Supplemental Digital Content is available in the text.
Keywords: drug combinations, drug therapy, heart failure, mortality, network meta-analysis
Abstract
Background—
Treatments that reduce mortality and morbidity in patients with heart failure with reduced ejection fraction, including angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), β-blockers (BB), mineralocorticoid receptor antagonists (MRA), and angiotensin receptor–neprilysin inhibitors (ARNI), have not been studied in a head-to-head fashion. This network meta-analysis aimed to compare the efficacy of these drugs and their combinations regarding all-cause mortality in patients with heart failure with reduced ejection fraction.
Methods and Results—
A systematic literature review identified 57 randomized controlled trials published between 1987 and 2015, which were compared in terms of study and patient characteristics, baseline risk, outcome definitions, and the observed treatment effects. Despite differences identified in terms of study duration, New York Heart Association class, ejection fraction, and use of background digoxin, a network meta-analysis was considered feasible and all trials were analyzed simultaneously. The random-effects network meta-analysis suggested that the combination of ACEI+BB+MRA was associated with a 56% reduction in mortality versus placebo (hazard ratio 0.44, 95% credible interval 0.26–0.66); ARNI+BB+MRA was associated with the greatest reduction in all-cause mortality versus placebo (hazard ratio 0.37, 95% credible interval 0.19–0.65). A sensitivity analysis that did not account for background therapy suggested that ARNI monotherapy is more efficacious than ACEI or ARB monotherapy.
Conclusions—
The network meta-analysis showed that treatment with ACEI, ARB, BB, MRA, and ARNI and their combinations were better than the treatment with placebo in reducing all-cause mortality, with the exception of ARB monotherapy and ARB plus ACEI. The combination of ARNI+BB+MRA resulted in the greatest mortality reduction.
Mortality in patients with heart failure and reduced ejection fraction (HFrEF) has improved over time because of the step-wise introduction of a variety of pharmacological treatments. For years, recommended treatments for patients with HFrEF included the combination of an angiotensin-converting enzyme inhibitor (ACEI; or an angiotensin II receptor blocker [ARB] if an ACEI is not tolerated), a β-blocker (BB), and a mineralocorticoid receptor antagonist (MRA).1 Despite these recommended treatments being evidence based, the mortality rate for patients with HFrEF remains high.2–4
Sacubitril/valsartan, a first-in-class angiotensin receptor–neprilysin inhibitor (ARNI), was recommended as a new treatment option for patients with HFrEF in the 2016 European Society for Cardiology guidelines5 and the 2016 American College of Cardiology/American Heart Association guidelines.6 These recommendations were based on the results of the PARADIGM-HF trial (Prospective Comparison of ARNI With ACE to Determine Impact on Global Mortality and Morbidity in Heart Failure), which showed sacubitril/valsartan to be superior to enalapril in reducing the risks of cardiovascular and all-cause mortality when added to a BB (in most patients) and a MRA (in many), as well as a diuretic and digoxin.7
There are now 5 types (ACEI, ARB, BB, MRA, and ARNI) of life-saving pharmacological therapies available to treat patients with HFrEF. Given that most trials in HFrEF have compared newer agents to placebo, which has included alternative background treatments as recommendations have evolved, there is a need to understand how the efficacy of these individual treatments and various combinations compare in terms of all-cause mortality. If all trials have at least one intervention in common with another, it is possible to develop a network of randomized controlled trials (RCTs), allowing for indirect comparisons of interventions not studied in a head-to-head fashion using network meta-analysis (NMA).8 The validity of any NMA relies on whether there are systematic differences across RCTs in terms of patient or disease characteristics that are treatment effect modifiers.8–11 Consequently, it is important to identify the relevant network of RCTs and to assess the feasibility of performing a valid NMA.
The objective of this study was to systematically identify RCTs evaluating recommended drug classes and combinations for HFrEF in terms of all-cause mortality and to perform a valid NMA assessing the comparative efficacy of these therapies.
Methods
Identification and Selection of Studies
A systematic literature review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.12 Medline, EMBASE, and Cochrane CENTRAL were searched to identify studies published between January 1987 and April 28, 2015. Search terms included a combination of free text and Medical Subject Heading terms (see Data Supplement). Two reviewers (H. Burnett and A. Earley) independently screened citations against the following predefined selection criteria.
Population
Studies evaluating adults (aged ≥18 years) with chronic HFrEF (left ventricular ejection fraction <45%) and New York Heart Association class II–IV of varying etiology (ischemic and dilated cardiomyopathy) who were outpatients were included. Studies were excluded if the entire study population had one of the following characteristics, which are known to impact treatment response or all-cause mortality: (1) acute heart failure, (2) hospitalized, (3) New York Heart Association class I, (4) clinical comorbidity (eg, chronic obstructive pulmonary disease, diabetes mellitus, or renal failure), (5) coronary heart disease, (6) post-myocardial infarction, (7) ischemia, (8) idiopathic dilated cardiomyopathy, (9) elderly (aged >70 years), or (10) from country outside of North America or Europe. Studies that included a proportion of patients with the characteristics described above were included.
Interventions
All guideline-recommended drug classes: ACEIs, BBs, ARBs, and MRAs and an ARNI, administered alone or in combination (see Table I in the Data Supplement for eligible drug molecules).
Comparators
Placebo or any intervention of interest of a different class; comparisons within the same class were excluded (eg, ACEI versus ACEI).
Outcomes
Death because of any cause reported as an efficacy or safety end point.
Study Design
Phase II or III RCTs published in English.
Data Extraction and Quality Assessment
For each included study, details were extracted on study design, patient characteristics, and interventions. The quality of the RCTs was assessed.13 For all-cause mortality, the total number of events was extracted for each arm, and the exposure time for each trial was extracted for the planned study duration, if reported, or else the mean or median follow-up time.
Feasibility Assessment
The feasibility of conducting a valid NMA was assessed using the process described by Cope et al,14 which involves an assessment of clinical heterogeneity in terms of the characteristics of the treatments, outcomes, study design, and patients and a comparison of differences within and across treatments in terms of baseline risk and the observed treatment effects. The following factors were identified a priori as potential treatment effect modifiers: use of concomitant treatments (eg, digoxin), duration of follow-up, year of publication, severity of included patients (eg, New York Heart Association class and left ventricular ejection fraction), heart failure etiology (eg, ischemic versus nonischemic), and history of myocardial infarction.
Network Meta-Analysis
Bayesian NMA models were used to simultaneously synthesize the results of the included studies and to obtain relative treatment effects.11,15–17 NMA within the Bayesian framework involves data, a hierarchical model or likelihood function with parameters, and prior distributions.18 The model relates data from RCTs to parameters reflecting the (pooled) relative treatment effect of each intervention compared with the reference treatment (eg, placebo). Data sets for the model were based on the reported number of patients with an event at the end of the trial per arm, the total number of patients randomized per arm, and the mean follow-up duration of the trial. The log mean follow-up time was used to transform the probability of an event into a constant rate for each trial arm by assuming an underlying Poisson process, and a complementary log–log (cloglog) link was used to model the event rates.10 Outputs from the model were presented as hazard ratios (HRs) for each treatment versus placebo. Goodness of fit was assessed using the residual deviance and deviance information criterion.19 Results of the random-effects model were presented unless the fixed-effect model resulted in a more parsimonious model. Noninformative prior distributions were used: a normal distribution for the difference measures (mean 0, var 104) and a uniform distribution for between-study standard deviation (range 0–5). The analysis was performed with published codes10 using OpenBuGS software20 (2 chains were used, including 100 000 burn-in iterations followed by 200 000 iterations).
Results of the NMA reflect the posterior distributions of the model parameters. In addition to point estimates of the HRs, 95% credible intervals (CrI), reflecting the range of true underlying effects with 95% probability, are presented. The rank probabilities and expected rank for all treatments are presented, as well as the probability that one treatment is better than a specific comparator.11 Means, standard deviations, and ranges were summarized for study and patient characteristics where possible.
Results
Study Selection
Fifty-seven RCTs were included (Figure 1) and are described in Table II in the Data Supplement.7,21–77 The majority were multicenter, double-blind, placebo-controlled trials, including between 28 and 8399 patients with a mean follow-up duration ranging from 8 weeks to 4 years. The treatment classes assessed included ACEI, BB, ARB, MRA, and ARNI. Patients were generally allowed concomitant therapies, such as diuretics, digoxin, and nitrates, as well as other permitted concomitant treatment classes.
Figure 1.
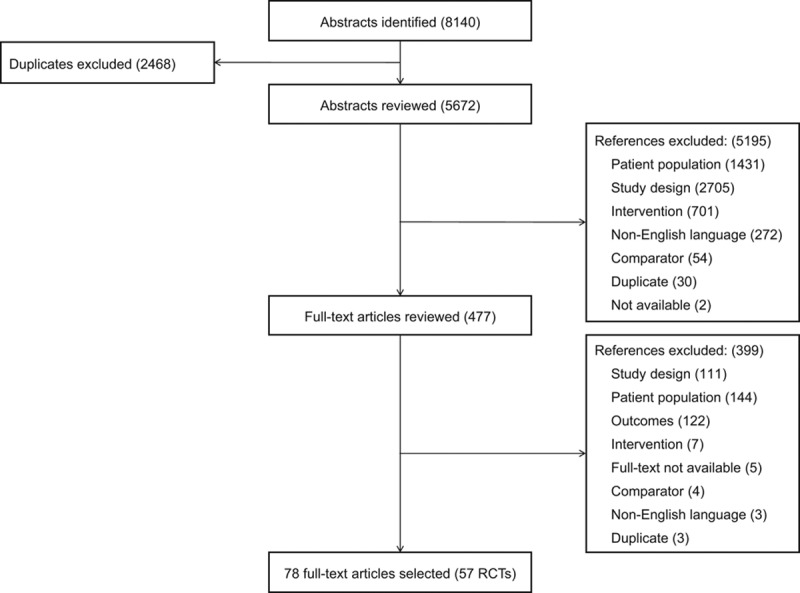
Flow diagram. RCT indicates randomized controlled trials.
Network of Evidence
In the network of connected RCTs (Figure 2), the thickness of the lines corresponds to the number of trials included per treatment comparison. The evidence was centralized around placebo and ACEI, with most RCTs informing the comparison of ACEI+BB versus ACEI. The treatment combination with ARNI was informed by a single RCT.
Figure 2.
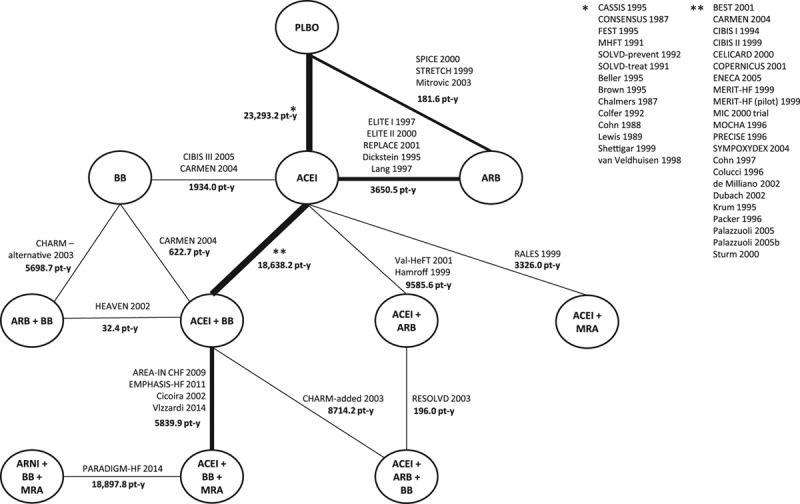
Network diagram of treatment classes and combinations reporting all-cause mortality. ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin-II receptor blocker; AREA-IN CHF, Anti-Remodelling Effect of Canrenone in Patients With Mild Chronic Heart Failure; ARNI, angiotensin receptor-neprilysin inhibitor; BB, beta blocker; BEST, Beta-Blocker Evaluation of Survival Trial; CARMEN, The Carvedilol and ACE-Inhibitor Remodelling Mild Heart Failure Evaluation Trial; CASSIS, Czech and Slovak Spirapril Intervention Study; CHARM-added, Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity–Added; CHARM-alternative, Candesartan in Heart Failure–Assessment of Mortality and Morbidity Alternative; CIBIS, Cardiac Insufficiency Bisoprolol Study; CONSENSUS, Cooperative North Scandinavian Enalapril Survival Study; COPERNICUS, Carvedilol Prospective Randomized Cumulative Survival; ELITE, Evaluation of Losartan in the Elderly Study; EMPHASIS-HF, Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure; ENECA, Efficacy of Nebivolol in the Treatment of Elderly Patients With Chronic Heart Failure as Add-On Therapy to ACE Inhibitors or Angiotensin II Receptor Blockers, Diuretics, and/or Digitalis; FEST, Fosinopril Efficacy/Safety Trial; HEAVEN, Heart Failure Valsartan Exercise Capacity Evaluation; MERIT-HF, Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure; MHFT, Munich Mild Heart Failure Trial; MIC, Metoprolol in Patients With Mild to Moderate Heart Failure: Effects on Ventricular Function and Cardiopulmonary Exercise Testing; MOCHA, Multicenter Oral Carvedilol Heart Failure Assessment; MRA, mineralocorticoid receptor antagonist; PARADIGM-HF, Prospective Comparison of ARNI (Angiotensin Receptor–Neprilysin Inhibitor) With ACEI (Angiotensin–Converting–Enzyme Inhibitor) to Determine Impact on Global Mortality and Morbidity in Heart Failure; PLBO, placebo; PRECISE, Prospective Randomized Evaluation of Carvedilol on Symptoms and Exercise; RALES, Randomized Aldactone Evaluation Study; REPLACE, Replacement of Angiotensin Converting Enzyme Inhibition; RESOLVD, Randomized Evaluation of Strategies for Left Ventricular Dysfunction; SOLVD-prevent, Studies of Left Ventricular Dysfunction–Prevention Trial; SOLVD-treat, Studies of Left Ventricular Dysfunction–Treatment Trial; SPICE, Study of Patients Intolerant of Converting Enzyme Inhibitors; STRETCH, Symptom, Tolerability, Response to Exercise Trial of Candesartan Cilexetil in Heart Failure; SYMPOXYDEX, Sympathetic and Oxydative Stress Kredex Study; and Val-HeFT, Valsartan Heart Failure Trial.7,21–77
Differences Within or Between Direct Treatment Comparisons That May Modify Treatment Effect
Treatment Definitions
There was a wide range in the types of individual and concomitant treatments (Table III in the Data Supplement). In fact, few trials included a true placebo arm because study patients were often permitted to receive or continue to receive the standard of care in addition to study drugs. An increase in the use of combination therapies was observed over the years, with the earliest trials being focused on ACEIs versus placebo, followed by the addition of BB (ACEI+BB versus ACEI studies), and then ARB and MRA containing therapies around the same time after their introduction. The combination ACEI+BB+MRA was first evaluated in 2002 compared with ACEI+BB. To take into account concomitant drug classes of interest and more accurately define placebo in the analysis, treatments were categorized to include the concomitant drug when the majority of patients in the study were receiving it at baseline. Specifically, if >50% of the trial patients received a concomitant drug of interest in the systematic review (eg, BB), the treatment was described as a combination therapy (the study drug class+the concomitant drug class(es), eg, ACEI+BB versus BB) in the analysis. The threshold to define concomitant therapy was based on expert opinion and involved an evaluation of different thresholds ranging from 50% to 60%.78
When the permitted concomitant drug was ACEI or ARB and the publication failed to report the distribution of patients receiving each class, it was assumed that patients were taking ACEI (Table IV in the Data Supplement). A sensitivity analysis was also performed that ignored concomitant therapies and evaluated how ARNI monotherapy was compared with ACEI and ARB monotherapies (Figure 3).7,21–45
Figure 3.
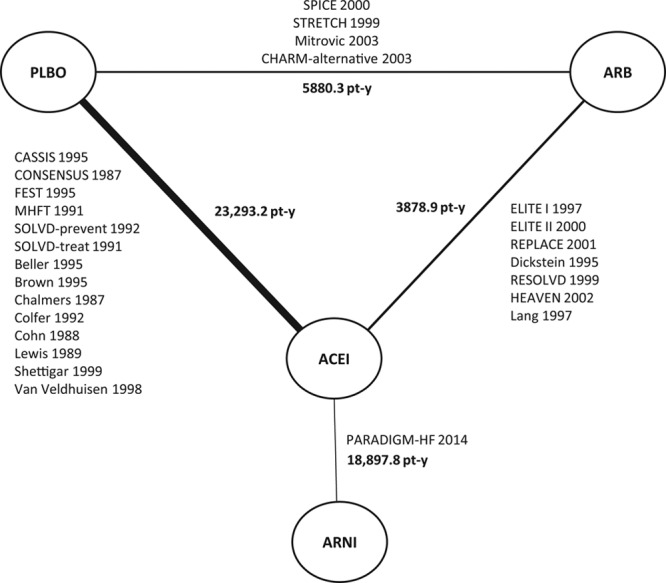
Sensitivity analysis evidence network of ARNI, ACEI, ARB and placebo for all-cause mortality ignoring background treatments. ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin-II receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; CASSIS, Czech and Slovak Spirapril Intervention Study; CHARM-alternative, Candesartan in Heart Failure–Assessment of Mortality and Morbidity Alternative; CONSENSUS, Cooperative North Scandinavian Enalapril Survival Study; ELITE, Evaluation of Losartan in the Elderly Study; FEST, Fosinopril Efficacy/Safety Trial; HEAVEN, Heart Failure Valsartan Exercise Capacity Evaluation; MHFT, Munich Mild Heart Failure Trial; PARADIGM-HF, Prospective Comparison of ARNI (Angiotensin Receptor–Neprilysin Inhibitor) With ACEI (Angiotensin–Converting–Enzyme Inhibitor) to Determine Impact on Global Mortality and Morbidity in Heart Failure; PLBO, placebo; REPLACE, Replacement of Angiotensin Converting Enzyme Inhibition; RESOLVD, Randomized Evaluation of Strategies for Left Ventricular Dysfunction; SOLVD-prevent, Studies of Left Ventricular Dysfunction–Prevention Trial; SOLVD-treat, Studies of Left Ventricular Dysfunction–Treatment Trial; SPICE, Study of Patients Intolerant of Converting Enzyme Inhibitors; and STRETCH, Symptom, Tolerability, Response to Exercise Trial of Candesartan Cilexetil in Heart Failure.7,21–45
Despite some variation in individual treatment doses and schedules (Table V in the Data Supplement), all analyses assumed that treatments within a given class were comparable in terms of their ability to prevent death. All trials were included, even though some variation was also observed in the proportion of patients receiving concomitant digoxin.
Outcome Definition
All-cause mortality is an objective end point and is usually reported as a primary or secondary outcome, although the NMA also included 28 trials that reported mortality as an adverse event or a reason for study withdrawal (Figure 4A). Because the quality of these 28 studies did not differ greatly from that of the other included trials, the broadest evidence base was included.
Figure 4.
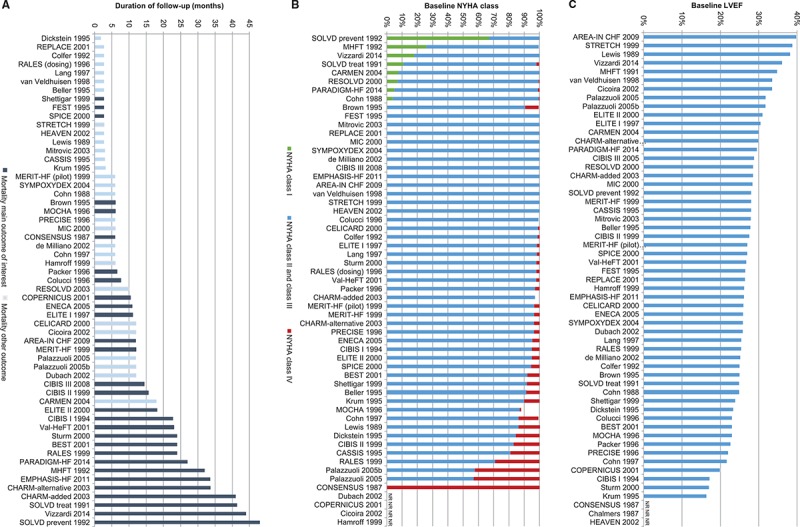
Distribution of potential treatment effect modifiers: A, Duration of follow-up7,21–77; B, NYHA class at baseline7,21–77; C, LVEF at baseline.7,21–77 AREA-IN CHF indicates Anti-Remodelling Effect of Canrenone in Patients With Mild Chronic Heart Failure; BEST, Beta-Blocker Evaluation of Survival Trial; CARMEN, The Carvedilol and ACE-Inhibitor Remodelling Mild Heart Failure Evaluation Trial; CASSIS, Czech and Slovak Spirapril Intervention Study; CHARM-added, Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity–Added; CHARM-alternative, Candesartan in Heart Failure–Assessment of Mortality and Morbidity Alternative; CIBIS, Cardiac Insufficiency Bisoprolol Study; CONSENSUS, Cooperative North Scandinavian Enalapril Survival Study; COPERNICUS, Carvedilol Prospective Randomized Cumulative Survival; ELITE, Evaluation of Losartan in the Elderly Study; EMPHASIS-HF, Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure; ENECA, Efficacy of Nebivolol in the Treatment of Elderly Patients With Chronic Heart Failure as Add-On Therapy to ACE Inhibitors or Angiotensin II Receptor Blockers, Diuretics, and/or Digitalis; FEST, Fosinopril Efficacy/Safety Trial; HEAVEN, Heart Failure Valsartan Exercise Capacity Evaluation; LVEF, left ventricular ejection fraction; MERIT-HF, Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure; MHFT, Munich Mild Heart Failure Trial; MIC, Metoprolol in Patients With Mild to Moderate Heart Failure: Effects on Ventricular Function and Cardiopulmonary Exercise Testing; MOCHA, Multicenter Oral Carvedilol Heart Failure Assessment; NR, not reported; NYHA, New York Heart Association; PARADIGM-HF, Prospective Comparison of ARNI (Angiotensin Receptor–Neprilysin Inhibitor) With ACEI (Angiotensin–Converting–Enzyme Inhibitor) to Determine Impact on Global Mortality and Morbidity in Heart Failure; PRECISE, Prospective Randomized Evaluation of Carvedilol on Symptoms and Exercise; RALES, Randomized Aldactone Evaluation Study; REPLACE, Replacement of Angiotensin Converting Enzyme Inhibition; RESOLVD, Randomized Evaluation of Strategies for Left Ventricular Dysfunction; SOLVD-prevent, Studies of Left Ventricular Dysfunction–Prevention Trial; SOLVD-treat, Studies of Left Ventricular Dysfunction–Treatment Trial; SPICE, Study of Patients Intolerant of Converting Enzyme Inhibitors; STRETCH, Symptom, Tolerability, Response to Exercise Trial of Candesartan Cilexetil in Heart Failure; SYMPOXYDEX, Sympathetic and Oxydative Stress Kredex Study; and Val-HeFT, Valsartan Heart Failure Trial.
Study and Patient Characteristics
The RCTs were broadly comparable in terms of study design, despite a wide range in publication year (1987–2014). There were some differences in study quality, although overall the risk of bias was low (Figure I in the Data Supplement). Given the differences in the duration of follow-up across trials (Figure 4A), patient exposure was accounted for in the analysis.
Enrolled patients were predominantly male (mean 76%, range 49%–90%) and between the ages of 52 and 73 years (mean 62 years; Table II in the Data Supplement). Most patients were classified as New York Heart Association class II–III (mean 86%), although 8 (14%) trials included a proportion of patients in class I and 36 (63%) trials included patients in class IV (Figure 4B). Baseline left ventricular ejection fraction ranged between 15% and 40% (mean 27%; Figure 4C). In terms of heart failure etiology, 57% of the included patients had ischemic heart disease (range 10%–83%). It was not possible to consistently assess differences in the duration of heart failure, the use of pacemakers or implantable devices, or the history of myocardial infarction because of inconsistent reporting.
Baseline Risk and Observed Treatment Effects
Given variation in publication year across trials, differences attributable to changes in clinical practice over time may result in differences in baseline risk that could influence the treatment effect. However, the network of evidence does not provide a well-connected common comparator (placebo or standard of care), partly because of the treatment categorization based on concomitant therapy used to account for differences in placebo. Figure II in the Data Supplement reports the rates of death per patient year for all treatment arms and RCTs by publication year. Although the rates varied across included RCTs, it was unclear whether these differences were driven by changes in practice over time or acted as a treatment effect modifier.
Network Meta-Analysis Results
All identified RCTs were included in the NMA and provided comparative evidence on all-cause mortality in patients with HFrEF.
Table 1 presents the results of the random effect NMA for all head-to-head comparisons and illustrates the HRs, the 95% CrIs, and the probability of a treatment being better than the comparator. We found significant between-study heterogeneity in the network of evidence (SD 0.18, 95% CrI 0.06–0.35; Table 1), which was expected given the differences observed in the included studies.
Table 1.
Results of Random Effect Network Meta-Analysis for All-Cause Mortality Rates: Difference in Intervention Versus the Comparator, 95% Credible Intervals (CrI), and Probability That the Intervention Is Better Than the Comparator [P(better)]
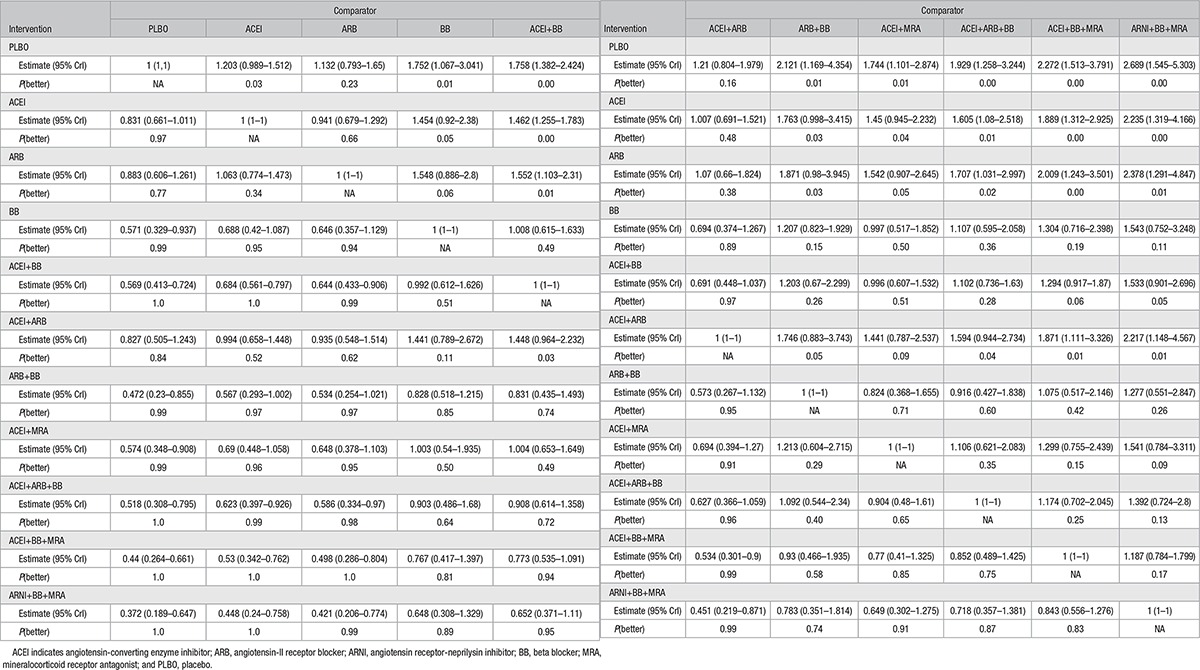
Figure 5 illustrates the HRs for each treatment class versus placebo for all-cause mortality. The combination of ACEI+BB+MRA was associated with a 56% reduction in mortality versus placebo (HR 0.44, 95% CrI 0.26–0.66), while ARNI+BB+MRA was associated with the greatest reduction in all-cause mortality versus placebo (HR 0.37, 95% CrI 0.19–0.65). Figure III in the Data Supplement summarizes the rank probabilities for all interventions.
Figure 5.
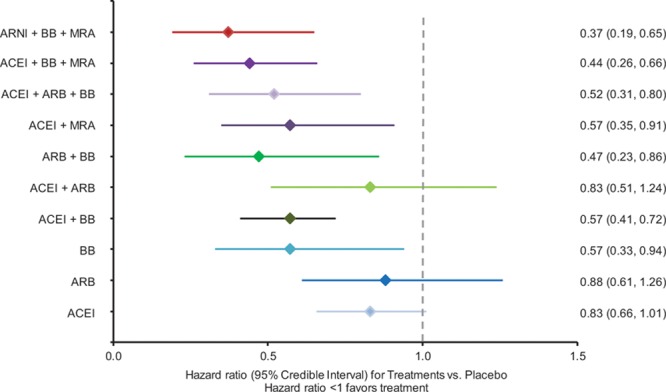
Results of random effect network meta-analysis for all-cause mortality: hazard ratios for intervention versus placebo for all-cause mortality and 95% credible intervals. ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin-II receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; BB, beta blocker; and MRA, mineralocorticoid receptor antagonist.
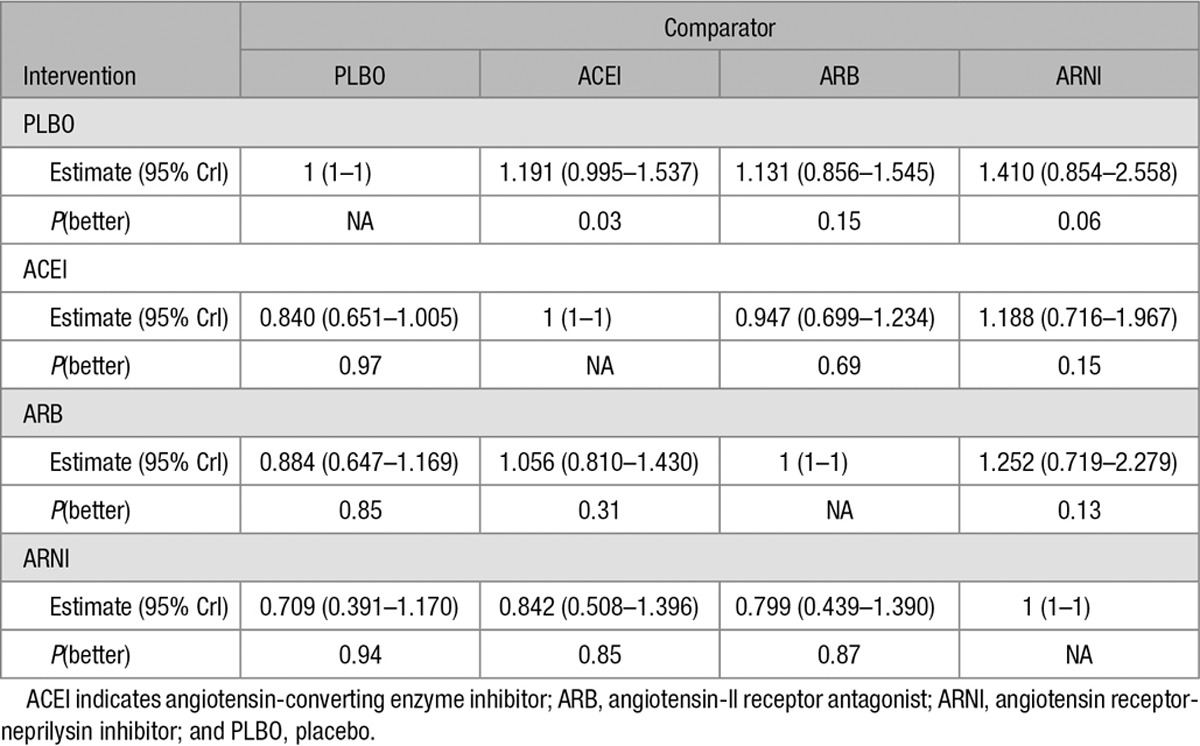
Table 2 presents the results from the sensitivity analysis that ignored concomitant therapies and evaluated how ARNI monotherapy was compared with ACEI and ARB monotherapies. The random-effects model suggests that all active treatments are likely to be more efficacious than placebo, although with more uncertainty than the base case analysis. The sensitivity analysis showed that in comparison to placebo, ARNI was associated with a 29% reduction in mortality (HR 0.71, 95% CrI 0.39–1.17); ACEI, a 16% reduction (HR 0.84, 95% CrI 0.65–1.01); and ARB, a 12% reduction (HR 0.88, 95% CrI 0.65–1.17).
Table 2.
Results of Random Effect Sensitivity Analysis Network Meta-Analysis for All-Cause Mortality Rates: Difference in Intervention Versus the Comparator, 95% Credible Intervals (CrI), and Probability That the Intervention Is Better Than the Comparator [P(better)]
Discussion
New trials build on the evidence from previous trials and therefore, test new drugs in addition to existing ones; as a result, it becomes increasingly difficult for clinicians to maintain a perspective on the relative efficacy of the treatments they are advised to use or to fully appreciate the cumulative benefit of combining treatments. To provide this perspective, the relative efficacy of recommended drug classes and combinations in reducing mortality of HFrEF were estimated. This is the first NMA to consider the totality of RCT evidence for recommended treatment classes and combinations, including 57 trials conducted over the past 30 years in patients with HFrEF.
Our results provide insight regarding the comparative efficacy of treatments for which no head-to-head trials exist and suggest that ARNI+BB+MRA and ACEI+BB+MRA are the most efficacious treatment combinations in terms of reducing all-cause mortality. These findings validate global guidelines, which recommend first-line treatment of HFrEF with ACEI+BB (ARB+BB for those unable to tolerate ACEI), followed by the addition of an MRA as second-line therapy and ARNI to replace ACEI in patients able to tolerate ACEI (or ARB) that remain symptomatic.5,6
Our findings also illustrate the step-wise reductions in mortality made possible by the incremental use of combinations of disease-modifying therapies. The NMA results suggest that ARNI+BB+MRA is the most efficacious therapy, reducing all-cause mortality by 63% compared with placebo. The magnitude of this benefit represents substantial progress in terms of treatments developed over the last 30 years (since the first report of an ACEI treatment). Although this finding depends on a single trial, PARADIGM-HF was the largest trial in the network, representing 18 898 patient-years of treatment exposure.7 It is also important to note that although BB monotherapy is included in the network and, therefore, can be compared with other monotherapies using NMA, data to support this comparison are based on 2 small, short-duration trials (CIBIS III [Cardiac Insufficiency Bisoprolol Study III]46 and CARMEN trial [Carvedilol and ACE-Inhibitor Remodelling Mild Heart Failure Evaluation]47). The majority of available evidence regarding the efficacy of BB therapy is based on studies where patients were also receiving an ACEI (and MRA in more recent trials).
Our study is the result of a comprehensive and detailed NMA performed jointly by clinicians and methodologists. The available data and underlying assumptions have been clearly illustrated to allow other researchers and decision-makers to critically analyze each choice and to update the analysis using a different approach. The way this study categorized the recommended drug combinations and concomitant drugs (eg, ACEIs, ARBs, BBs, and MRAs) reflects a methodological development necessary to assess the comparative efficacy of these treatment combinations. The threshold approach allowed for differences in placebo to be defined and yielded clinically meaningful results, with monotherapies being less effective than combination therapies and regimens, including 3 treatment classes likely to be most efficacious. The importance of this approach is highlighted by results of the sensitivity analysis where concomitant therapies were ignored: ARNI was associated with a 29% reduction in mortality compared with placebo, whereas the base case illustrated a 63% reduction with the combination of ARNI+BB+MRA. The difference relates to definition of placebo (as well as ACEI and ARB arms) in the sensitivity analysis, which included a wide range of concomitant drugs (eg, in CHARM-alternative trial [Candesartan in Heart Failure–Assessment of Mortality and Morbidity Alternative],43 an ARB versus placebo trial, 55% of patients were receiving a BB), which were ignored or in some cases pooled with true placebo studies. In the base case analysis, placebo more closely represents the baseline risk of the patient population of interest because treatments were categorized based on the study drugs and concomitant drugs of interest.
Overall, findings were generally consistent with other published (network) meta-analyses evaluating all-cause mortality that compared monotherapies within a single class to placebo in addition to standard of care.79–85 A recent putative placebo analysis by McMurray et al86 found that ARNI was associated with a 28% reduction in all-cause mortality, which was similar to the sensitivity analysis performed that ignored background therapy (ie, 29% reduction in all-cause mortality).
Direct comparisons of results from other published studies are limited by differences in included studies and the classification of concomitant drugs, which were often ignored or led to the exclusion of several trials. Therefore, the attempt in the current study to classify trials based on the background therapies may provide more valid insight regarding treatment classes used in combination in clinical practice.
Limitations
One limitation was the identification of concomitant therapy, which was based on data reported at baseline, which may have differed from treatments used during follow-up and certainly varied across the included trials. In addition, we assumed a class-effect, that is, all drugs in the same pharmacological class had similar efficacy, which may not be true. The same consideration applies to the dose of treatments used.
Most notably, differences were identified in terms of study duration, which may imply differences in the study purpose or type of mortality analysis. The length of follow-up in each trial was accounted for in the analysis assuming a proportional hazards model, which allowed for an assessment of the broadest evidence base. A comparison of alternative scales and statistical models may be of interest to explore alternative underlying assumptions and the consistency of direct and indirect evidence.
Despite differences identified, no inconsistencies were identified, and adjustment for patient characteristics did not have substantial impact on the results. However, it should be recognized that there is a risk of ecological bias as study-level data were used to estimate the treatment effects. Individual patient data would be required to better explore differences in treatment effect modifiers.9,11,87 In addition, some information was not consistently reported across the trials, limiting either the assessment of potential differences or the potential to adjust for differences (ie, duration of heart failure, etiology, use of devices, or history of myocardial infarction).
To our knowledge, this review includes the broadest evidence base. However, generalizability may be limited by including only English language studies and by excluding studies enrolling patients exclusively outside of North America and Europe. Based on the available data, it was not possible to assess some comparisons, such as MRA versus placebo, as well as the combination of a BB and MRA versus placebo. Although this study provides insight regarding all-cause mortality for patients with HFrEF, other important efficacy and safety outcomes should also be considered by decision-makers, including death because of cardiovascular causes and heart failure, hospitalizations, and health-related quality of life.
Conclusions
This report provides a comprehensive analysis of the comparative efficacy of the individual drug classes and combinations known to reduce mortality in patients with HFrEF. It was possible to pool and indirectly compare evidence from RCTs published over the last 34 years using NMA, providing insight into treatment comparisons in the absence of head-to-head trials. The threshold approach used to account for concomitant therapy provides a more accurate representation of the treatment comparisons evaluated in RCTs, often reflecting standard of care at the time. Our results show that the most efficacious combinations for reducing all-cause mortality are in line with the most recent guideline recommendations.
Acknowledgments
We thank Nick Miller for his assistance with the preparation of the article and Stefano Corda for his insight regarding the design and results of the study.
Sources of Funding
This work was supported by Novartis Pharma AG. The publication of this study was not contingent on the sponsor’s approval or censorship of the article.
Disclosures
H. Burnett, A. Earley, and S. Cope prepared this article based on funding from Novartis Pharma AG. C. Deschaseaux is an employee of Novartis Pharma AG. A.A. Voors has received consultancy fees and research grants from AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Cardio3Biosciences, Celladon, GSK, Johnson and Johnson, Merck/MSD, Novartis, Servier, Simgulex, Sphingotec, Stealth, Trevena, and Vifor. M. Senni has received consultancy fees from Novartis, Bayer, Merck/MSD, and Servier. J. McMurray's employer, Glasgow University, has been paid for his time spent consulting for Novartis Pharma AG. The other authors report no conflicts.
Supplementary Material
Footnotes
The Data Supplement is available at http://circheartfailure.ahajournals.org/lookup/suppl/doi:10.1161/CIRCHEARTFAILURE.116.003529/-/DC1.
CLINICAL PERSPECTIVE
Over the past 30 years, much progress has been made regarding the treatment of patients with heart failure and reduced ejection fraction. Mortality has reduced over time, and there are now 5 main classes of life-saving pharmacological therapies recommended for the treatment of patients with heart failure and reduced ejection fraction, including angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, β-blockers, mineralocorticoid receptor antagonists, and the angiotensin receptor–neprilysin inhibitor, sacubitril/valsartan. Given that new trials build on evidence from previous trials, and the fact that new drugs have mainly been tested on top of existing ones, it becomes increasingly difficult for clinicians to maintain a perspective on the relative efficacy of the separate treatments and their combinations. This study systematically identified 57 trials conducted over the past 34 years evaluating recommended treatment classes and combinations in patients with heart failure and reduced ejection fraction. Results from the systematic review were used to estimate the relative efficacy of these therapies with regards to survival, by means of network meta-analysis, providing insight into treatment comparisons in the absence of head-to-head trials. The network meta-analysis showed that all available treatment classes and combinations were more efficacious than placebo, with the exception of angiotensin II receptor blockers monotherapy and angiotensin II receptor blockers plus angiotensin-converting enzyme inhibitors. The combination of an angiotensin receptor–neprilysin inhibitor+β-blockers+mineralocorticoid receptor antagonists resulted in the greatest mortality reduction. Overall, these findings help illustrate the step-wise reductions in mortality made possible by the incremental use of combinations of disease-modifying therapies and validate the most recent global guideline recommendations.
References
- 1.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology; ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 3.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 4.Zannad F, Agrinier N, Alla F. Heart failure burden and therapy. Europace. 2009;11(suppl 5):v1–v9. doi: 10.1093/europace/eup304. doi: 10.1093/europace/eup304. [DOI] [PubMed] [Google Scholar]
- 5.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 6.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Colvin MM, Drazner MH, Filippatos G, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2016;134:e282–e293. doi: 10.1161/CIR.0000000000000435. doi: 10.1161/CIR.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 7.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 8.Jansen JP, Naci H. Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers. BMC Med. 2013;11:159. doi: 10.1186/1741-7015-11-159. doi: 10.1186/1741-7015-11-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper NJ, Sutton AJ, Morris D, Ades AE, Welton NJ. Addressing between-study heterogeneity and inconsistency in mixed treatment comparisons: application to stroke prevention treatments in individuals with non-rheumatic atrial fibrillation. Stat Med. 2009;28:1861–1881. doi: 10.1002/sim.3594. doi: 10.1002/sim.3594. [DOI] [PubMed] [Google Scholar]
- 10.Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33:607–617. doi: 10.1177/0272989X12458724. doi: 10.1177/0272989X12458724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, Lee K, Boersma C, Annemans L, Cappelleri JC. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011;14:417–428. doi: 10.1016/j.jval.2011.04.002. doi: 10.1016/j.jval.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 13.CRD’s Guidance for Undertaking Reviews in Health Care. Layerthorpe, York, UK: Centre for Reviews and Dissemination, University of York; 2008. [Google Scholar]
- 14.Cope S, Zhang J, Saletan S, Smiechowski B, Jansen JP, Schmid P. A process for assessing the feasibility of a network meta-analysis: a case study of everolimus in combination with hormonal therapy versus chemotherapy for advanced breast cancer. BMC Med. 2014;12:93. doi: 10.1186/1741-7015-12-93. doi: 10.1186/1741-7015-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331:897–900. doi: 10.1136/bmj.331.7521.897. doi: 10.1136/bmj.331.7521.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen JP, Crawford B, Bergman G, Stam W. Bayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons. Value Health. 2008;11:956–964. doi: 10.1111/j.1524-4733.2008.00347.x. doi: 10.1111/j.1524-4733.2008.00347.x. [DOI] [PubMed] [Google Scholar]
- 17.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–3124. doi: 10.1002/sim.1875. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 18.Sutton AJ, Jones DR, Abrams KR, Sheldon TA, Song F. Systematic reviews and meta-analysis: a structured review of the methodological literature. J Health Serv Res Policy. 1999;4:49–55. doi: 10.1177/135581969900400112. [DOI] [PubMed] [Google Scholar]
- 19.Spiegelhalter DJ, Abrams KR, Myles JP. Bayesian Approaches to Clinical Trials and Health-Care Evaluation. West Sussex, England: John Wiley & Sons; 2004. [Google Scholar]
- 20.Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: evolution, critique and future directions. Stat Med. 2009;28:3049–3067. doi: 10.1002/sim.3680. doi: 10.1002/sim.3680. [DOI] [PubMed] [Google Scholar]
- 21.Widimský J, Kremer HJ, Jerie P, Uhlír O. Czech and Slovak spirapril intervention study (CASSIS). A randomized, placebo and active-controlled, double-blind multicentre trial in patients with congestive heart failure. Eur J Clin Pharmacol. 1995;49:95–102. doi: 10.1007/BF00192366. [DOI] [PubMed] [Google Scholar]
- 22.Swedberg K The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 23.Erhardt L, MacLean A, Ilgenfritz J, Gelperin K, Blumenthal M. Fosinopril attenuates clinical deterioration and improves exercise tolerance in patients with heart failure. Fosinopril Efficacy/Safety Trial (FEST) Study Group. Eur Heart J. 1995;16:1892–1899. doi: 10.1093/oxfordjournals.eurheartj.a060844. [DOI] [PubMed] [Google Scholar]
- 24.Kleber FX, Niemöller L. Long-term survival in the Munich Mild Heart Failure Trial (MHFT). Am J Cardiol. 1993;71:1237–1239. doi: 10.1016/0002-9149(93)90657-x. [DOI] [PubMed] [Google Scholar]
- 25.Yusuf S. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigators. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 26.Yusuf S The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 27.Beller B, Bulle T, Bourge RC, Colfer H, Fowles RE, Giles TD, Grover J, Whipple JP, Fisher MB, Jessup M. Lisinopril versus placebo in the treatment of heart failure: the Lisinopril Heart Failure Study Group. J Clin Pharmacol. 1995;35:673–680. doi: 10.1002/j.1552-4604.1995.tb04107.x. [DOI] [PubMed] [Google Scholar]
- 28.Brown EJ, Jr, Chew PH, MacLean A, Gelperin K, Ilgenfritz JP, Blumenthal M. Effects of fosinopril on exercise tolerance and clinical deterioration in patients with chronic congestive heart failure not taking digitalis. Fosinopril Heart Failure Study Group. Am J Cardiol. 1995;75:596–600. doi: 10.1016/s0002-9149(99)80624-9. [DOI] [PubMed] [Google Scholar]
- 29.Chalmers JP, West MJ, Cyran J, De La Torre D, Englert M, Kramar M, Lewis GR, Maranhao MF, Myburgh DP, Schuster P. Placebo-controlled study of lisinopril in congestive heart failure: a multicentre study. J Cardiovasc Pharmacol. 1987;9(suppl 3):S89–S97. doi: 10.1097/00005344-198700003-00021. [DOI] [PubMed] [Google Scholar]
- 30.Colfer HT, Ribner HS, Gradman A, Hughes CV, Kapoor A, Laidlaw JC. Effects of once-daily benazepril therapy on exercise tolerance and manifestations of chronic congestive heart failure. The Benazepril Heart Failure Study Group. Am J Cardiol. 1992;70:354–358. doi: 10.1016/0002-9149(92)90618-9. [DOI] [PubMed] [Google Scholar]
- 31.Comparative effects of therapy with captopril and digoxin in patients with mild to moderate heart failure. The Captopril-Digoxin Multicenter Research Group. JAMA. 1988;259:539–544. [PubMed] [Google Scholar]
- 32.Lewis GR. Comparison of lisinopril versus placebo for congestive heart failure. Am J Cardiol. 1989;63:12D–16D. doi: 10.1016/0002-9149(89)90411-6. [DOI] [PubMed] [Google Scholar]
- 33.Shettigar U, Hare T, Gelperin K, Ilgenfritz JP, Deitchman D, Blumenthal M. Effects of fosinopril on exercise tolerance, symptoms, and clinical outcomes in patients with decompensated heart failure. Congest Heart Fail. 1999;5:27–34. [PubMed] [Google Scholar]
- 34.van Veldhuisen DJ, Genth-Zotz S, Brouwer J, Boomsma F, Netzer T, Man In ‘T Veld AJ, Pinto YM, Lie KI, Crijns HJ. High- versus low-dose ACE inhibition in chronic heart failure: a double-blind, placebo-controlled study of imidapril. J Am Coll Cardiol. 1998;32:1811–1818. doi: 10.1016/s0735-1097(98)00464-1. [DOI] [PubMed] [Google Scholar]
- 35.Granger CB, Ertl G, Kuch J, Maggioni AP, McMurray J, Rouleau JL, Stevenson LW, Swedberg K, Young J, Yusuf S, Califf RM, Bart BA, Held P, Michelson EL, Sellers MA, Ohlin G, Sparapani R, Pfeffer MA. Randomized trial of candesartan cilexetil in the treatment of patients with congestive heart failure and a history of intolerance to angiotensin-converting enzyme inhibitors. Am Heart J. 2000;139:609–617. doi: 10.1016/s0002-8703(00)90037-1. [DOI] [PubMed] [Google Scholar]
- 36.Riegger GA, Bouzo H, Petr P, Münz J, Spacek R, Pethig H, von Behren V, George M, Arens H. Improvement in exercise tolerance and symptoms of congestive heart failure during treatment with candesartan cilexetil. Symptom, Tolerability, Response to Exercise Trial of Candesartan Cilexetil in Heart Failure (STRETCH) Investigators. Circulation. 1999;100:2224–2230. doi: 10.1161/01.cir.100.22.2224. [DOI] [PubMed] [Google Scholar]
- 37.Mitrovic V, Willenbrock R, Miric M, Seferovic P, Spinar J, Dabrowski M, Kiowski W, Marks DS, Alegria E, Dukát A, Lenz K, Arens HA. Acute and 3-month treatment effects of candesartan cilexetil on hemodynamics, neurohormones, and clinical symptoms in patients with congestive heart failure. Am Heart J. 2003;145:E14. doi: 10.1067/mhj.2003.161. doi: 10.1067/mhj.2003.161. [DOI] [PubMed] [Google Scholar]
- 38.Pitt B, Segal R, Martinez FA, Meurers G, Cowley AJ, Thomas I, Deedwania PC, Ney DE, Snavely DB, Chang PI. Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE) Lancet. 1997;349:747–752. doi: 10.1016/s0140-6736(97)01187-2. [DOI] [PubMed] [Google Scholar]
- 39.Pitt B, Poole-Wilson PA, Segal R, Martinez FA, Dickstein K, Camm AJ, Konstam MA, Riegger G, Klinger GH, Neaton J, Sharma D, Thiyagarajan B. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial–the Losartan Heart Failure Survival Study ELITE II. Lancet. 2000;355:1582–1587. doi: 10.1016/s0140-6736(00)02213-3. [DOI] [PubMed] [Google Scholar]
- 40.Dunselman PH Replacement of Angiotensin Converting Enzyme Inhibition (REPLACE) Investigators. Effects of the replacement of the angiotensin converting enzyme inhibitor enalapril by the angiotensin II receptor blocker telmisartan in patients with congestive heart failure. The Replacement of Angiotensin Converting Enzyme Inhibition (REPLACE) investigators. Int J Cardiol. 2001;77:131–138; discussion 139. doi: 10.1016/s0167-5273(00)00426-5. [DOI] [PubMed] [Google Scholar]
- 41.Dickstein K, Chang P, Willenheimer R, Haunsø S, Remes J, Hall C, Kjekshus J. Comparison of the effects of losartan and enalapril on clinical status and exercise performance in patients with moderate or severe chronic heart failure. J Am Coll Cardiol. 1995;26:438–445. doi: 10.1016/0735-1097(95)80020-h. [DOI] [PubMed] [Google Scholar]
- 42.Lang RM, Elkayam U, Yellen LG, Krauss D, McKelvie RS, Vaughan DE, Ney DE, Makris L, Chang PI. Comparative effects of losartan and enalapril on exercise capacity and clinical status in patients with heart failure. The Losartan Pilot Exercise Study Investigators. J Am Coll Cardiol. 1997;30:983–991. doi: 10.1016/s0735-1097(97)00253-2. [DOI] [PubMed] [Google Scholar]
- 43.Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772–776. doi: 10.1016/S0140-6736(03)14284-5. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- 44.Willenheimer R, Helmers C, Pantev E, Rydberg E, Löfdahl P, Gordon A Heart Failure Valsartan Exercise Capacity Evaluation Study Group. Safety and efficacy of valsartan versus enalapril in heart failure patients. Int J Cardiol. 2002;85:261–270. doi: 10.1016/s0167-5273(02)00154-7. [DOI] [PubMed] [Google Scholar]
- 45.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 46.Dobre D, van Veldhuisen DJ, Goulder MA, Krum H, Willenheimer R. Clinical effects of initial 6 months monotherapy with bisoprolol versus enalapril in the treatment of patients with mild to moderate chronic heart failure. Data from the CIBIS III trial. Cardiovasc Drugs Ther. 2008;22:399–405. doi: 10.1007/s10557-008-6116-9. doi: 10.1007/s10557-008-6116-9. [DOI] [PubMed] [Google Scholar]
- 47.Komajda M, Lutiger B, Madeira H, Thygesen K, Bobbio M, Hildebrandt P, Jaarsma W, Riegger G, Rydén L, Scherhag A, Soler-Soler J, Remme WJ CARMEN Investigators and Co-Ordinators. Tolerability of carvedilol and ACE-Inhibition in mild heart failure. Results of CARMEN (Carvedilol ACE-Inhibitor Remodelling Mild CHF Evaluation). Eur J Heart Fail. 2004;6:467–475. doi: 10.1016/j.ejheart.2003.12.019. doi: 10.1016/j.ejheart.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 48.Cohn JN, Tognoni G Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 49.Hamroff G, Katz SD, Mancini D, Blaufarb I, Bijou R, Patel R, Jondeau G, Olivari MT, Thomas S, Le Jemtel TH. Addition of angiotensin II receptor blockade to maximal angiotensin-converting enzyme inhibition improves exercise capacity in patients with severe congestive heart failure. Circulation. 1999;99:990–992. doi: 10.1161/01.cir.99.8.990. [DOI] [PubMed] [Google Scholar]
- 50.Eichhorn EJ. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–1667. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 51.Lachat P. A randomized trial of beta-blockade in heart failure. The Cardiac Insufficiency Bisoprolol Study (CIBIS). CIBIS Investigators and Committees. Circulation. 1994;90:1765–1773. doi: 10.1161/01.cir.90.4.1765. [DOI] [PubMed] [Google Scholar]
- 52.Lechat P. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 53.Witchitz S, Cohen-Solal A, Dartois N, Weisslinger N, Juste K, Darmon JY. Treatment of heart failure with celiprolol, a cardioselective beta blocker with beta-2 agonist vasodilatory properties. The CELICARD Group. Am J Cardiol. 2000;85:1467–1471. doi: 10.1016/s0002-9149(00)00796-7. [DOI] [PubMed] [Google Scholar]
- 54.Krum H, Roecker EB, Mohacsi P, Rouleau JL, Tendera M, Coats AJ, Katus HA, Fowler MB, Packer M Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effects of initiating carvedilol in patients with severe chronic heart failure: results from the COPERNICUS Study. JAMA. 2003;289:712–718. doi: 10.1001/jama.289.6.712. [DOI] [PubMed] [Google Scholar]
- 55.Edes I, Gasior Z, Wita K. Effects of nebivolol on left ventricular function in elderly patients with chronic heart failure: results of the ENECA study. Eur J Heart Fail. 2005;7:631–639. doi: 10.1016/j.ejheart.2004.10.015. doi: 10.1016/j.ejheart.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 56.Hjalmarson A. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 57.Goldstein S, Kennedy HL, Hall C, Anderson JL, Gheorghiade M, Gottlieb S, Jessup M, Karlsberg RP, Friday G, Haskell L. Metoprolol CR/XL in patients with heart failure: a pilot study examining the tolerability, safety, and effect on left ventricular ejection fraction. Am Heart J. 1999;138(6 pt 1):1158–1165. doi: 10.1016/s0002-8703(99)70083-9. [DOI] [PubMed] [Google Scholar]
- 58.Genth-Zotz S, Zotz RJ, Sigmund M, Hanrath P, Hartmann D, Böhm M, Waagstein F, Treese N, Meyer J, Darius H. MIC trial: metoprolol in patients with mild to moderate heart failure: effects on ventricular function and cardiopulmonary exercise testing. Eur J Heart Fail. 2000;2:175–181. doi: 10.1016/s1388-9842(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 59.Bristow MR, Gilbert EM, Abraham WT, Adams KF, Fowler MB, Hershberger RE, Kubo SH, Narahara KA, Ingersoll H, Krueger S, Young S, Shusterman N. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation. 1996;94:2807–2816. doi: 10.1161/01.cir.94.11.2807. [DOI] [PubMed] [Google Scholar]
- 60.Packer M, Colucci WS, Sackner-Bernstein JD, Liang CS, Goldscher DA, Freeman I, Kukin ML, Kinhal V, Udelson JE, Klapholz M, Gottlieb SS, Pearle D, Cody RJ, Gregory JJ, Kantrowitz NE, LeJemtel TH, Young ST, Lukas MA, Shusterman NH. Double-blind, placebo-controlled study of the effects of carvedilol in patients with moderate to severe heart failure. The PRECISE Trial. Prospective Randomized Evaluation of Carvedilol on Symptoms and Exercise. Circulation. 1996;94:2793–2799. doi: 10.1161/01.cir.94.11.2793. [DOI] [PubMed] [Google Scholar]
- 61.Cohen Solal A, Jondeau G, Beauvais F, Berdeaux A. Beneficial effects of carvedilol on angiotensin-converting enzyme activity and renin plasma levels in patients with chronic heart failure. Eur J Heart Fail. 2004;6:463–466. doi: 10.1016/j.ejheart.2003.12.007. doi: 10.1016/j.ejheart.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 62.Cohn JN, Fowler MB, Bristow MR, Colucci WS, Gilbert EM, Kinhal V, Krueger SK, Lejemtel T, Narahara KA, Packer M, Young ST, Holcslaw TL, Lukas MA. Safety and efficacy of carvedilol in severe heart failure. The U.S. Carvedilol Heart Failure Study Group. J Card Fail. 1997;3:173–179. doi: 10.1016/s1071-9164(97)90013-0. [DOI] [PubMed] [Google Scholar]
- 63.Colucci WS, Packer M, Bristow MR, Gilbert EM, Cohn JN, Fowler MB, Krueger SK, Hershberger R, Uretsky BF, Bowers JA, Sackner-Bernstein JD, Young ST, Holcslaw TL, Lukas MA. Carvedilol inhibits clinical progression in patients with mild symptoms of heart failure. US Carvedilol Heart Failure Study Group. Circulation. 1996;94:2800–2806. doi: 10.1161/01.cir.94.11.2800. [DOI] [PubMed] [Google Scholar]
- 64.de Milliano PA, de Groot AC, Tijssen JG, van Eck-Smit BL, Van Zwieten PA, Lie KI. Beneficial effects of metoprolol on myocardial sympathetic function: Evidence from a randomized, placebo-controlled study in patients with congestive heart failure. Am Heart J. 2002;144:E3. doi: 10.1067/mhj.2002.121807. [DOI] [PubMed] [Google Scholar]
- 65.Dubach P, Myers J, Bonetti P, Schertler T, Froelicher V, Wagner D, Scheidegger M, Stuber M, Luchinger R, Schwitter J, Hess O. Effects of bisoprolol fumarate on left ventricular size, function, and exercise capacity in patients with heart failure: analysis with magnetic resonance myocardial tagging. Am Heart J. 2002;143:676–683. doi: 10.1067/mhj.2002.121269. [DOI] [PubMed] [Google Scholar]
- 66.Krum H, Sackner-Bernstein JD, Goldsmith RL, Kukin ML, Schwartz B, Penn J, Medina N, Yushak M, Horn E, Katz SD. Double-blind, placebo-controlled study of the long-term efficacy of carvedilol in patients with severe chronic heart failure. Circulation. 1995;92:1499–1506. doi: 10.1161/01.cir.92.6.1499. [DOI] [PubMed] [Google Scholar]
- 67.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 68.Palazzuoli A, Quatrini I, Vecchiato L, Calabria P, Gennari L, Martini G, Nuti R. Left ventricular diastolic function improvement by carvedilol therapy in advanced heart failure. J Cardiovasc Pharmacol. 2005;45:563–568. doi: 10.1097/01.fjc.0000159880.12067.34. [DOI] [PubMed] [Google Scholar]
- 69.Palazzuoli A, Quatrini I, Vecchiato L, Scali C, De Paola V, Iovine F, Martini G, Nuti R. Effects of carvedilol on left ventricular diastolic function and chamber volumes in advanced heart failure. Minerva Cardioangiol. 2005;53:321–328. [PubMed] [Google Scholar]
- 70.Sturm B, Pacher R, Strametz-Juranek J, Berger R, Frey B, Stanek B. Effect of beta 1 blockade with atenolol on progression of heart failure in patients pretreated with high-dose enalapril. Eur J Heart Fail. 2000;2:407–412. doi: 10.1016/s1388-9842(00)00120-3. [DOI] [PubMed] [Google Scholar]
- 71.McKelvie RS, Rouleau JL, White M, Afzal R, Young JB, Maggioni AP, Held P, Yusuf S. Comparative impact of enalapril, candesartan or metoprolol alone or in combination on ventricular remodelling in patients with congestive heart failure. Eur Heart J. 2003;24:1727–1734. doi: 10.1016/s0195-668x(03)00477-9. [DOI] [PubMed] [Google Scholar]
- 72.White M. Effects of metoprolol CR in patients with ischemic and dilated cardiomyopathy: the randomized evaluation of strategies for left ventricular dysfunction pilot study. Circulation. 2000;101:378–384. doi: 10.1161/01.cir.101.4.378. [DOI] [PubMed] [Google Scholar]
- 73.McMurray JJ, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, Olofsson B, Yusuf S, Pfeffer MA CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767–771. doi: 10.1016/S0140-6736(03)14283-3. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- 74.Boccanelli A, Mureddu GF, Cacciatore G, Clemenza F, Di Lenarda A, Gavazzi A, Porcu M, Latini R, Lucci D, Maggioni AP, Masson S, Vanasia M, de Simone G AREA IN-CHF Investigators. Anti-remodelling effect of canrenone in patients with mild chronic heart failure (AREA IN-CHF study): final results. Eur J Heart Fail. 2009;11:68–76. doi: 10.1093/eurjhf/hfn015. doi: 10.1093/eurjhf/hfn015. [DOI] [PubMed] [Google Scholar]
- 75.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [Google Scholar]
- 76.Cicoira M, Zanolla L, Rossi A, Golia G, Franceschini L, Brighetti G, Marino P, Zardini P. Long-term, dose-dependent effects of spironolactone on left ventricular function and exercise tolerance in patients with chronic heart failure. J Am Coll Cardiol. 2002;40:304–310. doi: 10.1016/s0735-1097(02)01965-4. [DOI] [PubMed] [Google Scholar]
- 77.Vizzardi E, Nodari S, Caretta G, D’Aloia A, Pezzali N, Faden G, Lombardi C, Raddino R, Metra M, Dei Cas L. Effects of spironolactone on long-term mortality and morbidity in patients with heart failure and mild or no symptoms. Am J Med. Sci. 2014;347:271–276. doi: 10.1097/MAJ.0b013e31829dd6b1. doi: 10.1097/MAJ.0b013e31829dd6b1. [DOI] [PubMed] [Google Scholar]
- 78.Burnett H, Cope S, Vieira MC, Sagkriotis A, Senni M, Deschaseaux C. The importance of treatment classifications that account for concomitant treatments in the context of a network meta-analysis comparing pharmacological treatments for chronic heart failure. Value Health. 2014;17:A327. doi: 10.1016/j.jval.2014.08.595. doi: 10.1016/j.jval.2014.08.595. [DOI] [PubMed] [Google Scholar]
- 79.Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure. A Bayesian meta-analysis. Ann Intern Med. 2001;134:550–560. doi: 10.7326/0003-4819-134-7-200104030-00008. [DOI] [PubMed] [Google Scholar]
- 80.Chatterjee S, Biondi-Zoccai G, Abbate A, D’Ascenzo F, Castagno D, Van Tassell B, Mukherjee D, Lichstein E. Benefits of β blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ. 2013;346:f55. doi: 10.1136/bmj.f55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu LJ, Chen YQ, Deng SB, Du JL, She Q. Additional use of an aldosterone antagonist in patients with mild to moderate chronic heart failure: a systematic review and meta-analysis. Br J Clin Pharmacol. 2013;75:1202–1212. doi: 10.1111/bcp.12012. doi: 10.1111/bcp.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jong P, Demers C, McKelvie RS, Liu PP. Angiotensin receptor blockers in heart failure: meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2002;39:463–470. doi: 10.1016/s0735-1097(01)01775-2. [DOI] [PubMed] [Google Scholar]
- 83.Kuenzli A, Bucher HC, Anand I, Arutiunov G, Kum LC, McKelvie R, Afzal R, White M, Nordmann AJ. Meta-analysis of combined therapy with angiotensin receptor antagonists versus ACE inhibitors alone in patients with heart failure. PLoS One. 2010;5:e9946. doi: 10.1371/journal.pone.0009946. doi: 10.1371/journal.pone.0009946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Solomon SD, Claggett B, McMurray JJ, Hernandez AF, Fonarow GC. Combined neprilysin and renin-angiotensin system inhibition in heart failure with reduced ejection fraction: a meta-analysis. Eur J Heart Fail. 2016;18:1238–1243. doi: 10.1002/ejhf.603. doi: 10.1002/ejhf.603. [DOI] [PubMed] [Google Scholar]
- 85.Xie W, Zheng F, Song X, Zhong B, Yan L. Renin-angiotensin-aldosterone system blockers for heart failure with reduced ejection fraction or left ventricular dysfunction: network meta-analysis. Int J Cardiol. 2016;205:65–71. doi: 10.1016/j.ijcard.2015.12.010. doi: 10.1016/j.ijcard.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 86.McMurray J, Packer M, Desai A, Gong J, Greenlaw N, Lefkowitz M, Rizkala A, Shi V, Rouleau J, Solomon S, Swedberg K, Zile MR, Andersen K, Arango JL, Arnold M, Bělohlávek J, Böhm M, Boytsov S, Burgess L, Cabrera W, Chen CH, Erglis A, Fu M, Gomez E, Gonzalez A, Hagege AA, Katova T, Kiatchoosakun S, Kim KS, Bayram E, Martinez F, Merkely B, Mendoza I, Mosterd A, Negrusz-Kawecka M, Peuhkurinen K, Ramires F, Refsgaard J, Senni M, Sibulo AS, Jr, Silva-Cardoso J, Squire I, Starling RC, Vinereanu D, Teerlink JR, Wong R PARADIGM-HF Committees and Investigators. A putative placebo analysis of the effects of LCZ696 on clinical outcomes in heart failure. Eur Heart J. 2015;36:434–439. doi: 10.1093/eurheartj/ehu455. doi: 10.1093/eurheartj/ehu455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jansen JP. Network meta-analysis of survival data with fractional polynomials. BMC Med Res Methodol. 2011;11:61. doi: 10.1186/1471-2288-11-61. doi: 10.1186/1471-2288-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]


