Abstract
Immunosuppression after liver transplantation (LT) is presently based on use of calcineurin inhibitors (CNI), although they are associated with an increased incidence of renal dysfunction, cardiovascular complications, and de novo and recurrent malignancies. Over the past decade, mammalian target of rapamycin inhibitors have received considerable attention as immunosuppressants because they are associated with a more favorable renal profile versus CNI, as well as antiproliferative activity in clinical studies. Comprehensive guidelines on use of everolimus (EVR) in LT are still lacking. In Italy, a project, named Everolimus: the road to long-term functioning, was initiated to collect the experience on EVR after LT with the aim of providing guidance for transplant clinicians. Herein, recommendations by this national consensus group, based on Delphi methodology, are presented. Consensus was reached on 20 of the 23 statements proposed, and their level of evidence, grade of recommendation, and percent of agreement are reported. Statements are grouped into 4 areas: (A) renal function; (B) time of EVR introduction, CNI reduction and elimination, and risk for graft rejection; (C) antiproliferative effects of EVR; and (D) management of EVR-related adverse events. The high level of consensus shows that there is good agreement on the routine use of EVR in predefined clinical scenarios, especially in light of posttransplant nephrotoxicity and other adverse events associated with long-term administration of CNIs.
The authors summarize the recommendations reached by an Italian National Consensus group using the Delphi methodology on the use of everolimus in liver transplantation, particularly its role in renal function, antiproliferative effects, adverse events, timing of introduction, and rejection risk.
Immunosuppression after liver transplantation (LT) is currently based on use of calcineurin inhibitors (CNI) with a resulting increased incidence of renal dysfunction, cardiovascular complications, and de novo and recurrent malignancies.1-5 Calcineurin inhibitor–related nephrotoxicity is the most frequent cause of renal dysfunction after LT and is associated with a 4.55-fold increased risk of death.2,6,7 Strategies of CNI reduction and withdrawal have recently been introduced in clinical practice in view of preventing or limiting CNI-related toxicities and commonly include use of antimetabolites (azathioprine and mycophenolic acid derivatives), the anti–interleukin-2 receptor antibody basiliximab, and the mammalian target of rapamycin inhibitors (mTORi), everolimus (EVR), and sirolimus (SIR), alone or in different combination schedules.7
Mammalian target of rapamycin inhibitors and CNI act on different sites of the T cell activation pathway.8 Mammalian target of rapamycin inhibitors are selective inhibitors of the mTOR complex, which is a serine-threonine kinase with a key role for cell metabolism.8 The activity of mTOR is upregulated in a number of different cancers, and EVR reduces levels of vascular endothelial growth factor, which potentiates angiogenic processes. In addition to its effects on T cells, EVR is a potent inhibitor of growth and proliferation of tumor cells, endothelial cells, fibroblasts, and blood vessel–associated smooth muscle cells.
The pharmacokinetic profile of EVR has a shorter terminal half-life versus SIR (about 30 vs 60 hours), and steady-state trough levels are reached quicker (4 vs 6 days). This results in loading doses not being required and easier dose adjustments in clinical practice.9-12 Studies investigating the exposure-response relationship have shown that EVR has a relatively narrow therapeutic window of 3 to 8 ng/mL, and monitoring of drug trough levels is warranted to adjust dose levels.11,12
Recent studies on mTORi have shown that they are associated with superior renal function versus CNI in de novo and maintenance LT recipients.13,14 Table 1 summarizes the most relevant clinical studies on EVR in de novo LT.
TABLE 1.
Summary of clinical studies on EVR in de novo liver transplant recipients
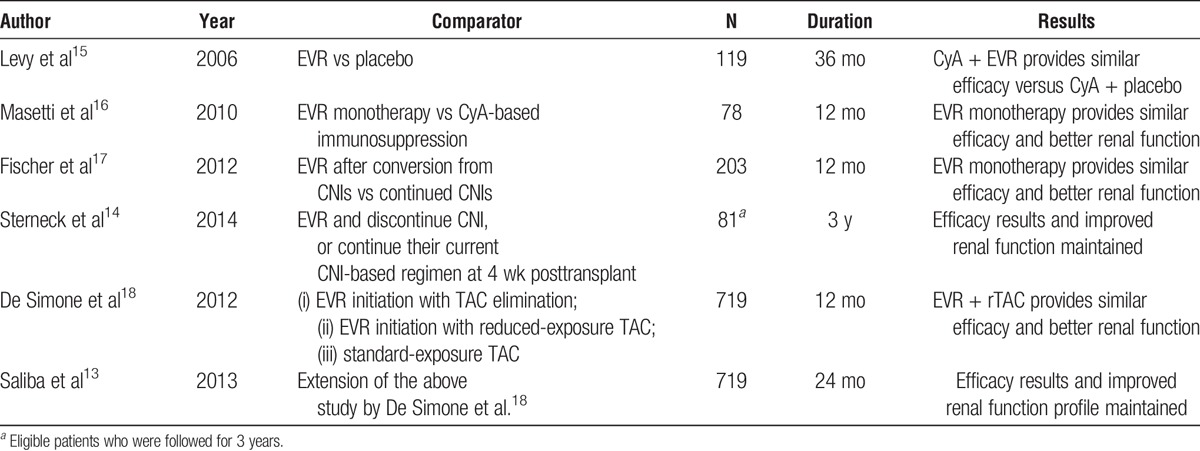
The use of EVR in de novo LT recipients has been recently approved based on the results of De Simone et al.18 in which EVR was introduced at day 30 (±5) after surgery. The 2-year results of the extension period have recently demonstrated that early introduction of EVR with reduced-exposure tacrolimus (TAC) at 1 month significantly improved renal function versus standard TAC exposure.13 The 3-year results of the multicenter, Preservation of Renal Function in Liver Transplant Recipients with Certican Therapy study showed the possibility to discontinue CNI between 8 and 16 weeks after surgery and confirmed sustained preservation of renal function vs. patients on CNI.14
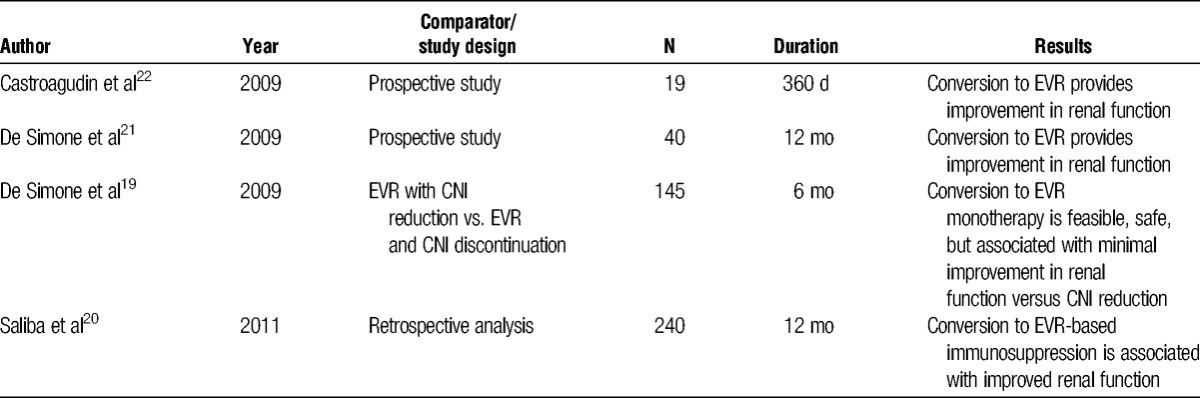
Evidence on conversion from CNI- to EVR-based immunosuppression is reported in Table 2, with late conversion referring to patients 6 months or longer after transplantation.19 In 145 maintenance (≥12 months) LT patients randomized to EVR introduction with CNI reduction or discontinuation (n = 72) versus CNI reduction (≤50%) (n = 73), 80% of patients on EVR could discontinue CNI 6 months thereafter.19 A multicenter, retrospective study on EVR in real-life clinical practice reported on 240 patients converted from CNI to EVR in France.20 About 60% of patients were converted to EVR monotherapy with a very low risk of acute rejection and an acceptable safety profile. Collateral studies in selected maintenance LT recipients confirmed that conversion to EVR monotherapy was feasible in 75% of cases and associated with improvement in renal function.21
TABLE 2.
Summary of clinical studies on conversion to EVR during the maintenance period in liver transplant recipients
Because of the recent approval of EVR for LT and the relatively limited number of available studies, comprehensive guidelines are still lacking. Compared with many other countries worldwide, Italy has a solid and long-standing experience in the optimal strategies on the practical clinical use of EVR, which began in 2006. In Italy, off-label use of EVR in LT was allowed before its official registration thanks to specific legislation (law 648/96) and based on evidence of its efficacy in this setting. This educational initiative included preparation of a consensus document with evidence-based recommendations on use of EVR after LT, in view of guiding transplant clinicians in general and special clinical settings. The current article reports the set of recommendations that were issued by this national consensus group in Italy.
MATERIALS AND METHODS
Study Design
In November 2014, the educational initiative, Everolimus: the road to long-term functioning, was set up in Italy to collect all national experiences on use of EVR in kidney and LT, address priorities and challenges with use of EVR in clinical practice, and introduce practice guidelines. In the setting of LT, the scientific committee (SC) members (see Appendix A) opted to draft a set of evidence-based consensus statements and recommendations to guide use of EVR and help clinicians manage immunosuppression-related complications after LT. The SC of the project was composed of Italian experts in the field of LT as demonstrated by their publication records, participation in national meetings and clinical trials, overall expertise, and academic rank, in addition to experience with EVR.
Development of Consensus Statements and Recommendations
The consensus methodology is illustrated in Figure 1, consisting of a 3-step process incorporating a modified Delphi method, which took place between November 2014 and January 2015.
FIGURE 1.
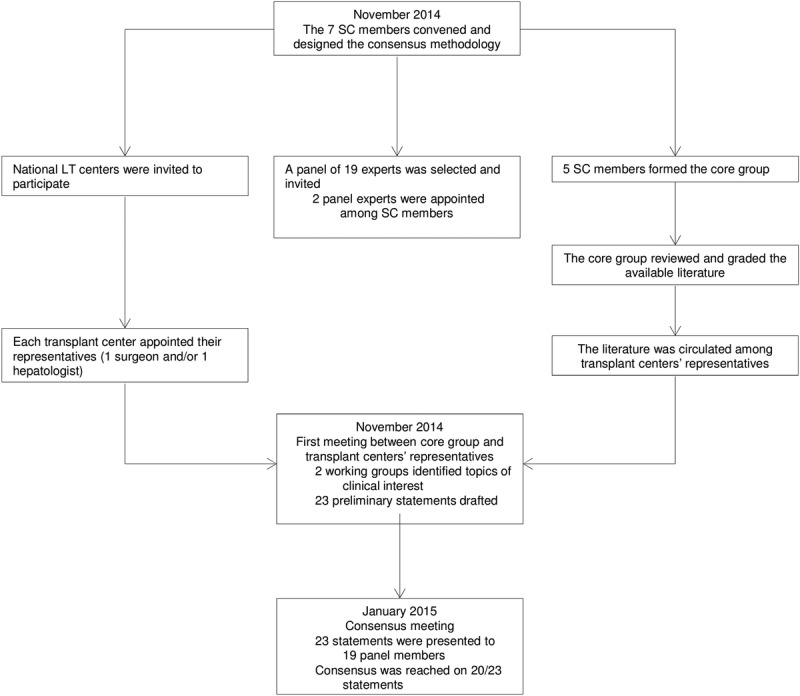
The consensus methodology is illustrated. This was a 3-step process incorporating a modified Delphi method and based on the National Plan Guide for Consensus Meeting.23
Step 1
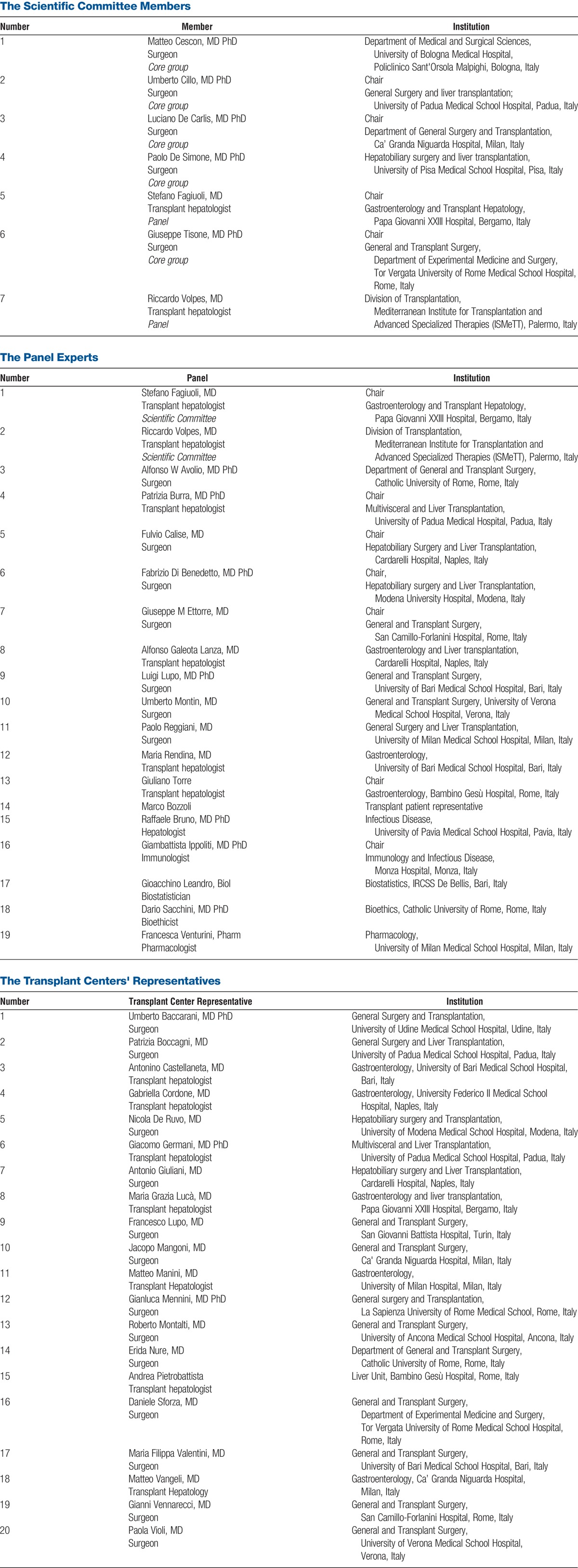
In November 2014, in the wake of the official approval of EVR in adult LT, a group of transplant physicians (ie, the SC; see Appendix A) invited all of the Italian liver transplant centers to participate in a consensus meeting to define recommendations on use of EVR-incorporating immunosuppression. For this initiative, the SC: (1) designed the consensus methodology according to the National Plan Guide for Consensus Meeting, and based on what was published elsewhere23,24; (2) appointed a multidisciplinary panel of experts (see Appendix A); and (3) invited the directors of each transplant center to appoint either a surgeon or a transplant hepatologist to be part of 2 working groups for selection of topics relevant to clinical practice (see Appendix A).
According to the guidelines for consensus recommendations,23 the SC was divided into 2 groups: 2 members were part of the expert panel, whereas 5 acted as coordinators (core group) of the consensus methodology. Although 5 to 10 experts are considered adequate for content validation,25 19 experts were contacted and asked to participate in consensus development. All 19 provided consent and agreed to participate. Panel experts were chosen to represent professional groups that directly influence patient care and would benefit from clinical practice guidelines. Panel members were identified from national institutions and selected based on their clinical and research expertise in the management of immunosuppression. Eligibility criteria for transplant physicians included at least 2 of the following: 10-year experience or longer in liver transplant surgery or transplant hepatology, direct responsibility in management of immunosuppression, previous participation in consensus meetings, serving as national and/or international SC members, serving as editor for transplant journals, and participation in phase 2 or phase 3 immunosuppressive trials. Nontransplant experts were selected from previous national consensus meetings.26 The panel consisted of 7 transplant surgeons, 6 transplant hepatologists, 1 experienced hepatologist, 1 immunologist, 1 biostatistician, 1 bioethicist, 1 hospital pharmacologists, and 1 patients' representative (see Appendix A). Panel members were not involved in the process of selecting or drafting the statements.
In November 2014, the core group carried out a literature search. The PubMed database was searched with no language limitations until October 31, 2014. Multiple searches were performed using combinations of the following terms: liver transplant, transplant, immunosuppression, mTOR, mTORi, rapamycin, EVR, SIR, renal dysfunction, renal failure, chronic kidney disease, diabetes mellitus, hyperlipidemia, dyslipidemia, hypercholesterolemia, hypertriglyceridemia, hepatic artery thrombosis, oral sores, oral ulcers, mucositis, stomatitis, pneumonitis, interstitial lung disease, wound dehiscence, proteinuria, leukopenia, thrombocytopenia, malignancy, neoplasm, cancer, skin cancer, Kaposi sarcoma, hepatocellular carcinoma (HCC), and cholangiocarcinoma. The reference lists of all articles were checked manually for additional citations and gray literature.
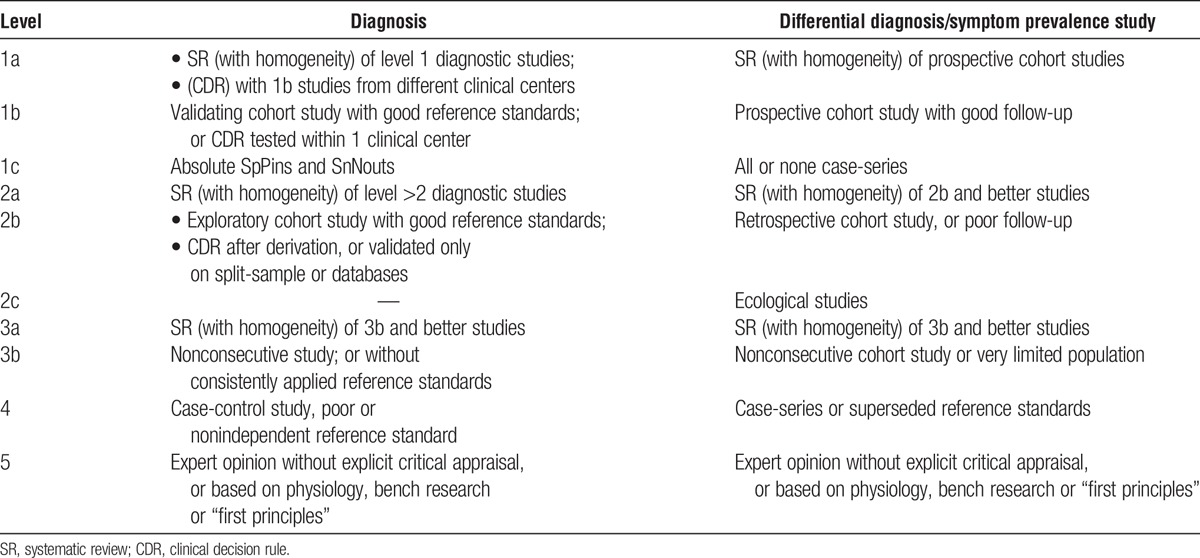
Two members of the core group screened all titles and abstracts to discard irrelevant ones. A third member of the core group resolved conflicts. Articles from the literature search were included if they described use of EVR-based immunosuppression in de novo or maintenance adult LT recipients. Full texts of relevant studies were retrieved and reviewed for eligibility. Each reference was graded according to the quality of their content (Table 3). All retrieved references were circulated among the transplant center representatives before the face-to-face meeting.
TABLE 3.
Levels of evidence based on the Oxford Centre for Evidence-Based Medicine
Step 2
On November 26, 2014, the transplant center representatives and the core group convened in Milan, Italy, for a face-to-face meeting (see Appendix A). The participants were split into 2 working groups. The groups provided feedback on the retrieved references and identified 4 areas of interest: (A) renal function; (B) time of EVR introduction, CNI reduction and elimination, and risk for graft rejection; (C) antiproliferative effects of EVR; and (D) EVR-related adverse events. At the end of the day, the participants arranged a set of 23 preliminary statements to be presented at the final consensus meeting.
In December 2014, a draft document containing the list of statements was circulated by email among the core group members with the intention to clarify any redundancy or issues regarding comprehension or syntax of each statement.
Step 3
The consensus meeting took place on January 28, 2015, in Milan, Italy. The participants included the 19 panel members and the SC. Each scientific coordinator involved in drafting of the recommendations presented the background evidence and proposed the relative statements (Table 4). Panel experts were given the opportunity to provide comments and suggest additional items that might not have been included when developing the initial list of statements. Eventually, the consensus meeting coordinators amended the statements as per the experts' advice in a separate meeting room.
TABLE 4.
Grades of recommendation
Modified Delphi Consensus Process
Each of the 19 panel members scored the proposed consensus statements by a show of hands on a Likert scale from 1 to 5 as per the following: 1, strongly disagree; 2, disagree; 3, neutral; 4, agree; and 5, strongly agree. For the purposes of the consensus statement, agreement among respondents of 70% or greater for each statement (expressed as the percentage of respondents scoring an item either 4 [somewhat agree] or 5 [agree]) was considered to represent consensus. This degree of agreement is based on standards in previously reported Delphi studies.27,28
Role of Pharmaceutical Industry
Novartis Italia, SpA (Origgio, Varese, Italy) provided financial support for development of field work for the 2 consensus meetings. The Novartis staff did not take part in selecting and recruiting panel experts and participants to the consensus process; nor in the assignment of tasks among SC and working group members; nor in the analysis and interpretation of literature data, nor in the development and drafting of the recommendations. The Novartis staff was not involved in writing the article or in the decision to submit the article for publication. All of the authors were significantly involved in these activities.
RESULTS
Consensus was reached on 20 (86.9%) of the 23 statements proposed. The approved statements, their level of evidence, grade of recommendation (if applicable), and percent of agreement are herein reported. The statements are grouped into 4 areas: (A) renal function; (B) time of EVR introduction, CNI reduction and elimination, and risk for graft rejection; (C) antiproliferative effects of EVR; and (D) management of EVR-related adverse events.
(A) Renal Function
Level of evidence, 1b; grade of recommendation, A; agreement, 88%.
Statement 1: Renal-sparing immunosuppressive strategies for LT recipients include the following options13,14,16-18,29:
A triple or a quadruple regimen with use of induction agents in association with antimetabolites and delayed introduction of CNI (within 5-10 days after surgery) ± steroids;
EVR-facilitated CNI reduction starting 30 (±5) days after transplantation;
Early (≤10 days) use of EVR to reduce CNI exposure.
Comment
Several studies have investigated the efficacy, safety of implementation of renal-sparing immunosuppressive strategies usually including a triple or quadruple regimen with use of antimetabolites, and induction agents in combination with CNI and steroids.29 Recently, 2 large multicenter, randomized, open-label trials have investigated EVR-facilitated CNI reduction and stepwise withdrawal from 1 to 4 months after transplantation and showed improved renal function at 3 years for patients on reduced CNI exposure13,18 or on EVR monotherapy.14,17 One recent single-center experience suggested the feasibility of stepwise elimination of CNI starting 10 days after surgery.16
Statement 2: EVR-facilitated reduction of CNI early (30±5 days) or very early (≤10 days) after transplantation improves renal function at 1 and 3 years.13,14,16-18
Level of evidence, 1b; grade of recommendation, A; agreement, 88%.
Comment
Based on current evidence, patients receiving renal-sparing immunosuppression at an early (30 ± 5 days) or very early (≤10 days) time after transplantation are expected to get 8 to 12 mL/min per 1.73 m2 improvement in renal function at 12 months after transplantation.13,14,16-18 Such improvement is confirmed throughout longer follow-up periods (3 years) in studies on EVR.14,30 However, it is important to note that this evidence is derived from studies enrolling patients with high renal function at transplantation. In the H2304 study trial, mean estimated glomerular filtration rate at transplantation was about 80 mL/min per 1.73 m2,18 whereas it was about 76 mL/min per 1.73 m2 in both the PROTECT trial17 and in the study by Masetti et al.16 Currently, evidence in large group of patients with deteriorated renal function at transplantation is still lacking.
Statement 3: Delaying renal-sparing intervention strategies until glomerular filtration <60 mL/min per 1.73 m2 is associated with only minor improvement in renal function.19,31,32
Level of evidence, 2; grade of recommendation, B; agreement, 94%.
Comment
Withholding renal-sparing immunosuppressive interventions until renal function drops below 60 mL/min per 1.73 m2 is associated with little improvement in renal function in 2 series on conversion from CNI to EVR19 and SIR.31
(B) Time of EVR Introduction, CNI Reduction and Elimination, and Risk for Graft Rejection
Statement 4: In LT, early (30 ± 5 days) CNI reduction with EVR introduction is as effective and safe as standard-exposure CNI immunosuppression. CNI reduction facilitated by EVR can be implemented.13,18
Level of evidence, 1b; grade of recommendation, A; agreement, 90%.
Comment
Based on recent evidence, CNI reduction facilitated by EVR initiation can be safely implemented from day 30 after LT, even in the presence of CNI-related renal dysfunction (as per serum creatinine >1.5 mg/dL, an estimated glomerular filtration rate <60 mL/min per 1.73 m2 and/or any other decline in renal function deemed clinically relevant).13,18
Statement 5: CNI withdrawal is associated with a 10− 20% risk of acute rejection of the liver graft depending on time of discontinuation after LT.13,14,16-19,21,30,31
Level of evidence, 2; no recommendation applicable; agreement, 88%.
Comment
Current data suggest that the risk of acute rejection of the liver graft upon CNI withdrawal facilitated by EVR introduction depends mainly on the time since transplant. In one of the largest studies on de novo use of EVR after LT (H2304), the cumulative 12-month incidence of acute rejection was 4.1% among patients on EVR+ reduced TAC (rTAC) versus 10.7% for patients on TAC versus 19.9% for patients who discontinued TAC. Incidence of treated biopsy-proven acute rejection (tBPAR) was 2.9% in EVR + rTAC patients versus 7.0% for TAC versus 16.5% for patients who discontinued TAC.18 At 24 months, the cumulative incidence of acute rejection was 6.1% for EVR + rTAC versus 13.3% for TAC control versus 26.4% for patients who discontinued TAC, whereas tBPAR was reported in 4.8%, 7.7%, and 19.9% of EVR + rTAC, TAC control, and TAC elimination patients, respectively.13 In the 36-month follow-up study (H2304E1), the cumulative incidence of acute rejection was 7.3%, 17.7%, and 26.8% for EVR + rTAC, TAC control, and TAC elimination, whereas tBPAR was 4.8%, 9.2%, and 21.5%, respectively.30 Maintenance studies report a variable acute rejection risk from almost nil to 15%,19-21 according to the interval from transplantation and mode of tapering. To date, no information is available on the impact of native liver disease on the risk of acute rejection upon CNI withdrawal both in de novo and in maintenance transplant populations.
Statement 6: Conversion to EVR monotherapy for CNI-related renal toxicity is feasible in 80% of patients at ≥12 months after liver transplantation. The impact on renal function of conversion strategies is dependent on the severity of renal impairment and timing of conversion.19-22,33
Level of evidence, 2; no recommendation applicable; agreement, 76%.
Comment
Data from randomized trials in maintenance (≥12 months) LT recipients confirm that feasibility of conversion from CNI-based to EVR monotherapy is 80% at 6 and 12 months, but that the impact on renal function is poor for patients with deteriorated renal function, for example, calculated creatinine clearance <60 mL/min.19 Consistent information is derived from a randomized study on conversion to SIR,31 and from single-center retrospective series and cohorts.22,33
Statement 7: Due to different pharmacokinetic interactions, TAC should not be reduced before EVR is in the target blood range (≥3 ng/mL), whereas cyclosporine A (CyA) should be reduced upon administration of EVR.11,15
Level of evidence, 2; grade of recommendation, B; agreement, 95%.
Comment
Whereas there is virtually no pharmacokinetic interaction between EVR and TAC, EVR produces a 4-fold increase in CyA area under the concentration-time curve (AUC). Upon introduction of EVR in patients on CyA, concomitant CyA reduction is therefore warranted.11,12,15
(C) Antiproliferative Effects of EVR
Statement 8: EVR and SIR share similar antiproliferative properties both in vitro and in vivo, with EVR presenting advantages due to its shorter half-life.9-11,34-37
Level of evidence, 2; no recommendation applicable; agreement, 76%.
Comment
Sirolimus and EVR have similar antiproliferative properties.9-11,34-37 The 2 drugs share a common molecular structure, with EVR having a shorter terminal half-life and quicker steady state versus SIR.9-12 These latter properties might represent an advantage for EVR versus SIR in clinical practice, although there are no direct comparative studies, with trough blood level dose adjustments being quicker for EVR versus SIR.12
Statement 9: Use of mTORi is associated with a reduced incidence of de novo malignancies after kidney, heart, and LT.38-41
Level of evidence, 3; no recommendation applicable; agreement, 95%.
Comment
Experimental and clinical evidence confirm the antiproliferative properties of the mTORi SIR and EVR. Although no randomized, controlled trial has, to date, demonstrated superiority of mTORi-based immunosuppression in reducing the incidence of de novo and recurrent malignancies after solid organ transplantation in general, and LT in particular, use of mTORi is supported by retrospective, cohort, and registry data analysis.38-40 The expert panel agreed that all LT recipients at high risk of de novo malignancies (ie, human herpesvirus-8-positive patients; alcoholic cirrhosis patients; patients with concurrent inflammatory bowel disease; recipients of grafts from donors at risk of transmission of malignancies; Epstein Barr virus-DNA–negative recipients, and recipients developing Epstein Barr virus-DNA positivity after transplantation) may warrant mTORi-based immunosuppression.
Statement 10: In LT, mTORi can be used as immunosuppressants to reduce the risk of posttransplant HCC recurrence.40-42
Level of evidence, 3; grade of recommendation, C; agreement, 95%.
Comment
There is sufficient preclinical and clinical evidence on the role of mTORi to control recurrence and growth of HCC. Based on currently available data, patients at high risk for HCC recurrence as per explant histology (ie, tumor grading, vascular infiltration, and so on) represent the ideal target population for a policy of prophylaxis of tumor recurrence with mTORi. Although multivariate analysis of a registry population of adult LT recipients comparing 2491 HCC patients with 12 167 non-HCC recipients has shown that mTORi are associated with improved posttransplant patient survival among patients with HCC at transplant,40 recent reports have mitigated these findings.43,44 A prospective, randomized clinical trial on SIR-based immunosuppression has reported a benefit only for patients within Milan criteria,43 and a recent analysis of the Scientific Registry of Transplant Recipients on patients with SIR-based maintenance immunosuppression has suggested reductions in recurrence and cancer-specific mortality, but effects of SIR seem to be modified by age at transplantation.44
Statement 11: Use of mTORi is recommended for patients with de novo malignancies after LT.40,41,45-47
Level of evidence, 2; grade of recommendation, B; agreement 95%.
Comment
Mammalian target of rapamycin inhibitors are indicated in LT patients with de novo malignancies after transplantation. Current evidence supports use of either mTORi monotherapy or combined immunosuppression with reduced-exposure CNI, depending on individual risk (eg, time since transplant, indication to transplantation, age at transplantation, and so on).40,41,45-47
Statement 12: In patients with recurrent HCC after LT, it is recommended to introduce EVR together with CNI reduction or withdrawal, due to its combined immunosuppressive and antiproliferative properties.40,41,48-50
Level of evidence, 5; grade of recommendation, B; agreement, 80%.
Statement 13: In patients with recurrent HCC after LT, use of EVR is recommended unless clinically contraindicated and irrespective of implementation of other treatment modalities (eg, surgery, radiology-guided tumor ablation, transarterial chemoembolization, or transarterial radioembolization).40,41,48-50
Level of evidence, 5; grade of recommendation, B; agreement, 95%.
Comment on Statements 12 and 13
Recurrence of HCC after LT is still associated with dismal outcomes.41 Everolimus has antiproliferative activity and retrospective series, registry data analysis and case reports suggest that mTORi should be introduced for patients with recurrent HCC in the posttransplant course to slow disease progression.40,41,48-50 In this regard, the recent Sirolimus after Liver Transplantation study investigated whether mTORi-based immunosuppression improves outcomes in LT candidates with HCC.43 In this long-term, prospective clinical trial, 525 LT recipients with HCC initially receiving mTORi-free immunosuppression were randomized 4 to 6 weeks after transplantation to mTORi-free immunosuppression or SIR. The trial showed no difference in overall survival between the 2 groups after 8 years, although there was significant benefit of mTORi-based immunosuppression in improving recurrence-free survival and overall survival in the first 3 to 5 years, especially in low-risk patients.43
There is no evidence regarding the role of mTORi as neoadjuvant or adjuvant agents in combination with surgery or radiology-based treatment modalities for posttransplant recurrent HCC, but literature data suggest patients can be treated with either modality unless mTORi are clinically contraindicated (eg, coexisting proteinuria, severe dyslipidemia, neutropenia, and so on).40,41,48-50
Statement 14: For LT patients with recurrent HCC not amenable to surgical or radiological treatment, a combination regimen with EVR and sorafenib shows a pathophysiological rationale.40,41,48-50
Level of evidence, 5; no recommendation applicable; agreement, 78%.
Comment
This recommendation is based on the complementary effect of the 2 agents on the molecular pathways involved in HCC.48 However, use of such a regimen is recommended only for experienced LT specialists or oncologists due to the potential for sorafenib-related and EVR-related side effects.48-50
(D) Management of EVR-Related Adverse Events
Statement 15: In transplant patients, EVR-related dyslipidemia is dose-dependent.15,51
Level of evidence, 1; no recommendation applicable; agreement, 100%.
Statement 16: When dyslipidemia is observed in LT recipients on treatment with EVR at trough levels higher than the recommended ranges (>8 ng/mL), prompt reduction of EVR oral dose is warranted.13,18
Level of evidence, 2; grade of recommendation, B; agreement, 94%.
Statement 17: Dyslipidemia occurring in LT recipients should be treated (with EVR dose reduction if trough levels are >8 ng/mL) irrespective of the time from transplantation.51-53
Level of evidence, 5; grade of recommendation, C; agreement, 94%.
Comment on Statements 15, 16, and 17
There is solid evidence in the literature that use of mTORi in transplant patients is associated with increased levels of total cholesterol and triglycerides, and that this adverse event is dose-dependent.15,51 In published randomized, controlled trials dyslipidemia was treated with reduction of EVR exposure, especially for patients with higher than the recommended trough levels (3-8 ng/mL).13,18 It is important to note that the literature data show no increased use of statins among patients on EVR-based immunosuppression versus patients on CNI, nor does any evidence exist suggesting an increased risk of cardiovascular events for patients on EVR.52
Statement 18: The risk of EVR-related proteinuria (as per >1 g/d) in LT recipients is about 3% at 3 years.13,18,30
Level of evidence, 1b; no recommendation applicable; agreement, 94%.
Comment
Mammalian target of rapamycin inhibitor treatment is associated with a net increase in protein urinary excretion. In the H2304 study, the overall incidence of proteinuria as an adverse event was higher in the EVR plus reduced TAC group than in the standard TAC group (3.7% vs 0.8%, respectively; P = 0.063), and proteinuria was the leading cause of study drug discontinuation (8 vs 1 patient).13,18,30 However, the mean urine protein-to-creatinine ratio remained below the 500 mg/d threshold until 36 months after transplantation.13,18,30 This is in part related to the high baseline renal function of the population included the trial.13,18,30 After CNI withdrawal the incidence of proteinuria can be higher due to both selection of patients with worse baseline renal function and lack of the vasoconstrictor effect of CNI. Proteinuria greater than 3 g/d was 9.5% (2/21) in a conversion study,32 whereas in the PROTECT trial, proteinuria was reported in 9.9% of EVR-treated patients versus 2.0% of the patients in the CNI group (P = 0.05).14,17 None of the cases of proteinuria were considered severe or serious. In the study of maintenance patients by De Simone et al,19 2 patients (2.7%) discontinued EVR because of proteinuria, which in both cases was suspected to be related to study medication. However, there is a suggestion that mTORi-related proteinuria may well be the harbinger of underlying renal disease presenting upon CNI elimination or reduction and due to loss of CNI-related glomerular vasoconstriction, but also that it may accelerate progression of renal dysfunction.19,22,54-56 Patients on mTORi and with proteinuria greater than 800 mg/d warrant constant monitoring of renal function and EVR discontinuation should be considered for overt nephrotic proteinuria.19
Statement 19: In LT recipients with severe neutropenia (<1000 mm3), leukopenia (<2000 mm3), or thrombocytopenia (<50 000 mm3) dose adjustments of EVR or withdrawal are recommended.13,18,30
Level of evidence, 5; grade of recommendation, C; agreement, 94%.
Comment
In published randomized, controlled trials EVR-related hematological adverse events were treated with EVR dose reduction or withdrawal, especially for patients with higher than the recommended trough levels (3-8 ng/mL).13,18,30
Statement 20: EVR-based immunosuppressive regimens are not associated with an increased risk of infections compared with standard CNI-based immunosuppression.13,14,17,18,30
Level of evidence, 1b; no recommendation applicable; agreement, 94%.
Comment
The largest trials on EVR after LT did not show a statistically significant increase in the incidence of infectious complications for patients on EVR-facilitated TAC reduction.13,14,17,18,30
DISCUSSION
The national consensus group was organized with the major objective of consolidating national experience on EVR after LT, based on Delphi methodology, to obtain a framework for the clinical management of transplant recipients. The national consensus group in Italy reached broad consensus on 20 statements. However, consensus was not reached on wound healing adverse events, time to EVR reduction, or posttransplant proteinuria.
Considering wound healing adverse events, the rates of incisional hernia and lymphocele have been reported with mTORi when patients received a high loading dose.57 However, more recent studies using lower doses of an mTORi without a loading dose have shown that rates of wound-healing complications do not differ significantly between mTORi and other immunosuppressive therapies.58 In 2 randomized trials, the incidence of wound complications and incisional hernia was similar in liver transplant recipients receiving EVR or CNIs.13,14,17,18,30 A third study reported a higher rate of incisional hernia in 52 liver transplant recipients receiving EVR compared with 26 recipients on cyclosporine (46.1% vs 26.9%), although the difference was not significant.59
Most complications develop during the first few postoperative months. At 30 days or longer posttransplantation, wounds should be somewhat healed and wound strength should be improving; the risks of wound complications associated with newly initiated mTORi therapy at this stage should be minimal. It was felt that further studies are needed to explore the impact of EVR on wound healing in case of an anticipated (<30 days) use after LT.
Any decision on use of EVR in patients with prior surgeries and/or at higher risk for wound healing complications should be tailored on the individual risk-to-benefit ratio.
No consensus could also be reached on the time to EVR reduction even though “very early introduction of EVR posttransplantation (<10 days) has been reported as effective and safe in two randomized clinical trials.”15,16 This was deemed to be mostly attributable to differences in interpretation of what is meant by ‘very early’.
No consensus could also be reached on monitoring of renal function. Although several studies have provided evidence in this regard,13,14,17,18,30 it should be considered that clinically significant proteinuria (>2 g/day) likely requires evaluation of EVR dose adjustments and/or withdrawal, in association with introduction of antiproteinuric drugs (angiotensin converting enzyme inhibitors and CNIs) and referral to renal care specialists.
Not all topics related to the use of EVR-incorporating immunosuppression after LT could be addressed by the current initiative. Some issues—such as hepatic artery thrombosis, diabetes mellitus and cytomegalovirus infection, and whose incidence is not increased among patients on EVR versus those—on CNI in large, prospective, randomized trials13,14,17,18,30—were considered less relevant by the transplant centers' representatives during the initial face-to-face meeting. Participants in the consensus initiative were asked to address issues they perceived as challenging or that deserved clarification in light of the available literature evidence. Similarly, the antifibrotic properties of EVR and its potentially favorable impact on hepatitis C virus progression after transplantation60,61 were not explored by the current consensus group due to the introduction of novel direct antiviral agents for hepatitis C virus infection in the same period. The issue of EVR-related interstitial pneumonia, which was reported in 2% to 5% of patients on EVR in the H2304 and PROTECT trials,13,14,17,18,30 was not addressed because its incidence is lower than what was reported in other solid organ transplant populations (ie, kidney) or in cancer patients.62,63
A consensus was reached for the majority of statements, including ongoing treatments, renal function, and de novo tumors. Everolimus provides a valid therapeutic option for LT recipients, particularly considering posttransplant nephrotoxicity. Additional studies are needed, especially regarding the early introduction of EVR and its use in recurrent HCC.
ACKNOWLEDGMENTS
The authors wish to thank HPS—Health Publishing & Services Srl for providing methodological and logistic support, and Ray Hill, an independent medical writer, who assisted with journal styling before submission on behalf of Health Publishing & Services Srl.
Acknowledgments
The consensus panel included: Alfonso W Avolio, MD PhD, Department of General and Transplant Surgery, Catholic University of Rome, Rome, Italy; Patrizia Burra, MD PhD, Multivisceral and Liver Transplantation, University of Padua Medical Hospital, Padua, Italy; Fulvio Calise, MD, Hepatobiliary Surgery and Liver Transplantation, Cardarelli Hospital, Naples, Italy; Fabrizio Di Benedetto, MD PhD, Hepatobiliary surgery and Liver Transplantation, Modena University Hospital, Modena, Italy; Giuseppe M Ettorre, MD, General and Transplant Surgery, San Camillo-Forlanini Hospital, Rome, Italy; Alfonso Galeota Lanza, MD, Gastroenterology and Liver transplantation, Cardarelli Hospital, Naples, Italy; Luigi Lupo, MD PhD, General and Transplant Surgery, University of Bari Medical School Hospital, Bari, Italy; Umberto Montin, MD, General and Transplant Surgery, University of Verona Medical School Hospital, Verona, Italy; Paolo Reggiani, MD, General Surgery and Liver Transplantation, University of Milan Medical School Hospital, Milan, Italy; Maria Rendina, MD, Transplant hepatologist; Gastroenterology, University of Bari Medical School Hospital, Bari, Italy; Giuliano Torre, MD PhD, Gastroenterology, Bambino Gesù Hospital, Rome, Italy; Marco Bozzoli, transplant patient representative; Raffaele Bruno, MD PhD, Infectious Disease, University of Pavia Medical School Hospital, Pavia, Italy; Giambattista Ippoliti, MD PhD, Immunology and Infectious Disease, Monza Hospital, Monza, Italy Gioacchino Leandro, Biostatistics, IRCSS De Bellis, Bari, Italy; Dario Sacchini, MD PhD, Bioethics, Catholic University of Rome, Rome, Italy; Francesca Venturini, Pharmacology, University of Milan Medical School Hospital, Milan, Italy.
Appendix 1.
Footnotes
This initiative was funded by Novartis Italy, Origgio (VA), Italy.
P.D.S. has served as advisor and has received speaker’s fees from Novartis. The other authors declare no conflicts of interest.
P.D.S., S.F., M.C., and U.C. participated in writing the initial draft of the article. All authors reviewed the draft, provided expertise for critical revisions, and approved the final version of the article.
Contributor Information
Collaborators: Consensus Panel
REFERENCES
- 1.Felga G, Evangelista AS, Salvalaggio PR, et al. Hepatocellular carcinoma recurrence among liver transplant recipients within the Milan criteria. Transplant Proc. 2012;44:2459–2461. [DOI] [PubMed] [Google Scholar]
- 2.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. [DOI] [PubMed] [Google Scholar]
- 3.Tjon AS, Sint Nicolaas J, Kwekkeboom J, et al. Increased incidence of early de novo cancer in liver graft recipients treated with cyclosporine: an association with c2 monitoring and recipient age. Liver Transpl. 2010;16:837–846. [DOI] [PubMed] [Google Scholar]
- 4.Welker MW, Bechstein WO, Zeuzem S, et al. Recurrent hepatocellular carcinoma after liver transplantation - an emerging clinical challenge. Transpl Int. 2013;26:109–118. [DOI] [PubMed] [Google Scholar]
- 5.Wimmer CD, Angele MK, Schwarz B, et al. Impact of cyclosporine versus tacrolimus on the incidence of de novo malignancy following liver transplantation: a single center experience with 609 patients. Transpl Int. 2013;26:999–1006. [DOI] [PubMed] [Google Scholar]
- 6.Gonwa TA, Mai ML, Melton LB, et al. End-stage renal disease (ESRD) after orthotopic liver transplantation (oltx) using calcineurin-based immunotherapy: risk of development and treatment. Transplantation. 2001;72:1934–1939. [DOI] [PubMed] [Google Scholar]
- 7.Pham PT, Pham PC, Wilkinson AH. Management of renal dysfunction in the liver transplant recipient. Curr Opin Organ Transplant. 2009;14:231–239. [DOI] [PubMed] [Google Scholar]
- 8.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–2729. [DOI] [PubMed] [Google Scholar]
- 9.Kirchner GI, Meier-Wiedenbach I, Manns MP. Clinical pharmacokinetics of everolimus. Clin Pharmacokinet. 2004;43:83–95. [DOI] [PubMed] [Google Scholar]
- 10.Schuler W, Sedrani R, Cottens S, et al. SDZ RAD, a new rapamycin derivative: pharmacological properties in vitro and in vivo. Transplantation. 1997;64:36–42. [DOI] [PubMed] [Google Scholar]
- 11.Shihab F, Christians U, Smith L, et al. Focus on mTOR inhibitors and tacrolimus in renal transplantation: Pharmacokinetics, exposure-response relationships, and clinical outcomes. Transpl Immunol. 2014;31:22–32. [DOI] [PubMed] [Google Scholar]
- 12.Shipkova M, Hesselink DA, Holt DW, et al. Therapeutic drug monitoring of everolimus: a consensus report. Ther Drug Monit. 2016;38:143–169. [DOI] [PubMed] [Google Scholar]
- 13.Saliba F, De Simone P, Nevens F, et al. Renal function at two years in liver transplant patients receiving everolimus: results of a randomized, multicenter study. Am J Transplant. 2013;13:1734–1745. [DOI] [PubMed] [Google Scholar]
- 14.Sterneck M, Kaiser GM, Heyne N, et al. Everolimus and early calcineurin inhibitor withdrawal: 3-year results from a randomized trial in liver transplantation. Am J Transplant. 2014;14:701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy G, Schmidli H, Punch J, et al. Safety, tolerability, and efficacy of everolimus in de novo liver transplant recipients: 12- and 36-month results. Liver Transpl. 2006;12:1640–1648. [DOI] [PubMed] [Google Scholar]
- 16.Masetti M, Montalti R, Rompianesi G, et al. Early withdrawal of calcineurin inhibitors and everolimus monotherapy in de novo liver transplant recipients preserves renal function. Am J Transplant. 2010;10:2252–2262. [DOI] [PubMed] [Google Scholar]
- 17.Fischer L, Klempnauer J, Beckebaum S, et al. A randomized, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation – PROTECT. Am J Transplant. 2012;12:1855–1865. [DOI] [PubMed] [Google Scholar]
- 18.De Simone P, Nevens F, De Carlis L, et al. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. Am J Transplant. 2012;12:3008–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Simone P, Metselaar HJ, Fischer L. Conversion from a calcineurin inhibitor to everolimus therapy in maintenance liver transplant recipients: A prospective, randomized, multicenter trial. Liver Transpl. 2009;15:1262–1269. [DOI] [PubMed] [Google Scholar]
- 20.Saliba F, Dharancy S, Lorho R, et al. Conversion to everolimus in maintenance liver transplant patients: A multicenter, retrospective analysis. Liver Transpl. 2011;17:905–913. [DOI] [PubMed] [Google Scholar]
- 21.De Simone P, Carrai P, Precisi A, et al. Conversion to everolimus monotherapy in maintenance liver transplantation: Feasibility, safety, and impact on renal function. Transpl Int. 2009;22:279–286. [DOI] [PubMed] [Google Scholar]
- 22.Castroagudin JF, Molina E, Romero R, Otero E, Tome S, Varo E. Improvement of renal function after the switch from a calcineurin inhibitor to everolimus in liver transplant recipients with chronic renal dysfunction. Liver Transpl. 2009;15:1792–1797. [DOI] [PubMed] [Google Scholar]
- 23.Lomas J. Words without action? The production, dissemination, and impact of consensus recommendations. Annu Rev Public Health. 1991;12:41–65. [DOI] [PubMed] [Google Scholar]
- 24.Rostom A, Moayyedi P, Hunt R, Canadian Association of Gastroenterology Consensus G Canadian consensus guidelines on long-term nonsteroidal anti-inflammatory drug therapy and the need for gastroprotection: Benefits versus risks. Aliment Pharmacol Ther. 2009;29:481–496. [DOI] [PubMed] [Google Scholar]
- 25.Lynn MR. Determination and quantification of content validity. Nurs Res. 1986;35:382–382. [PubMed] [Google Scholar]
- 26.Cillo U, Burra P, Mazzaferro V, et al. A multistep, consensus-based approach to organ allocation in liver transplantation: toward a "Blended Principle Model". Am J Transplant. 2015;15:2552–2561. [DOI] [PubMed] [Google Scholar]
- 27.Mahler DA, Selecky PA, Harrod CG, et al. American College of Chest Physicians Consensus Statement on the management of dyspnea in patients with advanced lung or heart disease. Chest. 2010;137:674–691. [DOI] [PubMed] [Google Scholar]
- 28.Zafar SY, Currow DC, Cherny N, Strasser F, Fowler R, Abernethy AP. Consensus-based standards for best supportive care in clinical trials in advanced cancer. Lancet Oncol. 2012;13:e77–e82. [DOI] [PubMed] [Google Scholar]
- 29.Neuberger JM, Mamelok RD, Neuhaus P, et al. Delayed introduction of reduced-dose tacrolimus, and renal function in liver transplantation: The ‘RESPECT’ study. Am J Transplant. 2009;9:327–336. [DOI] [PubMed] [Google Scholar]
- 30.Fischer L, Saliba F, Kaiser GM, et al. Three-year outcomes in de novo liver transplant patients receiving everolimus with reduced tacrolimus: follow-up results from a randomized, multicenter study. Transplantation. 2015;99:1455–1462. [DOI] [PubMed] [Google Scholar]
- 31.Abdelmalek MF, Humar A, Stickel F, et al. Sirolimus conversion regimen versus continued calcineurin inhibitors in liver allograft recipients: a randomized trial. Am J Transplant. 2012;12:694–705. [DOI] [PubMed] [Google Scholar]
- 32.Watt KD, Coss E, Pedersen RA, Dierkhising R, Heimbach JK, Charlton MR. Pretransplant serum troponin levels are highly predictive of patient and graft survival following liver transplantation. Liver Transpl. 2010;16:990–998. [DOI] [PubMed] [Google Scholar]
- 33.De Simone P, Precisi A, Petruccelli S, et al. The impact of everolimus on renal function in maintenance liver transplantation. Transplant Proc. 2009;41:1300–1302. [DOI] [PubMed] [Google Scholar]
- 34.Afinitor (everolimus). Summary of prescribing characteristics. Novartis Pharmaceuticals. Available from: https://www.pharma.us.novartis.com/product/pi/pdf/afinitor.pdf. Accessed 23 January 2015.
- 35.Villanueva A, Chiang DY, Newell P, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135:1972–1983, 1983 e1971-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. [DOI] [PubMed] [Google Scholar]
- 38.Kauffman HM, Cherikh WS, Cheng Y, et al. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation. 2005;80:883–889. [DOI] [PubMed] [Google Scholar]
- 39.Piselli P, Serraino D, Segoloni GP, et al. Risk of de novo cancers after transplantation: results from a cohort of 7217 kidney transplant recipients, Italy 1997–2009. Eur J Cancer. 2013;49:336–344. [DOI] [PubMed] [Google Scholar]
- 40.Toso C, Merani S, Bigam DL, et al. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology. 2010;51:1237–1243. [DOI] [PubMed] [Google Scholar]
- 41.Cholongitas E, Mamou C, Rodriguez-Castro KI, et al. Mammalian target of rapamycin inhibitors are associated with lower rates of hepatocellular carcinoma recurrence after liver transplantation: a systematic review. Transpl Int. 2014;27:1039–1049. [DOI] [PubMed] [Google Scholar]
- 42.Treiber G. mTOR inhibitors for hepatocellular cancer: a forward-moving target. Expert Rev Anticancer Ther. 2009;9:247–261. [DOI] [PubMed] [Google Scholar]
- 43.Geissler EK, Schnitzbauer AA, Zülke C, et al. Sirolimus use in liver transplant recipients with hepatocellular carcinoma: a randomized, multicenter, open-label phase 3 trial. Transplantation. 2016;100:116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanik EL, Chinnakotla S, Gustafson SK, et al. Effects of maintenance immunosuppression with sirolimus after liver transplant for hepatocellular carcinoma. Liver Transpl. 2016;22:627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bilbao I, Sapisochin G, Dopazo C, et al. Indications and management of everolimus after liver transplantation. Transplant Proc. 2009;41:2172–2176. [DOI] [PubMed] [Google Scholar]
- 46.Jiménez-Romero C, Manrique A, Marqués E, et al. Switching to sirolimus monotherapy for de novo tumors after liver transplantation. A preliminary experience. Hepatogastroenterology. 2011;58:115–121. [PubMed] [Google Scholar]
- 47.Vivarelli M, Dazzi A, Cucchetti A, et al. Sirolimus in liver transplant recipients: a large single-center experience. Transplant Proc. 2010;42:2579–2584. [DOI] [PubMed] [Google Scholar]
- 48.Bhoori S, Toffanin S, Sposito C, et al. Personalized molecular targeted therapy in advanced, recurrent hepatocellular carcinoma after liver transplantation: a proof of principle. J Hepatol. 2010;52:771–775. [DOI] [PubMed] [Google Scholar]
- 49.De Simone P, Crocetti L, Pezzati D, et al. Efficacy and safety of combination therapy with everolimus and sorafenib for recurrence of hepatocellular carcinoma after liver transplantation. Transplant Proc. 2014;46:241–244. [DOI] [PubMed] [Google Scholar]
- 50.Gomez-Martin C, Bustamante J, Castroagudin JF, et al. Efficacy and safety of sorafenib in combination with mammalian target of rapamycin inhibitors for recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2012;18:45–52. [DOI] [PubMed] [Google Scholar]
- 51.Kasiske BL, de Mattos A, Flechner SM, et al. Mammalian target of rapamycin inhibitor dyslipidemia in kidney transplant recipients. Am J Transplant. 2008;8:1384–1392. [DOI] [PubMed] [Google Scholar]
- 52.Holdaas H, Potena L, Saliba F. mTOR inhibitors and dyslipidemia in transplant recipients: a cause for concern? Transplant Rev (Orlando). 2014;29:93–102. [DOI] [PubMed] [Google Scholar]
- 53.Singh S, Watt KD. Long-term medical management of the liver transplant recipient: what the primary care physician needs to know. Mayo Clin Proc. 2012;87:779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pillebout E, Nochy D, Hill G, et al. Renal histopathological lesions after orthotopic liver transplantation (OLT). Am J Transplant. 2005;5:1120–1129. [DOI] [PubMed] [Google Scholar]
- 55.Cozzolino M, Gentile G, Mazzaferro S, et al. Blood pressure, proteinuria, and phosphate as risk factors for progressive kidney disease: a hypothesis. Am J Kidney Dis. 2013;62:984–992. [DOI] [PubMed] [Google Scholar]
- 56.Iwatsuki S, Esquivel CO, Klintmalm GB, et al. Nephrotoxicity of cyclosporine in liver transplantation. Transplant Proc. 1985;17:191–195. [PMC free article] [PubMed] [Google Scholar]
- 57.Pengel LH, Liu LQ, Morris PJ. Do wound complications or lymphoceles occur more often in solid organ transplant recipients on mTOR inhibitors? A systematic review of randomized controlled trials. Transpl Int. 2011;24:1216–1230. [DOI] [PubMed] [Google Scholar]
- 58.Nashan B, Citterio F. Wound healing complications and the use of mammalian target of rapamycin inhibitors in kidney transplantation: a critical review of the literature. Transplantation. 2012;94:547–561. [DOI] [PubMed] [Google Scholar]
- 59.Montalti R, Mimmo A, Rompianesi G, et al. Early use of mammalian target of rapamycin inhibitors is an independent risk factor for incisional hernia development after liver transplantation. Liver Transpl. 2012;18:188–194. [DOI] [PubMed] [Google Scholar]
- 60.Saliba F, Brown RS, Metselaar HJ, et al. Everolimus based immunosuppression in hepatitis C virus positive de novo liver transplant recipients: 24-month results from a randomized controlled trial. Liver Transpl. 2013;19:S100. [Google Scholar]
- 61.Villamil FG, Gadano AC, Zingale F, et al. Fibrosis progression in maintenance liver transplant patients with hepatitis C recurrence: a randomised study of everolimus vs. calcineurin inhibitors. Liver Int. 2014;34:1513–1521. [DOI] [PubMed] [Google Scholar]
- 62.Baas MC, Struijk GH, Moes DJ, et al. Interstitial pneumonitis caused by everolimus: a case-cohort study in renal transplant recipients. Transpl Int. 2014;27:428–436. [DOI] [PubMed] [Google Scholar]
- 63.Willemsen AE, Grutters JC, Gerritsen WR, et al. mTOR inhibitor-induced interstitial lung disease in cancer patients: comprehensive review and a practical management algorithm. Int J Cancer. 2016;138:2312–2321. [DOI] [PubMed] [Google Scholar]


