Abstract
The phenotypic axis of invasion and proliferation in malignant glioma cells is a well-documented phenomenon. Invasive glioma cells exhibit a decreased proliferation rate and a resistance to apoptosis, and invasive tumor cells dispersed in brain subsequently revert to proliferation and contribute to secondary tumor formation. One miRNA can affect dozens of mRNAs, and some miRNAs are potent oncogenes. Multiple miRNAs are implicated in glioma malignancy, and several of which have been identified to regulate tumor cell motility and division. Using rat 9 L gliosarcoma and human U87 glioblastoma cell lines, we investigated miRNAs associated with the switch between glioma cell invasion and proliferation. Using micro-dissection of 9 L glioma tumor xenografts in rat brain, we identified disparate expression of miR-9 between cells within the periphery of the primary tumor, and those comprising tumor islets within the invasive zone. Modifying miR-9 expression in in vitro assays, we report that miR-9 controls the axis of glioma cell invasion/proliferation, and that its contribution to invasion or proliferation is biphasic and dependent upon local tumor cell density. In addition, immunohistochemistry revealed elevated hypoxia inducible factor 1 alpha (HIF-1α) in the invasive zone as compared to the primary tumor periphery. We also found that hypoxia promotes miR-9 expression in glioma cells. Based upon these findings, we propose a hypothesis for the contribution of miR-9 to the dynamics glioma invasion and satellite tumor formation in brain adjacent to tumor.
Keywords: Glioma, Glioblastoma, Mirna, Mir-9, Invasion
Introduction
Glioma cell migration and proliferation are stochastically mutually exclusive processes, and the tumor cells defer cell division in order to migrate and vice versa [1, 2]. Due to the phenotypic switch between motility and growth, invasive glioma cells exhibit a decreased proliferation rate and a resistance to apoptosis, which may contribute to chemotherapy and radiation resistance [3]. Ultimately, invasive tumor cells that evade surgical debulking and treatment subsequently revert to proliferation and contribute to secondary tumor formation [4].
Over 60% of human protein-coding genes are conserved targets of miRNAs [5]. Each miRNA can affect a number of mRNAs, thus depending upon its targets, each miRNA can function as a potent oncogene or tumor suppressor [6]. Aberrant gene expression is the primary mechanism of miRNA dysfunction in cancer, and miRNAs are differentially expressed in gliomas relative to normal tissue [7, 8]. Consequently, miRNAs have rapidly emerged as potential biomarkers in patients with glioma [9, 10]. Multiple miRNAs have now been linked to glioma malignancy, and several have been identified to regulate tumor cell motility and division [9, 10].
Here, we investigated miRNAs associated with the switch between glioma cell invasion and proliferation. To this end, we first compared expression levels of 172 miRNAs between tumor cells of 9 L intracranial xenografts that resided within the outer periphery of the primary tumor, and those that were in tumor cell islets within the invasive region. This experiment revealed that miR-9 was the most disparately expressed miRNA between the two cell populations. Guided by this finding, we performed a series of in vitro experiments to elucidate the influence of miR-9 upon the proliferation and invasion of glioma cells. miR-9 can be promoted by hypoxia [11]. As we observed a relative increase in HIF-1α within the invasive zone in brain, we also tested the effect of hypoxia upon miR-9 in glioma cells. We report that miR-9 defines the axis of glioma cell invasion/proliferation, and its contribution to invasion or proliferation is biphasic, dependent upon tumor cell density. In light of our findings, we have developed a hypothesis for the role of miR-9 in the process invasion and secondary tumor formation in malignant glioma in brain.
Materials and Methods
Growth Curve
Cells were plated in each well of a 96-well plate containing DMEM with FBS at a concentration of 10%. Every 24 h, total adherent and non-adherent cells in each experimental well were quantified using a hematocytometer. Cell counts of three wells per time point per group were averaged. The growth curve experiment was performed three times with similar results.
Migration and Invasion Assays
Matrigel invasion assays were used to assess tumor cell invasion. Invasion was determined using 24-well BD invasion chambers (8.0 μm pore size; BD Biosciences, Cowley, UK) as described previously, with the modification that 9 L, 9 L–m9, U87, or U87-m9 cells were initially plated at 5000 or 50,000 cells/cm2 [12]. Cells were stained with CellTracker Green (Molecular Probes, OR) and fixed in 4% paraformaldehyde. Three fields of cells on the lower membrane surface were counted in each well at 10× magnification. The invasion experiment was performed twice with similar results. 9 L and 9 L–m9 spheroids were established by culturing cells in suspension on noble agar coated flasks. Individual cell spheres (~200 μm diameter) were cultured in 1 ml of medium in a 24-well culture plate (Corning, Lowell, MA). Culture wells are modified polystyrene, hydrophilic and negatively charged when medium is added, to enable cell attachment and spreading. Migration was quantified using a micrometer as the mean difference between the leading edge of migrating cells and the original tumor sphere diameter 3 spheres per group were measured, and mean migration between groups was compared.
PCR and Western Blot
To profile gene expression associated with tumor cell invasion in vivo, we employed a RT2 Profiler PCR Array (SuperArray, MD) upon cells isolated from 8 μm frozen coronal sections using LCMD. We restricted our analysis to two functional gene groups in the array, those associated with angiogenesis and those associated with invasion and metastasis. PCR was performed using the SYBR Green system on an ABI 7000 PCR instrument. RT for miRNAs was performed using a hsa-miR-9 TaqMan MicroRNA Assay which detects both human or rat miR-9. RNU43 or U6 primers were used as house-keeping controls. PCR for individual miRNAs was performed with TaqMan Universal Master Mix (Applied Biosystems, CA). Western blot was performed to detect E-cadherin (Abcam, MA) and β-actin (Santa Cruz, CA). Protein concentration was quantified using a BCA protein assay kit (Pierce, IL), and samples were loaded normalized to total protein.
Cells and miRNA Transfection
9 L and U87-MG cells were obtained from the American Type Culture Collection (Manassas, VA). Cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS). For miR-9 expressing cell lines 9 L–m9 and U87-m9, hsa-miR-9 expression plasmid (GenScript) was used. Transfection was performed using electroporation. 2 × 106 9 L or U87-MG cells were suspended in 150 μl of Ingenio Electroporation Solution (Mirus) with 2 μg of plasmid DNA. The Amaxa Nucleofector Device was used for electroporation with Program A-33. Transfected cells were resuspended in 10 ml complete culture medium, centrifuged, and then plated. Puromycin was used for clone selection. We verified miR-9 over-expression using a hsa-miR-9 TaqMan® miRNA assay (Applied Biosystems).
Laser-Capture Microdissection of 9 L Glioma Model in Fisher Rat
Male Fischer rats (250–275 g) were used for this study. A 2 mm diameter craniotomy was made on the right hemisphere anterior to the coronal suture. Using a Hamilton syringe, cells were injected 3.5 mm deep, 3.0 mm to the right and 1.0 mm anterior of the bregma. Rats were implanted with 2.5 × 105 9 L cells (5 μl PBS) over a 15-min interval. The craniotomy was covered with Horsley’s bone wax, and the incision was closed with 4–0 silk suture (Ethicon). Rats were sacrificed 10 days after implantation under anesthesia with i.p. administration of ketamine (100 mg/kg) and xylazine (10 mg/kg). 8 μm frozen coronal sections were used for laser-capture experiments. Using LCMD (DM6000M, Leica, Germany), we excised 9 L xenograph cells from two distinct regions in brain: 1) from the tumor periphery (within the primary tumor mass at least 100 μm from normal brain tissue and 2) from invasive tumor (tumor cells or islets at least 100 μm from the tumor mass, completely surrounded by brain parenchyma). Following LCMD, total RNA was isolated from tumor tissue, and using specific primers, miR-146b-5p and control RNU43 was measured.
Conditioned Medium Assay
Cells were plated at 5 k/cm2 or 250 k/cm2 and cultured for 24 h. Conditioned medium from these wells was then transferred into wells of cells in experimental (all 5 k/cm2) wells and cultured for 24 h.
Results
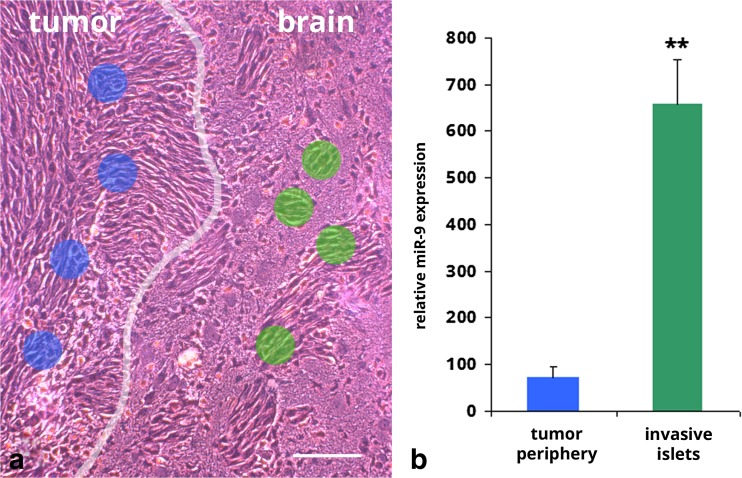
To measure miRNAs differentially expressed between proliferative and invasive glioma cells, we employed laser capture micro-dissection (LCMD) and a miRNA PCR array to test miRNA expression levels in glioma cells of 9 L xenographs in Fisher rat 2 weeks post-implantation. Total RNA was isolated from tumor cells in two distinct regions: 1) >100 μm within the primary tumor, and 2) from tumor cells >100 μm from the bulk tumor boundary (Fig. 1a). Measuring 172 miRNAs from cells in these two distinct regions, we found miR-9 in transplanted 9 L cells was most disparate: miR-9 was more than 10-fold (657.9 ± 97.8 vs. 60.6 ± 5.4) higher in invasive tumor cell islets as compared to the bulk tumor mass (Fig. 1b). Thus, in our intracranial xenograft model of malignant glioma, invasive tumor cells expressed higher levels of miR-9. These findings appeared in agreement with a previous study by Tan et al., who reported that a balance between cyclic AMP response element-binding protein (CREB) and miR-9 determines the proliferation or migration status of glioma cells, whereby miR-9 promotes migration and CREB induces proliferation [13]. Moreover, as miR-9 expression is an independent prognostic factor for overall survival in glioma patients [14], we elected to further investigate this miRNA.
Fig. 1.
Expression of miR-9 is relatively higher in invasive tumor islets compared to those in the tumor periphery. a 9 L gliosarcoma xenograft in Fisher rat at 2-weeks post-implantation. Blue and green dots show target areas for LCMD; primary tumor periphery (blue) and invasive tumor islets (green). White line highlights border of primary tumor mass. Bar =100 μm. b Expression of miR-9 in tissue isolated from 9 L xenografts from primary tumor periphery or invasive tumor islets. **p < 0.01 vs. primary tumor tissue
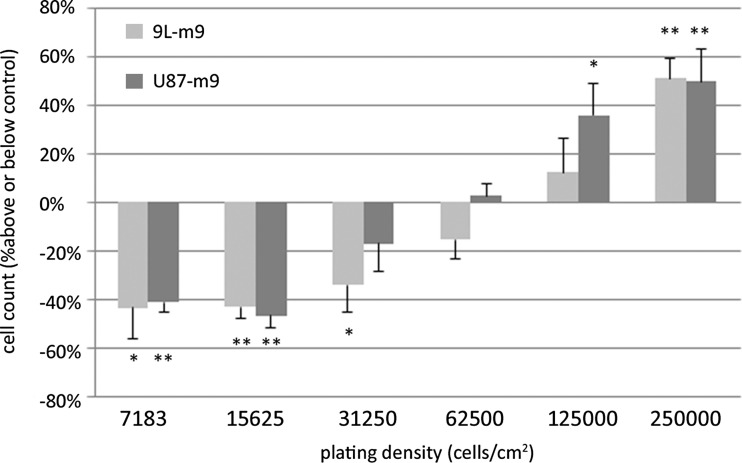
Two identical tumor cells may become phenotypically diverge due to stochastic variation in gene expression levels or differences in their local micro-environment, including tumor cell density [15–18]. As an example, cell density modulates focal adhesion kinase (Fak) activation, a regulator of glioma invasion [19, 20]. Deisboeck et al., have suggested that the onset of invasion marks the time point when the tumor’s cell density reaches a compaction maximum [21]. One difference between cells within invasive tumor cell islets and those in the bulk tumor we sampled with LCMD was relative local tumor cell density. Therefore, we sought to test the effects of miR-9 expression in 9 L glioma cells cultured at different densities. To this end, we first established a 9 L glioma cell line that stably over-expressed miR-9 (9 L–m9). We then plated both control 9 L cells and 9 L–m9 cells at six densities ranging from 7812.5 cells/cm2 to 250,000 cells/cm2 and cultured them for 24 h, at which point growth rates were determined. As shown in Fig. 2, glioma cells that over-expressed miR-9 proliferated at a significantly lower rate at low densities as compare to control, but proliferated at a significantly higher rate compare to control at high densities. This unexpected result suggested that miR-9 not only influences tumor cell proliferation, but that miR-9 has a biphasic effect upon proliferation, which itself was dependent upon cell density. To determine if this effect was specific to the 9 L cell line, we then performed the same experiment with U87 human glioblastoma cells. We first established a U87 cell line that stably over-expressed miR-9 (U87-m9), and then repeated the experiment. As with 9 L cells, U87-m9 proliferated at a significantly lower rate at low densities as compare to U87 control, but proliferated at a significantly higher compare as to U87 control at high cell densities (Fig. 2). Thus, our data indicate that miR-9 has a biphasic density-dependent effect upon glioma cell proliferation.
Fig. 2.
Over-expression of miR-9 in 9 L or U87 glioma cells reduces cell growth at low plating densities, and increases cell growth at high densities. Data are presented as the percentage above or below of the mean cell count 9 L–m9 cells compared to 9 L control cells or U87-m9 cells compared to U87 control cells plated at that density. *p < 0.05, **p < 0.01
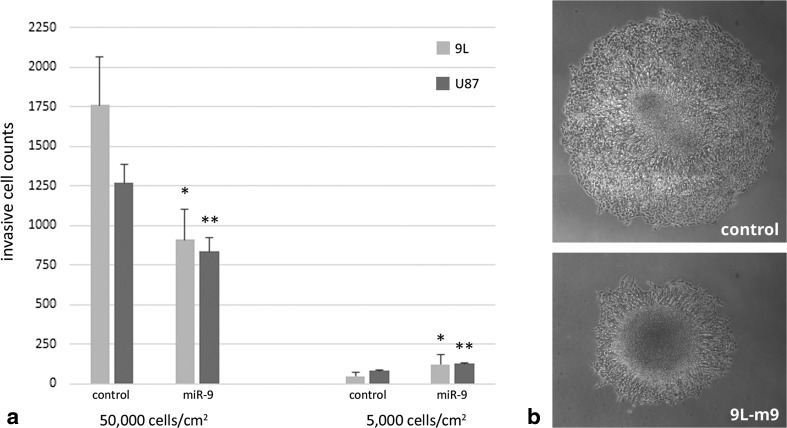
It is known that invasive cells have low proliferation rate, but high motility. We have shown that invasive cells have high miR-9 expression, which is consistent with their decreased proliferation in a low-density environment. Therefore, next we sought to test the effect of miR-9 upon cell motility. Is the invasion of glioma cells also altered by miR-9 in a density-dependent manner? To test this hypothesis, we performed a Matrigel invasion chamber assay employing 9 L and 9 L–m9 cells plated at low (5000 cells/cm2) or high (50,000 cells/cm2) density. Tumor cells were plated, and those that invaded through the extra-cellular protein matrix after 24 h were imaged and counted. Once again, we found that miR-9 over-expression elicited a biphasic effect. However, converse to the effect that miR-9 expression had upon proliferation, cells that over-expressed miR-9 invaded through the extracellular matrix at a significantly higher rate at low densities as compared to control, but at a significantly lower rate as compared to control at high densities (Fig. 3a). To test the effect of miR-9 upon glioma cell motility independent of matrix degradation, we then plated 9 L and 9 L–m9 tumor spheres (high density) and measured cell migration out of the tumor spheres. Here, we found that miR-9 over-expression reduced migration of cells out of the tumor spheres in vitro (Fig. 3b). These invasion and migration data indicate that the effect of miR-9 upon invasiveness and migration of tumor cells is density-dependent and inverse of the effect that miR-9 had upon tumor cell proliferation. Taken together, our experiments demonstrate that miR-9 expression does regulate the phenotypic axis of invasion and proliferation in glioma cells, but whether miR-9 promotes or suppresses motility or growth depends upon cell density. To our knowledge, this is the first report of a density-dependent biphasic effect of miR-9 upon glioma cell invasion and proliferation.
Fig. 3.
Over-expression of miR-9 in glioma cells increases cell invasion at low plating densities, and decreased cell invasion and migration at high densities. a Invasion of 9 L, 9 L–m9, U87, or U87-m9 cells in matrigel invasion chamber assays. 9 L–m9 and U87-m9 cells invaded more at 5000 cells/cm2, and invaded less at 50000 cells/cm2 compared to 9 L or U87 control, respectively. *p < 0.05, **p < 0.01 vs. control. b Tumor spheroid migration assay with 9 L or 9 L–m9 cells imaged at 96 h. Radial cell migration away from the tumor spheroid was higher in 9 L cells compared to 9 L–m9 cells (956.0 ± 84.0 μm vs. 433.3 ± 155.0 μm, **p < 0.01)
Our in vitro experiments demonstrated a biphasic effect of miR-9 upon motility and growth, whereas our in vivo measurement of miR-9 in primary tumor or invasive glioma cells suggest that miR-9 expression is correlated with the same phenotypic properties that are observed for invasive cells: high motility and low proliferation. We therefore postulated that this biphasic effect be important for the eventual switch from an invasive to proliferative phenotype far from the primary tumor. One phenomenon that has been reported to affect this switch is hypoxia [22].
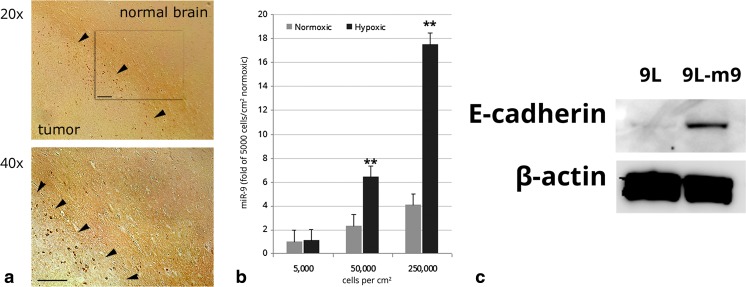
Hypoxia contributes to glioma invasiveness [23, 24]. Previously, we demonstrated that hypoxia contributes to glioma migration by decreasing cell-to-cell adhesion rather than increasing individual cell motility [25]. It was recently reported that hypoxia promotes hypoxia inducible factor 1α (HIF-1α) -mediated up-regulation of miR-9 in pulmonary artery smooth muscle cells, however the effect of hypoxia upon miR-9 in tumor has yet to be investigated [11]. We therefore tested the effect of hypoxia upon miR-9 signaling in glioma cells. First, in an effort to determine regional differences in hypoxia in our 9 L xenograft model, we performed immunohistochemical staining for HIF-1α in coronal tissue of 9 L tumor-bearing rats. HIF-1α is a master regulator that coordinates the cellular response to hypoxia [26]. In this experiment, we found HIF-1α immunoreactivity was most pronounced in the brain adjacent to tumor, the region that contained the invasive tumor islets which we isolated by LCMD (Fig. 4a). Next, to determine if hypoxia induced miR-9 expression in glioma cells, we cultured 9 L cells in normoxic and hypoxic conditions, and compared their miR-9 expression. As we previously established miR-9 influences proliferation and motility at different densities, we performed this experiment at plating densities of 5000, 50,000, and 250,000 cells/cm2 (Fig. 4b). Here, we found that miR-9 was significantly higher in hypoxic compared to normoxic cells in cultures plated at 50000 and 250,000 cells/cm2. These data indicate that miR-9 is increased by hypoxia in 9 L cells in moderate to high density culture conditions.
Fig. 4.
a Hif-1α immunoreactivity in Fisher rat brain bearing 9 L gliosarcoma xenograft. Expression of Hif-1α was relatively elevated in the invasive zone of brain-adjacent to tumor as compared to primary tumor periphery or non-tumor brain. DAB (dark brown) indicates Hif-1α immunoreactivity. Arrows indicate tumor border. Bar =100 μm. b Hypoxia induces miR-9 expression in 9 L tumor cells in vitro. 24 h of hypoxic culture conditions significantly up-regulated miR-9 in 9 L cells cultured at 50000 cells/cm2 or 250,000 cells/cm2. **p < 0.01. c Protein expression of E-cadherin is increased in 9 L cells over-expressing miR-9 as compared to 9 L control
The loss of E-cadherin in tumor cells results in a more invasive phenotype, resistance to apoptosis, and loss of adhesion at the pre-metastatic niche, all controlled by factors under the influence of HIF-1 [27]. In a previous study, we demonstrated that hypoxia promoted the invasiveness of glioma cells by reducing cell-to-cell adherence, with coincident decrease in the adhesion protein E-cadherin [25]. The effect of miR-9 upon E-cadherin expression in glioma has not been studied; however Liu et al., have reported that miR-9 over-expression induced E-cadherin expression in melanoma via inhibition of the NF-κB1-Snail1 pathway [28]. In contrast, Song et al., reported that miR-9 suppresses E-cadherin in esophageal squamous cell carcinoma by directly targeting E-cadherin mRNA, and miR-9 has been found to suppress E-cadherin in other cell types [29–31]. To determine if miR-9 affected E-cadherin expression in glioma cells, we performed Western blot to measure the relative expression levels of E-cadherin between miR-9 over-expressing and control 9 L cells. Here we found that 9 L–m9 cells expressed more E-cadherin than 9 L control, suggesting that miR-9 up-regulates E-cadherin in 9 L glioma cells (Fig. 4c).
Our findings demonstrate that miR-9 regulates a density-dependent biphasic effect upon the phenotypic switch between invasion and proliferation. Furthermore, we provide evidence that hypoxia induces expression of miR-9, and that miR-9 increases expression of the cell-to-cell junction protein E-cadherin. In light of the regionally distinct expression of HIF-1α that we found in the tumor-bearing rat brain and the regionally distinct miR-9 expression in xenograft tumor cells that we measured, we have formulated a hypothesis for the contribution of miR-9 to glioma cell dynamics in the brain adjacent to tumor. In brief, we propose that hypoxia increases the propensity for cells to detach from the primary tumor, and assume an invasive phenotype due to hypoxia-induced miR-9 expression in low density conditions. As these cells migrate away from the tumor, conditions become less hypoxic, which leads to tumor cell clustering [32]. Tumor cell clustering reestablishes miR-9 expression, which promotes cell proliferation and the formation of satellite tumor islets. This hypothesis is detailed in the discussion of this investigation.
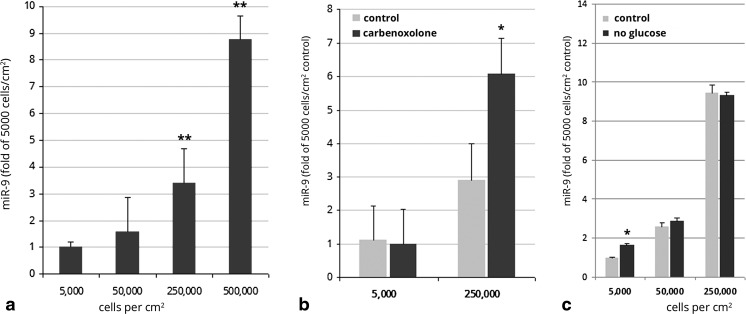
The tumor microenvironment is structurally heterogeneous with regions of varying levels of nutrients, mechanical pressures, and vascular densities [21]. miR-9 has been implicated in promoting glioma invasion, thus it was unsurprising that invasive islets expressed higher levels of miR-9 as compared to the bulk tumor. However, if miR-9 expression underpins a phenotypic switch between proliferation and invasion, one might expect a down-regulation of miR-9 in tumor cells within the invasive zone as they divide and establish satellite tumor islets. As miR-9 levels were much higher in clusters of invasive tumor cells as compared to those in the primary tumor, we surmised that the density of tumor cells might influence the expression of miR-9. Indeed, in our hypoxic culture experiments (Fig. 4b), expression of miR-9 under normoxic conditions increased with cell density. To test this hypothesis, we measured miR-9 in 9 L cells plated at different densities and cultured for 24 h. These experiments revealed that tumor cell expression of miR-9 in vitro correlated with cell plating density: miR-9 levels in cells plated at high density (5 × 105 cells/cm2) were more than 8 fold higher than in cells plated at low density (5 × 103 cells/cm2; Fig. 5a). To our knowledge, this the first report of density-dependent expression of miR-9.
Fig. 5.
Expression of miR-9 increased with cell density in cultured 9 L glioma cells. a miR-9 expression was significantly higher in 9 L cells plated at 50000 or 250,000 cells/cm2 compared to cells plated at 5000 cells/cm2. **p < 0.01 vs. 5000 cells/cm2 control. b Inhibition of gap junctions with carbenoxolone did not decrease miR-9 expression in 9 L cells plated at 50000 or 250,000 cells/cm2 compared to untreated control. *p < 0.01 vs. untreated control. C, Effect of glucose deprivation on miR-9 expression in 9 L cells plated at 5000, 50,000, or 250,000 cells/cm2 compared to cells cultured in normal glucose conditions. *p < 0.01 vs. normal glucose control
We have shown that in our in vivo experiment, cells in the invasive region have a higher expression of miR-9, compared to cells in the primary tumor. We then revealed that high cell density in vitro led to high miR-9 expression. Since the density of tumor cells in the invasive region is not comparably higher than within the primary tumor mass, we face an apparent paradox. However, the conditions of cell density experienced by tumor cells in vitro cannot be simply extrapolated to those conditions experienced by the tumor cells in brain, and our in vitro experiments did not test which components of high-density culture affect individual cells and induce miR-9 expression. That is, in contrast to high-density in vitro culture, cells at lower in vitro density experience multiple differences, such as: 1) more soluble endogenous factors, 2) fewer cell-to-cell contacts, 3) lower nutrient availability and 4) less physical restriction. If endogenous factors, cell-to-cell contacts, nutrient availability, and/or physical restriction underpinned the density-dependent expression of miR-9, then the role of miR-9 in glioma invasion in brain is both dynamic and influenced by the tumor microenvironment. Therefore, in an effort to extend our in vitro findings to conditions experienced by glioma cells in brain, we sought to isolate the physiological components of high-density culture, and determined their individual effect(s) upon miR-9 expression and signaling in glioma cells.
High-density cultures more quickly alter the culture medium by metabolizing nutrients, and releasing soluble factors and waste [33]. Thus, we tested whether medium conditioned by high density cultures would increase miR-9 expression in lower density cultures exposed to that medium. For this experiment, 9 L cells were plated at low (5 k/cm2) or high (250 k/cm2) densities and cultured for 24 h. Conditioned medium from these wells was then transferred into wells with 9 L cells plated at low density which were then cultured (5 k/cm2) for 24 h. No significant difference was found between miR-9 expression in 9 L cells cultured in conditioned medium from either low or high density culture conditions. These findings indicate that the density-dependent increase of miR-9 in 9 L tumor cells in vitro is not significantly mediated by soluble factors.
Cells grown in high density conditions experience more cell-to-cell contacts than those grown in low density conditions. Adjacent glioma cells physically interact via adhesion proteins, microtubes, membrane fusing, microvesicles, and by gap junctions [34–37]. Although the mechanisms by which adjacent tumor cells interact are numerous, substantial evidence indicates that gap junctions play key roles in intercellular communication, and can regulate both glioma proliferation and invasion [36, 38–40]. Indeed, even cell-to-cell interactions via microtubes, adherens, and microvesicles are modulated by gap junctions [34, 41, 42]. We previously demonstrated that functional miRNAs can be shuttled between adjacent tumor cells by gap junctions [43]. More recently, Hong et al., demonstrated that gap junction exchanged miRNAs promoted glioma invasion [36]. Thus, as miR-9 expression increased with cell density, we investigated the influence of gap junction communication upon density-dependent expression of miR-9 in 9 L glioma cells. To test the role of gap junction communication in density-dependent miR-9 expression in glioma cells, we incubated 9 L cells at 5 k/cm2 or 250 k/cm2 with the broad-spectrum gap junction antagonist carbenoxolone, or PBS vehicle for 24 h. After incubation with carbenoxolone, there was no significant difference between miR-9 expression in the low density cultures (Fig. 5b). However, in high density cultures, carbenoxolone treatment increased miR-9 expression 2-fold compared to untreated control. miR-9 expression increases with 9 L cell density (and consequently more cell-to-cell contacts) in vitro, thus we surmised that if gap junctions contributed to density-dependent miR-9 expression, then blocking gap junctions with carbenoxolone would decrease miR-9 at high cell densities. However, we found that carbenoxolone treatment had the opposite effect of further increasing miR-9. Therefore, these experiments suggest that gap junction intercellular communication does not drive the density dependent increase in miR-9 that we observe in vitro.
Tan et al., previously reported that CREB enhances the transcription of miR-9 by direct binding [13]. CREB expression is stimulated by glucose deprivation [44, 45]. In glucose-containing culture medium, due to higher local glucose metabolism, cells grown at high density should experience relatively less glucose availability than those grown at lower density. Therefore we sought to determine whether the density-dependent increase in miR-9 observed in vitro might be dependent upon glucose. To test if glucose availability underpinned density-dependent miR-9 expression in glioma cells, we incubated 9 L cells at 5 k/cm2, 50 k/cm2, or 250 k/cm2 in normal glucose or low glucose culture medium. Here, we found that in low-density (5 k/cm2) culture, glucose deprivation slightly increased miR-9 expression (1.6 fold), however, in moderate- or high-density cultures (50 k/cm2 and 250 k/cm2), glucose deprivation had no significant effect upon miR-9 expression (Fig. 5c). Taken together, these results suggest that the density-dependent increase in miR-9 observed in vitro is not dependent upon glucose availability.
Growing evidence indicates that mechanical cues including stiffness and topography, regulate mechanosensitive subcellular pathways and influence cellular functions such as motility, proliferation, and differentiation [46–48]. As cells experience more physical restriction in high-density cultures compared to cells in low-density cultures, we aimed to test whether increased in physical restriction altered miR-9 expression in glioma cells in vitro. To this end, we added glass microbeads (53-56 μm) to glioma cell cultures in an effort to increase the physical restriction of the cells whilst not increasing the overall cell number. Here, we added 200 k glass beads/ cm2 to cells grown at a density of 200 k/cm2, and compared them to cells grown without glass beads at 200 k/cm2. We then measured miR-9 expression 24 h after plating the cells. These experiments were inconclusive. Here, we found a modest decrease in miR-9 in cultures containing glass beads. However, upon inspection, the cells attached to glass beads in addition to the culture flask, which likely increased the available culture area, and reduced physical restriction. Thus, were unable to isolate the contribution of physical restriction to the density dependent induction of miR-9 that we measured in vitro.
Taken together, we were unable to determine the component of density-dependent induction of miR-9 in 9 L glioma cells in vitro. Our experiments that tested the contribution of soluble factors, gap junction communication, or glucose availability, did not isolate the mechanism by which an increase in cell density in vitro resulted in increased miR-9 expression. Nevertheless, our experiments revealed that miR-9 expression in cultured glioma cells was positively correlated with density. Most notably, our findings demonstrate that over-expression of miR-9 has a biphasic effect upon glioma cell proliferation and invasion that depends upon cell density. Finally, we found that miR-9 expression was increased by hypoxia in 9 L cells at moderate to high culture densities.
Discussion
Numerous mechanisms have been implicated in the transition of glioma cells from proliferation to invasion; however the phenotypic dichotomy of cell proliferation and migration suggests coordinated regulation of the switch. Most miRNAs simultaneously regulate multiple targets, thereby conducting multifaceted changes in protein expression [5, 49, 50]. Several miRNAs have been identified that correlate to clinical outcomes and modulate glioblastoma proliferation and invasion [9, 50–52]. In an effort to elucidate the signaling mechanisms that regulate the switch from proliferation to invasion in malignant glioma, we performed LCMD to isolate glioma cells from the primary tumor and from invasive tumor islets located >100 μm from the tumor boundary. We then assayed 172 miRNAs in these two cell regionally distinct populations, compared relative expression levels, and found that miR-9 was the most disparately expressed miRNA among those assayed, at levels more than 10-fold higher in invasive islets compared to cells in the primary tumor.
Previous investigations have indicated that miR-9 promotes glioma migration, however opposing effects of miR-9 upon migration and proliferation have been reported in other tumors [13, 53, 54]. To clarify the role of miR-9 in the invasion and proliferation in glioma, we tested the effect of miR-9 over-expression in cultured glioma cells. As miR-9 was differentially expressed in primary tumor cells or those in islets in brain adjacent to tumor, we chose to vary the cell plating density in our experiments. These experiments revealed the unexpected result that the effect of miR-9 over-expression upon proliferation and invasion was dependent upon the relative cell density; at high cell densities, miR-9 over-expression decreased migration, and increased proliferation of glioma cells, whereas at low cell densities (like those found in the invasive region around the primary tumor), miR-9 over-expression increased migration and decreased proliferation of glioma cells. We confirmed this effect in both 9 L and U87 glioma cell lines.
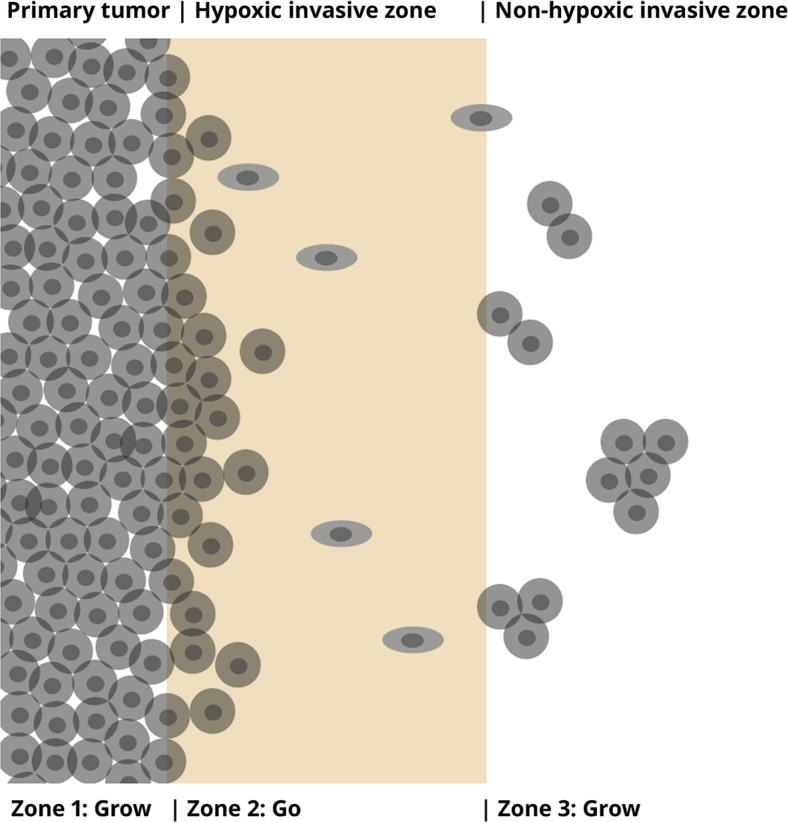
Our results demonstrated the following: 1) miR-9 increases glioma cell migration and decreases proliferation at low densities, but has the opposite effect at high densities, 2) a region of hypoxia is found in brain-adjacent to tumor, but the region far from the tumor which includes the invasive zone, is less hypoxic. These findings, taken together with the finding that hypoxia decreases cell-cell adhesion, have led us to develop the following hypothesis regarding the contribution of miR-9 and hypoxia to glioma invasion and satellite tumor islet formation in glioma (Fig. 6): Cells at the primary tumor/brain interface are hypoxic. As a result of this hypoxia, cell-to-cell adhesion is reduced and thus, cells have a propensity to detach from the surface of a primary tumor. Cells in the invasive region have a high miR-9 expression. Once detached, individual glioma cells near the primary tumor experience both hypoxia and low tumor cell density, which promotes an invasive phenotype (low proliferation, high migration). As these invasive cells migrate away from the tumor, conditions become less hypoxic, which leads to an increase in cell-to-cell adhesion, and thus an increased tendency for tumor cells to cluster [32]. Clustering results in increased local cell density, and density-driven miR-9 expression promotes cell proliferation. Thus, the biphasic effects of miR-9, and the effects of hypoxia upon adhesion may contribute to the phenotypic switch from invasion to proliferation, leading to formation of satellite tumors.
Fig. 6.
Schematic outlining the possible contribution of miR-9 to invasion and satellite tumor formation in brain. Zone 1: Primary tumor. Zone 2: Hypoxic invasive zone (close to the primary tumor). Zone 3: Non-hypoxic invasive zone (distant from the primary tumor). Cells at the primary tumor/brain interface are hypoxic. Due to hypoxia, cell-to-cell adhesion is reduced and tumor cells detach from the primary tumor. Tumor cells in the invasive region are at relatively low tumor cell density, but high miR-9 expression promotes an invasive phenotype (high migration, low proliferation). As invasive tumor cells migrate away from the primary tumor, conditions become less hypoxic, which leads to an increase in cell-to-cell adhesion and decrease in migration, and thus an increased tendency to cluster. Tumor cell clustering results in density-driven miR-9 expression and thus, cell proliferation. Thus, the biphasic effects of miR-9, and the effects of hypoxia upon adhesion may contribute to the phenotypic switch from invasion to proliferation, leading to formation of satellite tumors
We investigated what could promote expression of miR-9 in tumor cells in the invasive region. Density-dependent signaling mechanisms have been identified in tumor cells, including glioma [55–57]. We tested if cell density would affect expression of miR-9 in vitro. To our surprise, cell density positively correlated with increased miR-9 expression in cultured 9 L and U87 glioma cells; glioma cells plated at low densities expressed significantly less miR-9 than cells plated at higher densities. Taken together with the density-dependent biphasic effects of miR-9 that we observed in cells over-expressing miR-9, we surmise that the effect of miR-9 upon the phenotypic axis of invasion and proliferation in glioma cells is multi-potent and dynamically modulated by the tumor microenvironment.
Hypoxia promotes glioma invasion [23, 24]. Indeed, it is considered that hypoxia contributes to the pseudopalisading of glioma cells at the tumor periphery by down-regulating proliferation and increasing tumor cell migration [22, 58, 59]. Hypoxia promotes glioma cell migration and decreases proliferation, whereas oxygenation has the opposite effect [22, 58]. Thus, hypoxia has been identified as a key component in orchestration of the metabolic grow-to-go transition of individual tumor cells that underpins the invasive nature of glioma [58, 60]. Work by Brat et al. provides evidence that satellite cell accumulation is the result of motile hypoxic cells which are less proliferative and more apoptotic than those in the adjacent parent tumor [59]. In agreement with previous findings that pseudopalisading cells are severely hypoxic and over-express HIF-1α [22, 59], our immunohistochemistry revealed HIF-1α was most highly expressed in the invasive periphery of 9 L xenografts in brain.
In our in vitro experiments, we found that hypoxia increased miR-9 expression in glioma cells cultured at moderate or high densities. We previously revealed that hypoxia decreases the adhesion molecule E-cadherin in glioma cells, and that decreased cell-to-cell adhesion contributed to migration of glioma cells under hypoxic conditions rather than an increase in motility [25]. In this study, we tested the effect of miR-9 over-expression upon E-cadherin in cultured glioma cells, and found that miR-9 increased E-cadherin protein expression.
Infiltration of tumor cells into surrounding normal brain tissue confounds clinical management of malignant glioma. Hypoxia and resulting anaerobic glycolysis plays a dual role in regulating tumor proliferation and invasion, and compelling evidence suggests that hypoxic stress upon pseudopalisading cells at the tumor periphery drives the invasive process [22, 58–61]. Numerous pro-invasive molecules have been shown to be inducible by hypoxia and HIF-1, including miR-9 [11, 23, 62]. Sequential switching between proliferation and invasion characterizes malignant glioma progression [61]. Tan et al., provided evidence for a role for miR-9 in the grow or go phenomenon in malignant glioma cells, reporting a proliferation-inhibitory role for miR-9 by targeting CREB and a migration-enhancive role by suppressing NF1 [13]. In this investigation, we found that the effect of miR-9 over-expression upon invasion and proliferation was biphasic, and dependent upon cell density. To our knowledge the experiments by Tan et al., were not carried out at multiple cell densities, and it would be interesting to test if miR-9/CREB feedback was altered by different cell seeding numbers. Indeed, reports of the influence of miR-9 upon tumor migration and proliferation are conflicting [13, 54, 63]. Our data suggests that some of the differences observed might be due to different cell densities in experiments.
The processes of proliferation and invasion of malignant glioma cells are presumed to be mutually exclusive and the term “go or grow” has commonly been used to describe this behavior [22, 58, 64]. In previous investigations of this phenotypic axis, signaling molecules such as miRNAs and proteins have been characteristically revealed as monotonic regulators of growth or invasion [1, 12, 22, 65]. Here, we identify miR-9 as a molecule with the capacity to drive either invasion or growth, dependent upon local cell density. As miR-9 targets numerous mRNAs, it is probable that the biphasic effect of miR-9 represents a shift in the available target profile, which in turn may be influenced by cell density. However, we were unable to isolate the aspect of cell density that drives miR-9 expression, and further experiments are warranted. Hypoxia promotes miR-9 expression in glioma cells, and invasive cells on the tumor periphery are characteristically hypoxic. We found that miR-9 expression was highly disparate between cells within the primary tumor and those within the invasive zone in vivo. Thus, we surmise that miR-9 is a regulator of satellite tumor formation in malignant glioma, making it a compelling target for therapeutic intervention.
Acknowledgements
The authors would like to thank Dr. Xuguang Zheng for and Dr. Ben Buller for assistance and helpful discussions in preparation of this manuscript.
References
- 1.Dhruv HD, McDonough Winslow WS, Armstrong B, Tuncali S, Eschbacher J, Kislin K, Loftus JC, Tran NL, Berens ME. Reciprocal activation of transcription factors underlies the dichotomy between proliferation and invasion of glioma cells. PLoS One. 2013;8(8):e72134. doi: 10.1371/journal.pone.0072134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giese A, Loo MA, Tran N, Haskett D, Coons SW, Berens ME. Dichotomy of astrocytoma migration and proliferation. Int J Cancer. 1996;67(2):275–282. doi: 10.1002/(SICI)1097-0215(19960717)67:2<275::AID-IJC20>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21(8):1624–1636. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 4.Hassaneen W, Levine NB, Suki D, Salaskar AL, de Moura Lima A, McCutcheon IE, Prabhu SS, Lang FF, DeMonte F, Rao G, Weinberg JS, Wildrick DM, Aldape KD, Sawaya R. Multiple craniotomies in the management of multifocal and multicentric glioblastoma. clinical article. J Neurosurg. 2011;114(3):576–584. doi: 10.3171/2010.6.JNS091326. [DOI] [PubMed] [Google Scholar]
- 5.Friedman RC, Burge CB. MicroRNA target finding by comparative genomics. Methods Mol Biol. 2014;1097:457–476. doi: 10.1007/978-1-62703-709-9_21. [DOI] [PubMed] [Google Scholar]
- 6.Fabbri M, Croce CM, Calin GA. MicroRNAs. Cancer J. 2008;14(1):1–6. doi: 10.1097/PPO.0b013e318164145e. [DOI] [PubMed] [Google Scholar]
- 7.Nicoloso MS, Calin GA. MicroRNA involvement in brain tumors: from bench to bedside. Brain Pathol. 2008;18(1):122–129. doi: 10.1111/j.1750-3639.2007.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silber J, James CD, Hodgson JG. microRNAs in gliomas: small regulators of a big problem. Neruomol Med. 2009;11(3):208–222. doi: 10.1007/s12017-009-8087-9. [DOI] [PubMed] [Google Scholar]
- 9.Areeb Z, Stylli SS, Koldej R, Ritchie DS, Siegal T, Morokoff AP, Kaye AH, Luwor RB. MicroRNA as potential biomarkers in glioblastoma. J Neuro-Oncol. 2015;125(2):237–248. doi: 10.1007/s11060-015-1912-0. [DOI] [PubMed] [Google Scholar]
- 10.Bradley BS, Loftus JC, Mielke CJ, Dinu V. Differential expression of microRNAs as predictors of glioblastoma phenotypes. BMC Bioinformatics. 2014;15:21. doi: 10.1186/1471-2105-15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shan F, Li J, Huang QY. HIF-1 alpha-induced up-regulation of mir-9 contributes to phenotypic modulation in pulmonary artery smooth muscle cells during hypoxia. J Cell Physiol. 2014;229(10):1511–1520. doi: 10.1002/jcp.24593. [DOI] [PubMed] [Google Scholar]
- 12.Katakowski M, Zheng X, Jiang F, Rogers T, Szalad A, Chopp M. MiR-146b-5p suppresses egfr expression and reduces in vitro migration and invasion of glioma. Cancer Investig. 2010;28(10):1024–1030. doi: 10.3109/07357907.2010.512596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan X, Wang S, Yang B, Zhu L, Yin B, Chao T, Zhao J, Yuan J, Qiang B, Peng X. The CREB-miR-9 negative feedback minicircuitry coordinates the migration and proliferation of glioma cells. PLoS One. 2012;7(11):e49570. doi: 10.1371/journal.pone.0049570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Z, Wang L, Li G, Liu H, Fan F, Li Z, Li Y, Gao G. Increased expression of microRNA-9 predicts an unfavorable prognosis in human glioma. Mol Cell Biochem. 2013;384(1–2):263–268. doi: 10.1007/s11010-013-1805-5. [DOI] [PubMed] [Google Scholar]
- 15.Neildez-Nguyen TM, Parisot A, Vignal C, Rameau P, Stockholm D, Picot J, Allo V, Le Bec C, Laplace C, Paldi A. Epigenetic gene expression noise and phenotypic diversification of clonal cell populations. Differentiation. 2008;76(1):33–40. doi: 10.1111/j.1432-0436.2007.00219.x. [DOI] [PubMed] [Google Scholar]
- 16.Pham K, Chauviere A, Hatzikirou H, Li X, Byrne HM, Cristini V, Lowengrub J. Density-dependent quiescence in glioma invasion: instability in a simple reaction-diffusion model for the migration/proliferation dichotomy. J Biol Dyn. 2012;6(Suppl 1):54–71. doi: 10.1080/17513758.2011.590610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vultur A, Cao J, Arulanandam R, Turkson J, Jove R, Greer P, Craig A, Elliott B, Raptis L. Cell-to-cell adhesion modulates stat3 activity in normal and breast carcinoma cells. Oncogene. 2004;23(15):2600–2616. doi: 10.1038/sj.onc.1207378. [DOI] [PubMed] [Google Scholar]
- 18.Batt DB, Roberts TM. Cell density modulates protein-tyrosine phosphorylation. J Biol Chem. 1998;273(6):3408–3414. doi: 10.1074/jbc.273.6.3408. [DOI] [PubMed] [Google Scholar]
- 19.Azzalin A, Moretti E, Arbustini E, Magrassi L. Cell density modulates SHC3 expression and survival of human glioblastoma cells through fak activation. J Neuro-Oncol. 2014;120(2):245–256. doi: 10.1007/s11060-014-1551-x. [DOI] [PubMed] [Google Scholar]
- 20.Lindemann C, Hackmann O, Delic S, Schmidt N, Reifenberger G, Riemenschneider MJ. SOCS3 promoter methylation is mutually exclusive to EGFR amplification in gliomas and promotes glioma cell invasion through STAT3 and FAK activation. Acta Neuropathol. 2011;122(2):241–251. doi: 10.1007/s00401-011-0832-0. [DOI] [PubMed] [Google Scholar]
- 21.Deisboeck TS, Mansury Y, Guiot C, Degiorgis PG, Delsanto PP. Insights from a novel tumor model: indications for a quantitative link between tumor growth and invasion. Med Hypotheses. 2005;65(4):785–790. doi: 10.1016/j.mehy.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Horing E, Harter PN, Seznec J, Schittenhelm J, Buhring HJ, Bhattacharyya S, von Hattingen E, Zachskorn C, Mittelbronn M, Naumann U. The “go or grow” potential of gliomas is linked to the neuropeptide processing enzyme carboxypeptidase E and mediated by metabolic Stress. Acta Neuropathol. 2012;124(1):83–97. doi: 10.1007/s00401-011-0940-x. [DOI] [PubMed] [Google Scholar]
- 23.Joseph JV, Conroy S, Pavlov K, Sontakke P, Tomar T, Eggens-Meijer E, Balasubramaniyan V, Wagemakers M, den Dunnen WF, Kruyt FA. hypoxia enhances migration and invasion in glioblastoma by promoting a mesenchymal shift mediated by the HIF1alpha-ZEB1 axis. Cancer Lett. 2015;359(1):107–116. doi: 10.1016/j.canlet.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Zheng X, Jiang F, Katakowski M, Kalkanis SN, Hong X, Zhang X, Zhang ZG, Yang H, Chopp M. Inhibition of ADAM17 reduces hypoxia-induced brain tumor cell invasiveness. Cancer Sci. 2007;98(5):674–684. doi: 10.1111/j.1349-7006.2007.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khain E, Katakowski M, Hopkins S, Szalad A, Zheng X, Jiang F, Chopp M. Collective behavior of brain tumor cells: the role of hypoxia. Phys Rev E Stat Nonlinear Soft Matter Phys. 2011;83(3 Pt 1):031920. doi: 10.1103/PhysRevE.83.031920. [DOI] [PubMed] [Google Scholar]
- 26.Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meir EG. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro-Oncology. 2005;7(2):134–153. doi: 10.1215/S1152851704001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unwith S, Zhao H, Hennah L, Ma D. The potential role of HIF on tumour progression and dissemination. Int J Cancer. 2015;136(11):2491–2503. doi: 10.1002/ijc.28889. [DOI] [PubMed] [Google Scholar]
- 28.Liu S, Kumar SM, Lu H, Liu A, Yang R, Pushparajan A, Guo W, Xu X. MicroRNA-9 up-regulates E-cadherin through inhibition of NF-kappaB1-Snail1 pathway in melanoma. J Pathol. 2012;226(1):61–72. doi: 10.1002/path.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu M, Zhu H, Yang S, Wang Z, Bai J, Xu N. C-Myc suppressed E-cadherin through miR-9 at the post-transcriptional level. Cell Biol Int. 2013;37(3):197–202. doi: 10.1002/cbin.10039. [DOI] [PubMed] [Google Scholar]
- 30.MH L, Huang CC, Pan MR, Chen HH, Hung WC. Prospero homeobox 1 promotes epithelial-mesenchymal transition in colon cancer cells by inhibiting E-cadherin via miR-9. Clin Cancer Res. 2012;18(23):6416–6425. doi: 10.1158/1078-0432.CCR-12-0832. [DOI] [PubMed] [Google Scholar]
- 31.Song Y, Li J, Zhu Y, Dai Y, Zeng T, Liu L, Li J, Wang H, Qin Y, Zeng M, Guan XY, Li Y. MicroRNA-9 promotes tumor metastasis via repressing E-cadherin in esophageal squamous cell carcinoma. Oncotarget. 2014;5(22):11669–11680. doi: 10.18632/oncotarget.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khain E, Schneider-Mizell CM, Nowicki MO, Chiocca EA, Lawler SE, Sander LM (2009) Pattern formation of glioma cells: effects of adhesion. Epl 88(2). doi:10.1209/0295-5075/88/28006
- 33.Leontieva OV, Demidenko ZN, Blagosklonny MV. Contact inhibition and high cell density deactivate the mammalian target of rapamycin pathway, thus suppressing the senescence program. Proc Natl Acad Sci U S A. 2014;111(24):8832–8837. doi: 10.1073/pnas.1405723111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osswald M, Jung E, Sahm F, Solecki G, Venkataramani V, Blaes J, Weil S, Horstmann H, Wiestler B, Syed M, Huang L, Ratliff M, Karimian Jazi K, Kurz FT, Schmenger T, Lemke D, Gommel M, Pauli M, Liao Y, Haring P, Pusch S, Herl V, Steinhauser C, Krunic D, Jarahian M, Miletic H, Berghoff AS, Griesbeck O, Kalamakis G, Garaschuk O, Preusser M, Weiss S, Liu H, Heiland S, Platten M, Huber PE, Kuner T, von Deimling A, Wick W, Winkler F. Brain tumour cells interconnect to a functional and resistant network. Nature. 2015;528(7580):93–98. doi: 10.1038/nature16071. [DOI] [PubMed] [Google Scholar]
- 35.Lewis-Tuffin LJ, Rodriguez F, Giannini C, Scheithauer B, Necela BM, Sarkaria JN, Anastasiadis PZ. Misregulated E-cadherin expression associated with an aggressive brain tumor phenotype. PLoS One. 2010;5(10):e13665. doi: 10.1371/journal.pone.0013665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong X, Sin WC, Harris AL, Naus CC. Gap junctions modulate glioma invasion by direct transfer of microRNA. Oncotarget. 2015;6(17):15566–15577. doi: 10.18632/oncotarget.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Appolloni I, Barilari M, Caviglia S, Gambini E, Reisoli E, Malatesta P. A cadherin switch underlies malignancy in high-grade gliomas. Oncogene. 2015;34(15):1991–2002. doi: 10.1038/onc.2014.122. [DOI] [PubMed] [Google Scholar]
- 38.Bates DC, Sin WC, Aftab Q, Naus CC. Connexin43 enhances glioma invasion by a mechanism involving the carboxy terminus. Glia. 2007;55(15):1554–1564. doi: 10.1002/glia.20569. [DOI] [PubMed] [Google Scholar]
- 39.Zhang W, Nwagwu C, Le DM, Yong VW, Song H, Couldwell WT. Increased invasive capacity of connexin43-overexpressing malignant glioma cells. J Neurosurg. 2003;99(6):1039–1046. doi: 10.3171/jns.2003.99.6.1039. [DOI] [PubMed] [Google Scholar]
- 40.Sin WC, Crespin S, Mesnil M. Opposing roles of connexin43 in glioma progression. Biochim Biophys Acta. 2012;1818(8):2058–2067. doi: 10.1016/j.bbamem.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 41.SC Y, Xiao HL, Jiang XF, Wang QL, Li Y, Yang XJ, Ping YF, Duan JJ, Jiang JY, Ye XZ, SL X, Xin YH, Yao XH, Chen JH, Chu WH, Sun W, Wang B, Wang JM, Zhang X, Bian XW. Connexin 43 reverses malignant phenotypes of glioma stem cells by modulating E-cadherin. Stem Cells. 2012;30(2):108–120. doi: 10.1002/stem.1685. [DOI] [PubMed] [Google Scholar]
- 42.Soares AR, Martins-Marques T, Ribeiro-Rodrigues T, Ferreira JV, Catarino S, Pinho MJ, Zuzarte M, Isabel Anjo S, Manadas B, PGS J, Pereira P, Girao H. Gap junctional protein Cx43 is involved in the communication between extracellular vesicles and mammalian cells. Sci Report. 2015;5:13243. doi: 10.1038/srep13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katakowski M, Buller B, Wang X, Rogers T, Chopp M. Functional microRNA is transferred between glioma cells. Cancer Res. 2010;70(21):8259–8263. doi: 10.1158/0008-5472.CAN-10-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okoshi R, Ando K, Suenaga Y, Sang M, Kubo N, Kizaki H, Nakagawara A, Ozaki T. Transcriptional regulation of tumor suppressor p53 by cAMP-responsive element-binding protein/AMP-activated protein kinase complex in response to glucose deprivation. Genes Cells. 2009;14(12):1429–1440. doi: 10.1111/j.1365-2443.2009.01359.x. [DOI] [PubMed] [Google Scholar]
- 45.Fusco S, Leone L, Barbati SA, Samengo D, Piacentini R, Maulucci G, Toietta G, Spinelli M, McBurney M, Pani G, Grassi C. A CREB-Sirt1-Hes1 circuitry mediates neural stem cell response to glucose availability. Cell Rep. 2016;14:1195–1205. doi: 10.1016/j.celrep.2015.12.092. [DOI] [PubMed] [Google Scholar]
- 46.Friedl P, Sahai E, Weiss S, Yamada KM. New dimensions in cell migration. Nat Rev Mol Cell Biol. 2012;13(11):743–747. doi: 10.1038/nrm3459. [DOI] [PubMed] [Google Scholar]
- 47.Pathak A, Kumar S. Biophysical regulation of tumor cell invasion: moving beyond matrix stiffness. Integr Biol (Camb) 2011;3(4):267–278. doi: 10.1039/c0ib00095g. [DOI] [PubMed] [Google Scholar]
- 48.Faurobert E, Bouin AP, Albiges-Rizo C. Microenvironment, tumor cell plasticity, and cancer. Curr Opin Oncol. 2015;27(1):64–70. doi: 10.1097/CCO.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 49.Malzkorn B, Wolter M, Liesenberg F, Grzendowski M, Stuhler K, Meyer HE, Reifenberger G. Identification and functional characterization of microRNAs involved in the malignant progression of gliomas. Brain Pathol. 2010;20(3):539–550. doi: 10.1111/j.1750-3639.2009.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henriksen M, Johnsen KB, Olesen P, Pilgaard L, Duroux M. MicroRNA expression signatures and their correlation with clinicopathological features in glioblastoma multiforme. Neruomol Med. 2014;16(3):565–577. doi: 10.1007/s12017-014-8309-7. [DOI] [PubMed] [Google Scholar]
- 51.Haapa-Paananen S, Chen P, Hellstrom K, Kohonen P, Hautaniemi S, Kallioniemi O, Perala M. Functional profiling of precursor micrornas identifies MicroRNAs essential for glioma proliferation. PLoS One. 2013;8(4):e60930. doi: 10.1371/journal.pone.0060930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Godlewski J, Bronisz A, Nowicki MO, Chiocca EA, Lawler S. Microrna-451: a conditional switch controlling glioma cell proliferation and migration. Cell Cycle. 2010;9(14):2742–2748. doi: 10.4161/cc.9.14.12248. [DOI] [PubMed] [Google Scholar]
- 53.Zheng L, Qi T, Yang D, Qi M, Li D, Xiang X, Huang K, Tong Q. MicroRNA-9 suppresses the proliferation, invasion and metastasis of gastric cancer cells through targeting cyclin d1 and Ets1. PLoS One. 2013;8(1):e55719. doi: 10.1371/journal.pone.0055719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun Z, Han Q, Zhou N, Wang S, Lu S, Bai C, Zhao RC. MicroRNA-9 enhances migration and invasion through KLF17 in hepatocellular carcinoma. Mol Oncol. 2013;7(5):884–894. doi: 10.1016/j.molonc.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yao PS, Kang DZ, Wang XF, Lin RY, Ye ZC. Cell-density-dependent manifestation of partial characteristics for neuronal precursors in a newly established human gliosarcoma cell line. In Vitro Cell Dev Biol Anim. 2015;51(4):345–352. doi: 10.1007/s11626-014-9839-x. [DOI] [PubMed] [Google Scholar]
- 56.Nilsson GM, Akhtar N, Kannius-Janson M, Baeckstrom D. Loss of E-cadherin expression is not a prerequisite for c-erbB2-induced epithelial-mesenchymal transition. Int J Oncol. 2014;45(1):82–94. doi: 10.3892/ijo.2014.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morgenstern K, Hanson-Painton O, Wang BL, De Bault L. Density-dependent regulation of cell surface gamma-glutamyl transpeptidase in cultured glial cells. J Cell Physiol. 1992;150(1):104–115. doi: 10.1002/jcp.1041500115. [DOI] [PubMed] [Google Scholar]
- 58.Kathagen A, Schulte A, Balcke G, Phillips HS, Martens T, Matschke J, Gunther HS, Soriano R, Modrusan Z, Sandmann T, Kuhl C, Tissier A, Holz M, Krawinkel LA, Glatzel M, Westphal M, Lamszus K. Hypoxia and oxygenation induce a metabolic switch between pentose phosphate pathway and glycolysis in glioma stem-like cells. Acta Neuropathol. 2013;126(5):763–780. doi: 10.1007/s00401-013-1173-y. [DOI] [PubMed] [Google Scholar]
- 59.Brat DJ, Castellano-Sanchez AA, Hunter SB, Pecot M, Cohen C, Hammond EH, Devi SN, Kaur B, Van Meir EG. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004;64(3):920–927. doi: 10.1158/0008-5472.CAN-03-2073. [DOI] [PubMed] [Google Scholar]
- 60.Rong Y, Durden DL, Van Meir EG, Brat DJ. Pseudopalisading’ necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J Neuropathol Exp Neurol. 2006;65(6):529–539. doi: 10.1097/00005072-200606000-00001. [DOI] [PubMed] [Google Scholar]
- 61.Xie Q, Mittal S, Berens ME. Targeting adaptive glioblastoma: an overview of proliferation and invasion. Neuro-Oncology. 2014;16(12):1575–1584. doi: 10.1093/neuonc/nou147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fujiwara S, Nakagawa K, Harada H, Nagato S, Furukawa K, Teraoka M, Seno T, Oka K, Iwata S, Ohnishi T. Silencing hypoxia-inducible factor-1alpha inhibits cell migration and invasion under hypoxic environment in malignant gliomas. Int J Oncol. 2007;30(4):793–802. [PubMed] [Google Scholar]
- 63.Wang H, Zhang W, Zuo Y, Ding M, Ke C, Yan R, Zhan H, Liu J, Wang J. miR-9 promotes cell proliferation and inhibits apoptosis by targeting LASS2 in bladder cancer. Tumour Biol. 2015;36(12):9631–9640. doi: 10.1007/s13277-015-3713-7. [DOI] [PubMed] [Google Scholar]
- 64.Venur VA, Peereboom DM, Ahluwalia MS. Current medical treatment of glioblastoma. Cancer Treat Res. 2015;163:103–115. doi: 10.1007/978-3-319-12048-5_7. [DOI] [PubMed] [Google Scholar]
- 65.Paw I, Carpenter RC, Watabe K, Debinski W, Lo HW. Mechanisms regulating glioma invasion. Cancer Lett. 2015;362(1):1–7. doi: 10.1016/j.canlet.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]