theory and practice. 3. London: Butter Worths; 1980. [Google Scholar]
- Sharma M, Sharma PD, Bansal MP, Singh J. Lantadene A-induced apoptosis in human leukemia HL-60 cells. Indian J Pharmacol. 2007;39:140–144. doi: 10.4103/0253-7613.33433. [DOI] [Google Scholar]
- Shaymurat T, Gu J, Xu C, Yang Z, Zhao Q, Liu Y, Liu Y. Phytotoxic and genotoxic effects of ZnO nanoparticles on garlic (Allium sativum L.): a morphological study. Nanotoxicology. 2012;6:241–248. doi: 10.3109/17435390.2011.570462. [DOI] [PubMed] [Google Scholar]
- Srinivasan K, Kaul CL, Ramarao P. Partial protective effect of rutin on multiple low dose streptozotocin-induced diabetes in mice. Indian J Pharmacol. 2005;37:327–328. doi: 10.4103/0253-7613.16859. [DOI] [Google Scholar]
- Stintzing FC, Carle R. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci Technol. 2004;15:19–38. doi: 10.1016/j.tifs.2003.07.004. [DOI] [Google Scholar]
- Tatiya AU, Surana SJ, Khope SD, Gokhale SB, Sutar MP. Phytochemical investigation and immunomodulatory activity of Amaranthus spinosus Linn. IJPER. 2007;44:337–341. [Google Scholar]
- Vargas L, Victor M, F F. Plant cell culture protocols. Totowa: Humana Press; 2006. [Google Scholar]
- Zeashan H, Amresh G, Singh S, Rao CV. Hepatoprotective activity of Amaranthus spinosus in experimental animals. Food Chem Toxicol. 2008;46:3417–3421. doi: 10.1016/j.fct.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Zeashan H, Amresh G, Singh S, Rao CV. Hepatoprotective and antioxidant activity of Amaranthus spinosus against CCl4 induced toxicity. J Ethnopharmacol. 2009;125:364–366. doi: 10.1016/j.jep.2009.05.010. [DOI] [PubMed] [Google Scholar]