Abstract
Progenitors in the telencephalic subventricular zone (SVZ) remain mitotically active throughout life, and produce different cell types at embryonic, postnatal and adult stages. Here we show that Mash1, an important proneural gene in the embryonic telencephalon, is broadly expressed in the postnatal SVZ, in progenitors for both neuronal and oligodendrocyte lineages. Moreover, Mash1 is required at birth for the generation of a large fraction of neuronal and oligodendrocyte precursors from the olfactory bulb. Clonal analysis in culture and transplantation experiments in postnatal brain demonstrate that this phenotype reflects a cell-autonomous function of Mash1 in specification of these two lineages. The conservation of Mash1 function in the postnatal SVZ suggests that the same transcription mechanisms operate throughout life to specify cell fates in this structure, and that the profound changes in the cell types produced reflect changes in the signalling environment of the SVZ.
Keywords: adult neurogenesis, brain, oligodendrogenesis, transcription factor, transplantation
Introduction
Progenitor cells in the telencephalon, which generate the vast array of neurons and glia found in the adult cerebral cortex, basal ganglia and olfactory bulb, have unique characteristics. While in most regions of the central nervous system (CNS), neurogenesis and gliogenesis take place during embryonic development and cease before or soon after birth, new neurons, oligodendrocytes and astrocytes are generated in the telencephalon well into postnatal life. Indeed, stem cells in the telencephalon continuously proliferate and differentiate from embryonic stages to adulthood, although their cellular output changes dramatically over time. At embryonic stages, multipotent progenitors located in the telencephalic ventricular and subventricular zones produce a multitude of neuronal subtypes and a first wave of oligodendrocyte and astrocyte precursors. In particular, progenitors in the ventral telencephalon have been shown to produce both GABAergic interneurons and oligodendrocytes (He et al, 2001; Yung et al, 2002), while some progenitors in the dorsal telencephalon generate both cortical neurons and astrocytes (Williams and Price, 1995). Embryonic ventricular zone progenitors then give rise, soon before birth, to a distinct postnatal subventricular zone (SVZ) with unique features (Marshall et al, 2003; Pencea and Luskin, 2003; Tramontin et al, 2003).
A distinct characteristic of the postnatal SVZ is that it produces a second wave of astrocytic and oligodendrocytic precursors, destined to colonise the corpus callosum and cerebral cortex. Production of astrocytes and oligodendrocytes from SVZ precursors peaks during the first 2 weeks of postnatal life. Another important characteristic of the SVZ is that it contains specialised neuronal precursors, which migrate through the rostral migratory stream (RMS) into the olfactory bulb, where they differentiate into olfactory interneurons (Luskin, 1993). Olfactory interneurons are continuously produced by the SVZ from late embryogenesis onwards, and the adult SVZ is dedicated to the production of this cell type (Doetsch et al, 1999; Alvarez-Buylla and Garcia-Verdugo, 2002; Pencea and Luskin, 2003). Important differences therefore exist between progenitor cells in the embryonic and postnatal telencephalon, in that the former are largely multipotent and produce many types of neurons, while the latter are mostly lineage-restricted and produce essentially one neuronal subtype, olfactory interneurons.
Mechanisms controlling cell fate specification have been extensively investigated in the embryonic CNS, where basic helix–loop–helix (bHLH) transcription factors have a central role (Bertrand et al, 2002). In the embryonic ventral telencephalon, the proneural protein Mash1 is essential for the production of neuronal precursor cells (Casarosa et al, 1999; Horton et al, 1999). In the dorsal telencephalon, together with other proneural proteins of the Neurogenin family, Mash1 promotes the neuronal commitment of multipotent progenitors, while inhibiting their astrocytic differentiation (Nieto et al, 2001). In addition, Mash1 expression in embryonic telencephalic progenitors can activate a GABAergic subcortical differentiation programme that involves induction of the Dlx homeodomain (HD) protein family (Fode et al, 2000). Although GABAergic neurons and oliodendrocytes appear to originate from a common lineage in the telencephalon (He et al, 2001; Yung et al, 2002), a different family of bHLH proteins, Olig1 and Olig2, has been implicated in specification of telencephalic oligodendrocyte precursors (Lu et al, 2002).
Whether specification of neuronal and glial progenitors in the adult brain relies on the same transcription mechanisms as in the embryo is unknown. The limited differentiation potential of postnatal progenitors could reflect differences in the regulation of proneural factors operating in both embryonic and adult brain, or alternatively the recruitment of a different set of cell fate determinants to control neurogenesis and gliogenesis at postnatal stages (Kintner, 2002). In this paper, we have addressed the role of Mash1 in the postnatal SVZ, and we show that this gene is required at this stage for the specification of both neurons and oligodendrocytes. Thus, a common mechanism underlies the generation of two of the three cell types produced in the postnatal brain. Our results also indicate that, despite their limited differentiation potential, fate specification of progenitors in the postnatal brain relies on the same intrinsic mechanisms as in the embryo.
Results
Mash1 expression in the neonatal brain
We began investigating the mechanisms underlying cell fate specification in the postnatal SVZ by asking whether the proneural gene Mash1, which is essential for neurogenesis in the embryonic ventral telencephalon (Casarosa et al, 1999; Horton et al, 1999), remains expressed in the SVZ after birth.
In sections of brains harvested at birth (P0), numerous cells expressing Mash1 RNA and protein were found in the SVZ, as well as in the RMS, that extends from the lateral ventricles to the olfactory bulb (Figure 1A and data not shown). The neonatal SVZ contains numerous neuronal and glial precursors, but in double-labelling experiments, only a fraction of Mash1-positive cells expressed either the neuronal precursor marker, βIII-tubulin (14%; Figure 1B), or the oligodendrocyte precursor cell (OPC) markers NG2 (4%; Figure 1C), PDGFRα (4%; data not shown) and Olig2 (40%; Figure 1D). Most Mash1+ cells were not labelled with lineage-specific markers, possibly because they were too immature. To examine the fate of Mash1+ progenitors, we used a mouse transgenic line in which the LacZ reporter gene is under the control of Mash1 regulatory sequences (Mash1∷LacZ mice; Verma-Kurvari et al, 1996). At P0, the LacZ transgene was expressed, like Mash1, in the SVZ and the RMS, but also in the bulb where Mash1 RNA and protein is not detected, indicating that the βgal protein is stable enough to trace the fate of Mash1+ progenitors (Figure 1E). Double-labelling experiments in Mash1∷LacZ mice showed that the vast majority of βIII-tubulin+ neuronal precursors in the SVZ and RMS are βgal+ (Figure 1F), while a smaller number βgal+ cells express the OPC marker O4 (Figure 1G). Mash1∷LacZ mice were also analysed at P7, when the white matter is more differentiated than at birth. A large fraction of NG2+ OPCs expressed βgal in the corpus callosum (77%; Figure 1H), fimbria (75%), anterior commissure (67%) and olfactory bulb (45%). Thus, Mash1 is expressed in both olfactory interneuron precursors and oligodendrocyte precursors in the early postnatal SVZ.
Figure 1.
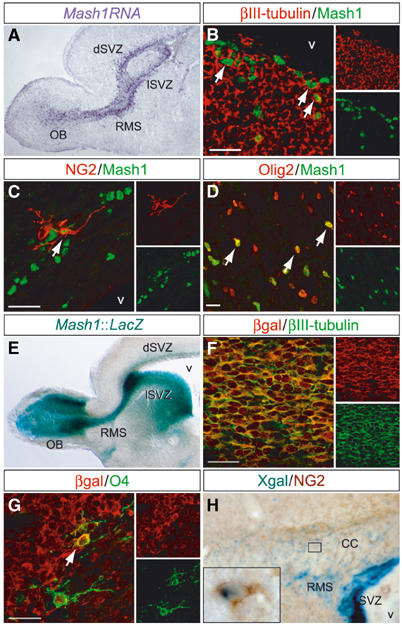
Mash1-expressing cells in the neonatal SVZ belong to two cell lineages. (A) Mash1 transcripts (purple) in a sagittal section through the rostral telencephalon of a P0 mouse are found in the dorsal and lateral parts of the SVZ (dSVZ and lSVZ, respectively), along the rostral migratory stream (RMS) and in the centre of the olfactory bulb. (B–D) Double antibody labelling of sagittal sections of a P0 brain showing Mash1+ cells (green) coexpressing the neuronal marker βIII-tubulin (B, red) and the oligodendrocyte precursor markers NG2 (C, red) and Olig2 (D, red). Double-labelled cells are marked by arrows. (E) X-gal staining of a sagittal brain section from a P3 Mash1∷LacZ transgenic mouse, showing the distribution of Mash1-βgal+ cells. (F, G) Double labelling of a sagittal brain section from a P0 transgenic mouse for βgal (red) and βIII-tubulin (F, green) or the OPC marker O4 (G, green, arrow). (H) Double labelling of a sagittal brain section from a P7 transgenic mouse for βgal (blue, X-gal precipitate) and NG2 (brown). The inset is an enlargement of the area outlined in the corpus callosum (CC). Scale bars: 20 μm.
Mash1 expression in the adult brain
To better define the stage in the olfactory interneuron lineage when Mash1 is expressed, we turned to the adult brain where the different types of progenitors in the lineage can be identified with specific antibodies (Alvarez-Buylla and Garcia-Verdugo, 2002). Mash1 expression in the adult rostral telencephalon was overall very similar to that observed at birth, with labelled cells located near the lateral ventricles and in the RMS and expressing proliferation markers (Figure 2A; Supplementary Figure 1A). The majority of Mash1+ cells in the SVZ had an antibody labelling profile characteristic of transit amplifying progenitors of the olfactory interneuron lineage, that is, positive for the HD proteins Dlx and negative for the neuroblast markers PSA-NCAM and mCD24 (56% of Mash1+ cells; Figure 2B; Doetsch et al, 2002). A smaller fraction of Mash1+ cells had characteristics of migrating neuroblasts (Dlx+, PSA-NCAM+, mCD24+; 26%; Figure 2B; Supplementary Figure 1B), while very few Mash1+ cells expressed GFAP, which marks SVZ astrocytes and stem cells (Supplementary Figure 1C; Doetsch et al, 1999). In adult Mash1∷LacZ transgenic mice (Supplementary Figure 1E), there was a excellent match between the expression of the LacZ transgene and the neuroblast markers PSA-NCAM and mCD24 in the SVZ and RMS, thus confirming that Mash1 is transiently expressed in all progenitors of the olfactory interneuron lineage (Figure 2C; Supplementary Figure 1F). βgal expression was also detected in oligodendrocyte precursors in the telencephalic white matter and grey matter (data not shown), but we have not yet attempted to define the exact stage of Mash1 expression in this lineage.
Figure 2.
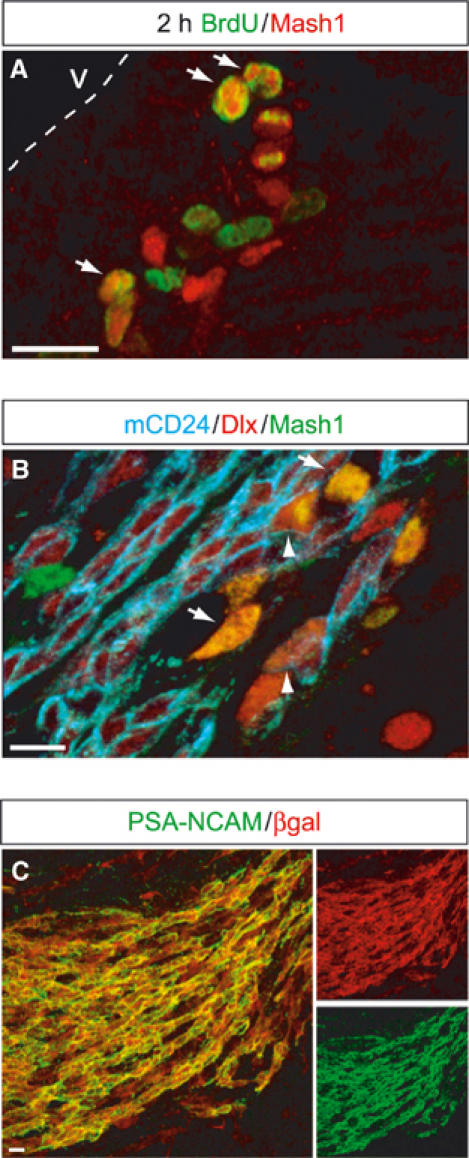
Mash1+ cells in the adult SVZ are progenitors of the olfactory interneuron lineage. (A) Double labelling of a sagittal section through the SVZ of an 8-week-old brain, for Mash1 (red) and BrdU (green) after 2 h of BrdU incorporation. Double-labelled cells are indicated by arrows. (B) Triple labelling for Mash1 (green), the progenitor and neuroblast marker Dlx (red) and the neuroblast marker mCD24 (blue). Mash1+ transit amplifying progenitors (Dlx−, mCD24− and Dlx+, mCD24−) are marked by arrows and Mash1+ neuroblasts (Dlx+, mCD24+) by an arrowhead. (C) Double labelling of a sagittal section through the RMS of an 8-week-old Mash1-LacZ transgenic mouse, for βgal (red) and the neuroblast marker PSA-NCAM (green). Most neuroblasts in the RMS are double labelled. Scale bars: 20 μm.
To confirm the position of Mash1+ cells in the adult neuronal lineage, we examined the time course of Mash1 expression during regeneration of the adult SVZ. Infusion of the antimitotic drug AraC into the adult brain eliminates rapidly dividing cells, including transit amplifying progenitors and neuroblasts, while leaving slow dividing stem cells unaffected (Doetsch et al, 1999). Almost all Mash1+ cells were eliminated by a 6-day-long AraC treatment (1.4% Mash1+ cell remaining 12 h after the end of the treatment, compared with control brains treated with vehicle only) and their number remained low after 24 h (3.2%). The number of Mash1+ cells reached control level 48 h after the end of the treatment (94.3%), while the number of PSA-NCAM+ neuroblasts remained low (1.8%). This time course of Mash1 expression in the regenerating SVZ closely matches that reported for transit amplifying progenitors or C cells (Doetsch et al, 1999).
Altogether, our results demonstrate that the majority of Mash1+ cells in the adult SVZ have characteristics of undifferentiated transit amplifying progenitors of the olfactory neuron lineage, and that Mash1 expression is only transiently maintained in differentiating neuroblasts.
Mash1 mutant phenotype at birth
The above data suggested that Mash1 might have a function in the postnatal SVZ. Since mice carrying a null mutation in Mash1 die soon after birth, they could be used to address the role of Mash1 in the generation of oligodendrocytes and olfactory interneurons by SVZ progenitors, which has already started at this stage (Spassky et al, 2001; Ivanova et al, 2003; Pencea and Luskin, 2003). We first compared the number and distribution of all dividing progenitors, marked by BrdU incorporation, in the telencephalon of Mash1 mutant and control newborns (Figure 3). The number of BrdU+ cells was significantly reduced in the lateral and dorsal SVZ and the RMS of Mash1 mutants (Figure 3D). There was no evidence of increased apoptosis (data not shown) and no accumulation of progenitor cells in other parts of the Mash1 mutant telencephalon (Figure 3D), suggesting that the reduction in the number of BrdU+ cells in the SVZ and RMS is due to a defect in the generation of progenitor cells rather than in their subsequent migration or survival.
Figure 3.
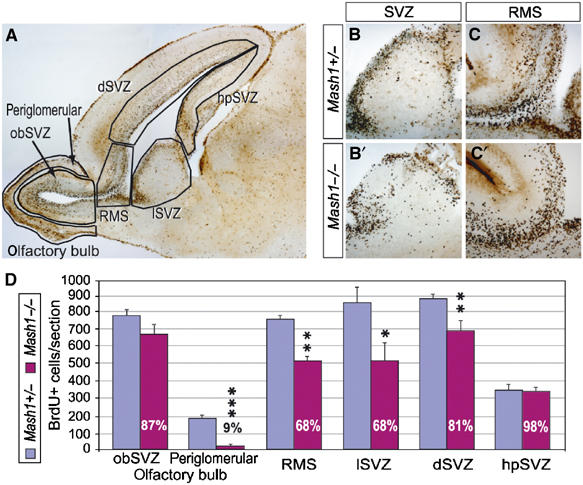
Less dividing progenitors are found in the SVZ and RMS of Mash1 mutants at birth. (A) Sagittal section of P0 brain showing the different zones used to quantify the defects in BrdU incorporation reported in (A). (B, B′, C, C′) Distribution of BrdU+ cells in the SVZ (B, B′) and proximal RMS (C, C′) of P0 control (B, C) and Mash1 mutants (B′, C′), after 30 min of BrdU incorporation. (D) Quantification of BrdU+ cells in different brain regions, as defined in (A), in P0 control (blue bars) and Mash1 mutants (purple bars). White numbers in purple bars are ratios of BrdU+ cell numbers in Mash1 mutants over controls. *P<0.05, **P<0.01, ***P<0.001, Student's t-test.
We then examined which types of cells were affected in Mash1 mutants. The olfactory bulb at birth contains neuronal precursors that have migrated along the RMS (Pencea and Luskin, 2003), and is also an active site of production of oligodendrocytes, generated locally from the rostral extension of the ventricular wall (Spassky et al, 2001). The overall size of the bulb was reduced by one-third in Mash1 mutant newborns (Figure 4C, C′ and F). There was a further reduction in the number of olfactory bulb neurons, marked by βIII-tubulin (Figure 4G), with granule cells more severely affected than periglomerular neurons (Figure 4H). To determine whether this neuronal deficit was due to a defect in neurogenesis at birth, rather than in interneuron production at earlier stages, we measured the fraction of neuronal precursors, marked by coexpression of βIII-tubulin and the mitotic marker Ki67, in acutely dissociated olfactory bulb cells (Figure 4A and A′). The percentage of double-labelled neuronal precursors was reduced by about two-thirds in mutant bulbs compared with controls (Figure 4G), indicating that neonatal neurogenesis is severely compromised in the absence of Mash1 function.
Figure 4.
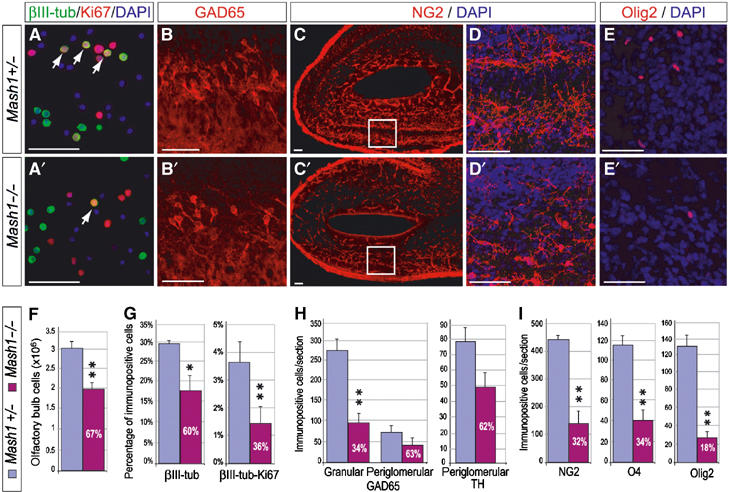
Reduced number of neuronal and oligodendrocyte precursors in the olfactory bulb of Mash1 mutants at birth. (A, A′) Double labelling for βIII-tubulin (green) and Ki67 (red) and counterstaining with DAPI (blue), to identify double-labelled neuronal precursors (arrows) in acutely dissociated olfactory bulb cells from P0 control (A) and Mash1 mutant (A′). (B–E, B′–E′) Sagittal sections of olfactory bulb from P0 control (B–E) and Mash1 mutant (B′–E′), labelled for the GABAergic neuron marker GAD65 (B, B′) and the OPC markers NG2 (C, C′, D, D′) and Olig2 (E, E′). (F, G) Quantification of all cells (F), and of neurons and neuronal precursors (G) in acutely dissociated control (blue bars) and Mash1 mutant (purple bars) bulb. (H, I) Quantification of GAD65+ neurons in the granular and periglomerular layers and of TH+ dopaminergic neurons (H), NG2+, O4+ and Olig2+ OPCs (I) in sagittal sections of control and Mash1 mutant bulb. White numbers in purple bars are ratios of numbers of labelled cells in Mash1 mutants over controls. *P<0.05, **P<0.01, Student's t-test. Scale bars: 20 μm.
As Mash1 is also expressed in the oligodendrocyte lineage at birth (Figure 1), we examined whether OPCs and oligodendrocytes were affected in Mash1 mutant newborns. As is the case for neuronal precursors, the number of NG2+, O4+ and Olig2+ OPCs was severely reduced in mutant bulbs (Figure 4C–E, C′–E′ and I). Oligodendrogenesis appeared to be less affected in other parts of Mash1 mutant brains, and we are currently examining whether particular subsets of oligodendrocyte precursors are missing in different brain regions in the absence of Mash1.
Together, these data indicate that Mash1 has a major role in the neonatal SVZ, where it is required for the generation of both neuronal and oligodendrocyte precursors. However, given the complexity of this structure, this study could not rule out that multiple defects contribute to the mutant phenotype in the postnatal SVZ, such as a defect in migration of precursors from other parts of the brain, or a defect in cell production at embryonic stages that would indirectly affect the postnatal SVZ. To better define the cellular function of Mash1 in the postnatal brain, we turned to a simpler model of differentiation of SVZ progenitors in culture.
Mash1 mutant phenotype in progenitor cultures
Neural progenitors can be propagated as ‘neurospheres' in cultures containing the mitogens EGF and FGF, and then differentiated into neurons, oligodendrocytes and astrocytes by switching cultures to serum-containing medium (Reynolds and Weiss, 1992; Morshead et al, 1994; Gritti et al, 1999). We first confirmed that Mash1 has a similar expression profile in neurosphere cultures established from the lateral ventricular walls of newborn mice (see Materials and methods and Supplementary Figure 2) as in vivo, and therefore that this culture system could be used to address the role of Mash1 in postnatal progenitors.
Mash1 was expressed in a large fraction of neurosphere cells cultivated with mitogens (55–70%; Supplementary Figure 2), and double-labelling experiments showed that these cells did not express neuronal- or glial-specific markers. The fraction of cells expressing Mash1 decreased rapidly as cultures were switched to a differentiation medium, and many of these cells transiently expressed NG2 or βIII-tubulin (Supplementary Figure 2G). These results indicate that Mash1 is expressed in SVZ-derived cultures in a manner very similar to that observed in the postnatal SVZ in vivo, that is, in immature, lineage marker-negative progenitors, and then transiently in differentiating neuronal and oligodendrocyte precursors.
We then established neurosphere cultures from the lateral ventricular walls of Mash1 mutants and control littermates at birth. Mash1 mutant and control neurospheres were propagated for up to four passages with similar efficiency (data not shown), suggesting that Mash1 does not have a significant role in the self-renewal of neural progenitors in culture. After 2–4 passages, neurospheres were dissociated and cultivated in differentiating conditions. Progenitors from control cultures produced large numbers of βIII-tubulin+ neurons, O4+ oligodendrocytes and GFAP+ astrocytes after 7 days in culture (Figure 5A, C and D). In striking contrast, Mash1 mutant cultures contained much fewer neurons and oligodendrocytes, while the number of astrocytes was increased (Figure 5A′, C and D). The number of nestin+ precursors was similar in mutant and control cultures throughout the course of the culture (Figure 5D), indicating that the reduction in the number of neurons and oligodendrocytes in mutant cultures is not due to a delay in precursor differentiation.
Figure 5.
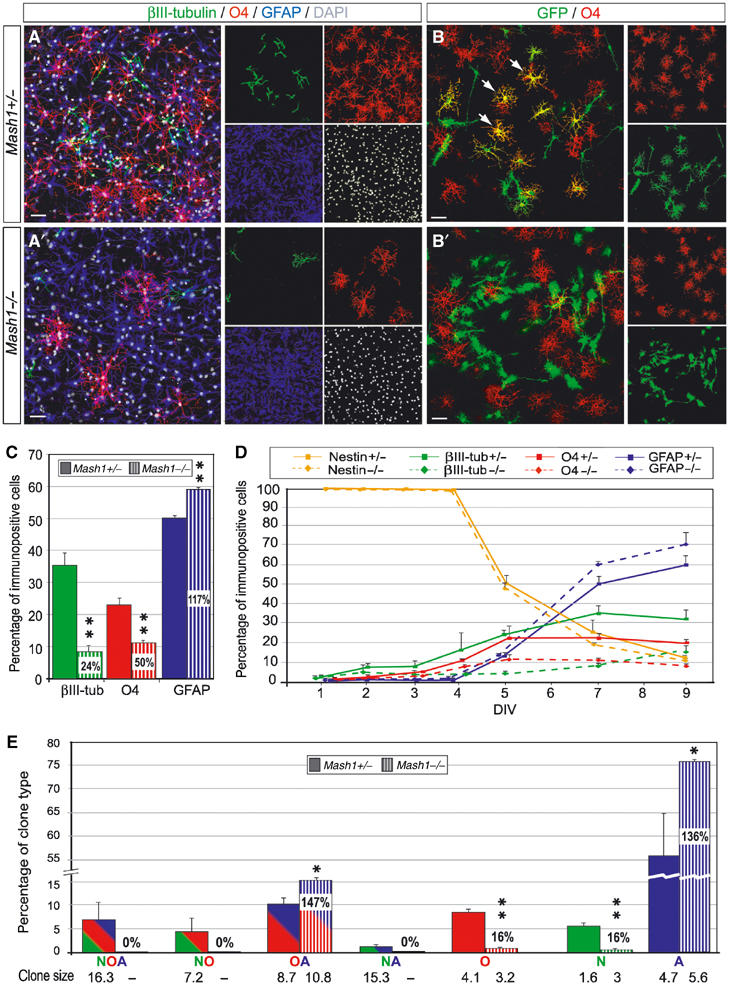
Defect in specification of neurons and oligodendrocytes in Mash1 mutant progenitor cultures. (A, A′) Triple labelling for βIII-tubulin (green), O4 (red) and GFAP (blue) and counterstaining with DAPI (white) in control (A) and mutant (A′) dissociated neurosphere cultures after 7 days. (B, B′) Double labelling for GFP (green) and O4 (red) in clonal cultures of GFP+ control (B) or mutant (B′) progenitors cocultivated with an excess of GFP-negative wild-type progenitors. The arrows mark GFP+ oligodendrocytes in the wild-type clone (B), which are absent in the mutant clone (B′). (C) Quantification of neurons (βIII-tubulin+), oligodendrocytes (O4+) and astrocytes (GFAP+) in control and mutant cultures after 7 days. White numbers in striped bars are ratios of numbers of labelled cells in mutants over control cultures. (D) Time course of the generation of neurons, oligodendrocytes and astrocytes in control and mutant progenitor cultures. (E) Quantification of the different types of clones generated in control and mutant progenitor cultures. Each pair of bars represents the frequency, in control and mutant cultures, of a particular type of clone, whose cell type composition is indicated by a colour code and letter code under the bars (N: neurons in green; O: oligodendrocytes in red; A: astrocytes in blue). The average size of each type of clone is indicated under the corresponding bar. *P<0.05, **P<0.01, Student's t-test. Scale bars: 10 μm.
The reduced production of neurons and oligodendrocytes in mutant cultures could reflect a defect in the specification of neuronal and oligodendrocytic precursors in these cultures, or alternatively the correct specification of precursors with reduced proliferation capacities. Moreover, the reduction in the number of OPCs may reflect an indirect role of Mash1-dependent neurons, rather than a cell autonomous role of Mash1, in the induction of oligodendrocytes. To distinguish between these hypotheses, we performed a clonal analysis of Mash1 mutant progenitors in culture, by cocultivating mutant or control neurosphere cells marked with green fluorescent protein (GFP) with a large excess of GFP-negative, wild-type neurosphere cells (Figure 5B and B′; see Materials and methods). In control experiments, GFP+ cells gave rise, after 7 days in culture, to a variety of clones of different size and cellular composition, including some clones containing neurons, oligodendrocytes and astrocytes and others containing only two of the three cell types (Figure 5E). When GFP+ Mash1 mutant progenitors were cultivated instead, the overall number of clones formed after 7 days was not significantly changed (data not shown), but their composition was dramatically different. There was a drastic reduction in the frequency of all types of clones containing neurons, and of most types of clones containing oligodendrocytes, and a parallel increase in the frequency of astrocytic clones (Figure 5E). Importantly, average clone size was not significantly different in mutant and control cultures after 3 days (wild-type cultures: 9.4±4.2, n=2; mutant cultures: 5.6±2.3, n=2), and clones of similar composition, including neurons- and oligodendrocytes-containing clones, were also of the same size in control and mutant cultures after 7 days (Figure 5E). Moreover, the number of apoptotic cells was not significantly increased in mutant cultures (data not shown). Together, these results ruled out that the loss of neurons and oligodendrocytes in mutant cultures could be due to a defect in precursor proliferation or survival. Rather, these experiments demonstrate that Mash1 is required for the specification of both neuronal and oligodendrocyte precursors, which in the absence of Mash1 function adopt instead an astrocytic fate.
Only a subset of oligodendrocytes was missing in Mash1 mutant clonal cultures, as already observed in nonclonal cultures (Figure 5C and D). Interestingly, oligodendrocytes present in mixed astros–oligos clones were spared by the Mash1 mutation, whereas oligodendrocytes found in other types of wild-type clones (i.e. neurons/astros/oligos clones, neurons/oligos clones and oligos-only clones) were mostly missing in mutant cultures. This suggests the existence of two distinct lineages of oligodendrocytes in SVZ progenitor cultures, only one of which requires Mash1 function.
In vivo transplantation of Mash1 mutant SVZ cells
In the above experiments, the reduction in the number of oligodendrocyte-containing clones generated by Mash1 mutant cells when cocultivated with wild-type cells demonstrated that Mash1 is required cell autonomously for the specification of oligodendrocytes in culture (Figure 5B and B′). To ask whether Mash1 is also required cell autonomously in vivo for the generation of oligodendrocytes, we transplanted Mash1 mutant and control SVZ progenitors into wild-type host brains. We used as donor tissue fragments of SVZ obtained from GFP+ P0 brains, rather than neurospheres, which fail to migrate correctly after transplantation in vivo (Soares and Sotello, 2004; see Materials and methods). To favour their differentiation into oligodendrocytes, SVZ cells were transplanted into the brain of 1- to 2-week-old mice, when gliogenesis reaches its peak. Host brains were harvested 4 weeks after transplantation, and eight of them, in which grafted GFP+ cells had settled in a gliogenic region (i.e. corpus callosum or cerebral cortex; Figure 6), were selected for further analysis. Three of these brains had been grafted with wild-type SVZ cells, which differentiated in the host environment into neurons, astrocytes and oligodendrocytes (Figure 6B). Although the fraction of cells having differentiated in each of the three cell types varied greatly between grafted brains (Figure 6B), possibly reflecting variations in the precise location of the graft and thereby in the composition of the niche of transplanted progenitors, a significant level of oligodendrogenesis was observed in these three control experiments. In contrast, in the five brains grafted with Mash1 mutant cells, a markedly lower fraction of transplanted cells had differentiated into oligodendrocytes (Figure 6B). Thus, Mash1 mutant cells present a defect in oligodendrogenesis that is not corrected by the wild-type brain environment, indicating that Mash1 is required cell autonomously in vivo for the differentiation of SVZ progenitors into oligodendrocytes.
Figure 6.
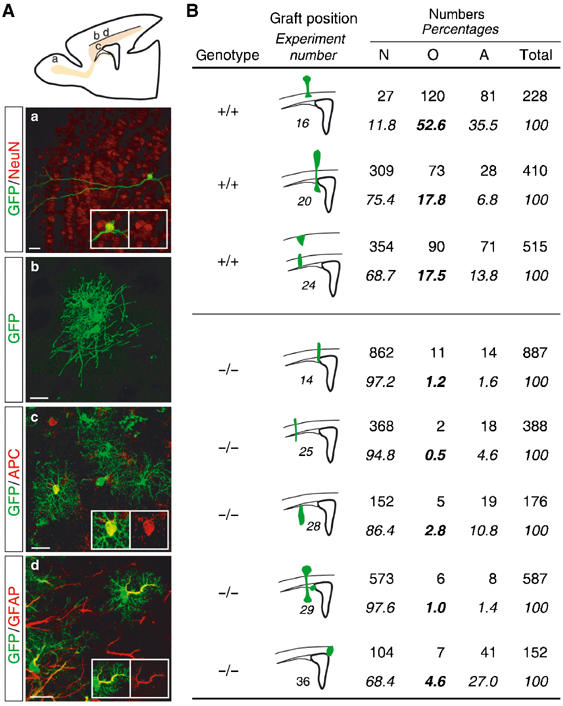
Transplanted Mash1 mutant SVZ cells produce less oligodendrocytes in vivo. (A) Identification of transplanted cells by GFP immunoreactivity (green, a–d) and characterisation of their cell type by their typical morphologies (a, neurons; b and c, oligodendrocytes; d, astrocytes) and by double labelling in red for the neuronal marker NeuN (a), the oligodendrocytic marker APC (c) or the astrocytic marker GFAP (d). The drawing of a brain shows where panels a–d are located. Neurons derived from transplanted SVZ cells are mostly located in the olfactory bulb, while oligodendrocytes and astrocytes are mostly located in the cerebral cortex and corpus callosum. (B) Quantification of the cell types of transplanted cells. The genotype of transplanted cells, the position of the grafts and the number and percentage of neurons (N), oligodendrocytes (O) and astrocytes (A) are indicated. The ratio of different cell types varies greatly among control transplanted brains as well as Mash1 mutant transplanted brains, but, overall, Mash1 mutant cells differentiate less in oligodendrocytes than wild-type cells. The lack of an apparent defect in neuronal differentiation of Mash1 mutant cells may be due to the location of the grafts in primarily gliogenic areas. Scale bars: 20 μm.
Discussion
In this article, we have examined the mechanisms governing the differentiation of progenitor cells in the postnatal SVZ. We have focused on the function of Mash1, a protein that has proneural activity in the embryonic telencephalon and remains abundantly expressed in postnatal telencephalic progenitors. Here we discuss our findings that Mash1 is required for the generation of both neurons and oligodendrocytes, and that similar mechanisms are employed for cell fate specification in the embryonic and postnatal brain.
Conservation of Mash1 proneural function in the postnatal SVZ
The majority of Mash1-expressing cells in the adult SVZ have characteristics of transit amplifying progenitors for olfactory interneurons. Specifically, these cells are mitotic and give rise to migrating neuroblasts, but do not express neuronal-specific markers themselves, and they are rapidly regenerated following an antimitotic treatment (Doetsch et al, 1999). This transient expression of Mash1 in the postnatal olfactory interneuron lineage is therefore very similar to its expression in the embryonic ventral telencephalon, that is, in intermediate progenitors located in the SVZ, and not in the self-renewing stem cells of the ventricular neuroepithelium or in postmitotic neurons (Torii et al, 1999; Yun et al, 2002).
Analysis of Mash1 mutants provides further evidence that similar genetic programmes control neurogenesis in the embryonic and postnatal telencephalon. Stem cells present in the mutant SVZ at birth produce few dividing neuronal precursors both in vivo or in culture, and clonal analysis indicates that this is due to a specification defect of neuronal precursors, which adopt an astrocytic fate in the absence of Mash1. This is reminiscent of the proneural defects observed in mutant embryos, characterised by a loss of neuronal precursors in the ventral telencephalon of Mash1 mutants, and by their replacement with astrocytic precursors in the dorsal telencephalon of Mash1;Ngn2 double mutants (Casarosa et al, 1999; Horton et al, 1999; Nieto et al, 2001; Yun et al, 2002). Thus, Mash1 also acts as a proneural factor for the specification of neuronal precursor cells in the postnatal SVZ. A defect in neuronal precursor migration has recently been reported in the olfactory bulb of Mash1 mutants (Murray et al, 2003). However, our observation of a reduced number of BrdU+ dividing cells in the SVZ in vivo, and our clonal analysis of mutant cells in culture, clearly shows that the primary cause of the neurogenesis defect and reduced size of the olfactory bulb in these mice is a proneural defect in the generation of olfactory neuron precursors, independent of a subsequent defect in cell migration.
We have shown, by analysing Mash1 mutants at birth, that this gene has an essential role in olfactory bulb neurogenesis, a process that extends continuously from late embryogenesis to adulthood. It is likely that the early postnatal function of Mash1 extends to adult neurogenesis, since Mash1 is also required for neuronal specification of SVZ progenitors harvested at birth and maintained for 3 weeks in culture, and Mash1 SVZ expression is maintained in the adult. However, it cannot be formally ruled out that olfactory bulb neurogenesis is governed by different mechanisms in the adult and at early postnatal stages, and a definitive demonstration of a role of Mash1 in the adult brain awaits the analysis of a conditional mutation in this gene.
Mash1 is required to promote oligodendrogenesis in the postnatal brain
The involvement of Mash1 in oligodendrogenesis, demonstrated here both in vivo and in culture, is a novel aspect of the function of this proneural gene. The finding that oligodendrocyte precursor production is reduced in Mash1 mutant clones cocultivated with wild-type cells, and in mutant cells transplanted in wild-type brains, demonstrates that Mash1 is required cell autonomously for the generation of OPCs. The expression of Mash1 by a large fraction of OPCs in SVZ cultures, as well as in the forebrain white matter (this work) and the optic nerve (Kondo and Raff, 2000), supports this notion. We also observed a significant reduction in the number of OPCs in the ventral spinal cord of Mash1 mutant embryos (N Bertrand, M Sugimori, M Nakafuku and F Guillemot, unpublished data), suggesting that Mash1 function in oligodendrogenesis is not restricted to postnatal stages and extends to embryonic development. This requirement for Mash1 function reflects a role in specification of oligodendrocyte precursors, as there is no apparent decrease in OPC proliferation or increase in cell death in Mash1 mutant cultures, and the reduction in the number of OPC-containing clones in these cultures is paralleled by an increase in the number of astrocytic clones. Therefore, Mash1 is required at a similar step in neuronal and oligodendrocyte lineages of the SVZ.
It is interesting that in both neonatal brain and SVZ cultures, only a fraction of OPCs is missing in the absence of Mash1. The loss of OPCs in P0 mutant brains appears to be more severe in the olfactory bulb than in other parts of the forebrain, including the subcortical white matter, fimbria and striatum (data not shown). This conclusion must however be mitigated by the finding that grafted wild-type SVZ cells generate oligodendrocytes that migrate into the cerebral cortex and corpus callosum of the host brain, while grafted Mash1 mutant cells largely fail to do so (Figure 6), suggesting that Mash1 also has a role in oligodendrogenesis outside of the olfactory bulb. In SVZ cultures, Mash1-dependent and -independent OPCs appear to belong to distinct lineages, with OPCs that originate from neuron–oligodendrocyte progenitors being almost completely eliminated in the absence of Mash1, whereas OPCs that originate from astrocyte–oligodendrocyte progenitors are unaffected. Oligodendrocytes generated in this in vitro model are therefore heterogeneous, in both cell lineage and genetic programmes. Whether OPCs that differ in their requirement for Mash1 in vivo also belong to different lineages is presently unknown. Oligodendrocyte precursors in the telencephalon are heterogeneous in many respects, including their cellular and molecular properties and time of origin (Spassky et al, 2001; Miller, 2002; Ivanova et al, 2003). In particular, it has been proposed that oligodendrocyte precursors in the olfactory bulb belong to a distinct lineage from other telencephalic OPCs, as they originate from a different part of the embryonic telencephalon, they do not depend on PDGF signalling and they express the myelin gene plp/dm20 at an earlier stage (Spassky et al, 2001). There is also evidence that some telencephalic oligodendrocytes derive in culture from bipotent neuron–oligodendrocyte progenitors present in the embryonic ventral telencephalon (He et al, 2001; Yung et al, 2002), while others derive from bipotent astrocyte–oligodendrocyte progenitors that have been identified by retroviral lineage tracing studies in the perinatal SVZ in vivo (Parnavelas, 1999; Marshall et al, 2003; Zerlin et al, 2004). Whether Mash1-dependent oligodendrocytes correspond to one of these previously defined lineages remains to be addressed.
Common mechanisms for the generation of GABAergic interneurons and oligodendrocytes
The idea that generation of oligodendrocytes and motor neurons is coupled in the spinal cord has recently received strong support from the analysis of mouse mutants (Kessaris et al, 2001; Lu et al, 2002; Zhou and Anderson, 2002). The finding that Mash1 is expressed, and required for the generation of precursors for olfactory interneurons and oligodendrocyte precursors, supports the idea that specification of neurons and oligodendrocytes is also coupled in the telencephalon. Interestingly, the bHLH gene Olig2 has also been implicated in the generation of oligodendrocytes and cortical interneurons (which share a GABAergic neurotransmission phenotype and a subpallial origin with olfactory bulb interneurons) in cultures of embryonic ventral telencephalon (Yung et al, 2002), suggesting that Mash1 and Olig2 may cooperate for the specification of these two cell types. Furthermore, the Dlx genes, which are expressed downstream of Mash1 in the ventral telencephalon, and are involved in specification of the GABAergic phenotype in neuronal precursors (Fode et al, 2000; Yun et al, 2002), are also expressed in telencephalic OPCs (He et al, 2001; Marshall et al, 2003), suggesting that a similar regulatory pathway, involving the sequential activity of Mash1/Olig2 and Dlx, underlies the differentiation of both GABAergic interneurons and oligodendrocytes.
Since Mash1 is involved in the specification of both neurons and oligodendrocytes, other mechanisms must be involved in the choice between these two fates. Combinations of the bHLH factors Neurogenins and Oligs have been proposed to specify the three fundamental neural cell types in the ventral spinal cord (Zhou and Anderson, 2002). However, a bHLH combinatorial code may not be sufficient to account for the choice between neuronal and oligodendrocytic fates in the telencephalon, since precursors for GABAergic interneurons and oligodendrocytes appear to share the same bHLH protein profile. Extrinsic signals have also been implicated in the specification of neuronal or oligodendrocytic precursors in different contexts. This is the case in particular of BMPs, which induce GABAeric neurons and inhibit oligodendrogenesis in the embryonic telencephalon (Yung et al, 2002), and Notch, which promotes oligodendrocytes at the expense of motor neurons in the zebrafish spinal cord (Park and Appel, 2003). Whether these signals are also active in the postnatal SVZ and how they influence transcriptional programmes specifying neuronal and oligodendrocytic fates remain to be investigated.
Similar mechanisms for cell fate specification in the embryonic and postnatal telencephalon
The telencephalic SVZ is a remarkable structure, unique in the nervous system in its capacity to produce new cells in large numbers throughout life. Mash1, one of the main proneural genes in the embryonic brain, remains expressed in the postnatal SVZ, where it is still required for the generation of neuronal and oligodendrocyte precursors. Thus, maintenance of Mash1 expression at postnatal stages appears to be an important property of the SVZ, which contributes to the persistence of a progenitor population capable of producing a differentiated progeny throughout life.
A striking characteristic of the SVZ is that its cellular output changes dramatically over time, from the generation of multiple neuronal subtypes in the embryo, to a burst of gliogenesis around birth, and a specialisation in olfactory interneuron production in the adult. The mechanisms driving these changes in SVZ progenitor differentiation are currently unknown, but they do not appear to involve significant changes in expression of Mash1 or other intrinsic components of the cell fate specification machinery characterised in the embryo (data not shown). The limited differentiation potential of adult progenitors may instead be due to a more restrictive signalling environment in the adult than in the embryonic brain. This conclusion raises the possibility that manipulating extrinsic signals in the brain will expand the differentiation potential of adult progenitors and thus improve the capacity of the brain for self-repair (Kruger and Morrison, 2002).
Materials and methods
Mash1 null mutants and reporter mice
Wild-type, heterozygous and homozygous Mash1 mutant animals were obtained from intercrosses of heterozygous Mash1 null mutant mice (Guillemot et al, 1993) and genotyped as described (Casarosa et al, 1999). The J1A20 mouse line is described by Verma-Kurvari et al (1996).
AraC treatment
Cytosine-β-D-arabinofuranoside (AraC; Sigma) diluted at 2% in vehicle (0.9% saline) or vehicle alone was infused into the brain using osmotic-minipumps (Alzet) implanted for 6 days on the surface of the brain of adult mice (8–10 weeks old), as described by Doetsch et al (1999). Animals were then killed by intracardiac perfusion of fixative, 12, 24 or 48 h after the end of the infusion, and brains were processed for immunostaining.
Neurosphere cultures
Neural stem cell cultures were established, as described by Reynolds and Weiss (1992), from the lateral ventricular walls of newborn wild-type animals. Details of the protocol are available in Supplementary data.
Mice carrying both the Mash1 mutant allele and an ubiquitously expressed GFP transgene (B5/EGFP; Hadjantonakis et al, 1998) were intercrossed to generate GFP+, homozygous and heterozygous Mash1 mutant animals, which were used to produce GFP+, Mash1 mutant and control neurospheres. Cocultures of Mash1 mutant or control, GFP+ cells with GFP-negative, wild-type cells were performed by harvesting passage 2–4, GFP+ and GFP− neurospheres, dissociating and mixing them at a ratio of 1 GFP+/2000 GFP− cells, and cultivating them in differentiating conditions as described in Supplementary data.
Transplantation experiments
Swiss mice (1–2 weeks old) were used as hosts. P0 brains from donor mice were sliced with a tissue chopper, and the SVZ was dissected out and dissociated in small tissue fragments using needles. SVZ fragments were injected with a Hamilton syringe into the SVZ of the lateral ventricle, using morphological landmarks to guide the injection. At the end of the experiments, animals were perfused intracardially with 4% paraformaldehyde, and brains were removed and processed for immunocytochemistry. Eight brains were analysed 1 week after transplantation and 22 brains were analysed 4 weeks after. Among these, eight brains had received a graft that had landed in the corpus callosum or the cerebral cortex (i.e. a gliogenic environment). Three of these brains (#16, 22, 24) have received a mixture of SVZ cells derived from three wild-type donors. The five others (#14, 25, 28, 29, 36) have received Mash1 mutant SVZ cells, obtained respectively from two newborn mutants (#14, 25, 28, 29) and from a third one (#36). In grafted brains, transplanted cells were identified by their GFP immunoreactivity, and their cell type was recognised primarily by their morphology (Figure 6B). Cell type identity was confirmed for a large fraction of the cells by double labelling for the following cell type-specific markers: NeuN (neurons), NG2 or APC (oligodendrocytes) and GFAP (astrocytes).
Immunohistochemistry, BrdU labelling and in situ hybridisation
Newborn and adult brains were perfused with 4% paraformaldehyde, cut at 30–100 μm with a vibratome and kept free-floating in PBS. For BrdU and NG2 immunostaining, brains were fixed as above, cryoprotected in 20% sucrose, embedded in OCT (Tissue-Tek, Miles) and cut at 30 μm on a cryostat. Sections were incubated in a blocking solution (PBS plus 10% normal goat serum and 0.1% Tween 20) and incubated with primary antibodies (listed in Supplementary data) overnight at 4°C. Secondary antibodies (listed in Supplementary data) were incubated for 1 h at room temperature. DAB reaction (Roche) was performed on sections incubated with biotinylated secondary antibodies (Vector Laboratories) and processed with the enhancing avidin/biotin system (Vector Laboratories). For BrdU labelling, sections were incubated for 30 min in 2 N HCl at 37°C and processed for antibody staining. In situ hybridisation with a Mash1 probe was performed as described by Casarosa et al (1999). Bright field images were taken with a Zeiss Axiophot microscope. Immunofluorescence was visualised with a Leica TC SP1 confocal microscope. Images were treated using an imaging software package created by JL Vonesh (IGBMC, Strasbourg) and Adobe Photoshop 6.0 programme.
Quantification of the data
Cells positive for BrdU or cell type-specific markers were counted in seven adjacent 30-μm-thick sections through the olfactory bulb and lateral ventricles of P0 brains. At least two animals were used for each genotype. Transplanted GFP+ cells were counted in all 40-μm-thick sections obtained from grafted half-brains. To count neurons and neuronal precursors in newborn olfactory bulbs, bulbs were dissected and mechanically dissociated to single cells. Total cell number was measured and compared in mutant and control bulbs. Cells were then plated at 105 cells per well on 10 mm coverslips coated with poly-D-lysine, and immediately fixed with 4% paraformaldehyde at room temperature for 15 min. Four animals were used for each genotype. Cell counting in neurosphere cultures was performed in 10 randomly selected fields (× 40 or × 63 objectives) for each coverslip. Two or three animals were used per genotype. The experiments illustrated are representative examples of at least three independent experiments.
Supplementary Material
Supplementary data
Supplementary Figure 1
Supplementary Figure 2
Acknowledgments
We gratefully acknowledge DJ Anderson, C Heldin, U Lendal, B Nait-Oumesmar, G Panganiban, G Rougon, JR Rubenstein and B Zalc for the gift of antibodies, D Hentsch and J-L Vonesh for their help with confocal microscopy, F Nothias for her encouragements to SS, and Jacky Smith and our colleagues at NIMR for their help with our recent move. The Developmental Studies Hybridoma Bank is maintained by the Iowa University, Department of Biological Sciences. CP was supported by an EMBO long-term postdoctoral fellowship, a Fondation pour la Recherche Médicale fellowship and by the MRC. This work was supported by grants from the European Community Research and Technological Development program (QLG3-CT-2000-01471), the Human Frontier Science Program, the Association pour la Recherche sur le Cancer and the ATC reseau cellules souche neurales from INSERM, to FG, and by Institutional funds from INSERM, CNRS and Hôpital Universitaire de Strasbourg (at IGBMC) and from the MRC (at NIMR).
References
- Alvarez-Buylla A, Garcia-Verdugo JM (2002) Neurogenesis in adult subventricular zone. J Neurosci 22: 629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, Guillemot F (2002) Proneural genes and the specification of neural cell types. Nat Rev Neurosci 3: 517–530 [DOI] [PubMed] [Google Scholar]
- Casarosa S, Fode C, Guillemot F (1999) Mash1 regulates neurogenesis in the ventral telencephalon. Development 126: 525–534 [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain [In Process Citation]. Cell 97: 703–716 [DOI] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A (2002) EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron 36: 1021–1034 [DOI] [PubMed] [Google Scholar]
- Fode C, Ma Q, Casarosa S, Ang SL, Anderson DJ, Guillemot F (2000) A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes Dev 14: 67–80 [PMC free article] [PubMed] [Google Scholar]
- Gritti A, Frolichsthal-Schoeller P, Galli R, Parati EA, Cova L, Pagano SF, Bjornson CR, Vescovi AL (1999) Epidermal and fibroblast growth factors behave as mitogenic regulators for a single multipotent stem cell-like population from the subventricular region of the adult mouse forebrain. J Neurosci 19: 3287–3297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL (1993) Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell 75: 463–476 [DOI] [PubMed] [Google Scholar]
- Hadjantonakis AK, Gertsenstein M, Ikawa M, Okabe M, Nagy A (1998) Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mech Dev 76: 79–90 [DOI] [PubMed] [Google Scholar]
- He W, Ingraham C, Rising L, Goderie S, Temple S (2001) Multipotent stem cells from the mouse basal forebrain contribute GABAergic neurons and oligodendrocytes to the cerebral cortex during embryogenesis. J Neurosci 21: 8854–8862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton S, Meredith A, Richardson JA, Johnson JE (1999) Correct coordination of neuronal differentiation events in ventral forebrain requires the bHLH factor MASH1. Mol Cell Neurosci 14: 355–369 [DOI] [PubMed] [Google Scholar]
- Ivanova A, Nakahira E, Kagawa T, Oba A, Wada T, Takebayashi H, Spassky N, Levine J, Zalc B, Ikenaka K (2003) Evidence for a second wave of oligodendrogenesis in the postnatal cerebral cortex of the mouse. J Neurosci Res 73: 581–592 [DOI] [PubMed] [Google Scholar]
- Kessaris N, Pringle N, Richardson WD (2001) Ventral neurogenesis and the neuron–glial switch. Neuron 31: 677–680 [DOI] [PubMed] [Google Scholar]
- Kintner C (2002) Neurogenesis in embryos and in adult neural stem cells. J Neurosci 22: 639–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Raff M (2000) Basic helix–loop–helix proteins and the timing of oligodendrocyte differentiation. Development 127: 2989–2998 [DOI] [PubMed] [Google Scholar]
- Kruger GM, Morrison SJ (2002) Brain repair by endogenous progenitors. Cell 110: 399–402 [DOI] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH (2002) Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell 109: 75–86 [DOI] [PubMed] [Google Scholar]
- Luskin MB (1993) Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 11: 173–189 [DOI] [PubMed] [Google Scholar]
- Marshall CA, Suzuki SO, Goldman JE (2003) Gliogenic and neurogenic progenitors of the subventricular zone: who are they, where did they come from, and where are they going? Glia 43: 52–61 [DOI] [PubMed] [Google Scholar]
- Miller RH (2002) Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol 67: 451–467 [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D (1994) Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron 13: 1071–1082 [DOI] [PubMed] [Google Scholar]
- Murray RC, Navi D, Fesenko J, Lander AD, Calof AL (2003) Widespread defects in the primary olfactory pathway caused by loss of Mash1 function. J Neurosci 23: 1769–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M, Schuurmans C, Britz O, Guillemot F (2001) Neural bHLH genes control the neuronal versus glial fate decision in cortical progenitors. Neuron 29: 401–413 [DOI] [PubMed] [Google Scholar]
- Park HC, Appel B (2003) Delta-Notch signaling regulates oligodendrocyte specification. Development 130: 3747–3755 [DOI] [PubMed] [Google Scholar]
- Parnavelas JG (1999) Glial cell lineages in the rat cerebral cortex. Exp Neurol 156: 418–429 [DOI] [PubMed] [Google Scholar]
- Pencea V, Luskin MB (2003) Prenatal development of the rodent rostral migratory stream. J Comp Neurol 463: 402–418 [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255: 1707–1710 [DOI] [PubMed] [Google Scholar]
- Soares S, Sotello C (2004) Adult neural stem cells from the mouse subventricular zone are limited in migratory ability compared with progenitor cells of similar origin. Neuroscience, available online 20 September 2004 [DOI] [PubMed] [Google Scholar]
- Spassky N, Heydon K, Mangatal A, Jankovski A, Olivier C, Queraud-Lesaux F, Goujet-Zalc C, Thomas JL, Zalc B (2001) Sonic hedgehog-dependent emergence of oligodendrocytes in the telencephalon: evidence for a source of oligodendrocytes in the olfactory bulb that is independent of PDGFRalpha signaling. Development 128: 4993–5004 [DOI] [PubMed] [Google Scholar]
- Torii M, Matsuzaki F, Osumi N, Kaibuchi K, Nakamura S, Casarosa S, Guillemot F, Nakafuku M (1999) Transcription factors Mash-1 and Prox-1 delineate early steps in differentiation of neural stem cells in the developing central nervous system. Development 126: 443–456 [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Garcia-Verdugo JM, Lim DA, Alvarez-Buylla A (2003) Postnatal development of radial glia and the ventricular zone (VZ): a continuum of the neural stem cell compartment. Cereb Cortex 13: 580–587 [DOI] [PubMed] [Google Scholar]
- Verma-Kurvari S, Savage T, Gowan K, Johnson JE (1996) Lineage-specific regulation of the neural differentiation gene MASH1. Dev Biol 180: 605–617 [DOI] [PubMed] [Google Scholar]
- Williams BP, Price J (1995) Evidence for multiple precursor cell types in the embryonic rat cerebral cortex. Neuron 14: 1181–1188 [DOI] [PubMed] [Google Scholar]
- Yun K, Fischman S, Johnson J, Hrabe de Angelis M, Weinmaster G, Rubenstein JL (2002) Modulation of the notch signaling by Mash1 and Dlx1/2 regulates sequential specification and differentiation of progenitor cell types in the subcortical telencephalon. Development 129: 5029–5040 [DOI] [PubMed] [Google Scholar]
- Yung SY, Gokhan S, Jurcsak J, Molero AE, Abrajano JJ, Mehler MF (2002) Differential modulation of BMP signaling promotes the elaboration of cerebral cortical GABAergic neurons or oligodendrocytes from a common sonic hedgehog-responsive ventral forebrain progenitor species. Proc Natl Acad Sci USA 99: 16273–16278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerlin M, Milosevic A, Goldman JE (2004) Glial progenitors of the neonatal subventricular zone differentiate asynchronously, leading to spatial dispersion of glial clones and to the persistence of immature glia in the adult mammalian CNS. Dev Biol 270: 200–213 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Anderson DJ (2002) The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell 109: 61–73 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary Figure 1
Supplementary Figure 2


