Abstract
Since it emerged in Brazil in May 2015, the mosquito-borne Zika virus (ZIKV) has raised global concern due to its association with a significant rise in the number of infants born with microcephaly and neurological disorders such as Guillain-Barré syndrome. We developed prototype subunit and adenoviral-based Zika vaccines encoding the extracellular portion of the ZIKV envelope gene (E) fused to the T4 fibritin foldon trimerization domain (Efl). The subunit vaccine was delivered intradermally through carboxymethyl cellulose microneedle array (MNA). The immunogenicity of these two vaccines, named Ad5.ZIKV-Efl and ZIKV-rEfl, was tested in C57BL/6 mice. Prime/boost immunization regimen was associated with induction of a ZIKV-specific antibody response, which provided neutralizing immunity. Moreover, protection was evaluated in seven-day-old pups after virulent ZIKV intraperitoneal challenge. Pups born to mice immunized with Ad5.ZIKV-Efl were all protected against lethal challenge infection without weight loss or neurological signs, while pups born to dams immunized with MNA-ZIKV-rEfl were partially protected (50%). No protection was seen in pups born to phosphate buffered saline-immunized mice. This study illustrates the preliminary efficacy of the E ZIKV antigen vaccination in controlling ZIKV infectivity, providing a promising candidate vaccine and antigen format for the prevention of Zika virus disease.
Keywords: Zika virus, ZIKV-E, Vaccine, Microneedles, Adenovirus
Highlights
-
•
Two ZIKV vaccines, named Ad5.ZIKV-Efl and ZIKV-rEfl, were tested in C57BL/6 mice.
-
•
Protective immunity was evaluated in passively immune seven-day-old pups after virulent ZIKV intraperitoneal challenge.
-
•
This study illustrates the efficacy of the ZIKV E antigen vaccination by promising candidate vaccines.
1. Introduction
Zika virus (ZIKV) is a mosquito-borne flavivirus of the Flaviviridae family that was first identified in Uganda in 1947. The virus has recently attracted global attention due to its rapid spread from Brazil to other countries in the Americas (Dick et al., 1952, Zanluca et al., 2015). The ZIKV outbreak in Brazil has been associated with a significant rise in the number of babies born with microcephaly (Zanluca et al., 2015) and neurological disorders such as Guillain-Barré syndrome and has been declared a “Global Emergency” by the World Health Organization (WHO 2016 http://www.who.int/mediacentre/factsheets/zika/en/; WHO 2016 http://www.who.int/mediacentre/news/statements/2016/1st-emergency-committee-zika/en/; CDC 2016 http://www.cdc.gov/zika/). Concern over the spread of ZIKV to the Northern Hemisphere with its concomitant morbidity is spurring the search for an effective vaccine. ZIKV is related to dengue, yellow fever, Japanese encephalitis, and West Nile viruses (WNV), all of which are arthropod-borne flaviviruses. Like other flaviviruses, ZIKV contains a positive, single-stranded, genomic RNA encoding a polyprotein that is proteolytically processed to yield three structural proteins: the capsid (C), the precursor of membrane (prM), and the envelope (E), and seven nonstructural proteins (NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5) (Dick et al., 1952).
The succesful development of flavivirus vaccines began 80 years ago in 1937 with the yellow fever YFV17D live-attenuated vaccine (Monath, 2008). Since then, > 600 million people have been vaccinated, with 98% protection and a > 10 year persistence of vaccine–induced immunity (Barrett and Teuwen, 2009). However, several severe adverse events associated with vaccine administration have been observed over the last 20 years. Thus, a purified, inactivated vaccine has been recently developed and its testing results suggest good immunogenicity and tolerability (Monath et al., 2011). A few weeks ago, two studies showing immunogenicity of a plasmid DNA or adenovirus (serotype 52) expressing virus-like particles in mice and non-human primates were published (Larocca et al., 2016, Abbink et al., 2016). Here, to build on these initial findings to develop an effective ZIKV vaccine, we describe the development of a recombinant adenoviral vector expressing codon-optimized ZIKV E antigen and a subunit recombinant ZIKV E vaccine delivered transcutaneously by carboxymethyl cellulose (CMC) microneedle arrays (MNAs) (Bediz et al., 2014, Korkmaz et al., 2015), investigate their ability to induce neutralizing immune responses, and assess their ability to passively protect against ZIKV challenge in a novel neonatal ZIKV infection mouse model.
2. Materials & Methods
2.1. Adenoviral Construction and Purification of Recombinant Protein
For construction of pAd/ZIKV-Efl, the gene encoding human secretory signal peptide hidden Markov model (SP-HMM, MWWRLWWLLLLLLLLWPMVWA), the extracellular portion of the ZIKV strain BeH815744 envelope gene (GenBank KU365780, defined as amino acids 216–794 of the polyprotein), BamH I-linked T4 fibritin foldon trimerization domain (GSGYIPEAPRDGQAYVRKDGEWVLLSTFL), Tobacco Etch Virus Protease (Tp) (ENLYFEG), and six histidine tag were codon-optimized for optimal expression in mammalian cells using the UpGene codon optimization algorithm (Gao et al., 2004). pAd/ZIKV-Efl was generated by subcloning the codon-optimized ZIKV-Efl gene into the shuttle vector, pAd (GenBank U62024) at SalI/NotI sites. Subsequently, replication-defective adenovirus 5, designated as Ad5.ZIKV-Efl, was generated by loxP homologous recombination. Moreover, we also purified recombinant proteins named ZIKV-rEfl from the supernatant using His60 Ni Superflow Resin (Clontech) under native conditions to be used as a subunit vaccine. Briefly, the supernatant of Human Embryonic Kidney (HEK) 293 cells infected with Ad5.ZIKV-Efl was heat-inactivated at 65 °C for 30 min and mixed with the same volume of binding buffer (40 mM imidazole, 900 mM NaCl, 100 mM sodium phosphate, pH 7.4). His60 Ni Superflow Resin (Clontech) previously equilibrated with equilibration buffer (20 mM imidazole, 500 mM NaCl, 50 mM sodium phosphate, pH 7.4) was added and the mixture was incubated overnight at 4 °C with rotation. The next day, the settled resin mix was packed into an empty column. The column was washed with 10 ml of equilibration buffer three times followed by 10 ml of wash buffer (40 mM imidazole, 500 mM NaCl, 50 mM sodium phosphate, pH 7.4) three times and eluted in 10 ml of elution buffer (500 mM imidazole, 500 mM NaCl, 50 mM sodium phosphate, pH 7.4). The eluate was concentrated and desalted with phosphate buffered saline (PBS) in an Amicon Ultra-15 filter (Millipore). This desalting step was repeated three times. The concentrations of the purified recombinant ZIKV-Efl were determined by the Bradford assay using bovine serum albumin (BSA) as a protein standard.
2.2. Virus Stock
ZIKV stocks were provided by Dr. Rober Tesh of University of Texas Medical Branch. Vero cells were infected with ZIKV DAKAR41542 at MOI of 0.01 and incubated until the monolayer showed significant cytopathic effect. Culture supernantant was clarified by centrifugation at 3000g for 15 min. Virus was precipitated overnight by addition of NaCl (0.4 M) and 6% polyethylene glycol. After centrifugation at 10,000g for 30 min, the viral pellet was re-dissolved to 1/100 of the original volume in PBS and centrifuged on a 5 to 50% sucrose gradient at 90,000g for 3 h, followed by dialysis with PBS buffer. The virus was diluted to a proper concentration with 5% Trehalose Buffer (20 mM Tris, pH 7.8, 75 mM NaCl, 2 mM MgCl2, 5% Trehalose, 0.0025% Tween 80) and kept at − 80 °C. For the virus titer, vero cells were seeded in a six-well plate at 1 × 105 cells per well. The next day, cells were infected with log dilutions of ZIKV for 1 h and overlayed with 1% methyl cellulose media containing 5% fetal bovine serum. After three days of infection, cells were stained with 1% crystal violet. Plaques were counted and titers were calculated by multiplying the number of plaques by the dilution and dividing by the infection volume.
2.3. Animal Experiments
Six- to eight-week-old C57BL/6 female mice (five animals per group) were inoculated subcutaneously (s.c.) with 1 × 1011 viral particles (v.p.) of Ad5.ZIKV-Efl or PBS as a negative control, and intradermally (i.d.) with MNA coated with 20 μg of ZIKV-rEfl. Two weeks after the primary immunization, mice were boosted intranasally (i.n.) or i.d. with the same dose of the respective immunogens. Mice were bled from the retro-orbital sinus at week 0, 2, 4, and 6, and serum samples were evaluated for ZIKV antibody by enzyme-linked immunosorbent assay (ELISA) and plaque reduction neutralization assay (PRNT). For the immunization study, a protocol approved by the University of Pittsburgh Institutional Animal Care and Use Committee was followed.
To evaluate passive protection by maternal antibody, pups were obtained by mating non-immunized males with immunized females at three weeks following booster vaccination. Pups were challenged intraperitoneally (i.p.) with ZIKV DAKAR41542 (105 pfu/50 μl) at seven days after birth. Two non-challenged pups from each litter were used as a control and bled at 28 days after birth to determine passive maternal antibodies. The physical condition of the pups was observed and their body weights were measured daily for 15 days. Exhibiting > 10% loss of body weight was defined as onset of disease. In addition to mice that were found dead, mice with weight loss exceeding 25% of their highest body weight were euthanized and recorded as dead. Severity of neurological signs was scored as described previously (Yoshii et al., 2014). Signs of paralysis and loss of balance associated with viral infection were scored as 0 (absent), 1 (present), or 2 (severe). Scoring for paralysis was assigned as follows: 0, normal; 1, dragging limbs or inversion of dorsum pedis; and 2, complete paralysis and no spontaneous movement. Scoring for loss of balance was assigned as follows: 0, normal; 1, leaning of head or trunk posture to one side; and 2, inability to retain posture and falling to one side or a circling movement to one side. Total scores were quantified and were expressed as means ± the standard errors of the mean.
2.4. ELISA Assay
Sera from the animals were collected every two weeks and tested for ZIKV-specific IgG by conventional ELISA. Briefly, ELISA plates were coated with 2 × 105 pfu of heat-inactivated ZIKV DAKAR4542 at 60 °C for 20 min per well overnight at 4 °C in carbonate coating buffer (100 mM, pH 9.5) and then blocked with PBS containing 0.05% Tween 20 (PBS-T) and 2% BSA for 1 h. Mouse sera were diluted 1:200 or 1:20 for pups sera in PBS-T with 1% BSA and incubated for 2 h. After the plates were washed, HRP-conjugated anti-mouse IgG (1:2000, Santacruz) was added to each well and incubated for 1 h. The plates were washed three times and developed with 3,3′5,5′-tetramethylbenzidine, and the reaction was stopped with 1 M H2SO4 and absorbance at 450 nm was determined using an ELISA reader (BIO-TEK instruments).
2.5. Plaque Reduction Neutralization Assay (PRNT)
To determine the plaque reduction neutralizing titer at week 6, 60 μl of the pooled sera or 30 μl of each mouse sera was diluted in twofold serial dilutions (from 1/16 to 1/516 or from 1/32 to 1/1024) and incubated with 100 pfu of ZIKV DAKAR41542 in 100 μl of serum-free media at 37 °C for 1 h and subsequently added to a Vero cell monolayer at a density of 5 × 104 cells grown in six-well tissue culture plates and further incubated at 37 °C for 1 h. After incubation, the inoculant was removed, the semisolid media was added, and the plates were incubated for an additional five days. Titers were expressed as the reciprocal of the highest serum dilution still giving a 50% reduction in plaque number (PRNT50) relative to samples incubated with pre-immunized control pooled sera.
2.6. Statistical Analysis
In vitro experiments in this paper were repeated at least twice and data shown are means of those replicates ± standard error. For the statistical analysis, the Student's t-test, one-way analysis of variance and Tukey's multiple comparison tests, and log-rank (Mantel-Cox) test were performed using Graph Pad Prism version 5.0 software (San Diego, California, USA). Results were considered statistically significant when the P value was < 0.05. Symbols *, **, and *** are used to indicate P values of < 0.05, < 0.01, and < 0.001, respectively.
3. Results
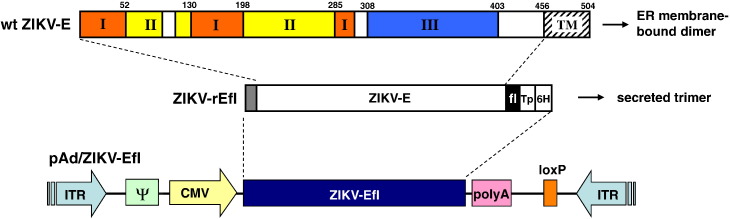
We generated recombinant E1/E3-deleted adenovirus serotype 5-based vectors that encode for the human secretory signal peptide hidden Markov model (SP-HMM) followed by the codon-optimized extracellular portion of the ZIKV BeH815744 E gene fused to the T4 fibritin foldon trimerization domain (ZIKV-Efl). Moreover, the ZIKV-Efl antigen was engineered with a polyhistidine-tag and a Tobacco Etch Virus (TEV) protease cleavage sequence to facilitate downstream purification (Fig. 1). The replication-defective adenovirus 5, designated as Ad5.ZIKV-Efl, was generated by loxP homologous recombination as previously described (Kim et al., 2014, Hardy et al., 1997). Recombinant ZIKV-rEfl protein was purified from the supernatant of a Ad5.ZIKV-Efl-infected HEK 293 cell line using His60 Ni Superflow Resin under native conditions and packaged as a subunit vaccine in an MNA (MNA-ZIKV-Efl).
Fig. 1.
Schematic representations of plasmid vector pAd/ZIKV-Efl. A shuttle vector carrying the gene encoding human secretory signal peptide hidden Markov model (SP-HMM), the extracellular portion of the ZIKV envelope gene (amino acids 216–794 of the polyprotein), BamH I-linked T4 fibritin foldon trimerization domain (fl), Tobacco Etch Virus Protease (Tp), and six histidine tag (6H) were designated as shown in the diagram. The three domains of ZIKV E are represented based on West Nile virus E: domain I is orange, domain II is yellow, and domain III is blue (Mou et al., 2013). The vector was used to generate recombinant replication-deficient adenoviruses by homologous recombination with the adenoviral genomic DNA. Abbreviations are as follows: ITR, inverted terminal repeat; TM, transmembrane domain.
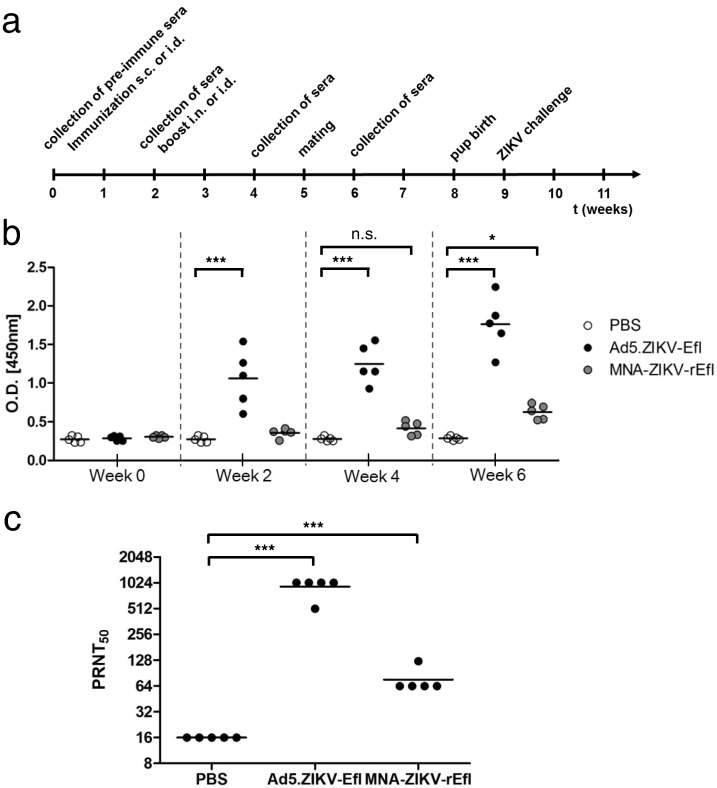
We then tested the ability of Ad5.ZIKV-Efl and MNA-ZIKV-rEfl to elicit a specific anti-ZIKV immune response in vivo. C57BL/6 mice were inoculated s.c. with 1011vp of Ad5.ZIKV-Efl or i.d. with 20 μg of MNA-ZIKV-rEfl, or with PBS on day 0 followed by booster immunization on day 14 with the same dose i.n. or i.d., respectively (Fig. 2a). At 0, 2, 4, and 6 weeks post prime immunization, sera were obtained from all mice and screened for the presence of ZIKV-specific antibodies using ELISA analysis. ZIKV-specific antibodies were detected as soon as two weeks after the first immunization in the sera of mice vaccinated with Ad5.ZIKV-Efl (P = 0.0002), while mice immunized MNA-ZIKV-rEfl showed significant titers at four weeks after the booster immunization (P < 0.05) when compared with the sera of mice immunized with PBS (Fig. 2b).
Fig. 2.
Characterization of ZIKV-specific immune responses induced by Ad5.ZIKV-Efl and MNA-ZIKV-rEfl. (a) Experimental schedule representing the immunization timeline. C57BL/6 mice (n = 5/group) were immunized subcutaneously with 1 × 1011 viral particles of Ad.ZIKV-Efl or PBS as a negative control and boosted intranasally with the same amount of adenovirus two weeks later. MNA-ZIKV-rEfl was administered through intradermal delivery. (b) ZIKV-specific IgG antibody levels were measured at the indicated time points using ELISA. (c) The ZIKV-neutralizing titers at week 6 post-immunization were measured using Vero cells by determining the reciprocal of the highest serum dilution still giving a 50% reduction in plaque number by 50% (PRNT50), relative to samples incubated with pre-immunized control pooled sera. Statically significant differences (Tukey's test) are marked by bars and asterisks. *, P < 0.05; ***, P < 0.001. The same mean of neutralization was detected in two independent neutralizing tests with combined mouse sera.
Furthermore, qualitative neutralizing activity of ZIKV antibodies was tested in a PRNT 50% assay. The presence of ZIKV-neutralizing antibodies was shown in both Ad5.ZIKV-Efl and MNA-ZIKV-rEfl, although the response in the mice immunized with MNA-ZIKV-rEfl was four- to 16-fold lower than the response achieved in the mice immunized with Ad5.ZIKV-Efl. As expected, no neutralizing antibody responses were observed in the control animal group (Fig. 2c). These findings support our premise that Ad5.ZIKV-Efl- and MNA-ZIKV-rEfl-based ZIKV E antigen vaccines are able to induce neutralizing ZIKV-specific immunity.
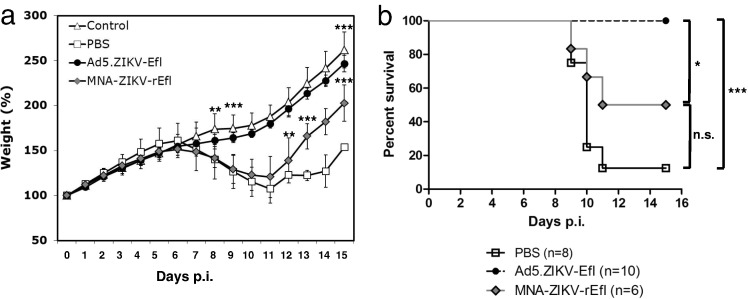
To further understand how the vaccine induced ZIKV E-specific immunity, neutralizing the ZIKV in vivo and protecting the animal from its pathogenic effects, we developed a passive protection suckling mouse model. Building upon the knowledge (Dick et al., 1952) that day 7- (but not day 14-) old suckling mice are susceptible to ZIKV infection via the i.p. route showing neurological signs, pups were obtained by mating immunized female with nonimmunized male mice at week 3 after booster immunization. Pups were challenged i.p. at seven days after birth with 105 pfu of ZIKV DAKAR41542, monitored daily for mortality, and weighed for 15 days. The mean time to disease onset (10% weight loss) was slightly earlier in the pups from PBS-immunized mice than in those from MNA-ZIKV-rEfl-immunized mice, although the difference was not significant (7.75 vs. 8.25 days, P = 0.1598) (Table 1). All pups born to PBS-immunized mice showed more than a 20% body weight loss in the 10 days postinfection. However, weight loss in the MNA-ZIKV-rEfl pups was reduced and a significant difference was found from day 12 (P < 0.01; P < 0.001, day 13–day 15) after challenge when compared to the PBS pups. No weight loss was observed in the pups born to the dams immunized with Ad5.ZIKV-Efl vaccine and no significant difference was measured between the pups of Ad5.ZIKV-Efl-immunized mice and the unchallenged control pups for the entire period. The significant difference started at day 8 (P < 0.01; P < 0.001, day 9–day 15) after challenge when compared to the PBS pups. (Fig. 3a). The survival rates of pups from two animals in each group were also monitored after challenge with ZIKV DAKAR41542. Survival rates of 100% (10/10) and 50% (3/6) were observed in the pups from Ad5.ZIKV-Efl- and MNA-ZIKV-rEfl-immunized dams, respectively, whereas a 12.5% (1/8) survival rate was seen in pups from PBS-immunized dams (Fig. 3b). The differences between the pups from Ad5.ZIKV-Efl- and those from PBS-immunized dams and between the pups from Ad5.ZIKV-Efl- and those from MNA-ZIKV-immunized dams were statistically significant (P = 0.0001 and P = 0.0136, respectively). When the pups from MNA-ZIKV-rEfl- and PBS-immunized dams were compared, no significant difference in survival rate was observed (P = 0.1493), indicating that the Ad5.ZIKV-Efl vaccine candidates were efficient in passively protecting neonatal mice against lethal ZIKV challenge.
Table 1.
Pathogenicity of Zika virus in a mouse model.
| Vaccine for dams | No. of pups | Mean time to onset of disease (days) ± SDa | Neurological disease (%)b | Neurological scorec |
|---|---|---|---|---|
| PBS | 8 | 7.75 ± 0.88 | 100 (8/8) | 4.62 ± 1.30 |
| Ad5.ZIKV-Efl | 10 | ND | 0 (0/10)d | – |
| MNA-ZIKV-rEfl | 6 | 8.25 ± 0.50 | 83.30 (5/6)e | 2.80 ± 0.83f |
Exhibiting > 10% loss of body weight was defined as onset of disease. There were no significant differences in the average onset of disease in each group (P = 0.1598). ND; not detected.
The percentage of mice showing neurological symptoms at disease onset. The number of mice showing neurological symptoms at day 10 post-infection/the number of mice showing onset of disease at day 10 post-infection.
Scores for the severity of neurological signs were quantified as described in Section 2.
Three out of 10 mice showed transient neurological signs (neurological score; 2.33 ± 0.57) at one time point. Significant difference from the percentage of PBS group (P < 0.0001).
No significant difference from the percentage of PBS group (P = 0.2482).
Significant difference from the score of PBS group (P < 0.05).
Fig. 3.
Protection from ZIKV infection in neonatal mice by maternal immunization with Ad5.ZIKV-Efl and MNZ.ZIKV-rEfl. Pups were obtained by mating nonimmunized males with immunized females at five weeks after prime vaccination. Pups were challenged intraperitoneally at seven days after birth with ZIKV DAKAR41542 (105 pfu/50 μl). Body weight (a) and survival (b) were monitored for 15 days post-infection. Statistically significant differences (Tukey's test or log-rank (Mantel-Cox) test) are marked by bars and asterisks. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
As expected, all pups of PBS-immunized dams showed neurological signs including loss of balance, paresis, and hindlimb paralysis, with 4.62 ± 1.30 of neurological score. However, five out of six pups of MNA-ZIKV-rEfl-immunized dams exhibited neurological illness (no significant difference from the percentage of PBS group; P = 0.2482), although the neurological severity score was significantly lower than that of pups from PBS-immunized mice (P < 0.05). In contrast, the pups from Ad5.ZIKV-Efl-immunized mice showed mild symptoms at one time point or no signs of neurological illness (Table 1).
Lastly, to detemine the relationship between survival rate and maternally-transferred antibody, the sera from 25-day-old non-challenged pups born from immunized dams were collected and tested for reactivity with ZIKV by ELISA. The level of maternal IgG ZIKV-specific antibodies measured in pups nursed by Ad5.ZIKV-Efl-immunized dams was significantly higher than that in pups nursed by PBS-immunized dams (P < 0.001). However, in the pups nursed by MNA-ZIKV-Efl-immunized dams, the level of IgG antibodies against ZIKV-rEfl was not significantly higher when compared with that in pups nursed by PBS-immunized dams (Fig. S1). These data suggest that the survival rate in pups correlated with the maternally-transferred antibody IgG titer, and although some of the animals immunized with MNA-ZIKV-Efl were protected, the level of ZIKV-specific IgG transferred to the newborns was suboptimal.
4. Discussion
In this study, we describe the construction and immunological evaluation of two ZIKV vaccine candidates. Our initial evaluations indicated that the ZIKV vaccines Ad5.ZIKV-Efl and MNA-ZIKV-rEfl elicited a humoral immune response in immunized C57BL/6 mice. The humoral response was characterized by high titers of antibodies to E antigen as confirmed by ELISA, as well as neutralizing titers confirmed by PRNT50 assay. Importantly, in pups born to immunized dams, ZIKV-specific immunity was passively transferred and protected them from day 7 challenge of 105 pfu of the ZIKV DAKAR41542 strain.
The Ad5.ZIKV-Efl and MNA-ZIKV-rEfl vaccines were engineered using the 2015 Brazil ZIKV strain BeH815744. The BeH815744 strain E protein differs from the DAKAR41542 strain E protein used for challenge in three amino acids (98% identity). In general, the ZIKV envelope protein is highly conserved.
Although in the presented studies the adenovirus-based Ad5.ZIKV-Efl vaccine was the most potent of the two tested ZIKV vaccine candidates, we acknowledge that is the least likely among the two candidates to be translated into a clinical product. This is because the prevalence of anti-adenovirus serotype 5-neutralizing antibodies in humans limits its use as suitable clinical vaccine platform. However, the experimental use of serotype 5 adenoviral-based vaccines, as shown in this study, is an invaluable tool for the antigen vaccine selection for any given pathogen. Conversely, the MNA-delivered ZIKV vaccine MNA-ZIKV-rEfl, although not optimized for inducing neutralizing immunity in the current format, is a clinically applicable vaccine platform to target infectious diseases such as ZIKV. The geometric design of the MNA-based vaccine platform affords unique advantages for efficient delivery and targeting to the superficial skin microenvironment, which is rich in antigen-presenting cells. While immunogenicity was lower than that observed in a previously reported adjuvented and inactivated whole virus vaccine (Larocca et al., 2016), the MNA-based vaccine offers the safety and clinical advantages of a defined recombinant subunit antigen and the potential for local co-delivery of adjuvants at very low doses. Co-delivery of TLR ligand adjuvants at very low concentrations can substantially increase the immunogenicity of an influenza subunit vaccine (Weldon et al., 2012). Importantly, the fabrication process of MNAs affords unique product advantages in reproducibility, safety, manufacturing, and distribution critical for widespread clinical deployment. Thus, our current and future efforts toward the clinical translation of ZIKV vaccine are directed toward the testing and comparison of different E antigen formats (dimeric vs. trimeric), and clinically relevant antigen-adjuvant formats that can be co-delivered in MNAs.
One thing that we learned in the presented ZIKV vaccine studies is that the yield of production of the ZIKV envelope E subunit protein was very low in the current format. This finding, also confirmed by a recently published ZIKV vaccine study (Larocca et al., 2016), is similar to what was previously observed for other flaviviruses (Taylor et al., 2016). The low yield of E protein is probably due to the absence of preM, which is important for protein stability. For instance, expression of WNV E protein alone showed proteolytic cleavage compared to the E protein produced in the presence of preM (Taylor et al., 2016). Thus, the inclusion of preM sequence seems to be an important prerequisite in ZIKV E-based vaccine development. Importantly, in this study, we used an immunocompetent mouse challenge model of ZIKV infection. This approach was inspired by a 1952 publication (Dick et al., 1952) in which ZIKV was shown to be pathogenic in newborn mice. Although this model does not recapitulate the ZIKV pathogenesis observed in humans, it is an effective model to evaluate the in vivo neutralizing activity of vaccine-induced ZIKV immunity. During the conduct of these experiments, many mouse models of ZIKV infection were established in interferon receptor-deficient mice and SJL mice (Cugola et al., 2016, Shah and Kumar, 2016, Miner et al., 2016, Dowall et al., 2016, Lazear et al., 2016, Rossi et al., 2016). Further investigations in SJL mice, the closest clinical model of fetal microcephaly, will be considered to evaluate the efficiency of vaccine candidates here in the future.
The following are the supplementary data related to this article.
Carnoxymethyl cellulose microneedle array fabrication.
Transfer of maternal ZIKV-E-specific IgG to pups. Two pups of each litter were bled at 28 days after birth to determine passive maternal antibodies and confirmed by ELISA coated with ZIKV. Statically significant differences (Tukey's test) are marked by bars and asterisks. ***, P < 0.001; n.s.; statistically not significant.
Conflict of Interest Statement
The authors declare no conflicts of interest.
Author Contributions
AG, LF, and EK designed the study. EK made adenoviral constructs, purified recombinant proteins, performed animal experiments and immunologic assays, and analyzed data. SH and TK prepared recombinant adenovirus and Zika virus stocks. GE manufactured the MNA patches. AG and EK wrote the manuscript.
Acknowledgments & Funding
We thank Ms. Christine Heiner for her assistance with manuscript preparation. We are grateful to Dr. Rober Tesh at the University of Texas Medical Branch for providing the Zika DAKAR41542 strain. UPMC and the UPMC/University of Pittsburgh Department of Surgery provided funding to support these preliminary studies.
References
- Abbink P., Larocca R.A., de la Barrera R.A., bricault C.A., Moseley E.T., Boyd M., Kirilova M., Li Z., Ng'Ang'A D., Nanayakkara O., Nityanandam R., Mercado N.B., Borducchi E.N., Agarwal A., Brinkman A.L., Cabral C., Chandrashekar A., Giglio P.B., Jetton D., Jimenez J., Lee B.C., Mojta S., Molloy K., Shetty M., Neubauer G.H., Stephenson K.E., Peron J.P., Zanotto P.M., Misamore J., Finneyfrock B., Lewis M.G., Alter G., Modjarrad K., Jarman R.G., Eckels K.H., Michael N.L., Thomas S.J., Barouch D.H. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 2016;353:1129–1132. doi: 10.1126/science.aah6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A.D., Teuwen D.E. Yellow fever vaccine - how does it work and why do rare cases of serious adverse events take place? Curr. Opin. Immunol. 2009;21:308–313. doi: 10.1016/j.coi.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Bediz B., Korkmaz E., Khilwani R., Donahue C., Erdos G., Falo L.D., Jr., Ozdoganlar O.B. Dissolvable microneedle arrays for intradermal delivery of biologics: fabrication and application. Pharm. Res. 2014;31:117–135. doi: 10.1007/s11095-013-1137-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cugola F.R., Fernandes I.R., Russo F.B., Freitas B.C., Dias J.L., GUIMARAES K.P., Benazzato C., Almeida N., Pignatari G.C., Romero S., Polonio C.M., Cunha I., Freitas C.L., Brandao W.N., Rossato C., Andrade D.G., Faria D.P., Garcez A.T., Buchpigel C.A., Braconi C.T., Mendes E., Sall A.A., Zanotto P.M., Peron J.P., Muotri A.R., Beltrao-Braga P.C. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534:267–271. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick G.W., Kitchen S.F., Haddow A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- Dowall S.D., Graham V.A., Rayner E., Atkinson B., Hall G., Watson R.J., Bosworth A., Bonney L.C., Kitchen S., Hewson R. A susceptible mouse model for Zika virus infection. PLoS Negl. Trop. Dis. 2016;10:e0004658. doi: 10.1371/journal.pntd.0004658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Rzewski A., Sun H., Robbins P.D., Gambotto A. UpGene: application of a web-based DNA codon optimization algorithm. Biotechnol. Prog. 2004;20:443–448. doi: 10.1021/bp0300467. [DOI] [PubMed] [Google Scholar]
- Hardy S., Kitamura M., Harris-Stansil T., Dai Y., Phipps M.L. Construction of adenovirus vectors through Cre-lox recombination. J. Virol. 1997;71:1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Okada K., Beeler J.A., Crim R.L., Piedra P.A., gilbert B.E., Gambotto A. Development of an adenovirus-based respiratory syncytial virus vaccine: preclinical evaluation of efficacy, immunogenicity, and enhanced disease in a cotton rat model. J. Virol. 2014;88:5100–5108. doi: 10.1128/JVI.03194-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkmaz E., Friedrich E.E., Ramadan M.H., Erdos G., Mathers A.R., O. B.O., Washburn N.R., Falo L.D., Jr. Therapeutic intradermal delivery of tumor necrosis factor-alpha antibodies using tip-loaded dissolvable microneedle arrays. Acta Biomater. 2015;24:96–105. doi: 10.1016/j.actbio.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocca R.A., Abbink P., Peron J.P., Zanotto P.M., Iampietro M.J., Badamchi-Zadeh A., Boyd M., Ng'Ang'A D., Kirilova M., Nityanandam R., Mercado N.B., Li Z., Moseley E.T., Bricault C.A., Borducchi E.N., Giglio P.B., Jetton D., Neubauer G., Nkolola J.P., Maxfield L.F., Barrera R.A., Jarman R.G., Eckels K.H., Michael N.L., Thomas S.J., Barouch D.H. Vaccine protection against Zika virus from Brazil. Nature. 2016 doi: 10.1038/nature18952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear H.M., Govero J., Smith A.M., Platt D.J., Fernandez E., Miner J.J., Diamond M.S. A mouse model of Zika virus pathogenesis. Cell Host Microbe. 2016;19:720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner J.J., Cao B., Govero J., Smith A.M., Fernandez E., Cabrera O.H., Garber C., Noll M., Klein R.S., Noguchi K.K., Mysorekar I.U., Diamond M.S. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell. 2016;165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monath T.P. Treatment of yellow fever. Antiviral Res. 2008;78:116–124. doi: 10.1016/j.antiviral.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Monath T.P., Fowler E., Johnson C.T., Balser J., Morin M.J., Sisti M., Trent D.W. An inactivated cell-culture vaccine against yellow fever. N. Engl. J. Med. 2011;364:1326–1333. doi: 10.1056/NEJMoa1009303. [DOI] [PubMed] [Google Scholar]
- Mou H., Raj V.S., van Kuppeveld F.J., Rottier P.J., Haagmans B.L., Bosch B.J. The receptor binding domain of the new Middle East respiratory syndrome coronavirus maps to a 231-residue region in the spike protein that efficiently elicits neutralizing antibodies. J. Virol. 2013;87:9379–9383. doi: 10.1128/JVI.01277-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S.L., Tesh R.B., Azar S.R., Muruato A.E., Hanley K.A., Auguste A.J., Langsjoen R.M., Paessler S., Vasilakis N., Weaver S.C. Characterization of a novel murine model to study Zika virus. Am.J.Trop. Med. Hyg. 2016;94:1362–1369. doi: 10.4269/ajtmh.16-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A., Kumar A. Zika virus infection and development of a murine model. Neurotox. Res. 2016;30:131–134. doi: 10.1007/s12640-016-9635-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor T.J., Diaz F., Colgrove R.C., Bernard K.A., Deluca N.A., Whelan S.P., Knipe D.M. Production of immunogenic West Nile virus-like particles using a herpes simplex virus 1 recombinant vector. Virology. 2016;496:186–193. doi: 10.1016/j.virol.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon W.C., Zarnitsyn V.G., Esser E.S., Taherbhai M.T., Koutsonanos D.G., Vassilieva E.V., Skountzou I., Prausnitz M.R., Compans R.W. Effect of adjuvants on responses to skin immunization by microneedles coated with influenza subunit vaccine. PLoS One. 2012;7:e41501. doi: 10.1371/journal.pone.0041501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii K., Sunden Y., Yokozawa K., Igarashi M., Kariwa H., Holbrook M.R., Takashima I. A critical determinant of neurological disease associated with highly pathogenic tick-borne flavivirus in mice. J. Virol. 2014;88:5406–5420. doi: 10.1128/JVI.00421-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanluca C., de Melo V.C., Mosimann A.L., dos Santos G.I., dos Santos C.N., Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem. Inst. Oswaldo Cruz. 2015;110:569–572. doi: 10.1590/0074-02760150192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Carnoxymethyl cellulose microneedle array fabrication.
Transfer of maternal ZIKV-E-specific IgG to pups. Two pups of each litter were bled at 28 days after birth to determine passive maternal antibodies and confirmed by ELISA coated with ZIKV. Statically significant differences (Tukey's test) are marked by bars and asterisks. ***, P < 0.001; n.s.; statistically not significant.