Abstract
There is strong evidence that mitochondrial dysfunction mediated oxidative stress results in aging and energy metabolism deficits thus playing a prime role in pathogenesis of Alzheimer’s disease, neuronal death and cognitive dysfunction. Evidences accrued in empirical studies suggest the antioxidant, anticancer and anti-inflammatory activities of the phytochemical pterostilbene (PTS). PTS also exhibits favourable pharmacokinetic attributes compared to other stilbenes. Hence, in the present study, we explored the neuroprotective role of PTS in ameliorating the intracerebroventricular administered streptozotocin (STZ) induced memory decline in rats. PTS at doses of 10, 30 and 50 mg/kg, was administered orally to STZ administered Sprague–Dawley (SD) rats. The learning and memory tests, Morris water maze test and novel object recognition test were performed which revealed improved cognition on PTS treatment. Further, there was an overall improvement in brain antioxidant parameters like elevated catalase and superoxide dismutase activities, GSH levels, lowered levels of nitrites, lipid peroxides and carbonylated proteins. There was improved cholinergic transmission as evident by decreased acetylcholinesterase activities. The action of ATPases (Na+ K+, Ca2+ and Mg2+) indicating the maintenance of cell membrane potential was also augmented. mRNA expression of battery of genes involved in cellular mitochondrial biogenesis and inflammation showed variations which extrapolate to hike in mitochondrial biogenesis and abated inflammation. The histological findings corroborated the effective role of PTS in countering STZ induced structural aberrations in brain.
Keywords: Pterostilbene, Streptozotocin, Fenofibrate, Brain, Learning and memory, Inflammation, AChE, ATPases, Protein carbonylation, PPARα, PGC1α, TNF-α, IL-6, Rats
Introduction
Alzheimer’s disease (AD) is the most common form of dementia which accounts for 50–75 % over the age of 60–65 years (Duthey 2013). The occurrence of familial Alzheimer’s disease (FAD) of all dementias is 5–10 % (Chartier-Harlin et al. 1991; Sisodia et al. 1999) while that of sporadic Alzheimer’s disease (SAD) represents 90–95 % of total cases (Meraz Ríos et al. 2013). AD is characterized by disturbed behavioral conditions, loss of the ability to think clearly and psychological symptoms of dementia, loss of brain cells and malfunctioning of neurons (White et al. 2000; Abdalla et al. 2012).
Two hypotheses have been postulated for AD, namely increased oxidative stress (OS) and abnormal mitotic signaling that serve as a initiator and propagator of this disease (Mariani et al. 2005). OS is known to cause inflammation and disruption of energy homeostasis in the brain at a cellular level. Inflammation as a response of stress and injury releases inflammatory mediators like tumor necrosis factor (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6) (Zhang et al. 2007). These mediators play a crucial role in the pathophysiology of AD, exerting deleterious effects to the progression of tissue damage and other precipitating factors. In addition, the accumulation of reactive oxygen species (ROS) results in damage to major cellular components including the nucleus, mitochondrial DNA, membranes and cytoplasmic proteins (Harman 1992). Interestingly, role of peroxisome proliferator activated receptors (PPARs) in energy homeostasis and ROS production/scavenging is well established (Moreno and Cerù 2015). These receptors are regulators of oxidative stress, inflammation and immune response, making them suitable targets for the treatment of chronic inflammatory diseases, diabetes, cancer and neurodegenerative disorders (Feige et al. 2006). A recent study has indicated the role of peroxisome proliferator activated receptor-alpha (PPAR-α) in modulation of neurotransmission by participating in the synthesis of signaling molecules (peroxides, lipids, ACh) (Chakravarthy et al. 2007). It has been reported that PPAR-α regulates the gene expression of catalase (CAT), superoxide dismutase 1 (SOD1) and acyl CoA oxidase 1 (ACOX1) (Fidaleo et al. 2014). These investigations strongly support a CNS role of PPAR-α in neuroprotection against oxidative damage, by controlling superoxide anion removal (by SOD1), and hydrogen peroxide generation (by SOD1 and ACOX1) and removal (by CAT). At the cellular level, the biogenesis or functionality of mitochondria, peroxisomes and even lysosomes are indeed regulated by PPARα and its cofactors particularly peroxisome proliferator activated receptor gamma co-activator 1 alpha (PGC1α) (Fidaleo et al. 2014; Ghosh et al. 2015).
Streptozotocin [2-deoxy-2-(3-(methyl-3-nitrosoureido)-d-glucopyranose; STZ], a glucosamine–nitrosourea compound has been used to develop a model of late onset, SAD (Kosaraju et al. 2014). The cytotoxicity caused by STZ is mainly due to DNA alkylation resulting in cellular necrosis (Szkudelski 2012). Systemic application of STZ not only damages insulin-producing pancreatic beta cells but also other organs expressing GLUT2 such as kidney and liver (Grieb 2015). Brain is not affected directly because blood–brain barrier (BBB) lacks this transporter protein. However, single or double intracerebroventricular (icv) STZ injection(s) in due course of time decreases cerebral glucose uptake and produces range of OS mediated deteriorating effects that resembles features of SAD (Grieb 2015). These findings point that glucose hypometabolism is an early and persistent hallmark of SAD and that AD brains reflect features of impaired insulin signaling.
Pterostilbene is polyphenolic, non-flavonoid compound that is primarily found in blueberries and Pterocarpus marsupium. Pterostilbene (trans-3,5-dimethoxy-4-hydroxystilbene; PTS) is a naturally derived antioxidant and anti-inflammatory agent (Drolet et al. 2009; Vauzour 2012). Several studies have demonstrated pleiotropic pharmacological activities of PTS including anti-inflammatory, antioxidant, anticancer, analgesic and including inhibition of histone deacetylase-1 (HDAC1) (Joseph et al. 2008; Remsberg et al. 2008; Bhaskaran and Vishwaraman 2009; Acharya and Ghaskadbi 2013). Pertaining to antidiabetic activity, P. marsupium extract and PTS alone have demonstrated ability to lower blood glucose levels (Manickam et al. 1997; Grover et al. 2005). Interestingly, PTS in a study in hamsters has shown hypolipidemic property by lowering levels of low density lipoprotein (LDL) and increasing levels of high density lipoprotein (HDL) in plasma which was postulated to be through the activation of PPAR-α (Rimando et al. 2005; Pyper et al. 2010). In yet another study PTS in mice has elicited inhibition of LPS induced nitric oxide (NO), TNF-α, IL-6 and microglia activation thus improving learning and memory (Hou et al. 2014). It has also been proven to suppress macrophage activation induced by lipopolysaccharide (LPS), which accounts for its anti-inflammatory actions (Pan et al. 2008). Moreover, a recent study in a model of senescence accelerated prone mouse (SAMP8) has confirmed that 2 months of dietary supplementation with PTS in mice lead to improved spatial memory in the radial arm water maze test. This provides robust evidence of the neuroprotective roles of PTS. In another study depicting OS induced signaling dysfunction PTS decreased the levels of phosphorylated c-Jun N-terminal kinases (JnK) and tau by activating PPAR-α, an upstream inducer of manganese superoxide dismutase (MnSOD) (Chang et al. 2012). This data prompted us to assume that PTS might have a role in improving energy homeostasis and may hold promise in obesity and metabolic syndrome associated cognition impairment (Naderali et al. 2009). PTS has added attributes like improved oral bioavailability (80 %), making it more suitable to administer in rodents than resveratrol with poor oral bioavailability (20 %) (Kapetanovic et al. 2011). Overall, the antioxidant and the anti-inflammatory capacity of PTS has depicted significant effects on neurological functions that may translate into clinical benefits in human subjects (McFadden 2013).
Fibric acid derivative, fenofibrate (FF) apart from its lipid lowering property has been reported to ameliorate the global cerebral ischemia induced learning and memory deficits in rats by activating PPAR-α (Xuan et al. 2014). Yet another study has demonstrated that FF can lower the proinflammatory mediators and effectors like inducible nitric oxide synthase (iNOS), cycloxegenase-2 (COX2) and matrix metallopeptidase-9 (MMP9) in rats suffering from acute traumatic brain injury (TBI) (Chew et al. 2006). This drove us to choose FF as a standard in this study as it is known that both FF and PTS induce PPAR-α and have anti-inflammatory properties.
Hence, the current study was planned to explore the impact of PTS in countering STZ induced memory decline in male rats. The effect was confirmed by learning and memory tests, brain antioxidant status, cholinergic transmission, ATPases activity and determining mRNA expression of genes involved in energy homeostasis and inflammation.
Materials and methods
Chemicals
The biochemicals i.e. Pterostilbene was obtained from Nanjing Zelang Medical Technology Co., Ltd (Nanjing, China). Fenofibrate was a gift sample (IOL Chemicals and Pharmaceutical Ltd, Punjab, India). Streptozotocin, ketamine, xylazine, sodium chloride (NaCl), sodium nitrite (NaNO2), sulphanilamide, bovine serum albumin (BSA), acetylthiocholine iodide, 2,4-Dinitrophenylhydrazine (DNPH), 5,5′-dithiobis (2-nitro-benzoic acid) (DTNB), 1,1,3,3-tetraethoxypropane (TEP), ammonium molybdate, glutathione (GSH), Tris HCl, trichloroacetic acid (TCA), 1-amino-2-naphthol-6-sulphonic acid (ANSA), N-(1-naphthyl) ethylenediamine dihydrochloride (NEDA) were purchased from S.D. Fine Chemicals Pvt. Ltd (Mumbai, India). 2-thiobabituric acid (TBA), 5,5′-dithiobis (2-nitro-benzoic acid) (DTNB) was obtained from Hi-media Laboratories Ltd (Mumbai, India). Adenosine triphosphate (ATP) was obtained from Sigma Aldrich Chemicals Co. (St. Louis, Missouri, USA).
PTS and FF were suspended in 1.0 % (w/v) sodium carboxy methyl cellulose (CMC) (Rubin and Rubin 2009; Ouk et al. 2014).
Animals preparation
Male Sprague–Dawley (SD) rats were purchased from Piramal Life Sciences Ltd, Mumbai, India. All the animals were housed in polypropylene plastic cage covered with metal grids in a temperature regulated environment (24–28 °C) and relative humidity (55 ± 10 %) The animals were subjected to a 12 h light-12 h dark cycle. They were maintained on a pelletized diet and water ad libitum. For the animal experimentation prior approval was obtained from ‘Institutional Animal Ethics Committee’, of Bombay College of Pharmacy, Mumbai. All studies were performed in accordance with ‘Committee for the Purpose of Control and Supervision on Experiments on Animals’ (CPCSEA) guidelines, India.
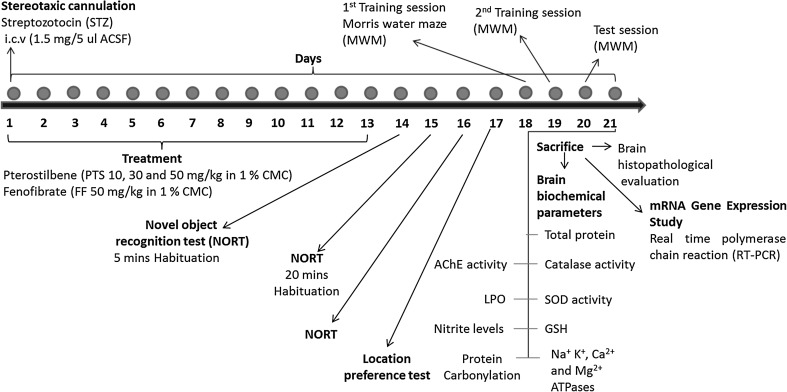
The experimental design (Fig. 1) espoused induction of SAD using STZ (1.5 mg/5 µl ACSF, icv) followed by treatment with PTS (10, 30 and 50 mg/kg, po) for 13 days in male SD rats. Towards the termination of the regimen various learning and memory tests viz. novel object recognition test (NORT) and Morris water maze test (MWM) were conducted. All the rats (n = 8) of each group were subjected to learning and memory tests (MWM and NORT). Post termination, brains of two rats per group were cryopreserved at −70 °C for histopathological examination while the remaining rat brains of each group were used for biochemical and gene expression studies. The biochemical estimations included catalase (CAT), superoxide dismutase (SOD), acetylcholinesterase (AChE) and Na+ K+, Ca2+ and Mg2+ ATPase activities, malondialdehyde (MDA), reduced glutathione (GSH), nitrite and carbonylated protein levels. The cryopreserved brains were used to estimate the mRNA expression of genes involved in inflammation, tumor necrosis factor (TNF), interleukin-6 (IL-6) and the energy homeostasis associated genes, PPAR-α and PGC1α.
Fig. 1.
Representative figure indicating induction and treatment regimen in rats. STZ streptozotocin, PTS pterostilbene, FF fenofibrate, NORT novel object recognition test, MWM Morris water maze test, SOD superoxide dismutase, GSH reduced glutathione, ATPases adenosine triphosphatase (Na+ K+, Ca2+, Mg2+), LPO lipid peroxidation, AChE acetylcholinesterase
In this study forty-eight male Sprague–Dawley (SD) rats were allocated into six groups.
Animal groups
Vehicle control: rats of this group were administered per oral (po) with 1.0 % (w/v) CMC in suitable volumes from day 1 to day 13 and artificial cerebrospinal fluid (ACSF) (5 µl, icv on day 1).
Positive control, STZ: rats of this group were injected with STZ (1.5 mg/5 µl ACSF, icv) on day 1 (n = 8).
STZ + PTS10: rats of this group were injected with STZ (1.5 mg/5 µl ACSF, icv) on day 1 and further, treated with pterostilbene 10 (PTS, 10 mg/kg, po) from day 1 to day 13 (n = 8).
STZ + PTS30: rats of this group were injected with STZ (1.5 mg/5 µl ACSF, icv) on day 1 and further, treated with pterostilbene 30 (PTS, 30 mg/kg, po) from day 1 to day 13 (n = 8).
STZ + PTS50: rats of this group were injected with STZ (1.5 mg/5 µl ACSF, icv) with STZ on day 1 and further, treated with pterostilbene 50 (PTS, 50 mg/kg, po) from day 1 to day 13 (n = 8).
Standard fenofibrate treated group, STZ + FF50: rats of this group were injected with STZ (1.5 mg/5 µl ACSF, icv) on day 1 and further, treated with fenofibrate 50 (FF, 50 mg/kg, po) from day 1 to day 13 (n = 8).
Stereotaxic surgery
Animals were anesthetized using a combination of ketamine (60 mg/kg, ip) and xylazine (6 mg/kg, ip) (10:1). The animals were secured in a stereotactic frame after they were deeply anesthetized. The scalp was shaved, a central incision on the scalp was made and burr hole were drilled on the either side of the skull with coordinates according to the stereotaxic atlas: −0.9 mm anterior, 1.8 mm lateral and −3.7 mm ventral from bregma (Weinstock et al. 2001; Paxinos et al. 2013). Animals were injected; 3.7 mm dorso-ventral from the skull, with streptozotocin (STZ, 1.5 mg/5 µl ACSF, icv on day 1) dissolved in freshly prepared artificial cerebrospinal fluid (ACSF, 147 mM NaCl, 2.9 mM KCl, 1.6 mM MgCl2, 1.7 mM CaCl2 and 2.2 mM dextrose) (Sharma and Gupta 2001; Saxena et al. 2010) into lateral ventricle using a Hamilton syringe needle attached to the Harward nanomite pump. The syringe was kept in place for 2 min for the proper diffusion of STZ. Saline was applied to both eyes to prevent drying of eyes and dental cement was applied to the incision following surgery, and transferred to a thermo-regulated pad to maintain normal body temperature until recovery. As a post-operative care, Betadine® ointment along with Neosporin® powder was applied daily to the surgical area. The surgical animals were kept under observation for any abnormality.
Learning and memory paradigms
Novel object recognition test (NORT)
The apparatus consisted of an evenly illuminated sound-proof box with a plexiglass box (25 cm × 25 cm × 25 cm) inside. The rat behavior is recorded with a video camera. The procedure includes 4 phases: pre-habituation, habituation, training, and testing. On the 1st day (day 14) from the day 1 STZ injection, animals were brought to the testing room 30 min before the experiment to familiarize with the environment. Rats were then allowed to freely explore the box in the absence of objects for 5 min. On the 2nd day (day 15), rats were habituated to the empty box for 20 min. On the 3rd day (day 16), each rat took a training trial followed by a testing trial. During the training trial, two identical objects were placed at two opposite positions within the box at same distance from the nearest corner. The rats were allowed to explore the identical objects for 10 min and then returned to their home cages. One h later, animals were placed back to the same box, where one of the two familiar objects was switched to a novel one, to start a 5 min testing phase. All objects used in this study were different in shapes and colors but identical in size. They were fixed on the floor of the box to avoid movement. To preclude the existence of olfactory cues, the entire box and objects were always thoroughly cleaned with 50 % alcohol after each trial. Ability to discriminate the novel object from familiar object was observed through the time taken to explore the objects. Object exploration time was defined as the length of time when animal directed its nose within 2 cm distance of the object, or sniffed or pawed the object. Sitting or standing on the object was not recognized as exploration. The exploration time was analyzed manually using smart video software. Recognition index (RI) in the testing phase and Location preference was calculated.
Location preference on day 17 was used as an environmental control, which should be 50 %, to rule out the influence of the location of the object. Animals with a total exploration time of less than 3 s during testing session were excluded from analysis (Carlini 2011; Zhanga et al. 2012).
Evaluation of spatial memory by Morris water maze test (MWM)
On the 18th day of the study, spatial learning and memory of the animals were tested in Morris water maze (Joseph et al. 2006; Li et al. 2012). MWM was employed to assess learning and memory of the animals, wherein the animal learned to escape on to a hidden platform. A large circular pool with dimensions (150 cm in diameter, 45 cm in height, filled to a depth of 30 cm with water at 28 ± 1 °C) was used. The water was made opaque with milk. The pool was divided into four equal areas (quadrant). A submerged platform (9 cm) was placed at the centre 1 cm below surface of the water during test session. The training environment was kept unaltered throughout the training period of 2 days (day 18 and 19). During the acquisition phase, each rat was subjected to four consecutive training trials on each day. The rat was gently placed in the water between the quadrants, facing the wall of the pool, with the drop location changing for each trial, and allowed 120 s to locate the platform. Then, the rat was allowed to stay on the platform for 20 s. If it failed to find the platform within 120 s, it was gently guided onto the platform and allowed to remain there for 20 s. Test session was conducted on day 20 with submerged platform and time taken to locate the platform during training and test session was measured as retention latency in seconds.
Brain tissue preparation
Post termination and isolation of the rat brains, homogenization of the whole brain tissues was performed on day 21. The tissues were homogenized in chilled 10 mM phosphate buffer saline (PBS, pH 7.4) to make 10 % homogenates. The homogenates were centrifuged at 4 °C at 5500 rpm for 10 min in cooling centrifuge (Remi C-30BL). The supernatants were used for the estimation of total protein, AChE, Na+ K+, Ca2+ and Mg2+ ATPases, catalase CAT, SOD activities, nitrite, protein carbonyl and LPO levels.
Biochemical parameters
Total proteins estimated in brain tissue homogenates
Protein content of brain samples were measured by the method of (Hartree 1972), a modified Lowry method using Bovine serum albumin (BSA) (10–160 µg/2.5 ml) as standard.
Catalase (CAT) activity
The CAT activity was assayed by the method described previously by Aebi et al. (1975). Briefly, the assay mixture consisted of 2.9 ml H2O2 and 0.1 ml brain tissue supernatant in a total volume of 3.0 ml. The observed change in absorbance was recorded spectrophotometrically at 240 nm for 3 min against blank, prepared by addition of 2.9 ml phosphate buffer and 0.1 ml distilled water without H2O2 addition. The CAT activity was expressed as μmole H2O2 consumed/min/mg protein.
Superoxide dismutase (SOD) activity
The SOD activity was assayed by the method described previously by Misra and Fridovich (1972). Briefly, the assay mixture consisted of 0.05 ml brain tissue supernatant, 0.5 ml EDTA and 2 ml carbonate buffer followed by initiation of reaction by addition of 0.5 ml of epinephrine (3 × 10−4 mM) solution. Auto oxidation of adrenaline (epinephrine) to adrenochrome at pH 10.5 was measured by following change in absorbance at 480 nm against blank for 3 min. Results were expressed as units of SOD activity (UA)/per mg tissue protein. One unit of SOD activity induces 50 % inhibition of adrenochrome formation.
Reduced glutathione (GSH) levels
The GSH content in the brain tissue supernatant was measured by the method described previously by Jollow et al. (1974) and Peixoto et al. (2013) where in 0.5 ml of brain tissue supernatant was mixed with 0.5 ml of 4 % sulphosalicylic acid and incubated at 4 °C for 1 h. Further, centrifugation was done at 3000 rpm at 4 °C for 15 min. To 27 µl of supernatant obtained after centrifugation, 145 µl of 0.1 M (pH 7.4) PBS and 27 µl of 100 mM 5,5′-Dithiobis-(2-nitrobenzoic acid) (Ellman’s reagent) was added. Absorbance was read immediately at 412 nm. GSH levels were expressed as nanomoles of DTNB conjugate formed/mg protein.
Lipid peroxidation (LPO) levels
Extent of lipid peroxidation product was analyzed as thiobarbituric acid reactive substances (TBARS) as described previously by Buege and Aust (1978) with certain adaptations. 0.1 ml of 150 mM Tris–HCl buffer, 0.1 ml of 1 mM FeSO4, 0.1 ml of 1.5 mM Ascorbic acid (2.642 mg in 10 ml) and 0.1 ml of brain tissue supernatant were taken in a glass tube. Further, the volume was made up to 1 ml with water and the reaction mixture was incubated at 37 °C for 15–20 min. After cooling to room temperature, 1 ml of 10 % trichloroacetic acid (TCA) and 2 ml of 0.375 % w/w thiobarbituric acid (TBA) was added and again the reaction mixture was subjected to incubation at 37 °C for 15 min. On further cooling the incubated mixture was centrifuged at 3000 rpm for 10 min, absorbance of the supernatant was read at 532 nm. TBARS was expressed as malondialdehyde (MDA) equivalents, µmoles of MDA/mg protein.
Acetylcholinesterase (AChE) activity
The AChE activity was assayed by the method described previously by Ellman et al. (1961). Briefly, the assay mixture consisted of 0.4 ml of brain tissue supernatant, 2.6 ml of phosphate buffer (pH 7.5, 0.1 M), 100 µl of DTNB reagent and 20 µl of acetylthiocholine. The change in absorbance was measured at 412 nm for 3 min. The AChE activity was expressed as µmol of thiocholine hydrolysed/min/mg brain tissue.
Nitrite levels
Nitrite was estimated by the method of (Green et al. 1982; Rai et al. 2014) using Griess reagent, which served as indicator of nitric oxide production. Equal volumes of brain tissue supernatant (500 µl) and Griess reagent (500 µl) were mixed. The mixture was further, incubated for 10 min at 37 °C and the absorbance was read spectrophotometrically at 540 nm. Nitrite levels were expressed as µg of nitrite/mg protein.
Protein carbonylation levels
Carbonylated protein was estimated by the method of (Castegna et al. 2003; Dalle-Donne et al. 2006; Yan 2009). The assay mixture consisted of 200 μl of DNPH solution and 1 ml of brain tissue supernatant. This mixture was incubated for 60 min at room temperature. Further the mixture was treated with 1.2 ml of 20 % TCA solution with incubation on ice for 10 min. The ice cold mixture was centrifuged for 10 min at 6000 rpm at room temperature. The pellet formed by centrifugation was washed by adding 3 ml 1:1 (v/v) ethanol: ethyl acetate mixture, followed by centrifugation for 10 min at 6000 rpm, room temperature. The washed pellet was sonicated at room temperature in a sonicator until the pellet was completely broken up. The washing step was repeated twice. The final pellet was then dissolved in 1 ml 0.2 % (w/v) SDS/20 mM Tris·Cl, pH 6.8. After the pellet was completely dissolved, 100 μl of the protein solution was pipetted and carbonylated protein was measured spectrophotometrically at 276 and 370 nm. The peak absorbance around 360 nm was calculated for the carbonyl content. Protein samples treated with 200 μl of 2 M HCl without DNPH were used as blanks following the same conditions. Carbonylated protein was expressed as nmol of carbonyl formed/mg protein. The extinction coefficient (ε) for DNPH is 22,000 M−1 cm−1. BSA was used as a protein standard. The carbonylated protein was expressed as nmol carbonyl formed/mg protein.
ATPases activity
Inorganic phosphorus (Pi)
Inorganic phosphorus was estimated by the method of (Fiske and Subbarow 1925). 5 ml of distilled water was added to 1 ml of brain tissue supernatant. Further, 1 ml of 2.5 % ammonium molybdate reagent and 0.5 ml of ANSA reagent was added. The colour developed after 20 min was read against blank containing water instead of sample at 620 nm. A standard graph was plotted taking different concentrations of standard phosphorus 16–80 µg. The values were expressed as µmol of inorganic phosphorus liberated/mg protein/min.
Na+ K+ ATPase
The assay mixture consisted of 1 ml of tris hydrochloride and 2 ml of each magnesium sulphate, sodium chloride, potassium chloride, EDTA and ATP in a test tube containing previously added 0.2 ml of brain tissue supernatant. This mixture was subjected to incubation (37 °C for 15 min). The incubated reaction mixture was arrested by addition of 1 ml of 10 % TCA. Further the mixture was mixed well and centrifuged at room temperature. The phosphorus content was estimated by determination of inorganic phosphorus (Pi) liberated (Kuijpers and Bonting 1970; Streck et al. 2001). The enzyme activity was expressed as µmol of inorganic phosphorus liberated/mg protein/min.
Ca2+ ATPase
The assay mixture consisted of 0.1 ml each of tris HCl buffer, calcium chloride and ATP in a test tube containing previously added 0.1 ml brain tissue supernatant. This mixture was subjected to incubation (37 °C for 15 min). The incubated reaction mixture was arrested by addition of 1 ml of 10 % TCA. Further the mixture was mixed well and centrifuged at room temperature. The phosphorus content was estimated by determination of inorganic phosphorus (Pi) liberated. The enzyme activity was expressed as µmol of inorganic phosphorus liberated/mg protein/min (Hjertén and Pan 1983).
Mg2+ ATPase
The assay mixture consisted of 0.1 ml each of tris HCl buffer, magnesium chloride and ATP was added to the test tube containing previously added 0.1 ml brain tissue supernatant. This mixture was subjected to incubation (37 °C for 15 min). The incubated reaction mixture was arrested by addition of 1 ml of 10 % TCA. Further the mixture was mixed well and centrifuged at room temperature. The phosphorus content was estimated by determination of inorganic phosphorus (Pi) liberated (Ohnishi et al. 1982). The enzyme activity was expressed as µmol of inorganic phosphorus liberated/mg protein/min.
Determination of expression of genes involved in mitochondrial biogenesis and inflammation
RNA was isolated by using a RNA isolation kit (Promega, SV Total RNA Isolation System®) from brain tissue (hippocampus) and the gene expressions of TNF-α, IL-6, PPAR-α, PGC-1α and 18S were estimated by using Real Time PCR method. Brain samples stored in liquid nitrogen at −70 °C were used for tissue processing. The samples were homogenized in RNA lysis buffer (RLA). Concentration of RNA was determined spectrophotometrically using gene quanta and purity of the RNA was determined by A260/A280 ratio. RNA was reverse transcribed using reverse transcriptase (Go Script™ RT mix for one step RT-qPCR) in a 25 μl mixture containing Go Taq® qPCR master mix 1×, Reference dye (CXR) 0.5 µM and Sample (RNA) (550 fg-100 ng) 114 µg. The resultant cDNA was amplified separately with specific primer for PGC-1α, PPAR-α, 18S, TNF-α and IL-6 using Go Taq® Probe 1-Step RT-qPCR System (Promega, USA). The gene expression analysis was performed by the comparative threshold cycle (Ct) method. The amplification of the 18S sequence was performed in parallel and was used to normalize the values obtained for the target genes.
Specific primers were synthesized commercially (Integrated DNA Technologies, Leuven Belgium) and the sequences are listed in Table 1.
Table 1.
Primers for RT-qPCR amplification of each gene
| Primer | Sense primer | Antisense primer |
|---|---|---|
| PGC-1α | 5′-CCA AAG CTGA AGC CCT CTT GC-3′ | 5′-GTT TAG TCT TCC TTT CCT CGT GTC C-3′ |
| PPAR-α | 5′-GAG AAA GCA AAA CTG AAA GCA GAG A-3′ | 5′-GAA GGG CGG GTT ATT GCT G-3′ |
| TNF-α | 5′-GAG GTC AAC CTG CCC AAG TA-3′ | 5′-CGT GTG TTT CTG AGC ATC GT-3′ |
| IL-6 | 5′-GGA GAC TTG CCT GGT GAA-3′ | 5′-CTG AGG TGC CCA TGC TAC-3′ |
| 18S | 5′-CAT CGA GCA GGT CTG TTC CC-3′ | 5′-TAG ATT GGC TTG ACG GAC TTG G-3′ |
PGC-1α peroxisome proliferator-activated receptor-gamma co-activator 1 alpha, PPAR-α peroxisome proliferator-activated receptor alpha, TNF-α tumor necrosis factor alpha, IL-6 interleukin 6, 18S reliable normalization gene
Histopathological examination
The rat brain tissues were collected. After collection, the brains were immediately preserved in 10 % neutral buffered formalin. The specimens were processed by routine method which included dehydration with increasing grades of alcohol i.e. 70, 80, 90 % and clearing with xylene embedding in paraffin. Thereafter the processed tissue were sectioned (at 5 μm) and taken on clean glass slides and stained with hematoxylin and eosin (H&E) and observed under microscopes at different magnifications.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 6 software. Studies involving two variables were analyzed by Two-way analysis of variance (ANOVA) with time and treatment as variables followed by post hoc Bonferroni test. One-way analysis of variance (ANOVA) followed by post hoc Bonferroni test was performed to compare groups in Novel Object Recognition Test (NORT), brain biochemical parameters and gene expression data. Results were reported as mean ± standard error of mean (SEM). p value <0.05 was considered statistically significant.
Results
Learning and memory paradigms
Effect of PTS and FF administration on memory performance in NORT
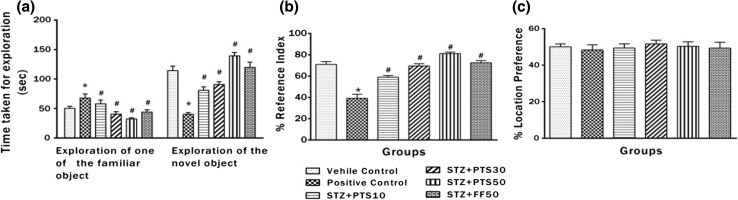
Figure 2a–c represents the results of NORT. The effect of PTS and FF on performance of positive control rats, measured by recognition index (RI) was significantly (*p < 0.05) impaired compared to the vehicle control. Average outcomes between the groups were significantly [p < 0.05, F(5, 42) = 37.52] different. The ability of the vehicle control group rats to discriminate the novel object from familiar ones was distinctly better as indicated by improved RI compared to positive control animals. Moreover, treatment with PTS (10, 30 and 50 mg/kg, po) and FF (50 mg/kg, po) showed dose dependent (# p < 0.05) improvement in the ability to discriminate novel object from familiar ones as compared to the positive control. Further, the RI was significantly (# p < 0.05) increased on treatment with PTS and FF. Location preference, to rule out the influence of the location of object showed no significant (*p > 0.05) difference between all the groups.
Fig. 2.
Effect of PTS and FF administration on memory performance in NORT. a Ability to discriminate between novel and familiar objects. b Recognition index (RI). c Location preference. There was a significant difference in performance between the rats who were treated with PTS (10, 30 and 50 mg/kg, po) and FF (50 mg/kg, po) dose when compared to positive control. Error bars represent mean ± SEM (n = 8). Hash indicates significant difference (# p < 0.05) from positive control. Asterisk indicates significant difference (*p < 0.05) from control group as assessed by (Bonferroni test)
Effect of PTS and FF administration on memory performance in MWM
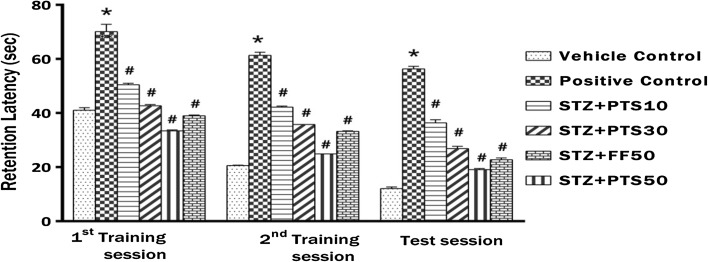
Figure 3 represents the retention latency (RL) during acquisition phase (training session) and test session of the MWM. Statistical analysis showed significant impact of time and treatment [p < 0.05, F(10, 126) = 13.84]. The performance of positive control rats to find the escape platform during 1st, 2nd training and test session (day 18, 19 and 20), as measured by RL was significantly (*p < 0.05) increased compared to the vehicle control rats. However, treatment with Pterostilbene (PTS 10, 30 and 50 mg/kg) showed dose dependent significant (# p < 0.05) decrease in their average retention latencies compared to positive control animals in all the three sessions (1st, 2nd training and test session). Furthermore, treatment with Fenofibrate (FF 50 mg/kg) also showed significant (# p < 0.05) decrease in retention latencies in all the three sessions (1st, 2nd training and test session) compared to positive control animals.
Fig. 3.
Effect of PTS and FF administration on memory performance in MWM
There was significant difference between the performance of rats who received different treatment doses of PTS (10, 30 and 50 mg/kg, po) and FF (50 mg/kg, po) compared to positive control rats. The positive control (icv STZ, 1.5 mg/5 µl ACSF) rats showed increased retention latency when compared to the vehicle control in the training and test sessions. Error bars represent mean ± SEM (n = 8). Hash indicates significant difference (# p < 0.05) from positive control. Asterisk indicates significant difference (*p < 0.05) from control group as assessed by (Bonferroni test).
Brain biochemical parameters
Effect of PTS and FF on brain CAT and SOD activities, GSH and MDA levels
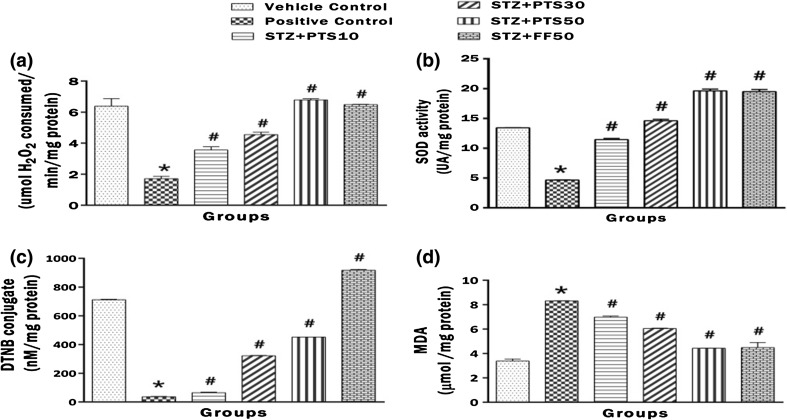
Figure 4a–d represents the brain activities of CAT, SOD and GSH, MDA levels. STZ administration induced significant (*p < 0.05) drop in brain CAT, SOD and GSH levels while leading to rise in MDA levels after day 1 instillation. These aberrations were significantly (# p < 0.05) countered by PTS (10, 30 and 50 mg/kg). Also, The standard FF (50 mg/kg) also significantly (# p < 0.05) reversed the adverse impact of STZ.
Fig. 4.
Effect of PTS and FF on brain CAT and SOD activities, GSH and MDA levels. a Change in the brain CAT activity. b Change in the brain SOD activity. c Change in the brain GSH level. d Change in the brain LPO level. There was significant difference in brain CAT and SOD activities, GSH and MDA levels between the STZ administered positive control group and the PTS and FF treated groups. Error bars represent mean ± SEM (n = 6). Hash indicates significant difference (# p < 0.05) from positive control. Asterisk indicates significant difference (*p < 0.05) from control group
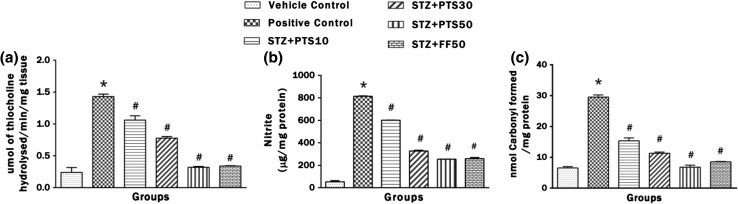
Effect of PTS and FF on brain AChE activity, nitrite and carbonylated protein levels
Figure 5a–d represents AChE activity, nitrite and carbonylated protein levels. Intracerebroventricular administration of STZ to the rats lead to significant (*p < 0.05) increase in AChE activity, nitrite and carbonylated protein levels in brain after instillation on day 1. Both PTS in doses of 10, 30 and 50 and FF (50 mg/kg) significantly (# p < 0.05) prevented this rise which is implicated in drop in cholinergic transmission and adverse impact of ROS.
Fig. 5.
Effect of PTS and FF on brain AChE activity, nitrite and carbonylated protein levels. a Change in the brain AChE activity. b Change in the brain nitrite levels. c Change in the brain carbonylated protein level. There was significant difference in brain AChE activity, nitrite and carbonylated protein levels between the STZ administered positive control group and the PTS and FF treated groups. Error bars represent mean ± SEM (n = 6). Hash indicates significant difference (# p < 0.05) from positive control. Asterisk indicates significant difference (*p < 0.05) from control group
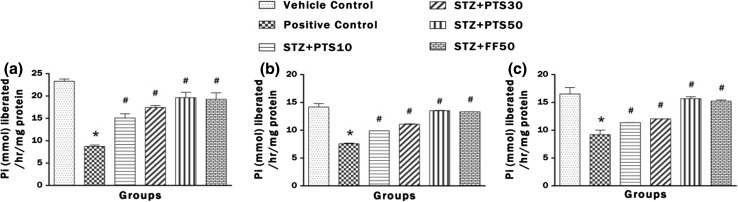
Effect of PTS and FF on brain activities of Na+ K+, Ca2+ and Mg2+ ATPases
Figure 6a–d represents the brain ATPases activity (Na+ K+, Ca2+ and Mg2+). Intracerebroventricular administration of STZ to the rats lead to significant (*p < 0.05) decrease in Na+ K+, Ca2+ and Mg2+ ATPases activities in brain after instillation on day 1. Both PTS in doses of 10, 30 and 50 and FF (50 mg/kg) significantly (# p < 0.05) improved ATPases activities which are implicated in restoring the membrane potential.
Fig. 6.
Effect of PTS and FF on brain activities of Na+ K+, Ca2+ and Mg2+ ATPases. a Change in the brain Na+ K+ ATPase activity. b Change in the brain Ca2+ ATPases activity. c Change in the brain Mg2+ ATPase activity. Treatment with PTS and FF has significantly improved ATPases activities. Error bars represent mean ± SEM (n = 6). Hash indicates significant difference (# p < 0.05) from positive control. Asterisk indicates significant difference (*p < 0.05) from control group
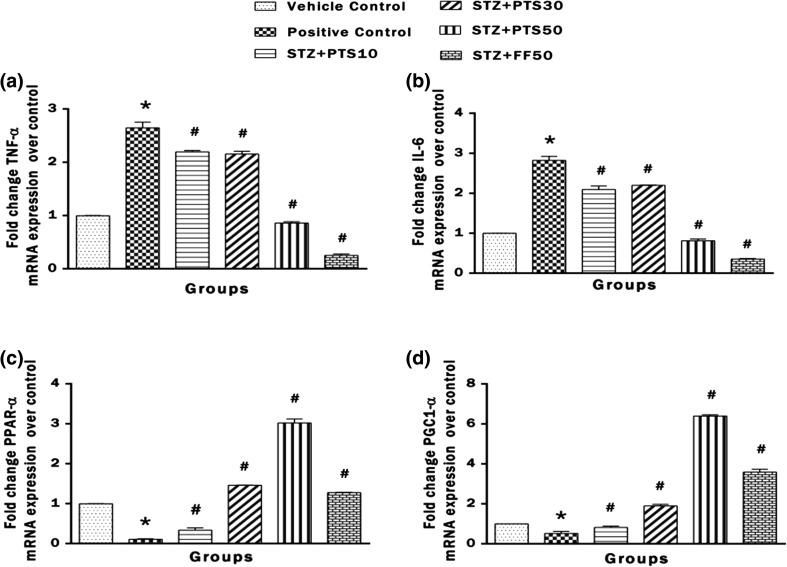
Effect of PTS and FF on brain mRNA expression of the genes TNF-α, IL-6, PPAR-α and PGC1-α
Figure 7a–d represents the mRNA expression of the genes TNF-α, IL-6, PPAR-α and PGC1-α in brain tissue of the rat. Statistical analysis showed that mRNA expression of TNF-α and IL-6 was increased as indicated by fold change when compared to control expressions. While the mRNA expression of PPAR-α and PGC1-α was decreased significantly (*p < 0.05) in the brains of positive control rats when compared to the vehicle control. Treatment with PTS (10, 30 and 50 mg/kg) showed dose dependent significant (# p < 0.05) decrease in mRNA expression of TNF-α, IL-6 and increase in mRNA expression of PPAR-α and PGC1-α as compared to positive control. Also, FF administration significantly (# p < 0.05) decreased the mRNA expression of TNF-α, IL-6 and increased mRNA expression of PPAR-α and PGC1-α as compared to positive control.
Fig. 7.
Effect of PTS and FF on brain mRNA expression of the genes TNF-α, IL-6, PPAR-α and PGC1-α. a Expression of TNF-α. b Expression of IL-6. c Expression of PPAR-α. d Expression of PGC1-α. Error bars represent mean ± SEM (n = 6). Hash indicates significant difference (# p < 0.05) from positive control. Asterisk indicates significant difference (*p < 0.05) from control group
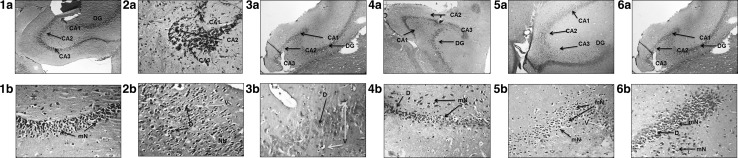
Histopathological examination
Representative hippocampal sections Fig. 8(2a, b) shows the damage inflicted by icv-STZ. It depicts distinct shrinkage of neurons (degenerated) with disturbed neuronal layer and vacuolation (degeneration of parenchymatous neurons) in CA3 region of hippocampus, shown with blue and red arrow respectively. Also, the presence of necrotic neurons (clustered) shown in yellow arrow was prominent. The representative hippocampal sections Fig. 8(3a, b; 4a, b; 5a, b; 6a, b) from rats treated with PTS (10, 30 and 50) and FF (50 mg/kg) respectively, shows myelinated and dense neurons. This indicates the PTS and FF prevented the detrimental pathological changes inflicted by STZ in the hippocampal region which is crucial for learning and memory.
Fig. 8.
1a–6a images (H&E ×100) of sagittal sections of rat brain hippocampus; 1b–6b images (H&E ×400) of CA3 region of rat brain hippocampus. CA1 Cornu ammonis1, CA2 Cornu ammonis2, CA3 Cornu ammonis3, DG dentate gyrus, mN myelinated neurons, D degeneration, V vacuolation, NN necrotic neurons
Figure 8 represents photomicrographs of H&E (100× and 400×) stained sections of hippocampus of (1a, b) Vehicle control rat showing intact neuronal layer, black arrow shows myelination of neurons with large vesicular nuclei and prominent nucleoli; (2a, b) STZ administered positive control rat brain hippocampus, the blue arrow indicating vacuolation (degeneration of parenchymatous neurons in hippocampus, red arrow indicates degeneration of parenchymatous neurons, yellow arrow indicates necrotic neurons and significant neuronal shrinkage; (3a, b) STZ + PTS10 administered rat brain hippocampus, the red arrow indicating the neurons are mild (+) degenerated and blue arrow indicating vacuolation with no significant neuronal shrinkage; (4a, b) STZ + PTS30 administered rat brain hippocampus, black arrow indicating the neurons are myelinated, red arrow indicating mild (+) degeneration of parenchymatous neurons and have large vesicular nuclei with prominent nucleoli and no neuronal shrinkage; (5a, b) STZ + PTS50 administered rat brain hippocampus, black arrow indicating myelination of neurons with large vesicular nuclei, prominent nucleoli with no neuronal shrinkage; (6a, b) STZ + FF50 administered rat brain hippocampus, black arrow indicating myelinated neurons with mild (+) degeneration of parenchymatous neurons, large vesicular nuclei with prominent nucleoli with no significant neuronal shrinkage.
Discussion
AD is a typical form of dementia characterized by memory impairment as a consequence of cholinergic neuronal degeneration in hippocampus (Saxena et al. 2011). There are preclinical as well as clinical evidences to indicate ACh as a major neurotransmitter responsible for memory function (Siddiqui and Levey 1999; Amenta et al. 2002; Jones 2003). Interestingly, mitochondrial abnormality progression is also, a major causative factor of AD (Prasad et al. 2002; Moreira et al. 2009, 2010). Recent molecular, cellular and gene expression studies in post mortem brain neurons from AD patients have revealed that mitochondrial ROS generation lead to oxidative damage (Castellani et al. 2002; Reddy 2006). Oxidative stress due to ROS generation in mitochondria has been suggested as a major factor of neuronal degeneration in AD. Investigators are focusing on target regulators of oxidative stress and inflammation, especially PPARs. PPARs are known to play a major role in energy homeostasis and ROS production/scavenging (Moreno and Cerù 2015). However, studies to understand the exact cause of AD, and progressive memory deterioration are still lacking. Therefore, the present investigation was undertaken to explore the antioxidant and antinflammatory effect of PTS on memory paradigms, biochemical markers of oxidative stress and activities of ATPases in rats after inducing brain aberrations with STZ. Although, there are several experimental models in rodents for dementia and associated disorders of neurodegeneration STZ induced dementia model in rats is commonly used in preclinical studies. Central or icv STZ causes neuronal damage in the brain by producing free radicals, thereby inducing the state of oxidative stress, impairment of glucose utilization and demyelination (Saxena et al. 2007, 2008, 2010).
In the present study STZ administration resulted in significant memory deficit as mirrored in the learning and memory tests performed namely, MWM and NORT. MWM test is used to test spatial memory by measuring retention latency in the terms of time to reach a hidden platform. Decrease in retention latency time in repeated trials demonstrates intact learning and memory function. Positive control rats showed increase in retention latency indicating cognitive impairment (Lannert and Hoyer 1998; Awasthi et al. 2010). Administration of ACSF (icv) in vehicle control group did not hinder the learning and memory status thus ruling out the possibility of interference of drug vehicle. Both PTS and FF treatment significantly prevented the memory decline indicated by the decrease in retention latency as observed in MWM.
NORT is based on the behavior of rodents to explore novelty and is a pure working memory test. It is used to test working memory by measuring the RI in terms of time taken to explore familiar and novel object. This test does not involve learning rule or reinforcement, and is thought to reflect working memory and visual memory in humans (Karasawa et al. 2008). Hence, by employing this test, the ability to discriminate between familiar and novel object can be observed. Ability to discriminate represents intact learning and memory function. Increase in RI in NORT demonstrates proper learning and memory function (Karasawa et al. 2008; Taglialatela et al. 2009; Greco et al. 2010; Zhanga et al. 2012). Positive control rats showed fall in RI while treatment with PTS and FF lead to increase in RI.
STZ-induced cholinergic dysfunction is attributed to oxidative stress and reduced brain cellular ATP levels (Lester-Coll et al. 2006). It has been reported that STZ-induced impairment of insulin function and increased oxidative stress were associated with increased expression of AChE in the rat brain (Pathan et al. 2006). In the present study, we found a significant increase in AChE activity in STZ inflicted brains, indicating alterations in cholinergic neurotransmissions as demonstrated in previous studies (Schmatz et al. 2009; Tota et al. 2011), suggesting an involvement of cholinergic hypofunction due to STZ (Tota et al. 2011). The present investigation revealed increased AChE activity in the positive control rats, which was ameliorated on PTS treatment. The brain AChE activities were significantly decreased in PTS and FF treated groups indicating improved availability of ACh at the synaptic cleft for proper cholinergic neurotransmission.
Several studies have postulated, oxidative stress as a key player in pathogenesis of neurodegenerative disorder like AD (Geula 1998; Zeevalk et al. 1998). Some other precipitating players hindering the brain functions are the carbonylated proteins. Proteins are major targets of oxidative modification by ROS (Yan 2009) resulting in its carbonylation. Oxidative damage often leads to a loss of protein function, which is considered a widespread indicator of disease-derived from protein dysfunction (Dalle-Donne et al. 2006). There was increase in carbonylated protein levels in icv STZ treated rats. This increase was stalled with PTS and FF treatments.
GSH, an important antioxidant that is involved in cellular detoxification of free radicals while, MDA is a biochemical marker of lipid peroxidation. Generation of free radicals is a major cause of STZ induced neurotoxicity. In the present study, decrease in GSH and increase in MDA level on STZ administration indicates increased production of free radicals. It is known that STZ induced ROS like nitrites, hydrogen peroxides cause neuronal degeneration (Saxena et al. 2010). Treatment with PTS and FF significantly increased GSH, decreased MDA and decreased nitrosative stress indicated by fall in nitrite production. Thus, PTS and FF treatment helped to overcome the icv STZ induced oxidative stress in brain. Additionally, it has been indicated that dysfunction of brain ATPases activities affects Na+, K+, Ca2+ and Mg2+ ions homeostasis and modulation of signal transduction (Pinsky et al. 2006). In the current study, activities of brain ATPases were significantly decreased after STZ administration. This adverse impact was prominently ameliorated with PTS and FF treatment, indicating their neuroprotective property. Yet, another critical factor associated with AD is mitochondrial dysfunction (Castellani et al. 2002). Impaired brain energy metabolism causes a decrease in acetylcholine (ACh) synthesis because of reduced availability of acetyl coenzyme A (Gibson and Blass 1976). A previous study has reported a significant reduction in brain ATP levels in STZ-injected rats and mice, indicating impairment of brain energy metabolism (Tota et al. 2011).
In this study mitochondrial biogenesis was adversely impacted on icv STZ instillation. Moreover, the mRNA expression of TNF-α and IL-6 was decreased while the mRNA expressions of PPAR-α and PGC1α was increased in PTS and FF treated groups showing there antioxidant and anti-inflammatory activity. This confounds that pterostilbene acts by increasing the expression of PPAR-α (Rimando et al. 2005; Chang et al. 2012). Brain hippocampus is known to play a major role in cognition (Farovik et al. 2010) histopathological evaluation of brain hippocampus corroborates the efficacy of pterostilbene by maintaining the non-pathologic brain morphology. Interestingly, the benefits of PTS on icv STZ induced memory decline is to that of Clitoria ternatea, Rutin and Curcumin as reported in earlier studies (Ishrat et al. 2009; Javed et al. 2012; Ahmed et al. 2013; Mehla et al. 2013).
The present study provides preclinical evidence on the antioxidant, anti-inflammatory action of PTS in a preclinical model of STZ induced memory decline. PTS administration abated memory decline and the effect was comparable with that of fenofibrate.
Conclusion
The present preclinical study has revealed a new dimension to the potential use of pterostilbene in treating memory decline associated with stressors simulating sporadic AD. The effect was mediated by its antioxidant effect in the brain which was further assisted by inhibition of AChE activity. The investigations also threw light on the impact of pterostilbene on decreasing gene expression of inflammatory mediators and improving expression of genes associated with cellular energy homeostasis. The effect was comparable to that of fenofibrate, an anti hyperlipidemic drug with proven nootropic activity.
References
- Abdalla B, Bisharat B, Abir M et al (2012) Traditional and modern medicine harmonizing the two approaches in the treatment of neurodegeneration (Alzheimer’s disease-AD). Complementary Therapies for the Contemporary Healthcare: Intech, pp 181–212
- Acharya JD, Ghaskadbi SS. Protective effect of Pterostilbene against free radical mediated oxidative damage. Complement Altern Med. 2013;13:238. doi: 10.1186/1472-6882-13-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebi H, Scherz B, Ben-Yoseph Y, et al. Dissociation of erythrocyte catalase into subunits and their re-association. Experientia. 1975;31:397–399. doi: 10.1007/BF02026338. [DOI] [PubMed] [Google Scholar]
- Ahmed ME, Khan MM, Javed H, et al. Amelioration of cognitive impairment and neurodegeneration by catechin hydrate in rat model of streptozotocin-induced experimental dementia of Alzheimer’s type. Neurochem Int. 2013;62:492–501. doi: 10.1016/j.neuint.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Amenta F, Di Tullio MA, Tomassoni D. The cholinergic approach for the treatment of vascular dementia: evidence from pre-clinical and clinical studies. Clin Exp Hypertens. 2002;24:697–713. doi: 10.1081/CEH-120015346. [DOI] [PubMed] [Google Scholar]
- Awasthi H, Tota S, Hanif K, et al. Protective effect of curcumin against intracerebral streptozotocin induced impairment in memory and cerebral blood flow. Life Sci. 2010;86:87–94. doi: 10.1016/j.lfs.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Bhaskaran S, Vishwaraman M (2009) process for obtaining purified Pterostilbene and methods of use thereof. US patent 20110144053 2009 July 30
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/S0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Carlini VP. The object recognition task: a new proposal for the memory performance study. Intech. 2011 [Google Scholar]
- Castegna A, Drake J, Pocernich C, et al. Protein carbonyl levels—an assessment of protein oxidation. In: Hensley K, Floyd RA, et al., editors. Methods in biological oxidative stress. Totowa, NJ: Humana Press Inc.; 2003. pp. 161–168. [Google Scholar]
- Castellani R, Hirai K, Aliev G, et al. Role of mitochondrial dysfunction in Alzheimer’s disease. J Neurosci Res. 2002;70:357–360. doi: 10.1002/jnr.10389. [DOI] [PubMed] [Google Scholar]
- Chakravarthy MV, Zhu Y, López M, et al. Brain fatty acid synthase activates PPARα to maintain energy homeostasis. J Clin Invest. 2007;117:2539–2552. doi: 10.1172/JCI31183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Rimando A, Pallas M, et al. Low-dose pterostilbene, but not resveratrol, is a potent neuromodulator in aging and Alzheimer’s disease. Neurobiol Aging. 2012;33:2062–2071. doi: 10.1016/j.neurobiolaging.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Crawford F, Hamandi K, et al. Screening for the β-amyloid precursor protein mutation (APP717: Val → Ile) in extended pedigrees with early onset Alzheimer’s disease. Neurosci Lett. 1991;129:134–135. doi: 10.1016/0304-3940(91)90738-F. [DOI] [PubMed] [Google Scholar]
- Chew LJ, Takanohashi A, Bell M. Microglia and inflammation: impact on developmental brain injuries. Ment Retard Dev Disabil Res Rev. 2006;12:105–112. doi: 10.1002/mrdd.20102. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Aldini G, Carini M, et al. Protein carbonylation, cellular dysfunction, and disease progression. J Cell Mol Med. 2006;10:389–406. doi: 10.1111/j.1582-4934.2006.tb00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet G, Laforest S, Bédard PJ, et al. Progress in neuro-psychopharmacology & biological psychiatry. Elsevier: Amsterdam. 2009;33:1289–1586. doi: 10.1016/j.pnpbp.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Duthey B (2013) Background paper 6.11: Alzheimer disease and other dementias. A public health approach to innovation. http://www.who.int/medicines/areas/priority_medicines/BP611Alzheimer.pdf. Accessed 8 Jun 2014
- Ellman GL, Courtney K, Andres V, et al. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Farovik A, Dupont LM, Eichenbaum H. Distinct roles for dorsal CA3 and CA1 in memory for sequential nonspatial events. Learn Mem. 2010;17:12–17. doi: 10.1101/lm.1616209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige JN, Gelman L, Michalik L, et al. From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog Lipid Res. 2006;45:120–159. doi: 10.1016/j.plipres.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Fidaleo M, Fanelli F, Paola Ceru M, et al. Neuroprotective properties of peroxisome proliferator-activated receptor alpha (PPARα) and its lipid ligands. Curr Med Chem. 2014;21:2803–2821. doi: 10.2174/0929867321666140303143455. [DOI] [PubMed] [Google Scholar]
- Fiske CH, Subbarow Y. The colorimetric determination of phosphorus. J Biol Chem. 1925;66:375–400. [Google Scholar]
- Geula C. Abnormalities of neural circuitry in Alzheimer’s disease Hippocampus and cortical cholinergic innervation. Neurol. 1998;51:S18–S29. doi: 10.1212/WNL.51.1_Suppl_1.S18. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Jana M, Modi K, et al. Activation of peroxisome proliferator-activated receptor α induces lysosomal biogenesis in brain cells implications for lysosomal storage disorders. J Biol Chem. 2015;290:10309–10324. doi: 10.1074/jbc.M114.610659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G, Blass J. Impaired synthesis of acetylcholine in brain accompanying mild hypoxia and hypoglycemia. J Neurochem. 1976;27:37–42. doi: 10.1111/j.1471-4159.1976.tb01540.x. [DOI] [PubMed] [Google Scholar]
- Greco SJ, Bryan KJ, Sarkar S, et al. Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis. 2010;19:1155. doi: 10.3233/JAD-2010-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrate, nitrite, and [15 N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- Grieb P. Intracerebroventricular streptozotocin injections as a model of Alzheimer’s disease: in search of a relevant mechanism. Mol Neurobiol. 2015;53:1–12. doi: 10.1007/s12035-015-9132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover J, Vats V, Yadav S. Pterocarpus marsupium extract (Vijayasar) prevented the alteration in metabolic patterns induced in the normal rat by feeding an adequate diet containing fructose as sole carbohydrate. Diab Obes Metab. 2005;7:414–420. doi: 10.1111/j.1463-1326.2005.00414.x. [DOI] [PubMed] [Google Scholar]
- Harman D. Free radical theory of aging. Mutat Res DNAging. 1992;275:257–266. doi: 10.1016/0921-8734(92)90030-S. [DOI] [PubMed] [Google Scholar]
- Hartree EF. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972;48:422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hjertén S, Pan H. Purification and characterization of two forms of a low-affinity Ca 2 + -ATPase from erythrocyte membranes. BBA Biomembr. 1983;728:281–288. doi: 10.1016/0005-2736(83)90480-7. [DOI] [PubMed] [Google Scholar]
- Hou Y, Xie G, Miao F, et al. Pterostilbene attenuates lipopolysaccharide-induced learning and memory impairment possibly via inhibiting microglia activation and protecting neuronal injury in mice. Prog Neuro Psychopharmacol Biol Psych. 2014;54:92–102. doi: 10.1016/j.pnpbp.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Ishrat T, Hoda MN, Khan MB, et al. Amelioration of cognitive deficits and neurodegeneration by curcumin in rat model of sporadic dementia of Alzheimer’s type (SDAT) Eur Neuropsychopharmacol. 2009;19:636–647. doi: 10.1016/j.euroneuro.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Javed H, Khan M, Ahmad A, et al. Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neurosci. 2012;210:340–352. doi: 10.1016/j.neuroscience.2012.02.046. [DOI] [PubMed] [Google Scholar]
- Jollow D, Mitchell J, Zampaglione N, et al. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacol. 1974;11:151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- Jones RW. Have cholinergic therapies reached their clinical boundary in Alzheimer’s disease? Int J Geriatr Psychiatry. 2003;18:S7–S13. doi: 10.1002/gps.936. [DOI] [PubMed] [Google Scholar]
- Joseph J, Fisher D, Bielinski D. Blueberry extract alters oxidative stress-mediated signaling in COS-7 cells transfected with selectively vulnerable muscarinic receptor subtypes. J Alzheimers Dis. 2006;9:35–42. doi: 10.3233/jad-2006-9103. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Rimando AM, Shukitt-Hale B (2008) Method to ameliorate oxidative stress and improve working memory via pterostilbene administration. US patent WO2009032870 A3
- Kapetanovic IM, Muzzio M, Huang Z, et al. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother Pharmacol. 2011;68:593–601. doi: 10.1007/s00280-010-1525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa J, Hashimoto K, Chaki S. D-Serine and a glycine transporter inhibitor improve MK-801-induced cognitive deficits in a novel object recognition test in rats. Behav Brain Res. 2008;186:78–83. doi: 10.1016/j.bbr.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Kosaraju J, Madhunapantula SV, Chinni S, et al. Dipeptidyl peptidase-4 inhibition by Pterocarpus marsupium and Eugenia jambolana ameliorates streptozotocin induced Alzheimer’s disease. Behav Brain Res. 2014;267:55–65. doi: 10.1016/j.bbr.2014.03.026. [DOI] [PubMed] [Google Scholar]
- Kuijpers W, Bonting S. The cochlear potentials. Pflugers Archiv. 1970;320:348–358. doi: 10.1007/BF00588213. [DOI] [PubMed] [Google Scholar]
- Lannert H, Hoyer S. Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav Neurosci. 1998;112:1199. doi: 10.1037/0735-7044.112.5.1199. [DOI] [PubMed] [Google Scholar]
- Lester-Coll N, Rivera EJ, Soscia SJ, et al. Intracerebral streptozotocin model of type 3 diabetes: relevance to sporadic Alzheimer’s disease. J Alzheimers Dis. 2006;9:13–33. doi: 10.3233/jad-2006-9102. [DOI] [PubMed] [Google Scholar]
- Li L, Zhang ZF, Holscher C, et al. (Val 8) glucagon-like peptide-1 prevents tau hyperphosphorylation, impairment of spatial learning and ultra-structural cellular damage induced by streptozotocin in rat brains. Eur J Pharmacol. 2012;674:280–286. doi: 10.1016/j.ejphar.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Manickam M, Ramanathan M, Farboodniay Jahromi M, et al. Antihyperglycemic activity of phenolics from Pterocarpus marsupium. J Nat Prod. 1997;60:609–610. doi: 10.1021/np9607013. [DOI] [PubMed] [Google Scholar]
- Mariani E, Polidori M, Cherubini A, et al. Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J Chromatogr B. 2005;827:65–75. doi: 10.1016/j.jchromb.2005.04.023. [DOI] [PubMed] [Google Scholar]
- McFadden D. A review of pterostilbene antioxidant activity and disease modification. Oxid Med Cell Longev. 2013;2013:1–15. doi: 10.1155/2013/575482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehla J, Pahuja M, Gupta P, et al. Clitoria ternatea ameliorated the intracerebroventricularly injected streptozotocin induced cognitive impairment in rats: behavioral and biochemical evidence. Psychopharmacol. 2013;230:589–605. doi: 10.1007/s00213-013-3185-7. [DOI] [PubMed] [Google Scholar]
- Meraz Ríos MA, Toral Rios D, Franco Bocanegra D, et al. Inflammatory process in Alzheimer’s disease. Front Integr Neurosci. 2013;7:741–749. doi: 10.3389/fnint.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- Moreira PI, Duarte AI, Santos MS, et al. An integrative view of the role of oxidative stress, mitochondria and insulin in Alzheimer’s disease. J Alzheimers Dis. 2009;16:741. doi: 10.3233/JAD-2009-0972. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Carvalho C, Zhu X, et al. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim Biophysica Acta Mol Basis Dis. 2010;1802:2–10. doi: 10.1016/j.bbadis.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Moreno S, Cerù MP. In search for novel strategies towards neuroprotection and neuroregeneration: is PPARα a promising therapeutic target? Neural Regen Res. 2015;10:1409. doi: 10.4103/1673-5374.165313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderali EK, Ratcliffe SH, Dale MC. Review: obesity and Alzheimer’s disease: a link between body weight and cognitive function in old age. Am J Alzheimers Dis Dementias. 2009;24:445–449. doi: 10.1177/1533317509348208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T, Suzuki T, Suzuki Y, et al. A comparative study of plasma membrane Mg2+-ATPase activities in normal, regenerating and malignant cells. Biochim Biophysica Acta Biomem. 1982;684:67–74. doi: 10.1016/0005-2736(82)90050-5. [DOI] [PubMed] [Google Scholar]
- Ouk T, Gautier S, Pétrault M, et al. Effects of the PPAR-α agonist fenofibrate on acute and short-term consequences of brain ischemia. J Cereb Blood Flow Metab. 2014;34:542–551. doi: 10.1038/jcbfm.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan MH, Chang YH, Tsai ML, et al. Pterostilbene suppressed lipopolysaccharide-induced up-expression of iNOS and COX-2 in murine macrophages. J Agri Food Chem. 2008;56:7502–7509. doi: 10.1021/jf800820y. [DOI] [PubMed] [Google Scholar]
- Pathan AR, Viswanad B, Sonkusare SK, et al. Chronic administration of pioglitazone attenuates intracerebroventricular streptozotocin induced-memory impairment in rats. Life Sci. 2006;79:2209–2216. doi: 10.1016/j.lfs.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Ashwell KW, Tork I. Atlas of the developing rat nervous system. 2. San Diego: Academic; 2013. [Google Scholar]
- Peixoto FP, Carrola J, Coimbra AM, et al. Oxidative stress responses and histological hepatic alterations in barbel, Barbus bocagei, from Vizela River, Portugal. Rev Int Contam Ambient. 2013;29:29–38. [Google Scholar]
- Pinsky MR, Brochard L, Mancebo J, et al. Applied physiology in intensive care medicine. Berlin: Springer; 2006. pp. 53–56. [Google Scholar]
- Prasad KN, Cole WC, Prasad KC. Risk factors for Alzheimer’s disease: role of multiple antioxidants, non-steroidal anti-inflammatory and cholinergic agents alone or in combination in prevention and treatment. J Am Coll Nutr. 2002;21:506–522. doi: 10.1080/07315724.2002.10719249. [DOI] [PubMed] [Google Scholar]
- Pyper SR, Viswakarma N, Yu S, et al. PPARα: energy combustion, hypolipidemia, inflammation and cancer. Nucl Recept Sig. 2010;8:1–21. doi: 10.1621/nrs.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai S, Kamat PK, Nath C, et al. Glial activation and post-synaptic neurotoxicity: the key events in streptozotocin (ICV) induced memory impairment in rats. Pharmacol Biochem Behav. 2014;117:104–117. doi: 10.1016/j.pbb.2013.11.035. [DOI] [PubMed] [Google Scholar]
- Reddy PH. Amyloid precursor protein-mediated free radicals and oxidative damage: implications for the development and progression of Alzheimer’s disease. J Neurochem. 2006;96:1–13. doi: 10.1111/j.1471-4159.2005.03530.x. [DOI] [PubMed] [Google Scholar]
- Remsberg CM, Yáñez JA, Ohgami Y, et al. Pharmacometrics of pterostilbene: preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity. Phytother Res. 2008;22:169–179. doi: 10.1002/ptr.2277. [DOI] [PubMed] [Google Scholar]
- Rimando AM, Nagmani R, Feller DR, et al. Pterostilbene, a new agonist for the peroxisome proliferator-activated receptor α-isoform, lowers plasma lipoproteins and cholesterol in hypercholesterolemic hamsters. J Agri Food Chem. 2005;53:3403–3407. doi: 10.1021/jf0580364. [DOI] [PubMed] [Google Scholar]
- Rubin D, Rubin T (2009) Method and compositions for administering resveratrol and pterostilbene. EP patent WO2009089338 A2
- Saxena G, Singh SP, Pal R, et al. Gugulipid, an extract of Commiphora whighitii with lipid-lowering properties, has protective effects against streptozotocin-induced memory deficits in mice. Pharmacol Biochem Behav. 2007;86:797–805. doi: 10.1016/j.pbb.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Saxena G, Singh SP, Agrawal R, et al. Effect of donepezil and tacrine on oxidative stress in intracerebral streptozotocin-induced model of dementia in mice. Eur J Pharmacol. 2008;581:283–289. doi: 10.1016/j.ejphar.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Saxena G, Bharti S, Kamat PK, et al. Melatonin alleviates memory deficits and neuronal degeneration induced by intracerebroventricular administration of streptozotocin in rats. Pharmacol Biochem Behav. 2010;94:397–403. doi: 10.1016/j.pbb.2009.09.022. [DOI] [PubMed] [Google Scholar]
- Saxena G, Patro IK, Nath C. ICV STZ induced impairment in memory and neuronal mitochondrial function: a protective role of nicotinic receptor. Behav Brain Res. 2011;224:50–57. doi: 10.1016/j.bbr.2011.04.039. [DOI] [PubMed] [Google Scholar]
- Schmatz R, Mazzanti CM, Spanevello R, et al. Resveratrol prevents memory deficits and the increase in acetylcholinesterase activity in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2009;610:42–48. doi: 10.1016/j.ejphar.2009.03.032. [DOI] [PubMed] [Google Scholar]
- Sharma M, Gupta Y. Effect of chronic treatment of melatonin on learning, memory and oxidative deficiencies induced by intracerebroventricular streptozotocin in rats. Pharmacol Biochem Behav. 2001;70:325–331. doi: 10.1016/S0091-3057(01)00611-6. [DOI] [PubMed] [Google Scholar]
- Siddiqui MF, Levey A. Cholinergic therapies in Alzheimer’s disease. Drugs Future. 1999;24:417–424. doi: 10.1358/dof.1999.024.04.668318. [DOI] [Google Scholar]
- Sisodia SS, Kim SH, Thinakaran G. Function and dysfunction of the presenilins. Am J Hum Genet. 1999;65:7–12. doi: 10.1086/302475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streck EL, Zugno AI, Tagliari B, et al. Inhibition of rat brain Na+, K+-ATPase activity induced by homocysteine is probably mediated by oxidative stress. Neurochem Res. 2001;26:1195–1200. doi: 10.1023/A:1013907104585. [DOI] [PubMed] [Google Scholar]
- Szkudelski T. Streptozotocin–nicotinamide-induced diabetes in the rat. Characteristics of the experimental model. Exp Biol Med. 2012;237:481–490. doi: 10.1258/ebm.2012.011372. [DOI] [PubMed] [Google Scholar]
- Taglialatela G, Hogan D, Zhang WR, et al. Intermediate-and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behav Brain Res. 2009;200:95–99. doi: 10.1016/j.bbr.2008.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tota S, Kamat PK, Shukla R, et al. Improvement of brain energy metabolism and cholinergic functions contributes to the beneficial effects of silibinin against streptozotocin induced memory impairment. Behav Brain Res. 2011;221:207–215. doi: 10.1016/j.bbr.2011.02.041. [DOI] [PubMed] [Google Scholar]
- Vauzour D, et al. Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects. Oxid Med Cell Longev. 2012;2012:1–16. doi: 10.1155/2012/914273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M, Kirschbaum SN, Lazarovici P, et al. Neuroprotective effects of novel cholinesterase inhibitors derived from rasagiline as potential anti-Alzheimer drugs. Ann N Y Acad Sci. 2001;939:148–161. doi: 10.1111/j.1749-6632.2001.tb03622.x. [DOI] [PubMed] [Google Scholar]
- White RF, Marans KS, Krengel M (2000) Psychological/behavioral symptoms in neurological disorders. In: Emergencies in mental health practice: evaluation and management, pp 312–331
- Xuan AG, Chen Y, Long DH, et al. PPARα agonist fenofibrate ameliorates learning and memory deficits in rats following global cerebral ischemia. Mol Neurobiol. 2014;52:1–9. doi: 10.1007/s12035-014-8882-7. [DOI] [PubMed] [Google Scholar]
- Yan LG, et al. Analysis of oxidative modification of proteins. Curr Protoc Protein Sci. 2009 doi: 10.1002/0471140864.ps1404s55. [DOI] [PubMed] [Google Scholar]
- Zeevalk GD, Bernard LP, Nicklas WJ. Role of oxidative stress and the glutathione system in loss of dopamine neurons due to impairment of energy metabolism. J Neurochem. 1998;70:1421–1430. doi: 10.1046/j.1471-4159.1998.70041421.x. [DOI] [PubMed] [Google Scholar]
- Zhang J-M, et al. Cytokines, inflammation and pain. Int Anesthesiol Clin. 2007;45(2):27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhanga R, Xuea G, Wanga S, et al. Novel object recognition as a facile behavior test for evaluating drug effects in APP/PS1 Alzheimer’s disease mouse model. J Alzheimers Dis. 2012;31:801–812. doi: 10.3233/JAD-2012-120151. [DOI] [PubMed] [Google Scholar]