Abstract
Several changes have been described in the stroma surrounding a tumor, including changes in cellular composition, altered extracellular matrix composition and organization, and increases in stiffness. Tumor cells are influenced by the composition, organization, and mechanical properties of the microenvironment, and by signals from stromal cells. Here we sought to test whether signaling from stromal fibroblasts and/or the small change in stiffness observed in vivo surrounding epithelial tumors regulates tumor cell invasion from a model of a tumor in situ. We generated a novel tumor in situ model system in which a tumor spheroid is encased within a collagen-IV containing membrane and further encased within a collagen-I matrix of in vivo stiffness with or without fibroblasts. Effects of the matrix, fibroblasts or fibroblast signals were determined by observing the invasion of tumor cells into the matrix. Effects of reciprocal tumor cell signaling upon fibroblasts were determined by observing markers of fibroblast activation. We found that a stiffened matrix led to increased dissemination of MDA-MB-231 cells from tumor spheroids when no fibroblasts were present and that MCF10A cells maintained a more normal organization with a stiffened matrix. The presence of fibroblasts, or fibroblast conditioned media, attenuated the effect upon MDA-MB-231 cells. We also observed an attenuation of fibroblast activation associated gene expression in the presence of MDA-MB-231 cells, with a paradoxical increase in activation associated contractile activity. Furthermore, we identified osteoprotegerin as a soluble factor released by fibroblasts in the stiffened environment that is key to the inhibition of cell invasion.
Electronic supplementary material
The online version of this article (doi:10.1007/s12307-016-0188-z) contains supplementary material, which is available to authorized users.
Keywords: Tumor in situ, 3D hydrogel, Extracellular matrix, Mechanotransduction, Invasion
Introduction
The tumor microenvironment (TME) surrounding mammary tumors is approximately four fold stiffer than normal tissue [1, 2]. This increase in stromal stiffness is dwarfed by the increase in stiffness of the solid tumor itself, in which stiffness may be increased 20 fold or more [1]. Both tumor cells and tumor associated cells have been shown to be sensitive to the mechanical properties of the extracellular matrix (ECM) and increasing ECM crosslinking in vivo, which increases stiffness, has been shown to drive tumor progression and invasion [1–5]. Many studies have investigated TME stiffness in directing epithelial morphology and control of invasive phenotypes, but these studies typically use models which do not adequately represent the physiological organization of the tissue. Many were done in 2D rather than 3D, others do not fully or appropriately control biophysical properties such as stiffness, and still others use mixed and/or randomly dispersed cell populations that do not recapitulate the in vivo tissue and/or ECM organization [1, 5–9]. Further, much of the in vitro research surrounding tumor invasion and metastasis does not take into consideration the basic anatomy of epithelial tumors – they begin as normal epithelium and typically progress to a tumor in situ, which remains encapsulated in a basement membrane (BM) and physically separated from the cells and extracellular matrix (ECM) of the stroma before invading the surrounding tissue. The ECM of the stroma differs in composition, structure and arrangement from the ECM of the basement membrane (BM) [10]. Each ECM component is recognized by a different subset of cell-matrix binding proteins that can differ significantly in their downstream signaling, so the makeup of the ECM can have diverse effects on anchorage-dependent signaling [11, 12].
Here we designed a model of a tumor in situ made up of a BM encapsulated tumor spheroid embedded in a collagen-I 3D hydrogel seeded with fibroblasts to mimic an in vivo set of ligands in a physiological arrangement. We varied the collagen-I hydrogel stiffness to mimic either normal breast (~200 Pa) or breast tumor adjacent stiffness (~800 Pa) using short polyethylene glycol polymers (12-atoms in length), functionalized with the addition of N-hydroxysuccinimide groups, which crosslinks the collagen-I matrix much like physiological crosslinks formed in vivo [13]. This allows us to alter the gel stiffness independently of gelation temperature, pH or collagen concentration, as each of these factors can alter the structure or ligand availability of the collagen-I matrix [14–16]. This stiffness controlled collagen-I hydrogel encapsulates both the BM coated tumor spheroids as well as fibroblasts to form a biomimetic tumor in situ.
We have previously shown that a modest 4X change in ECM stiffness leads to the activation of fibroblasts to a carcinoma-associated fibroblast-like state [17]. Carcinoma-associated fibroblasts (aka tumor associated fibroblasts; CAFs) play key roles in tumor growth and metastasis via both paracrine and juxtacrine interactions and are found surrounding and infiltrating tumors [18–23]. Multiple studies have shown that mixing CAFs with neoplastic cells prior to implantation in a mouse host results in larger tumors and more metastases when compared to injecting neoplastic cells alone – while recent studies with genetically engineered mice suggest that some components of the tumor stroma, possibly CAFs, may restrain tumor progression [24–27]. One possible paracrine signal which is secreted by CAFs is osteoprotegerin (OPG) [28, 29], a soluble decoy receptor for receptor activator of nuclear factor-κB ligand (RANKL). OPG has been shown to block RANKL induced migration in cells expressing the RANKL receptor (RANK), such as MDA-MB-231 cells [30, 31]. RANKL binding to RANK activates Src, Akt and MAPK/ERK signaling pathways to induce a migratory phenotype [30, 31], and OPG can sequester RANKL to inhibit RANK activation [32].
In this study, we examine the role of stromal stiffness and signals from fibroblasts in directing epithelial invasion and dissemination from a tumor in situ. We vary stromal stiffness and the availability of physical and paracrine signals from fibroblasts to interrogate the effect of these stromal features on tumor dissemination. We observed a dramatic increase in invasive behavior due to stromal stiffness when epithelial cells were cultured without stromal cells, but when stromal cells were included in the stiffened matrix, this effect was attenuated. We found that the stiffened matrix induced signaling from stromal fibroblasts that inhibited the stiffness-induced invasive behavior of the tumor cells, and further that this activity was mediated by osteoprotegerin. Together, these data suggest that this paracrine signal from stromal fibroblasts can override the effects of the mechanical environment on the tumor cells.
Methods
Cells and Cell Maintenance
MCF10A human mammary epithelial cells (product ATCC CRL-10,317, American Type Culture Collection) were cultured in DMEM/F12 (Corning) supplemented with 5 % horse serum (Atlanta Biologicals), 1 % L-glutamine (Corning), 1 % penicillin/streptomycin (Corning), 500 ng / mL hydrocortisone (Sigma Aldrich), 10 μg / mL insulin (Invitrogen), 100 ng / mL cholera toxin (Sigma Aldrich), and 20 ng / mL epidermal growth factor (Sigma Aldrich) at 37 °C under 5 % CO2. MCF10A cells in this study were used from passage 5–30. MDA-MB-231 human mammary epithelial tumor cells (product ATCC HTB-26, American Type Culture Collection) were cultured in DMEM (Corning) supplemented with 5 % fetal bovine serum (Atlanta Biologicals), 1 % L-glutamine (Corning) and 1 % penicillin/streptomycin (Corning) at 37 °C under 5 % CO2. MDA-MB-231 cells in this study were used from passage 20–40. Human breast fibroblasts (CCD-1065Sk, product ATCC CRL-2077, American Type Culture Collection) were cultured in MEM (Corning) supplemented by 10 % fetal bovine serum (Atlanta Biologicals), 1 % L-glutamine (Corning), 1 mM sodium pyruvate (Corning) and 1 % penicillin/streptomycin (Corning) and maintained at 37 °C under 5 % CO2. All cell lines were obtained within 6 months of experimentation and were authenticated by ATCC. Fibroblast cells were cultured from a minimum of 10 % to a maximum of 75 % confluence and only cells from passages 2–8 were used. Culture media suited for CCD-1065Sk fibroblast (HFb) cells was used in all experiments and does not alter cellular viability as both epithelial cell lines demonstrate an increased growth rate with HFb media (Fig. S1-c & d). All experimental conditions utilized the base media as defined for human breast fibroblasts (CCD-1065Sk) with any deviation (conditioned media, additional protein/antibody) described for each experiment.
Preparation of Spheroids and Hydrogels
Basement membrane coated epithelial spheroids were formed by adding 50,000 (MDA-MB-231) or 100,000 (MCF10A) cells in 50 μL of culture media to wells of a 96-well low attachment plate (product 7007, Corning). Plates were incubated for 2 days at 37 °C under 5 % CO2 in an incubator with a low intensity constant vibration (from incubator circulation fan and an orbital shaker) to induce cell coalescence. On day two, 25 μL growth factor reduced Matrigel® (product 354,230, Corning, Lots 27,690, 3,227,775, 4,260,001) diluted in OPTI-MEM I (product 31,985–070, Life Technologies) to 3 mg / mL was added to each well for a final GFR-Matrigel® concentration of 1 mg / mL (below the critical gelation concentration for Matrigel® which allows ECM components to be freely available for assembly). Plates were cultured for an additional 2 days before spheroids were harvested.
Soft and stiff hydrogels were prepared as before [17]. Briefly, collagen-I (product 150,026, MP Biomedicals) was re-suspended in 0.02 N acetic acid at 3 mg / mL. Collagen solution was combined with neutralizing solution (0.52 M Sodium Bicarbonate, 0.4 M HEPES and 0.08 N Sodium Hydroxide), cellular suspension media (DMEM +5 % BSA) with or without fibroblasts as indicated, and poly(ethylene glycol)-di(succinic acid N-hydroxysuccinimide ester) (PEG-diNHS) dissolved in DMSO (100 mg / mL, product E3257, Sigma-Aldrich, molecular weight 456.36) or DMSO control. The ratio for gel formation was 615 : 308 : 77 : 4 for collagen-I : suspension media: neutralizing solution: PEG-diNHS / DMSO. Hydrogel mixture was kept on ice.
To form fibroblast-only hydrogels, 100 μL droplets of collagen-I/fibroblast mixture were plated in 10 cm dishes (eight droplets per dish) and allowed to gel for 45 min at 37 °C. The surface tension of the collagen-I solution and hydrophobicity of the culture dish do not allow the droplets to spread, so they retain a uniform and replicable bead size. After initial gelation, 10 mL fibroblast culture media was added to the dish and the hydrogels were released from the surface with a plastic spatula. To make spheroid-containing hydrogels, pre-formed spheroids in 2 μL media droplets were transferred to 10 cm dishes (eight spheroids per dish). 100 μL of collagen-I solution was added to each spheroid droplet, briefly mixed in the pipette tip and re-deposited in the dish. Dishes were incubated for 45 min at 37 °C to allow gels to form, then 10 mL culture media was added to the dish and hydrogels were released from the surface with a spatula. To form spheroid-fibroblast hydrogels, the same procedure was employed, except that the collagen-I hydrogel mixture was pre-populated with fibroblasts as was done with the fibroblast-only hydrogels. In some cases, after formation, gels were transferred to 12-well plates (1 gel in 1.5 mL fibroblast culture media per well) to monitor each spheroid individually. All hydrogels were cultured on an orbital shaker to ensure they did not reattach to the culture vessel.
Microscopy
Confocal fluorescence and phase contrast images of live or fixed cells were taken with an inverted microscope (DMI 4000B Inverted Microscope, LEICA Microsystems), with a 10 X objective (N PLAN 10X/0.25 PH 1, ∞/−/B, LEICA Microsystems) utilizing an ORCA-ER digital camera (model C4742–95, Hamamatsu) and Volocity imaging software (Improvision/PerkinElmer). Images for dissemination and spheroid size measurements were taken using phase contrast. Multiple images were stitched together using Fiji (ImageJ) software [33] with the MosaicJ plugin. Disseminated cells were manually counted and the area and circularity of spheroids were measured by drawing a ROI around the spheroid body. Images for growth curves in differential media were taken at 10 X using phase contrast. Five random images were taken daily, manually counted and counts averaged. Count per image was converted to cells / cm2 utilizing the known dimensions of the culture dish.
Confocal Immunofluorescence
Hydrogels were prepared and cultured for 3 days. Gels were fixed with 37 °C, 4 % paraformaldehyde solution for 45 min, permeabilized with 0.25 % Triton-X solution for 45 min at room temperature except in the case of annexin V staining (see below), washed with PBS + 0.05 % sodium azide (PBS-NaAzide) and incubated for 2 h or overnight in blocking solution (5 % goat serum, 1 % BSA, 0.05 % NaN3 in PBS) at 4 °C. Samples were then incubated for 24 h with primary antibodies against proteins of interest (Collagen-IV @ 1:500, product GTX26311, GeneTex; α-SMA @ 1:200, product MA1–37,027, Thermo Scientific; Palladin @ 1:200, product A3986, Sigma-Aldrich; Ki-67 @ 1:100, product 550,609, BD Biosciences; Annexin V @ 1:500, product PA5–27,872, Thermo Scientific) followed by three 60 min washes with PBS-NaAzide. Samples were then incubated for 24 h with secondary antibodies (@ 1:300, Alexa Fluor, Life Technologies), rhodamine phalloidin for F-actin when indicated (product P1951, Sigma-Aldrich) and counterstained with DAPI, again followed by three 60 min washes with PBS-NaAzide. Immunostained hydrogels were stored and imaged in 8-chamber cell culture slides (product 154,534, Thermo Scientific) with 100 μL PBS-NaAzide. PBS-NaAzide was aspirated before imaging of hydrogel to reduce light scattering. All hydrogels for multiple semi-quantitative trials were stained and imaged concurrently with single dilutions of antibodies and confocal fluorescence microscopy laser exposure times were maintained constant for each protein. In our immunocytochemistry experiments, we examined fibroblasts both close to and far from the spheroid to account for any differences caused by spheroid proximity and for possible diffusion complications due to hydrogel compaction, although no differences were detected (data not shown). For proliferation and apoptosis experiments, spheroids were cultured as indicated or as a subconfluent monolayer in the case of 2D for 3 days. Samples were fixed as above – except in the case of Annexin V where no permeabilization step was done – and stained for Annexin V or Ki-67. Spheroids were imaged by confocal microscopy. Proliferative and apoptotic cells were counted and calculated as a percentage of the total cell count observed by DAPI staining.
Hydrogel Compaction Assay
Hydrogels were prepared as above and imaged with an 8-megapixel digital camera (XT894, Motorola). Hydrogel dimensions were measured with Fiji software [33], normalizing to the known size of the culture dish. Imaging of hydrogels in media causes a 15 % decrease in measured size in comparison to imaging without media, due to optical distortion. As hydrogels were imaged on day 0 without media and on day 3 in the presence of media, data was normalized to correct for this measurement differential.
Cytokine Identification
TIMPs and cytokines present in fibroblast conditioned media were identified using a semi-quantitative, sandwich-based, human cytokine array (product AAH-CYT-1000, RayBiotech) and quantified using Fiji (ImageJ) software [33]. Quantified intensity is normalized to unconditioned media, in which only MCP-1 was detected at a minimal level. Only those with a detectable level and a difference of more than 2-fold from unconditioned media are reported. Only one experimental replicate was completed. Three experimental replicates of conditioned media were combined and used as the experimental replicate. Error bars are derived from duplicate technical replicates included in the array.
Osteoprotegerin Assay
Spheroids were cultured as described above for individual spheroid monitoring. Recombinant human osteoprotegerin (product 6945-OS-025, R&D Systems) was added to culture media at a concentration of 100 ng / mL or neutralizing antibody to osteoprotegerin (product MAB805, R&D Systems) was added to culture media at a concentration of 2 μg / mL. Control conditions were treated with BSA or a GAPDH antibody at the same concentrations. Samples were grown for 3 days as before and evaluated by the same criteria as before.
Statistical Analysis
Statistical analysis was performed using Microsoft Excel with the Real Statistics Resource Pack (Release 2.14.1, http://www.real-statistics.com/). P-values <0.05 were considered significant. Each experiment utilized three independent trials unless otherwise noted. For initial spheroid characterization, 17 Matrigel® and 4 non-Matrigel® spheroids were considered for each cell line in 2 independent trials. For spheroid dissemination with no fibroblasts, 14 spheroids were considered for each cell line in each stiffness at each time point. For collagen-IV intensity measurements, 6 spheroids were used. For hydrogel compaction, 12 spheroids for each stiffness were considered for 50,000 fibroblast condition, 6 spheroids for each stiffness for 10,000 fibroblast condition, 9 spheroids for each stiffness for no fibroblast condition and 27 hydrogels for each stiffness for no spheroid condition. For fibroblast immunocytochemistry, 9 fibroblast populated hydrogels were used for each stiffness condition. For spheroid dissemination in the presence of fibroblasts, 12 spheroids for each stiffness with each fibroblast concentration were considered. For conditioned media experiments, 9 spheroids for each stiffness were considered. For OPG and antibody experiments, 12 spheroids for each stiffness were considered. Quantification of the concentration of factors in conditioned media was conducted once with duplicate spots on the array. Spheroid apoptosis and proliferation percentages were obtained from 3 spheroids for each stiffness. Cell growth rates in fibroblast media were obtained from 15 samples.
Results
Formation of Tumor Spheroids with a Collagen-IV Containing Basement Membrane
A tumor in situ is encapsulated within a basement membrane, so we sought to model this physiological organization in vitro. We used growth factor reduced Matrigel® (GFR-Matrigel®), a protein mixture secreted by Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells that is made up primarily of collagen-IV, laminin, and entactin, which are major components of the BM [34], as a source of BM proteins to jumpstart BM formation. We added dilute GFR-Matrigel® (below the gelation concentration of Matrigel®) to forming spheroids, which allowed the BM components to be adsorbed to the surface of the cells. This method led to the formation of tight, structurally stable spheroids from both the phenotypically normal MCF10A breast epithelial cells and the highly invasive MDA-MB-231 breast cancer cells within four days in vitro.
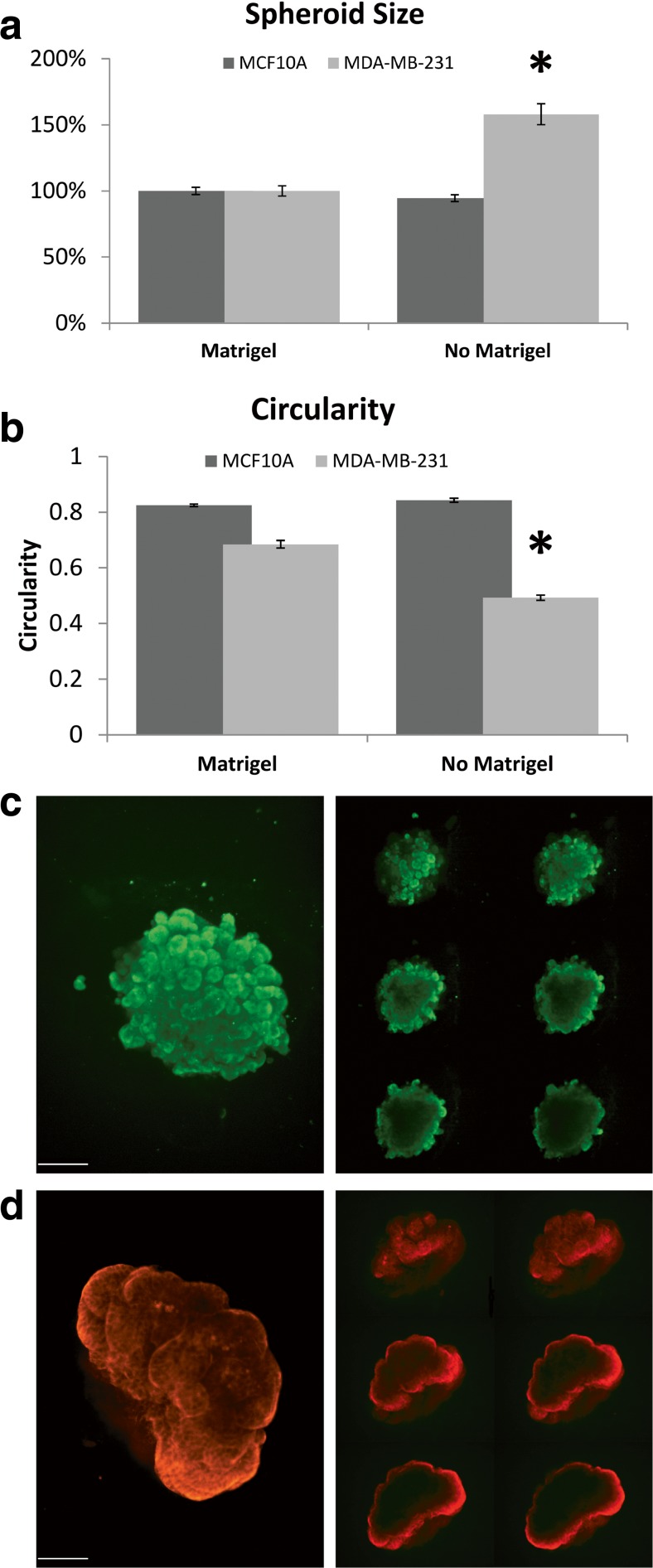
MCF10A spheroids were morphologically similar with or without the addition of GFR-Matrigel®, with no significant difference in cross-sectional area (Fig. 1-a) or circularity, which is a measure of spheroid roundness (Fig. 1-b). This suggests that the strong cell-cell contacts formed by non-invasive epithelial cells allow for spheroid formation without additional matrix components and/or that these cells secrete sufficient matrix to promote spheroid formation. The MCF10A spheroids formed in the absence of GFR-Matrigel®, however, were fragile and attempts to incorporate them into a larger hydrogel construct failed.
Fig. 1.
Spheroid characterization. a Spheroid size. Asterisk indicates significant difference. b Spheroid circularity as measured by Fiji (ImageJ) software [33]. Circularity is a measure of the fit of the measured shape to that of a circle, calculated as 4π(area/perimeter2). A value of 1.0 indicates a perfect circle whereas approaching 0 indicates an elongated polygon. Asterisk indicates significant difference. ANOVA p-values <0.05. Error bars show standard error of the mean. c & d Representative confocal images of MDA-MB-231 (c) and MCF10A (d) spheroids directly after incorporation into a collagen-I hydrogel and immunostained for collagen-IV. Right panels represent 5 μm optical sections in the Z dimension. Scale Bar =100 μm
MDA-MB-231 cells, on the other hand, pack loosely in the absence of GFR-Matrigel®, resulting in a larger cross-sectional area (Fig. 1-a) and oblong shapes and jutting protrusions which can be quantified as a decrease in circularity (Fig. 1-b), suggesting that the weak cell-cell contacts formed by invasive epithelial cells do not allow for tight spheroid formation without the addition of exogenous matrix components. The MDA-MB-231 spheroids formed without GFR-Matrigel® are also fragile and do not maintain coalescence during manipulation, which made it impossible to incorporate them into the complete model system. As the addition of GFR-Matrigel® to forming MDA-MB-231 spheroids results in more tightly packed and coherent spheroids (Fig. 1-a & b), these data suggest that BM components have a normalizing effect on the metastatic MDA-MB-231 cells, prompting them to acquire a less invasive, more normal epithelial phenotype.
We next incorporated the GFR-Matrigel®-10 A or −231 spheroids into a collagen-I hydrogel model of the tumor microenvironment (TME) stroma. We performed immunocytochemistry with an antibody to collagen-IV, one of the main constituents of the BM, and found that a layer of collagen-IV surrounds the spheroids, but is largely absent from the center (Fig. 1-c), indicating a BM-like coating. Together, these results suggest that we can form BM- encapsulated spheroids with both normal breast epithelial cells and highly invasive breast cancer cells. Further, they suggest a role for BM proteins in directing invasive cell types to assume a more normal cell-cell interactive phenotype.
Stromal Stiffness Induces Tumor Cell Invasion
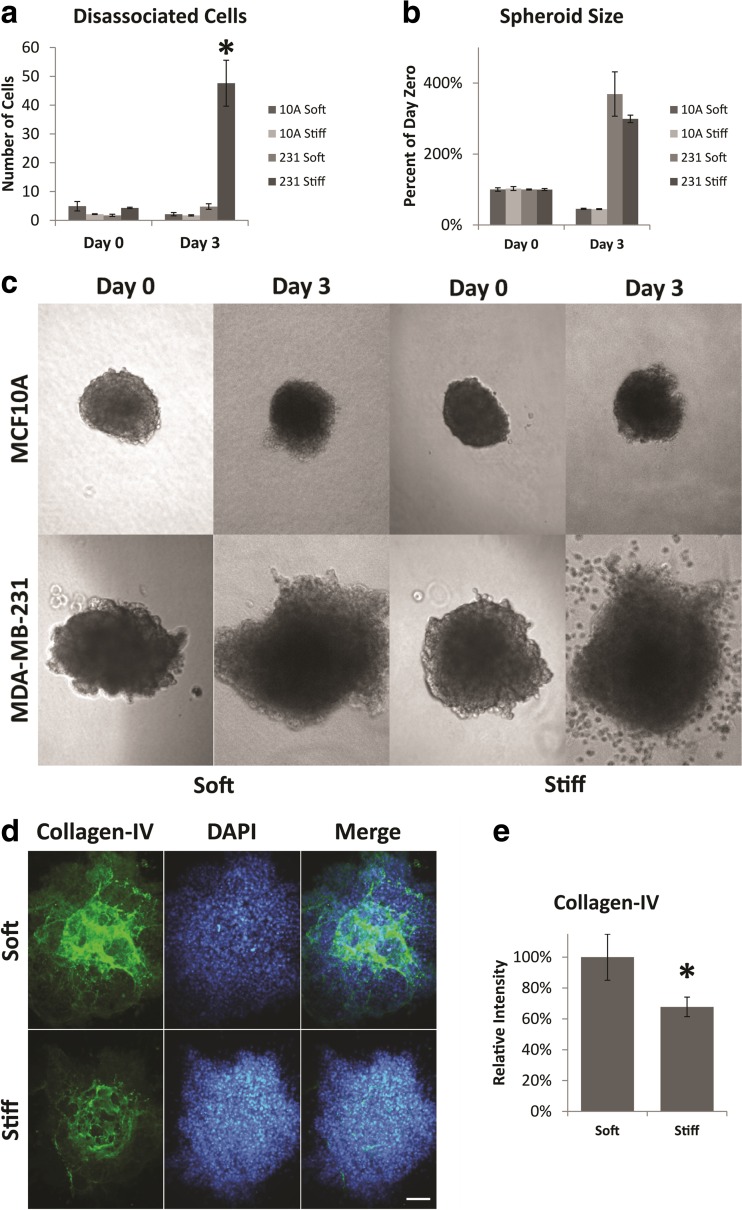
We next wanted to determine if the mechanical stiffness of the stromal environment alters the invasive response of cells within a BM encapsulated spheroid. We embedded spheroids in either ~200 Pa soft or ~800 Pa stiff collagen-I hydrogels (Young’s modulus) which recapitulates the mechanical properties of normal stroma or tumor-associated stroma [1, 2]. To do so, we formed either soft or stiffened (PEG-diNHS cross-linked) collagen-I hydrogels as previously demonstrated [17], placing the spheroids in the hydrogel before gelation. The composite hydrogels were then released from the substrate to avoid variations in stiffness due to the anchoring of the hydrogels, and incubated for 3 days. We then measured spheroid size and counted the number of cells that dissociated from the spheroid after 3 days. There was a slight decrease in the size of MCF10A spheroids over the 3 day time course in both soft and stiff gels, but there was no significant dissemination from the spheroid in either condition (Fig. 2-a, b & c). The MDA-MB-231 spheroids, however, showed a dramatic change in response to stromal stiffness. When the MDA-MB-231 spheroids were incorporated into a soft matrix, the spheroids increased significantly in size over the 3 day time course, but individual MDA-MB-231 cells did not dissociate from the spheroid body. When the MDA-MB-231 spheroids were incorporated into the stiffened matrix however, the spheroids increased in size and a large number of individual cells also migrated away from the spheroid body (Fig. 2-a, b & c). The integrity of the basement membrane around the MDA-MB-231 spheroids also appears to be diminished after 3 days in the stiff gels in comparison to the soft gels (Fig. 2-d), and there was significantly less collagen-IV staining around spheroids in the stiffened matrix in comparison to those in the softer matrix (Fig. 2-e). The change in size of both MCF10A and MDA-MB-231 spheroids is not likely to be due to differences in proliferation or apoptosis as the number of proliferative or apoptotic cells was unchanged for either cell type in soft or stiff gels, or when cells were grown as a 2D monolayer (Fig. S1-a & b). Together these data suggest that the spheroids comprised of non-invasive MCF10A cells do not undergo any significant morphological changes due to the stiffened stromal environment, but the stiffened stromal environment stimulates a switch to an invasive phenotype in the MDA-MB-231 cells, which can override the normalizing effect of the encapsulating BM.
Fig. 2.
Dissemination and size of spheroids. a Number of MCF10A or MDA-MB-231 cells that have dissociated from the main tumor spheroid after 3 days of culture. Disseminated cells were counted as cells which were not in contact with the main spheroid body – including disseminated clusters. b Final size of the tumor spheroid after 3 days of culture. c Representative phase contrast images of spheroids at day 0 and day 3. Images are of the same spheroid at day 0 and day 3. d Day 3 staining of collagen-IV is reduced in stiff hydrogels. e Quantification of collagen-IV staining. Asterisk indicates significant difference from day 0; Student’s t-test p-value <0.05. Error bars show standard error of the mean. All scale bars are 100 μm
Stiffness-Induced Fibroblast Activation Is Altered by Tumor Spheroids
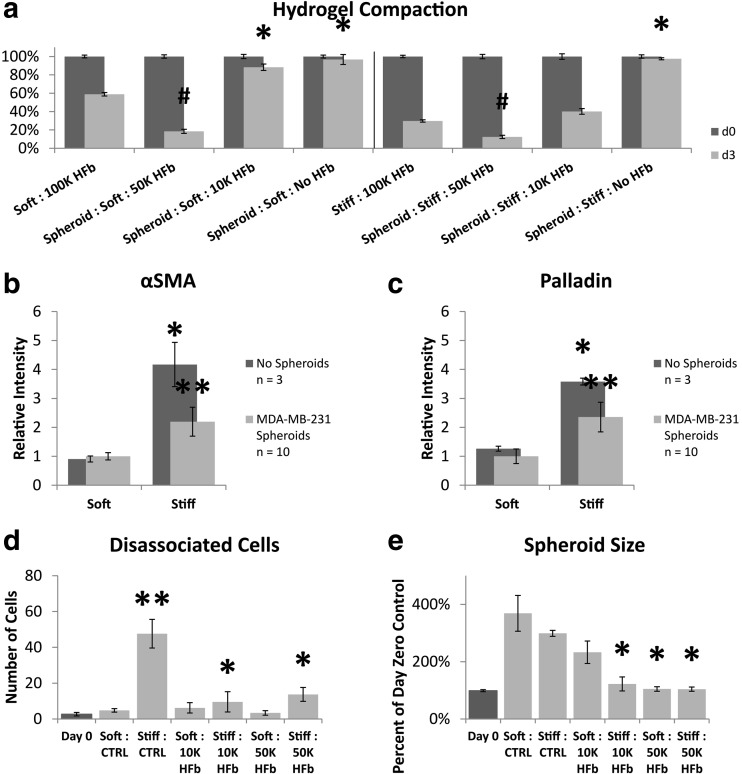
We have previously shown that growth in stiffened hydrogels can lead to the activation of fibroblasts to a carcinoma-associated fibroblast-like state, and others have shown that the tumor itself can affect stromal cell activation and recruitment [17, 35, 36]. Here, we sought to determine if the presence of a tumor spheroid had an effect on the stiffness-induced fibroblast activation we previously observed [17]. We measured the expression of two markers of fibroblast activation, α-smooth muscle actin (αSMA) and palladin [17], in fibroblasts in hydrogels with and without MDA-MB-231 spheroids. We also measured the compaction of the hydrogel, which is an indirect measure of fibroblast contractile activity. We saw that without fibroblasts (HFb) in the hydrogel, there was no compaction of either soft or stiff gels (Fig. 3-a). We also saw that the presence of the spheroid led to greater compaction than the fibroblasts (HFb) were able to accomplish alone in both soft and stiff gels. In fact, gels with spheroids and 50,000 fibroblasts (HFb) per mL compacted more than gels with twice as many fibroblasts but without spheroids. Furthermore, a relatively low density of fibroblasts in the stiff gel with a spheroid (10,000 fibroblasts / mL) led to the same amount of compaction as a 10 fold increase in the number of fibroblasts alone, but the same low amount of fibroblasts had almost no effect in the soft gel with spheroid (Fig. 3-a). These data suggest that signals from the spheroid are enhancing the contractile activity of the fibroblasts in this model system and to a greater extent in stiff hydrogels. Paradoxically, when we looked at two markers of fibroblast activation, we observed a decrease in expression when spheroids were present. Both α-smooth muscle actin and palladin expression increase when fibroblasts are cultured in stiff hydrogels, in comparison to growth in soft gels, but the presence of a MDA-MB-231 spheroid dampens this increase (Fig. 3-b & c). This suggests that signals from the epithelial spheroid may have a suppressive effect on myofibroblast-associated protein expression, although the cells show greater contractile activity. Taken together, these data suggest that the spheroid alters the response of fibroblasts to the environment in a complex manner, which decouples contractile activity from myofibroblast-associated protein expression.
Fig. 3.
Dissemination and spheroid size of MDA-MB-231 spheroids cultured with fibroblasts. a Hydrogel compaction over 3 days. Asterisk indicates significant increase in final size from 100 K; Hash indicates significant decrease in final size from 100 K; Student’s t-test p-value <0.05. b Semi-quantitative immunocytochemistry of αSMA. c Semi-quantitative immunocytochemistry of palladin. Values normalized to soft collagen-I hydrogel with spheroid present. Asterisk indicates significant difference from spheroid in soft gel. Double asterisk indicates significant difference from spheroid in soft gel and no spheroid in stiff gel; Student’s t-test p-value <0.05. d Number of cells which have dissociated from the main tumor spheroid. Asterisk indicates significant difference from day 0; Student’s t-test p-value <0.05. Double asterisk indicates significant difference from day 0; Student’s t-test p-value <0.005. (E) Final size of the tumor spheroid. Asterisk indicates significant difference from day 3 control; Student’s t-test p-value <0.05. Error bars signify standard error of the mean
Juxtacrine Signals from Stromal Fibroblasts Inhibit Tumor Cell Invasion
Increasing evidence supports a role for fibroblasts and other stromal cells in tumor progression [21]. In order to examine the effect of fibroblasts upon a tumor in situ, we incorporated a low (10,000 cells/mL) or medium concentration (50,000 cells/mL) of fibroblasts into the hydrogels when adding spheroids. Spheroids of MCF10A cells showed no response to the presence or absence of fibroblasts (Fig. S2), indicating that non-invasive epithelial cells are phenotypically unaffected by fibroblast signals. However, fibroblasts did have a significant effect on spheroids of MDA-MB-231 cells. When MDA-MB-231 spheroids were embedded in soft hydrogels, they showed little to no invasive behavior and the presence of fibroblasts did not change this. However, when the MDA-MB-231 spheroids were embedded in stiff hydrogels, the presence of fibroblasts at any concentration significantly decreased MDA-MB-231 invasive behavior (Fig. 3-d). The presence of fibroblasts also leads to a decrease in spheroid size in both soft and stiff gels (Fig. 3-e), but we suggest this is likely due to the compaction of the hydrogel by the fibroblasts (Fig. 3-a) [17]. Together, these data suggest that fibroblasts can suppress the invasive behavior of MDA-MB-231 cells.
Paracrine Signals from Stiffness Activated Fibroblasts Inhibit Tumor Cell Invasion
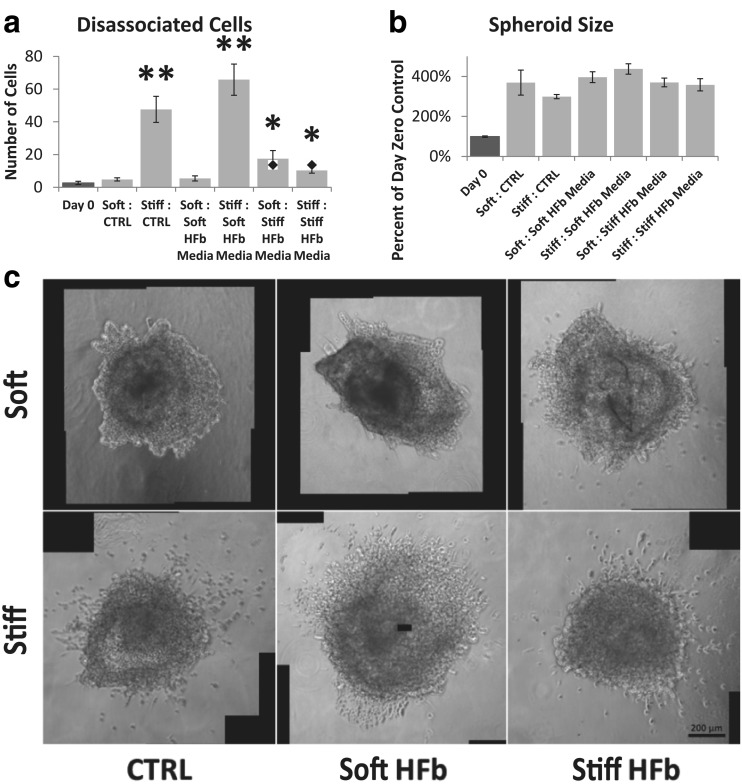
To determine if the effect that fibroblasts have on MDA-MB-231 invasive behavior in stiffened hydrogels is due to physical or paracrine interactions, we next cultured spheroids in hydrogels with fibroblast conditioned media. Conditioned media from fibroblasts cultured in soft hydrogels had little effect; the dissociation of cells from MDA-MB-231 spheroids was similar to those cultured without fibroblast conditioned media (Fig. 4-a & c). Conditioned media from fibroblasts cultured in stiff hydrogels, on the other hand, had a significant effect. Interestingly, incubation with stiff hydrogel conditioned media slightly increased the dissemination from spheroids embedded in soft stroma, but conversely, dramatically inhibited invasion from spheroids in stiff stroma (Fig. 4-a & c). Neither conditioned media caused a change in spheroid size in comparison to control (Fig. 4-b), confirming that the compaction seen in co-culture was due to the contractile activity of the fibroblasts themselves. Together, these data suggest that both physical and chemical signals generated by fibroblasts can affect tumor cell invasiveness and that these signals interact. Growth in a stiffened matrix alone can cause tumor cells to disseminate from the spheroid, but the presence of either fibroblasts or chemical signals from fibroblasts grown in a stiffened matrix can inhibit this invasive behavior. On the other hand, chemical signals from fibroblasts grown in a stiffened matrix can promote invasion in a soft matrix that is lacking the physical cues that promote invasion, although the magnitude of this effect is smaller than that seen within the stiffened matrix.
Fig. 4.
Fibroblast secreted cytokines override stiffness response. a Number of cells which have dissociated from the main tumor spheroid. For reference, diamond in plot is spheroid disassociation with 50 K fibroblasts present in hydrogel (Fig. 3). Asterisk indicates significant difference from day 0; Student’s t-test p-value <0.05. Double asterisk indicates significant difference from day 0; Student’s t-test p-value <0.005. b Final size of the tumor spheroid. There were no statistically significant differences on day 3; Student’s t-test p-value >0.05. c Representative phase contrast images of spheroids on day 3 with conditioned media treatment. Scale Bar =200 μm
Osteoprotegerin Inhibits Tumor Cell Invasion
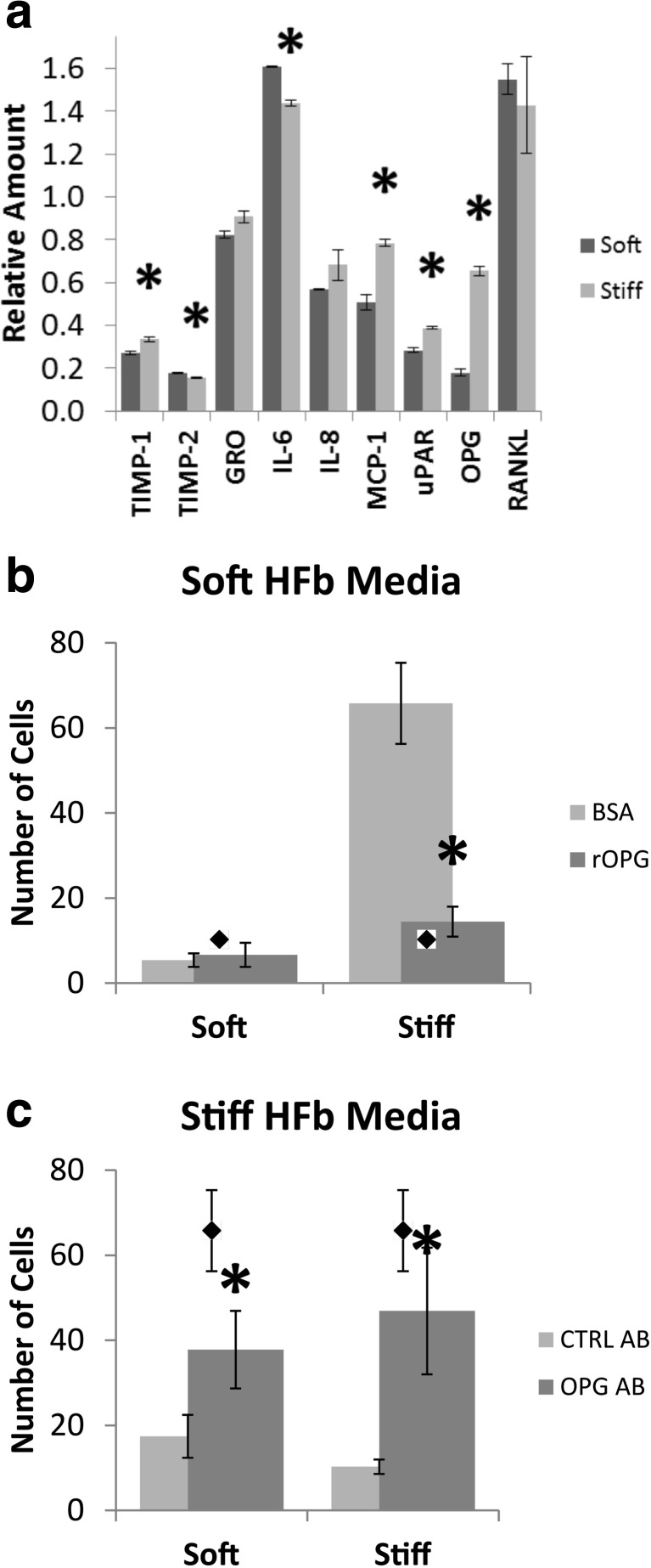
To determine which soluble factors secreted by fibroblasts may modulate invasive behavior, we screened fibroblast conditioned media for 120 candidate cytokines, and found that 30 were present in conditioned media (Table S1). Of those, eight were significantly higher in concentration in conditioned media than in unconditioned media (Fig. 5-a, Table S1). Osteoprotegerin (OPG), in particular, showed the greatest difference between conditioned media from stiff gels and that from soft gels – a 3.7-fold increase in stiff hydrogel media. OPG acts upon the RANK pathway as a decoy receptor for RANKL, therefore we probed for RANK via dot blot and saw no significant change in expression (Fig. 5-a). To determine if the difference in OPG concentration affects the invasive behavior of MDA-MB-231 cells, we cultured MDA-MB-231 spheroids in soft and stiff stromal hydrogels with either (1) stiff hydrogel fibroblast conditioned media to which neutralizing antibodies to OPG were added or (2) with soft hydrogel fibroblast conditioned media to which recombinant OPG was added. Soft hydrogel fibroblast conditioned media plus recombinant OPG (+rOPG) had little effect on spheroids in soft hydrogels; the dissemination of cells remained at baseline levels. +rOPG with stiff hydrogels however, significantly reduced the dissemination of cells from the spheroid to the same level as stiff hydrogel fibroblast conditioned media (Fig. 5-b). When we added an antibody to OPG to stiff hydrogel fibroblast conditioned media (OPG AB), the inhibition of invasiveness was lost and dissemination was increased to levels approaching those seen with soft hydrogel fibroblast conditioned media or unconditioned media in both soft and stiff hydrogels (Fig. 5-c). These data suggest that OPG can suppress tumor cell invasion and further that the expression of OPG by fibroblasts is regulated by their activation state, which is modulated by the mechanical properties of the stromal matrix. Further, they suggest that fibroblast paracrine signals controlling invasive behaviors override epithelial mechano-signaling observed when tumor cells are cultured alone.
Fig. 5.
Osteoprotegerin secretion is increased in activated fibroblasts and inhibits dissemination. a Quantification of cytokine array targets which were not present in or increased concentration from unconditioned media. Data normalized to unconditioned media by normalizing to array control and subtracting unconditioned media baselines. Asterisk indicates significant difference from soft gel. Student’s t-test p-value <0.05. Error bars show standard deviation. b Dissemination of cells from spheroids treated with soft hydrogel fibroblast media and additional recombinant OPG. Fewer cells disseminated from stiff stromal spheroids when OPG level was increased. Diamond markers represent spheroid dissemination when cultured within a stiff matrix with stiff matrix HFb conditioned media. c Dissemination of cells from spheroids treated with stiff hydrogel fibroblast media and an antibody to OPG. Cells disseminated from spheroids, regardless of hydrogel stiffness, when blocking OPG. Diamond markers represent spheroid dissemination when cultured within a stiff matrix with soft matrix HFb conditioned media. Asterisk indicates significant difference from conditioned media only. Student’s t-test p-value <0.05. Error bars show standard deviation of the mean
Discussion
Bidirectional communication between tumors and the TME has become an increasingly accepted paradigm in cancer biology [18, 37–39]. Fibroblasts are the major cell type in the stroma and they have been implicated in the growth, invasion and metastasis of tumors, therefore we focused on the interactions between fibroblasts and our model tumor in situ [35, 38, 40, 41]. We observed that a tumor spheroid composed of the invasive MDA-MB-231 cells does not exhibit a metastatic phenotype when encapsulated in a basement membrane and placed within a soft environment (Fig. 2-c). Further, we observed that without the addition of Matrigel® during the spheroid maturation process, the spheroids which formed did not strongly coalesce, were fragile and unable to be incorporated into the complete model. MDA-MB-231 cells have previously been shown to become more tumorigenic in xenograft models when Matrigel® was incorporated into the experimental system, but other data suggests that laminin, a major component of Matrigel®, has a normalizing effect on epithelial phenotype [9, 42, 43]. Our data supports this latter finding, as we observed that the presence of a GFR-Matrigel® nucleated BM suppresses the invasive phenotype. We also found that increasing the stiffness of the stromal matrix surrounding BM encapsulated MDA-MB-231 spheroids, but not spheroids composed of the more normal MCF10A epithelial cells, stimulates dissemination of individual cells (Fig. 2-c). MDA-MB-231 cells have been previously shown to be sensitive to matrix stiffness, with stiffness playing roles in growth, morphology and adhesion [44], and epithelial cells have also been shown to undergo EMT when cultured in stiff environments [4, 44]. Our data suggest that MDA-MB-231 spheroids can sense the mechanical properties of the stroma even when surrounded by a phenotypically normalizing basement membrane. Further investigations with this system may elucidate the transition from a tumor in situ to invasive metastatic disease.
When we added fibroblasts to the matrix surrounding our tumor in situ model, we observed a significant decrease in the number of cells that dissociated from the MDA-MB-231 tumor spheroid in stiff hydrogels (Fig. 3-d & e). This was unexpected as MDA-MB-231 cells have been previously shown to become more mesenchymal and increase expression of invasive markers such as MMP2 when cultured with fibroblasts vs cultured alone [45]. OPG inhibits NF-κB activation by sequestering RANKL from the RANK receptor and NF-κB activation regulates expression of invasion associated MMPs such as MMP2 [46]. MDA-MB-231 cells have also been shown to migrate individually, without the need to degrade matrix, through collagen-I hydrogels while they degrade the matrix to migrate through Matrigel® hydrogels [47]. This difference in migration method also switches immediately upon the interface of the different matrix type [47]. We hypothesize that the decreased dissemination of MDA-MB-231 cells – which are RANKL sensitive [30] – in stiff matrices is a result of the increased secretion of osteoprotegerin (OPG) by fibroblasts and stagnant expression of RANKL (Fig. 5-a), leading to a loss of NF-κB activation and therefore the ability to degrade the Matrigel® membrane to migrate away from the spheroid. Further that the increased secretion of OPG by fibroblasts is due to the stiffened matrix by altering mechanosensitive gene expression (Fig. 3-a, b & c).
Our model system also allows the matrix to be remodeled by the cells present within it and we observe this remodeling as a compaction of the hydrogel over the experimental time course. It is possible that the tumor cells become mechanically constrained by the compaction of the matrix, which could also inhibit cell motility (Fig. 3-a, d & e). However, we view the development of a mechanical constraint due to the compaction of the hydrogel as unlikely, as the number of disassociated cells remains constant between compacted stiff hydrogels (Fig. 3-d) and the uncompact, fibroblast free, stiff fibroblast conditioned media treated hydrogels (Fig. 4-a).
Experiments with conditioned media demonstrated that soluble signals from fibroblasts in stiff hydrogels, in which OPG levels were 3.7-fold higher than media from fibroblasts in soft hydrogels, led to a decrease in dissemination from MDA-MB-231 spheroids but did not limit spheroid size (Fig. 4-a). The addition of recombinant OPG to soft hydrogel fibroblast conditioned media inhibited dissemination to the same degree as media from cells in stiff hydrogels, and removing OPG from stiff hydrogel conditioned media via antibody treatment restored cell dissemination in stiff hydrogels (Fig. 5-b & c), suggesting that OPG can override mechanically induced tumor cell migration. Interestingly, the removal of OPG also led to an increase in dissemination in soft hydrogels. We hypothesize that this increase in dissemination is due to one of many factors known to elicit epithelial migration that are increased in both soft and stiff fibroblast conditioned media – including GRO (CXCL1), IL-6, IL-8, MCP-1 (CCL2) and μPAR – all of which interact with NF-kB signaling pathway to stimulate migration as does the RANKL-RANK / OPG system [36, 48–55].
Fibroblasts in the stiffened stromal hydrogel displayed an activated phenotype, as indicated by increased matrix modification as well as increased α-smooth muscle actin and palladin expression (Fig. 3-a, b & c), consistent with results from our lab and others [17]. When cultured with MDA-MB-231 spheroids, however, activation associated protein expression decreased, although matrix compaction increased. This may appear paradoxical at first glance – although protein markers of fibroblast activation are generally associated with a proportional change in matrix associated behaviors, a clear molecular mechanism linking activation associated behaviors such as matrix reorganization and degradation to activation associated protein expression has not been demonstrated. Tumor cells like the MDA-MB-231 cells used here also secrete cytokines such as transforming growth factor beta (TGFβ), connective tissue growth factor (CTGF) and IGF-2, which can drive myofibroblast differentiation independently [56–58] and it has been shown that myofibroblasts in the tumor environment are diverse with subpopulations that do not express canonical markers such as αSMA [59]. It is possible that the discrepancy we see between the decreases in these markers of activation and increases in matrix remodeling reflect interactions between these different signaling pathways and activated fibroblast subtypes and further experiments will be necessary to elucidate this dichotomy.
Conclusion
Here we demonstrated a new model system that mimics tissue organization of a tumor in situ, utilizes ligands and the organization of those ligands found in vivo, and matches the biophysical stiffness observed in normal and pathological states. With this physiologically representative system, we present results that suggest that paracrine signaling from stromal fibroblasts can override mechanical signals from the tumor stroma in directing invasive behaviors from tumor cells. These fibroblast paracrine signals, however, are induced by the mechanical signals of the stiffened stroma. Together, we conclude that the direct physical signal from the TME is instrumental in promoting an indirect fibroblast paracrine signal, which regulates invasive behavior.
BM, basement membrane;ECM, extracellular matrix; GFR-Matrigel®, growth factor reduced Matrigel®; OPG AB, stiff hydrogel fibroblast conditioned media plus an antibody to OPG; OPG, osteoprotegerin; PEG-diNHS, poly(ethylene glycol)-di(succinic acid N-hydroxysuccinimide ester); RANKL, nuclear factor-κB ligand; TME, tumor microenvironment; RANK, nuclear factor-κB ligand receptor; +rOPG, Soft hydrogel fibroblast conditioned media plus recombinant OPG.
Electronic supplementary material
(PDF 1733 kb)
(PDF 1171 kb)
(PDF 42 kb)
Acknowledgments
We would like to thank Mariah Hahn and Ryan Gilbert as well as members of their labs, Dany Munoz, Jon Zuidema and Chris McKay, for their assistance with biomaterials. This work has been supported by the American Cancer Society Research Scholar Grant (RSG-10-245-01-CSM).
Authors’ Contributions
JSM acquired all of the data and completed the data analysis. JSM and LAL jointly conceived and directed the project, interpreted the data, and drafted and revised the manuscript. Both authors read and approved the final manuscript.
Compliance with Ethical Standards
Competing Interests
The authors declare no competing interests.
Contributor Information
Joshua S. McLane, mclanejs@email.unc.edu
Lee A. Ligon, Phone: +1 (518) 276-3458, Email: ligonl@rpi.edu
References
- 1.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, SS M, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SFT, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar S, Weaver VM. Mechanics, Malignancy, and Metastasis: the Force Journey of a Tumor Cell. Cancer Metastasis Rev. 2009;28:113–127. doi: 10.1007/s10555-008-9173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gjorevski N, Boghaert E, Nelson CM. Regulation of epithelial-mesenchymal transition by transmission of mechanical stress through epithelial tissues. Cancer Microenviron. 2011;5:29–38. doi: 10.1007/s12307-011-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhimolea E, Maffini MV, Soto AM, Sonnenschein C. The role of collagen reorganization on mammary epithelial morphogenesis in a 3d culture model. Biomaterials. 2010;31:3622–3630. doi: 10.1016/j.biomaterials.2010.01.077. [DOI] [PubMed] [Google Scholar]
- 6.Carey SP, Kraning-Rush CM, Williams RM, Reinhart-King CA. Biophysical control of invasive tumor cell behavior by extracellular matrix microarchitecture. Biomaterials. 2012;33:4157–4165. doi: 10.1016/j.biomaterials.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wozniak MA Keely, PJ: Use of three-dimensional collagen gels to study mechanotransduction in t47d breast epithelial cells. Biol Proced Online 2005, 7:144–161. [DOI] [PMC free article] [PubMed]
- 8.Krause S, Maffini MV, Soto AM, Sonnenschein C. A novel 3d in vitro culture model to study stromal-epithelial interactions in the mammary gland. Tissue Eng Part C Methods. 2008;14:261–271. doi: 10.1089/ten.tec.2008.0030. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhuri O, Koshy ST, DA B, Cunha C, J-W S, CS V, KH A, DJ M. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat Mater. 2014;13(June):970–978. doi: 10.1038/nmat4009. [DOI] [PubMed] [Google Scholar]
- 10.Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119(Pt 19):3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harburger DS, Calderwood D a. Integrin signalling at a glance. J Cell Sci. 2009;122:159–163. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace D. Collagen gel systems for sustained delivery and tissue engineering. Adv Drug Deliv Rev. 2003;55:1631–1649. doi: 10.1016/j.addr.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Asadi A, Monroe MR, Douglas EP. pH effects on collagen fibrillogenesis in vitro: electrostatic interactions and phosphate binding. Mater Sci Eng C. 2009;29:1643–1649. doi: 10.1016/j.msec.2009.01.001. [DOI] [Google Scholar]
- 15.Yang Y-L, Leone LM, Kaufman LJ. Elastic moduli of collagen gels can be predicted from two-dimensional confocal microscopy. Biophys J. 2009;97:2051–2060. doi: 10.1016/j.bpj.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plant AL, Bhadriraju K, Spurlin T, JT E. Cell response to matrix mechanics: focus on collagen. Biochim Biophys Acta, Mol Cell Res. 2009;1793:893–902. doi: 10.1016/j.bbamcr.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 17.McLane JS, Ligon LA. Palladin mediates stiffness-induced fibroblast activation in the tumor microenvironment. Biophys J. 2015;109:249–264. doi: 10.1016/j.bpj.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 19.Marsh T, Pietras K, McAllister SS. Fibroblasts as architects of cancer pathogenesis. Biochim Biophys Acta. 1832;2012:1070–1078. doi: 10.1016/j.bbadis.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apostolopoulou M, Ligon L. Cadherin-23 mediates heterotypic cell-cell adhesion between breast cancer epithelial cells and fibroblasts. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. Am J Cancer Res. 2011;1:482–497. [PMC free article] [PubMed] [Google Scholar]
- 22.Ta B, Lai L a, Coleman J, MP B, Pan S, Chen R. Arousal of cancer-associated stroma: overexpression of palladin activates fibroblasts to promote tumor invasion. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xing F, Saidou J, Watabe K. Cancer associated fibroblasts (cafs) in tumor microenvironment. Front Biosci. 2010;15:166–179. doi: 10.2741/3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohuchida K, Mizumoto K, Murakami M, Qian L, Sato N, Nagai E, Matsumoto K, Nakamura T, Tanaka M. Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal. Interactions. 2004;1:3215–3222. doi: 10.1158/0008-5472.can-03-2464. [DOI] [PubMed] [Google Scholar]
- 25.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg R a. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 26.Goicoechea SM, García-Mata R, Staub J, Valdivia a, Sharek L, CG MC, RF H, Urrutia R, JJ Y, HJ K, Otey C a. Palladin promotes invasion of pancreatic cancer cells by enhancing invadopodia formation in cancer-associated fibroblasts. Oncogene. 2014;33:1265–1273. doi: 10.1038/onc.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhim ADD, Oberstein PEE, Thomas DHH, Mirek ETT, Palermo CFF, SA S, ENN D, Saunders T, CPP B, IWW T, CBB W, Kitajewski J, MGG F-B, MEE F-Z, Iacobuzio-Donahue C, KPP O, BZZ S. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuda E, Goto M, Mochizuki S, Yano K, Kobayashi F, Morinaga T, Higashio K. Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem Biophys Res Commun. 1997;234:137–142. doi: 10.1006/bbrc.1997.6603. [DOI] [PubMed] [Google Scholar]
- 29.Lyden D, Welch DR, Psaila B. Cancer metastasis: biologic basis and therapeutics. New York: Cambridge University Press; 2011. [Google Scholar]
- 30.Tang Z-N, Zhang F, Tang P, Qi X-W, Jiang J. RANKL-induced migration of mda-mb-231 human breast cancer cells via src and mapk activation. Oncol Rep. 2011;26:1243–1250. doi: 10.3892/or.2011.1368. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Teng Y, Zhang Y, Liu J, Xu L, Qu J, Hou K, Yang X, Liu Y, Qu X. C-Src-mediated RANKL-induced breast cancer cell migration by activation of the erk and akt pathway. Oncol Lett. 2012;3:395–400. doi: 10.3892/ol.2011.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 33.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes CS, Postovit LM, Lajoie G a. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886–1890. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- 35.Erez N, Truitt M, Olson P, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-κB-Dependent Manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 36.Lv M, Xu Y, Tang R, Ren J, Shen S, Chen Y, Liu B, Hou Y, Wang T. miR141-CXCL1-CXCR2 signaling-induced treg recruitment regulates metastases and survival of non-small cell lung cancer. Mol Cancer Ther. 2014;13:3152–3162. doi: 10.1158/1535-7163.MCT-14-0448. [DOI] [PubMed] [Google Scholar]
- 37.Cunha GR, Reese BA, Sekkingstad M. Induction of nuclear androgen-binding sites in epithelium of the embryonic urinary bladder by mesenchyme of the urogenital sinus of embryonic mice. Endocrinology. 1980;107:1767–1770. doi: 10.1210/endo-107-6-1767. [DOI] [PubMed] [Google Scholar]
- 38.Bhowmick N, Neilson E, Moses H. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432(November):332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orimo A, Weinberg R (2006) Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle (August):1597–1601 [DOI] [PubMed]
- 40.Król M, Pawłowski KM, Szyszko K, Maciejewski H, Dolka I, Manuali E, Jank M, Motyl T. The gene expression profiles of canine mammary cancer cells grown with carcinoma-associated fibroblasts (cafs) as a co-culture in vitro. BMC Vet Res. 2012;8:35. doi: 10.1186/1746-6148-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mullen P, Ritchie A, Langdon SP, Miller WR. Effect of matrigel on the tumorigenicity of human breast and ovarian carcinoma cell lines. Int J Cancer. 1996;67:816–820. doi: 10.1002/(SICI)1097-0215(19960917)67:6<816::AID-IJC10>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida S, Shimizu E, Ogura T, Takada M, Sone S. Stimulatory effect of reconstituted basement membrane components (matrigel) on the colony formation of a panel of human lung cancer cell lines in soft agar. J Cancer Res Clin Oncol. 1997;123:301–309. doi: 10.1007/BF01438305. [DOI] [PubMed] [Google Scholar]
- 44.Tilghman RW, Cowan CR, Mih JD, Koryakina Y, Gioeli D, Slack-Davis JK, Blackman BR, Tschumperlin DJ, Parsons JT. Matrix rigidity regulates cancer cell growth and cellular phenotype. PLoS One. 2010;5:1–13. doi: 10.1371/journal.pone.0012905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao M-Q, Kim BG, Kang S, Choi YP, Park H, Kang KS, Cho NH (2010) Stromal fibroblasts from the interface zone of human breast carcinomas induce an epithelial-mesenchymal transition-like state in breast cancer cells in vitro. J Cell Sci (September)):3507–3514 [DOI] [PubMed]
- 46.Yu M, Qi X, Moreno JL, Farber DL, Keegan AD. NF- B signaling participates in both RANKL- and IL-4-induced macrophage fusion: receptor cross-talk leads to alterations in NF- B pathways. J Immunol. 2011;187:1797–1806. doi: 10.4049/jimmunol.1002628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shin Y, Kim H, Han S, Won J, Jeong HE, Lee E-S, Kamm RD, Kim J-H, Chung S. Extracellular matrix heterogeneity regulates three-dimensional morphologies of breast adenocarcinoma cell invasion. Adv Healthc Mater. 2013;2:790–794. doi: 10.1002/adhm.201200320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Killian PH, Kronski E, Michalik KM, Barbieri O, Astigiano S, Sommerhoff CP, Pfeffer U, Nerlich AG, Bachmeier BE. Curcumin inhibits prostate cancer metastasis in vivo by targeting the inflammatory cytokines CXCL1 and − 2. Carcinogenesis. 2012;33:2507–2519. doi: 10.1093/carcin/bgs312. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y-H, Dong Y-Y, Wang W-M, Xie X-Y, Wang Z-M, Chen R-X, Chen J, Gao D-M, Cui J-F, Ren Z-G. Vascular endothelial cells facilitated HCC invasion and metastasis through the Akt and NF-κB pathways induced by paracrine cytokines. J Exp Clin Cancer Res. 2013;32:51. doi: 10.1186/1756-9966-32-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernando RI, Castillo MD, Litzinger M, Hamilton DH, Palena C. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res. 2011;71:5296–5306. doi: 10.1158/0008-5472.CAN-11-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yadav A, Kumar B, Datta J, Teknos TN, Kumar P. IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the jak-stat3-snail signaling pathway. Mol Cancer Res. 2011;9:1658–1667. doi: 10.1158/1541-7786.MCR-11-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Snyder M, Huang J, Huang X-Y, Zhang JJ. A signal transducer and activator of transcription 3·nuclear factor κB (Stat3·NFκB) complex is necessary for the expression of fascin in metastatic breast cancer cells in response to interleukin (IL)-6 and tumor necrosis factor (TNF)-α. J Biol Chem. 2014;289:30082–30089. doi: 10.1074/jbc.M114.591719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiu H-Y, Sun K-H, Chen S-Y, Wang H-H, Lee M-Y, Tsou Y-C, Jwo S-C, Sun G-H, Tang S-J. Autocrine CCL2 promotes cell migration and invasion via PKC activation and tyrosine phosphorylation of paxillin in bladder cancer cells. Cytokine. 2012;59:423–432. doi: 10.1016/j.cyto.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 54.Lester RD, Jo M, Montel V, Takimoto S, Gonias SL. uPAR induces epithelial-mesenchymal transition in hypoxic breast cancer cells. J Cell Biol. 2007;178:425–436. doi: 10.1083/jcb.200701092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gorantla B, Asuthkar S, Rao JS, Patel J, Gondi CS. Suppression of the uPAR-uPA system retards angiogenesis, invasion, and in vivo tumor development in pancreatic cancer cells. Mol Cancer Res. 2011;9:377–389. doi: 10.1158/1541-7786.MCR-10-0452. [DOI] [PubMed] [Google Scholar]
- 56.Lei X, Bandyopadhyay A, Le T, Sun L. Autocrine TGFbeta supports growth and survival of human breast cancer MDA-MB-231 cells. Oncogene. 2002;21:7514–7523. doi: 10.1038/sj.onc.1205966. [DOI] [PubMed] [Google Scholar]
- 57.Kondo S, Kubota S, Shimo T, Nishida T, Yosimichi G, Eguchi T, Sugahara T, Takigawa M. Connective tissue growth factor increased by hypoxia may initiate angiogenesis in collaboration with matrix metalloproteinases. Carcinogenesis. 2002;23:769–776. doi: 10.1093/carcin/23.5.769. [DOI] [PubMed] [Google Scholar]
- 58.Brünner N, Moser C, Clarke R, Cullen K. IGF-I and IGF-II expression in human breast cancer xenografts: relationship to hormone independence. Breast Cancer Res Treat. 1992;22:39–45. doi: 10.1007/BF01833332. [DOI] [PubMed] [Google Scholar]
- 59.Chauhan H, Abraham A, JRa P, JH P, Walker R a, JL J. There is more than one kind of myofibroblast: analysis of CD34 expression in benign, in situ, and invasive breast lesions. J Clin Pathol. 2003;56:271–276. doi: 10.1136/jcp.56.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1733 kb)
(PDF 1171 kb)
(PDF 42 kb)