Abstract
Some studies have shown that extracellular pH in tumors, which results in tumor progression, is less than that in normal tissues. The aim of this study was to investigate the effects of extracellular acidic pH on proliferation, invasion, and drug-induced apoptosis in acute lymphoblastic cells. The cells were cultured in different pH (pH 6.6 and pH 7.4) for 12 days. Cell proliferation was assessed by MTT assay and cell invasion was assayed by invasion assay and gene expression analysis of MMP-9. Drug-induced apoptosis was evaluated after exposure to doxorubicin for 24 hours by annexin V/PI staining and gene expression analysis of BAX pro-apoptotic protein. The results indicated the enhanced growth and invasion of leukemic cells at pH 6.6 (P ≤ 0.05). Furthermore, the cells at pH 6.6 were resistant to apoptosis by doxorubicin (P ≤ 0.05). It can be concluded that acidic pH increases the proliferation, invasion and reduces the drug-induced apoptosis in acute lymphoblastic leukemia. Extracellular acidity can influence the behavior of leukemic cells and therefore, the manipulation of extracellular liquid can be selected as a therapeutic strategy for leukemia, especially for acute lymphoblastic leukemia.
Keywords: Acidic pH, Proliferation, Invasion, Drug-induced apoptosis, Lymphoblastic leukemia
Introduction
Tumor progression can mean a reversal of normal cell development that involves a complex interaction of genetic and epigenetic factors in this progression [1–3]. Microenvironmental factors encompass all the physical interactions between cells, extracellular matrix and biochemical factors such as different types of nutrients [2].
Cancer cells increase glycolytic activity despite the presence of oxygen supply in the microenvironment; this event is called aerobic glycolysis or Warburg effect [3]. An inevitable consequence of aerobic glycolysis is the production of lactic acid [3, 4]. Studies have shown that the extracellular pH of normal cells is in the range of 7.2 to 7.5, whereas the extracellular pH of cancer cells is in the range of 6.2 to 6.9 which is caused by aerobic glycolysis [3–6].
The evidence shows that cellular phenotype is regulated by the acidic microenvironment [7]. Acid lactic produced by aerobic glycolysis inhibits dendritic cell activation, tumoricidal activity of cytotoxic T lymphocytes and natural killer cells and suppresses lymphocyte proliferation [4, 8]. Acidic condition has been reported to promote the transcription of growth factors such as vascular endothelial growth factor (VEGF), hypoxia-inducible transcription factor (HIF-1) and Interleukin (IL)-8 through nuclear factor- κB (NF- κB) and AP-1 pathways [9–15]. Equally important, acidic pH leads to an increase in OCT4, cyclin D1 and TGFα factors which play important roles in the proliferation of cancer cells [10, 14, 16].
It has been shown that cancer cells increase expression of proteases such as Cathepsin B, D, L and matrix Metalloproteinases 1, 2, 9 in the acidic microenvironment, in vitro and in vivo [6, 7, 14]. Acidity is shown to have a role in the resistance to chemotherapy drug absorption and weakly basic chemotherapeutic drugs such as doxorubicin [9, 17, 18].
T-cell acute lymphoblastic leukemia (T-ALL) is characterized by an overproduction of T-cell progenitor called lymphoblasts or leukemic blasts and mainly occurs in children and adolescents. T-ALL is known by its clinical and biological characteristics and is generally associated with more unfavorable clinical features such as bone marrow infiltration, leukocytosis, mediastinal enlargement and central nervous system (CNS) damage. Although treatment outcome in patients with T-ALL has improved in recent years, the majority of patients still relapse during the long term follow-up [19–21]. In previous publications, researchers evaluated the effects of extracellular acidic pH on solid tumors, including breast, ovarian and prostate cancer, in vitro and in vivo. In this study, we investigated the effects of extracellular acidic pH on proliferation, invasion and drug dependent apoptosis in Jurkat cell line as a model cancer cell for the experimental study of T-ALL.
Material and Methods
Cell Culture
Jurkat T-Leukemic cell line was obtained from national cell bank (Pasteur Institute, Tehran, Iran) and was maintained RPMI-1640 culture medium supplemented with 10 % Fetal Bovine Serum (FBS), 2 mM L-glutamine and 1× pen/strep antibiotic (Gibco; Carlbod, CA). The pH of medium was adjusted to 6.6 by 90 % Lactic Acid (Merck) [4, 7, 8].
Cell Proliferation Assay
The effect of acidic pH on Jurkat cells was assessed using MTT assay and direct cell counting. Initially, 104 Jurkat cells were cultured at pH 6.6 and 7.4 in 96 well plates for 12 days and MTT assay was performed using a cell growth determination kit (Sigma-Aldrich) in accordance to the manufacturer’s protocol.
Invasion Assay
In vitro invasion assay was performed with the 24-well culture insert plates with 8.0 μm pores (SPL Life Science, Pocheon, South Korea) and the upper chamber was covered by 100 μl Extracellular Matrix (Sigma-Aldrich; ST, Louis, MO). Then, the cells were plated in the upper chamber in 100 μl RPMI-1640 medium supplemented with 1 % FBS (Gibco) in both acidic and normal pH individually. The lower chamber was filled with 650 μl RPMI-1640 medium supplemented with 20 % FBS (Gibco). The cells were incubated for 24 h and the invaded cells into the lower chamber were counted by a hemocytometer.
Annexin V/PI Staining
Annexin V FITC Apoptosis Detection Kit (Sigma-Aldrich) was used according to the manufacturer’s guideline. The cells were treated with 0.3 μg/ml doxorubicin [22] (adriblastine® phamrmacia;Italia S.P.A) for 16 h. Then, the cells were washed with PBS and re-suspended in 1× binding buffer and then were incubated with 1 μl annexin V-FITC. Finally, the cells were incubated with 2 μl Propidium Iodide in 100 μl cell suspension with binding buffer for 10 min in darkness at room temperature. Afterwards, the cells were analyzed by Attune® Acoustic Focusing Cytometer (Applied Biosystems).
Quantitative RT- PCR
Total RNA was extracted from the cells at acidic and normal pH using TRIZOL (Life Technologies, USA) following the manufacturer’s instruction. Reverse transcription was performed using M-MuLV Reverse Transcriptase Kit (Fermentas, USA). cDNA was subjected to Real-Time PCR (StepOnePlus Real-Time PCR System; Applied Biosystems) using a SYBR Green Master Mix Kit (Ampliqon; Odense, Denmark). BAX and matrix metalloproteinase-9 (MMP-9) primers were selected to assess the apoptosis and cell invasion, respectively and Glyceraldehydes-3-phosphate dehydrogenase (GAPDH) was regarded as an endogenous control. Primers used for Real-Time-PCR analysis were presented in detail in Table 1.
Table 1.
The PCR primers sequences for quantitative Real-Time PCR
| Gene | Sequence (5`➔3`) | Product length (bp) |
|---|---|---|
| BAX- F | CAAACTGGTGCTCAAGGC | 178 |
| BAX -R | CACAAAGATGGTCACGGTC | |
| MMP-9- F | CGGACCAAGGATACAGTTTGT | 116 |
| MMP-9 -R | CTCAGTGAAGCGGTACATAGG | |
| GAPDH -F | CTCTCTGCTCCTCCTGTTCG | 114 |
| GAPDH -R | ACGACCAAATCCGTTGACTC |
Cell Staining
Giemsa (Sigma, Munich, Germany) staining was performed to evaluate the morphology of cells treated with doxorubicin. The slides were prepared using the Cytospin™ 4 Cytocentrifuge (ThermoFisher, USA ) with 2000 for 15 min.
Statistical Analysis
All tests were carried out in triplicate. Statistical analysis was performed using an unpaired student test while comparing two groups and conducting one-way ANOVA with Bonferroni’s multiple comparison tests for three groups or more by SPSS 18.0. Statistical differences were considered significant when P-value was less than 0.05.
Results
The Rate of Proliferation in Jurkat Cell Line at Acidic pH Was More than Normal pH
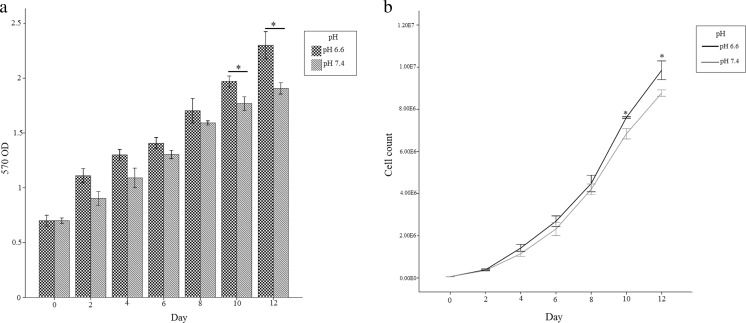
To determine the effect of acidic pH on proliferation of Jurkat cell, we used two methods: direct cell counting and MTT assay. The results demonstrated that the proliferation of Jurkat cell line has been increased at pH of 6.6 as compared to 7.4 specifically in the days of 10th and 12th (p < 0.05) (Fig. 1).
Fig. 1.
The graphs show the proliferation assay. a Cell growth determination by MTT assay. b Direct cell counting. Results comparing Jurkat cell line at pH 6.6 and pH 7.4. Values are shown as mean ± SEM. *: p < 0.05
Leukemic Cells at Acidic pH Medium Was more Invasive than Normal Medium
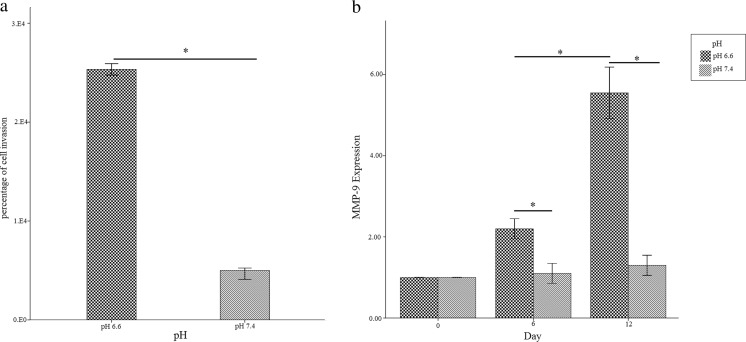
After 12 days of the Jurkat cell culture at normal and acidic pH, the cells were prepared for invasion assay. The results of invasion assay showed that invasion potential of Jurkat cells at pH 6.6 (25.1 %) was more than (4.3 folds) pH 7.4 (5.8 %). Since, it has been demonstrated that the presence of matrix metalloproteinases in tumor tissues was correlated with invasion properties of cancer cells, MMP9 gene expression was analyzed by real time PCR. At pH 6.6, Jurkat cells showed an increase in gene expression of MMP9 in compared to the cells which were cultured at pH 7.4 on the 6th and 12th day of culture (P < 0.05) (Fig. 2). These results showed that Jurkat cells at pH 6.6 was more invasive than Jurkat cells were cultured at pH 7.4 (P < 0.05).
Fig. 2.
Bar graphs show the invasion assay results comparing Jurkat cell line at pH 6.6 and pH 7.4. a Invasion assay results; b Real-Time PCR analysis of gene expression of MMP-9. Values are shown as mean ± SEM. *: p < 0.05
Jurkat Cell Line at pH 6.6 Was more Resistant to Apoptosis than pH 7.4
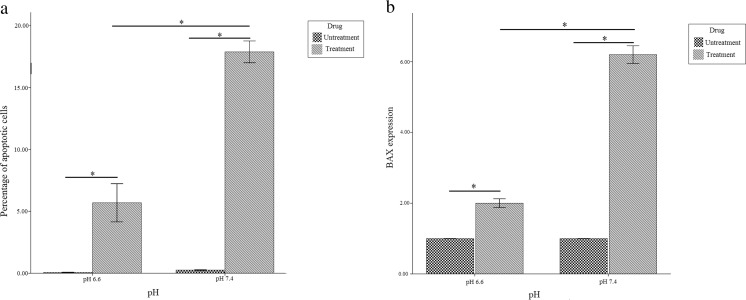
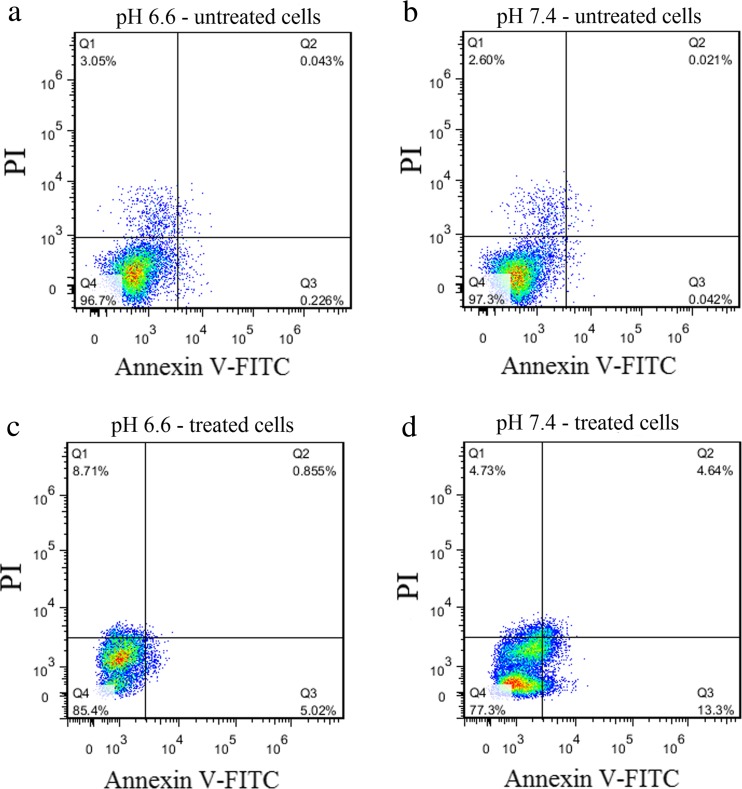
To evaluate the resistance of Jurkat cells cultured at pH 6.6 and 7.4 against doxorubicin, Annexin-PI staining was performed. The results showed that the level of apoptosis in Jurkat cells cultured at acidic and normal pH were 5.87 % and 17.94 %, respectively (Fig. 3a and Fig. 4).
Fig. 3.
Bar graphs show resistance to apoptosis induced by doxorubicin at pH 6.6 compared to pH 7.4 a Annexin V-PI staining; b Real time PCR analysis of gene expression of BAX. Values are shown as mean ± SEM. *: p < 0.05
Fig. 4.
Evaluation of apoptosis by Annexin V-FITC/PI staining and analysis by flow cytometry in Jurkat cell line. a Untreated cell with doxorubicin at pH 6.6; b Untreated cell with doxorubicin at pH 7.4; c) Treated cell with doxorubicin at pH 6.6; d)Treated cell with doxorubicin at pH 7.4
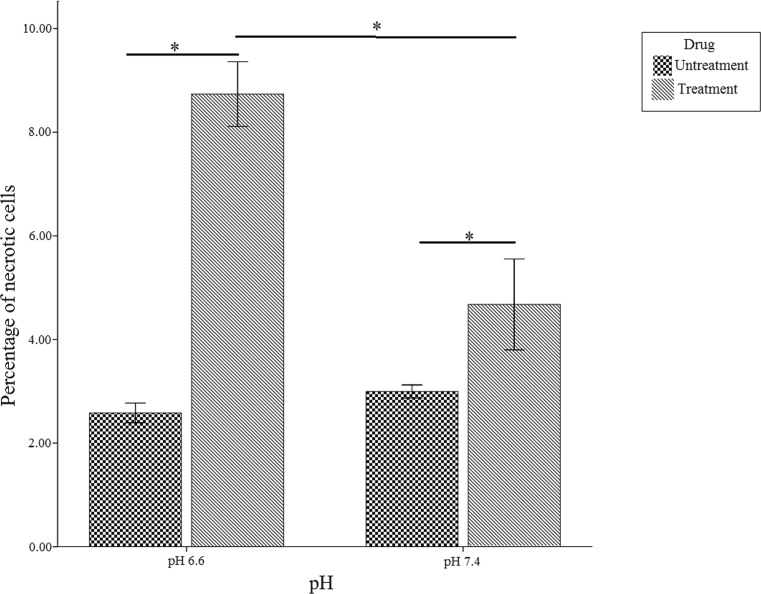
To confirm the Annexin-PI staining results, gene expression levels of BAX (anti-apoptotic protein) were also analyzed by real time PCR. Quantitative gene expression analysis of BAX showed increased expression of BAX in cells were cultured at normal pH (3.1 fold) (P < 0.05) in comparison to cells were cultured at acidic pH (Fig. 3b). Moreover, the percentage of necrotic cells in the acidic and normal medium was 8.71 % and 4.73 %, respectively (Fig. 5).
Fig. 5.
Bar graph shows an increase in the percentage of necrotic cell at pH 6.6 compared to pH 7.4. Values are shown as mean ± SEM. *:p < 0.05
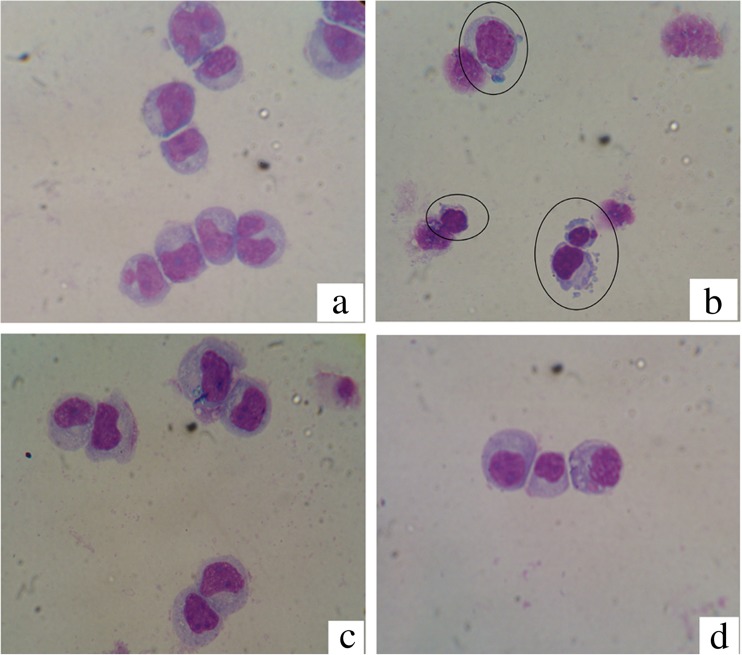
The morphological studies were assessed by Giemsa staining. The number of Jurkat cells which had some features of apoptotic cell (chromatin condensation, nuclear fragmentation, cell membrane blebbing and cell shrinkage) at normal pH was more than cells were cultured at acidic pH (Fig. 6).
Fig. 6.
The morphological changes of Jurkat cell line stained with Giemsa. a Untreated cell with doxorubicin at pH 7.4; b Treated cell with doxorubicin at pH 7.4; c Untreated cell with doxorubicin at pH 6.6; d Treated cell with doxorubicin at pH 6.6
Discussion
Genetic mutations and epigenetic alterations play crucial roles in cancers, but cell proliferation requires nutrients, energy, and biosynthetic activity to duplicate all the macromolecular components during each passage through the cell cycle [23–25]. It is not surprising that the cancerous cells alter its metabolism pathway from oxidative phosphorylation (oxphos) to aerobic glycolysis to meet their needs [3, 4, 7, 26]. The final product of aerobic glycolysis is lactic acid which leads to extracellular acidity. Decreasing tumor pH and hypoxia [8, 12, 27] are correlated with poor prognosis [9, 28].
In this study, we evaluated some properties of cancer cells. Invasion potential was evaluated by invasion assay, in vitro. The results showed that the invasion level of Jurkat cells at pH 6.6 (acidic pH) was more than that at pH 7.4. Also, MMP9 gene expression was correlated with invasion assays results. Previous studies demonstrated that the expression of genes involved in metastasis such as MMP-9 and cathapsin B increases at the acidic condition in the tumor tissue [6, 7, 26, 29–32]. Cathepsin L also amplifies the proteinase cascade through the activation of urokinase-type plasminogen activator (uPA) which is critical for metastatic cascade in tumor cells [26]. MMP-9 plays a vital role in promoting tumor invasion and tissue remodeling, and participates in the release of cell surface precursor-form of many growth factors by proteolysis of several ECM components [2, 29–31]. Glunde et al. (2003) reported filopodia formation which was observed more frequently in highly metastatic breast cancer cells maintained in acidic pH [32]. Raymond et al. (2008) reported that the acidosis promotes more invasive phenotypes of melanoma cells in vitro and in vivo [29].
Cell counting and MTT assay were performed to examine the proliferation and viability of Jurkat cells. The results indicated that cells which were cultured at pH 6.6 had more proliferation potential than that at pH 7.4. In many studies, it was reported that hypoxia caused by acidic extracellular increases the expression of HIF-α, C-myc, OCT4, Cyclin D1 and TGF α genes that play essential roles in the proliferation of cancer cells [9, 11, 15, 16, 33]. These studies have reported that acidic conditions in the tumor microenvironment promote autophagy which occurs as a survival adaptation in these conditions [34, 35]. Hashim et al.(2011) showed that non-volatile basic buffer inhibit proliferation and invasion of cancer cells in a mouse cancer model [36].
Metabolic reprogramming of cancer cells tolerates stress and induces drug resistance [37, 38]. Hexokinase II (HK II) is a key glycolytic enzyme that is transcriptionally enhanced in many cancer types [27]. HK II can bind to mitochondria and interact with the mitochondrial permeability transition pore Voltage-Dependent Anion Channel (VDAC) to serve as a cell survival molecule by preventing BAX/BAK oligomerization on the mitochondrial membrane and thus suppressing the apoptosis while preserving the mitochondrial integrity [27, 39, 40].Therefore, we evaluated the quantitative gene expression of BAX as an anti-apoptotic protein. According to apoptosis results; the gene expression of BAX in the cells were cultured in normal medium was more than the cells which were cultured at acidic medium. Annexin V-PI staining showed that the cells which were cultured in acidic medium were more resistant to doxorubicin compared to normal medium. Raghunand et al. (1999) reported that the extracellular pH of human breast tumor is acidic, which reduces the cytotoxicity of doxorubicin [17]. Thews et al. (2006) showed that acidic extracellular microenvironment can induce activity of p-glycoprotein which leads to reduce the concentration and cytotoxicity of chemotherapeutic drug by pumping entered drug to outside [41].
Lan et al. (2007) demonstrated microenvironmental pH can control the type of cell death. They mentioned normal pH kept an intracellular ATP level sufficient to let apoptotic mechanisms, while the acidic pH induced ATP depletion that directly causes necrosis [42]. In this regard, we also showed that the percentage of necrotic cells at pH 6.6 was more than that at pH 7.4. Necrosis is associated with breakdown of cancer tissues with the release of nucleic acid and other intra-cellular components which result in life threatening complications such as renal failure, arrhythmias, and seizures [43, 44].
To conclude, acidic pH as a result of aerobic glycolysis leads to the increased proliferation, invasion, necrosis and reduced drug-induced apoptosis in Jurkat cell line. Thus, it has been suggested that the leukemia progression should be limited by controlling the level of pH in the microenvironment of cancer cells.
Acknowledgments
The authors thank Stem Cell Technology Research Center for technical assistance supports.
References
- 1.López-Lázaro M. A New View of Carcinogenesis and an Alternative Approach to Cancer Therapy. Mol Med. 2010;16(3–4):144–153. doi: 10.2119/molmed.2009.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merl D, Chen JL-Y, Chi J-T, West M. An Integrative Analysis of Cancer Gene Expression Studies Using Bayesian Latent Factor Modeling. The annals of applied statistics. 2009;3(4):1675. doi: 10.1214/09-AOAS261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calderon-Montano JM, Burgos-Moron E, Perez-Guerrero C, Salvador J, Robles A, Lopez-Lazaro M (2011) Role Of The Intracellular pH In The metabolic switch between oxidative phosphorylation and aerobic glycolysis-relevance to cancer
- 4.Choi SYC, Collins CC, Gout PW, Wang Y. Cancer-Generated Lactic Acid: a Regulatory, Immunosuppressive Metabolite? J Pathol. 2013;230(4):350–355. doi: 10.1002/path.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva AS, Yunes JA, Gillies RJ, Gatenby RA. The Potential Role of Systemic Buffers in Reducing Intratumoral Extracellular pH and Acid-Mediated Invasion. Cancer Res. 2009;69(6):2677–2684. doi: 10.1158/0008-5472.CAN-08-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, Hashim AI, Morse DL, Raghunand N, Gatenby RA. Bicarbonate Increases Tumor pH and Inhibits Spontaneous Metastases. Cancer Res. 2009;69(6):2260–2268. doi: 10.1158/0008-5472.CAN-07-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato Y, Ozawa S, Miyamoto C, Maehata Y, Suzuki A, Maeda T, Baba Y. Acidic Extracellular Microenvironment and Cancer. Cancer Cell Int. 2013;13(1):1. doi: 10.1186/1475-2867-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCarty MF, Whitaker J. Manipulating Tumor Acidification as a Cancer Treatment Strategy. Altern Med Rev. 2010;15(3):264–272. [PubMed] [Google Scholar]
- 9.Fukumura D, Xu L, Chen Y, Gohongi T, Seed B, Jain RK. Hypoxia and Acidosis independently up-Regulate Vascular Endothelial Growth Factor Transcription in Brain Tumors in Vivo. Cancer Res. 2001;61(16):6020–6024. [PubMed] [Google Scholar]
- 10.López-Lázaro M. HIF-1: Hypoxia-Inducible Factor or Dysoxia-Inducible Factor? FASEB J. 2006;20(7):828–832. doi: 10.1096/fj.05-5168hyp. [DOI] [PubMed] [Google Scholar]
- 11.Gordan JD, Bertout JA, Hu C-J, Diehl JA, Simon MC. HIF-2α Promotes Hypoxic Cell Proliferation by Enhancing c-Myc Transcriptional Activity. Cancer Cell. 2007;11(4):335–347. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masson N, Ratcliffe PJ. Hypoxia Signaling Pathways in Cancer Metabolism: the Importance of Co-Selecting Interconnected Physiological Pathways. Cancer & metabolism. 2014;2(1):1. doi: 10.1186/2049-3002-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu L, Fidler IJ. Acidic pH-Induced Elevation in Interleukin 8 Expression by Human Ovarian Carcinoma Cells. Cancer Res. 2000;60(16):4610–4616. [PubMed] [Google Scholar]
- 14.Rofstad EK, Mathiesen B, Kindem K, Galappathi K. Acidic Extracellular pH Promotes Experimental Metastasis of Human Melanoma Cells in Athymic Nude Mice. Cancer Res. 2006;66(13):6699–6707. doi: 10.1158/0008-5472.CAN-06-0983. [DOI] [PubMed] [Google Scholar]
- 15.Xu L, Fukumura D, Jain RK. Acidic Extracellular pH Induces Vascular Endothelial Growth Factor (VEGF) in Human Glioblastoma Cells via ERK1/2 MAPK Signaling Pathway MECHANISM OF LOW pH-INDUCED VEGF. J Biol Chem. 2002;277(13):11368–11374. doi: 10.1074/jbc.M108347200. [DOI] [PubMed] [Google Scholar]
- 16.Gordan JD, Lal P, Dondeti VR, Letrero R, Parekh KN, Oquendo CE, Greenberg RA, Flaherty KT, Rathmell WK, Keith B. HIF-α Effects on c-Myc Distinguish two Subtypes of Sporadic VHL-Deficient Clear Cell Renal Carcinoma. Cancer Cell. 2008;14(6):435–446. doi: 10.1016/j.ccr.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raghunand N, He X, Van Sluis R, Mahoney B, Baggett B, Taylor C, Paine-Murrieta G, Roe D, Bhujwalla Z, Gillies R. Enhancement of Chemotherapy by Manipulation of Tumour pH. Br J Cancer. 1999;80(7):1005. doi: 10.1038/sj.bjc.6690455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Milito A, Fais S (2005) Tumor acidity, chemoresistance and proton pump inhibitors [DOI] [PubMed]
- 19.Alimoghaddam K, Jahani M, Mousavi S, Bahar B, Hamidieh A, Vaezi M, Ghaffari F, Jalali A, Sharifi-Aliabadi L, Ghavamzadeh A. Allogeneic Stem Cell Transplantation Outcome in Acute Lymphoblastic Leukemia Patients. International Journal of Hematology-Oncology and Stem Cell Research. 2015;6(4):1–4. [Google Scholar]
- 20.Onciu M. Acute Lymphoblastic Leukemia. Hematol Oncol Clin N Am. 2009;23(4):655–674. doi: 10.1016/j.hoc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Aifantis I, Raetz E, Buonamici S. Molecular Pathogenesis of T-Cell Leukaemia and Lymphoma. Nat Rev Immunol. 2008;8(5):380–390. doi: 10.1038/nri2304. [DOI] [PubMed] [Google Scholar]
- 22.ÀÏÎÏÒÎÇÀ ÑÀ, ÐÀÇËÈ È, ÏÐÅÏÀÐÀÒÀÌÈ ÍÏ. Comparative Analysis of Apoptosis Induced by Various Anticancer Drugs in Jurkat Cells. Exp Oncol. 2001;23:46–50. [Google Scholar]
- 23.Baxter E, Windloch K, Gannon F, Lee JS. Epigenetic Regulation in Cancer Progression. Cell & bioscience. 2014;4(1):1. doi: 10.1186/2045-3701-4-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The Biology of Cancer: Metabolic Reprogramming Fuels Cell Growth and Proliferation. Cell Metab. 2008;7(1):11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 25.You JS, Jones PA. Cancer Genetics and Epigenetics: two Sides of the Same Coin? Cancer Cell. 2012;22(1):9–20. doi: 10.1016/j.ccr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calorini L, Peppicelli S, Bianchini F. Extracellular Acidity as Favouring Factor of Tumor Progression and Metastatic Dissemination. Exp Oncol. 2012;34(2):79–84. [PubMed] [Google Scholar]
- 27.Lu W, Logsdon CD, Abbruzzese JL (2013) Cancer Metabolism and its Therapeutic Implications. Journal of Cell Science & Therapy 4(2):143–152
- 28.Schornack PA, Gillies RJ. Contributions of Cell Metabolism and H+ Diffusion to the Acidic pH of Tumors. Neoplasia. 2003;5(2):135–145. doi: 10.1016/S1476-5586(03)80005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moellering RE, Black KC, Krishnamurty C, Baggett BK, Stafford P, Rain M, Gatenby RA, Gillies RJ. Acid Treatment of Melanoma Cells Selects for Invasive Phenotypes. Clinical & experimental metastasis. 2008;25(4):411–425. doi: 10.1007/s10585-008-9145-7. [DOI] [PubMed] [Google Scholar]
- 30.Yu Q, Stamenkovic I. Cell Surface-Localized Matrix Metalloproteinase-9 proteolytically Activates TGF-β and Promotes Tumor Invasion and Angiogenesis. Genes Dev. 2000;14(2):163–176. [PMC free article] [PubMed] [Google Scholar]
- 31.Koujan SE, Gargarib BP, Khalili M (2015) Matrix Metalloproteinases and Breast Cancer. Thrita 4(1):e21959
- 32.Glunde K, Guggino SE, Solaiyappan M, Pathak AP, Ichikawa Y, Bhujwalla ZM. Extracellular Acidification Alters Lysosomal Trafficking in Human Breast Cancer Cells. Neoplasia. 2003;5(6):533–545. doi: 10.1016/S1476-5586(03)80037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones RG, Thompson CB. Tumor Suppressors and Cell Metabolism: a Recipe for Cancer Growth. Genes Dev. 2009;23(5):537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marino ML, Pellegrini P, Di Lernia G, Djavaheri-Mergny M, Brnjic S, Zhang X, Hägg M, Linder S, Fais S, Codogno P. Autophagy Is a Protective Mechanism for Human Melanoma Cells under Acidic Stress. J Biol Chem. 2012;287(36):30664–30676. doi: 10.1074/jbc.M112.339127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wojtkowiak JW, Rothberg JM, Kumar V, Schramm KJ, Haller E, Proemsey JB, Lloyd MC, Sloane BF, Gillies RJ. Chronic Autophagy Is a Cellular Adaptation to Tumor Acidic pH Microenvironments. Cancer Res. 2012;72(16):3938–3947. doi: 10.1158/0008-5472.CAN-11-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hashim AI, Cornnell HH, Ribeiro MLC, Abrahams D, Cunningham J, Lloyd M, Martinez GV, Gatenby RA, Gillies RJ. Reduction of Metastasis Using a Non-Volatile Buffer. Clinical & experimental metastasis. 2011;28(8):841–849. doi: 10.1007/s10585-011-9415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Y, Butler EB, Tan M. Targeting Cellular Metabolism to Improve Cancer Therapeutics. Cell Death Dis. 2013;4(3):e532. doi: 10.1038/cddis.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Wang X. The Expanding Role of Mitochondria in Apoptosis. Genes Dev. 2001;15(22):2922–2933. [PubMed] [Google Scholar]
- 40.Azoulay-Zohar H, Israelson A, Salah A-H, Shoshan-Barmatz V. In Self-Defence: Hexokinase Promotes Voltage-Dependent Anion Channel Closure and Prevents Mitochondria-Mediated Apoptotic Cell Death. Biochem J. 2004;377(2):347–355. doi: 10.1042/bj20031465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thews O, Gassner B, Kelleher DK, Schwerd G, Gekle M. Impact of Extracellular Acidity on the Activity of P-Glycoprotein and the Cytotoxicity of Chemotherapeutic Drugs. Neoplasia. 2006;8(2):143–152. doi: 10.1593/neo.05697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lan A, Lagadic-Gossmann D, Lemaire C, Brenner C, Jan G. Acidic Extracellular pH Shifts Colorectal Cancer Cell Death from Apoptosis to Necrosis upon Exposure to Propionate and Acetate, Major End-Products of the Human Probiotic Propionibacteria. Apoptosis. 2007;12(3):573–591. doi: 10.1007/s10495-006-0010-3. [DOI] [PubMed] [Google Scholar]
- 43.Bose P, Qubaiah O. A Review of Tumour Lysis Syndrome with Targeted Therapies and the Role of Rasburicase. J Clin Pharm Ther. 2011;36(3):299–326. doi: 10.1111/j.1365-2710.2011.01260.x. [DOI] [PubMed] [Google Scholar]
- 44.Cairo MS, Bishop M. Tumour Lysis Syndrome: New Therapeutic Strategies and Classification. Br J Haematol. 2004;127(1):3–11. doi: 10.1111/j.1365-2141.2004.05094.x. [DOI] [PubMed] [Google Scholar]