Supplemental Digital Content is available in the text
Keywords: antidepressants, endometrial cancer, Taiwan national insurance
Abstract
To our knowledge, the association between antidepressant exposure and endometrial cancer has not been previously explored. Herein, we aim to investigate the association between antidepressant prescription, including novel antidepressants, and the risk for endometrial cancer in a population-based study.
Data for the analysis were derived from National Health Insurance Research Database. We identified 8392 cases with a diagnosis of endometrial cancer and 82,432 matched controls. A conditional logistic regression model was used, with adjusting for potentially confounding variables (e.g., comorbid psychiatric diseases, comorbid physical diseases, and other medications). Risk for endometrial cancer in the population-based study sample was categorized by, and assessed as a function of, antidepressant prescription and cumulative dosage.
We report no association between endometrial cancer incidence and antidepressant prescription, including those prescribed either selective serotonin reuptake inhibitors (adjusted odds ratio [OR] = 0.98; 95% confidence interval [CI], 0.84–1.15) or serotonin norepinephrine reuptake inhibitors (adjusted OR = 1.14; 95% CI, 0.76–1.71). We also did not identify an association between higher cumulative doses of antidepressant prescription and endometrial cancer.
There was no association between antidepressant prescription and endometrial cancer.
1. Introduction
Antidepressants are the most commonly prescribed pharmacological agents for the treatment of mood disorders and are frequently prescribed off-label for the treatment of associated symptoms (e.g., insomnia). In addition, it has been suggested that adjunctive antidepressants with hormone replacement therapy are effective in the treatment of peri- and postmenopausal depressive women.[1,2] However, some studies have previously reported that antidepressant use is associated with increased risk for cancer, with reproductive system and gastrointestinal cancers being the most studied malignancies.[3,4]
A recently published meta-analysis identified a modest increase in the risk for breast and ovarian cancer with the use of antidepressants. The pooled odds ratio (OR) representing the association between antidepressant use and breast/ovarian cancer was 1.11 (95% confidence interval [CI], 1.03–1.20).[5] Parsing mechanistic pathways potentially linking antidepressants to breast/ovarian cancer needs to consider separate lines of evidence suggesting that selective serotonin reuptake inhibitors (SSRIs) have an inhibitory effect on tumor growth.[6,7] Moreover, other studies have reported no association between risk for ovarian cancer and the use of antidepressants.[8] Several studies have reported no conclusive evidence of breast cancer risk associated with the use of SSRIs after adjusting for the degree of serotonin reuptake inhibition and duration of use.[9,10]
Endometrial cancer is one of the leading causes of death amongst female patients with cancer worldwide. The age-standardized population incidence of endometrial cancer is 1.6 per 100,000.[11] Few studies have investigated the effects of antidepressant use on endometrial cancer. For example, Kato et al[12] reported that the use of antidepressants increases the risk for hormone-related cancers (i.e., breast, endometrial, and ovarian cancers) (relative risk [RR] = 1.8; 95% CI, 1.15–2.81). Fortuny et al[13] reported no significant effect of SSRIs use (i.e., paroxetine and fluoxetine) on risk for endometrial cancer in a population-based case–control study in the United States. To our knowledge, no prior study has investigated the effect of novel antidepressants, such as serotonin-norepinephrine reuptake inhibitors (SNRIs) on endometrial cancer.
Herein, we assessed the association between antidepressant prescription, including SSRIs and novel classes of agents, and incidence of endometrial cancer diagnosis in a nationwide population-based registry database in Taiwan.
2. Methods
2.1. Data source
The National Health Insurance (NHI) program was launched on March 1, 1995 by Taiwan's government. Approximately 99.5% of the Taiwanese population was enrolled in the NHI program in December 2008.[14] The National Health Insurance Research Database (NHIRD), derived from the original claims data of the NHI program, includes ambulatory care, hospital inpatient care, and prescription claims data between January 1, 1997 and December 31, 2008. The study was approved by the Institution Review Board of Cheng-Shan Medical University.
Patients with cancer, including endometrial cancer, are eligible to register with the Catastrophic Illness Registry and apply for a catastrophic illness certificate in Taiwan. The diagnosis must be confirmed by tissue pathology. The issuance of the certificate requires a diagnosis of catastrophic illness by physicians and a formal review by the Bureau of National Health Insurance, conducted by a panel of related medical experts.
The International Classification of Diseases, Ninth Revision, Clinical Modification, was used, and endometrial cancer was coded as 182.xx. A diagnosis of cancer was confirmed with the Catastrophic Illness Registry Dataset. The index date was operationalized as the date of the first endometrial cancer claim. The units of analysis were incident diagnosis of endometrial cancer between January 1, 1997 and December 31, 2008 and antidepressant prescription within 365 days before the index date.
For each endometrial cancer case, we used an incidence density sampling method[15] and randomly selected 10 matched controls at index date. The control population of 1 million individuals was randomly selected from the NHI dataset. To be included in the control population, an individual was required to have been without a current or history of cancer on the index date. Controls were individually age-matched to the case by birth year (Fig. 1).
Figure 1.
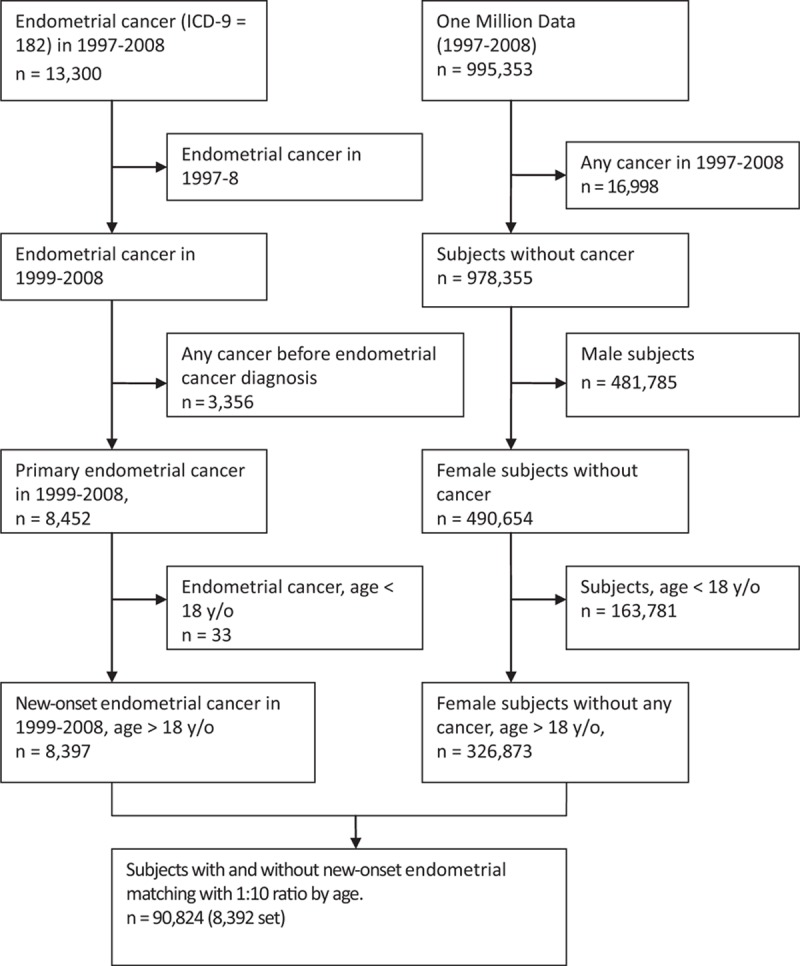
Flowchart of selection subjects.
2.2. Exposure assessment
We identified antidepressants (N06A) according to the Anatomical Therapeutic Chemical classification system (table S1, Supplemental content). Antidepressants were categorized as SSRIs (i.e., citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline), SNRIs (i.e., duloxetine and venlafaxine), tricyclic antidepressants (TCA; i.e., amitriptyline, clomipramine, dosulepin, doxepin, imipramine, maprotiline, and melitracen), monoamine oxidase inhibitors (MAOIs; i.e., moclobemide and rasagiline), noradrenergic and specific serotonergic antidepressants (i.e., mirtazapine), serotonin antagonist and reuptake inhibitor (i.e., trazodone), and norepinephrine-dopamine reuptake inhibitor (i.e., bupropion). Prescription data to proxy exposure to an antidepressant were obtained from the NHIRD. Antidepressant prescription data after the index date were excluded from analysis.
The defined daily dose (DDD), as defined by the World Health Organization, was used to assess the amount of antidepressant.[16] Cumulative dose values were stratified into 4 categories: ≧28 DDD, ≧84 DDD, ≧168 DDD, and ≧336 DDD.
We adjusted for the use of pharmacological agents with potentially confounding effects (e.g., estrogen, estrogen/progesterone, aspirin, nonsteroid anti-inflammatory drugs [NSAIDs], and statins) prescribed before the index date. The presence of comorbid medical conditions (e.g., depressive disorder, anxiety disorder, type 2 diabetes mellitus [type 2 DM], hypertension, hypercholesterolemia, and obesity) was also assessed.
2.3. Statistical analyses
We used the SAS version 9.2 software packages (SAS Institute, Cary, NC) to carry out conditional logistic regression models to investigate the association between 7 classes of antidepressant exposure and endometrial cancer risk. In each class of antidepressants, the crude OR and the adjusted OR were stratified into 4 cumulative dosages (≧28 DDD, ≧84 DDD, ≧168 DDD, and ≧336 DDD).
Corrected ORs are calculated after getting adjusted for demographic data and confounding variables including depressive disorders, anxiety disorder, type 2 DM, hypertension, hypercholesterolemia and obesity, and confounding drugs. The statistical significance of associations was assessed by P value <0.05 or a 95% CI.
3. Results
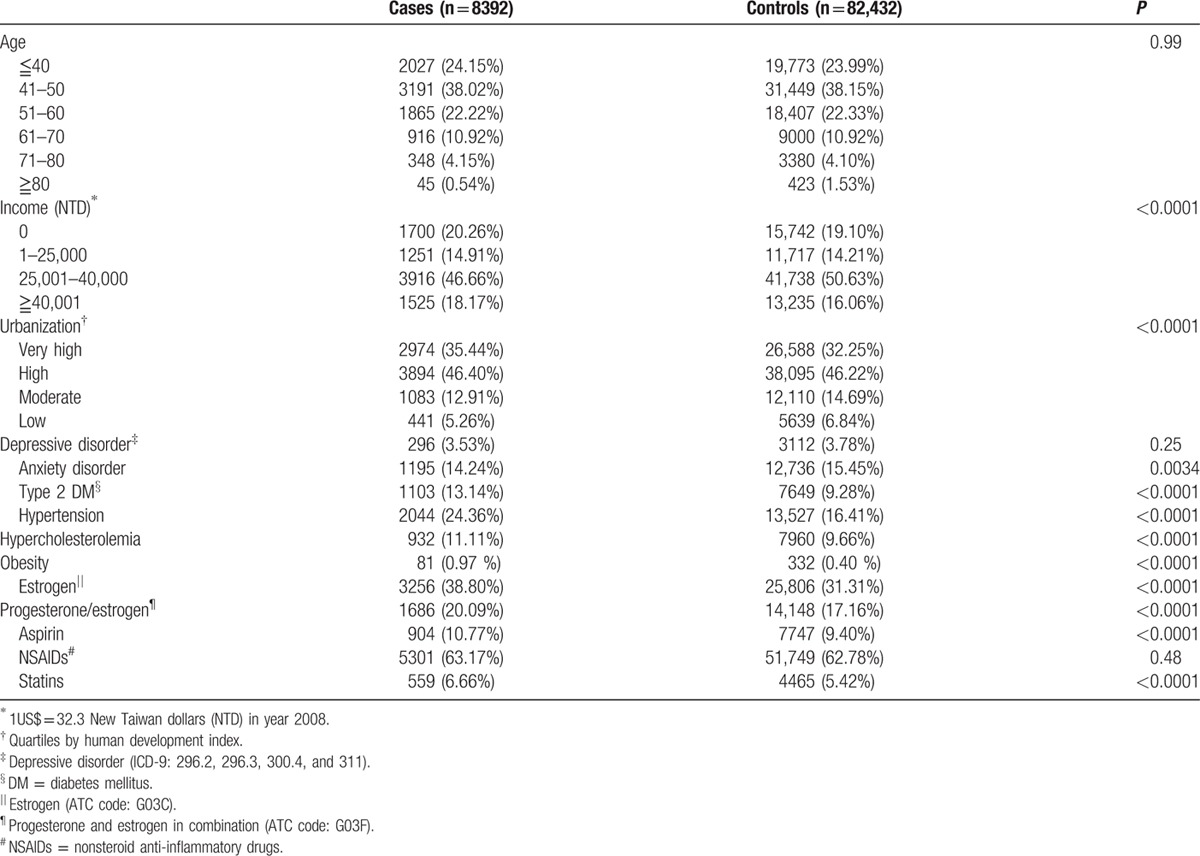
We identified 8392 cases with a diagnosis of endometrial cancer and 82,432 age-matched controls. Sociodemographic data of the study population are reported (e.g., age, income, and urbanization) in Table 1. Comorbid medical disorders, physical diseases, and exposure to potentially confounding concomitantly administered drugs are also reported in Table 1. The differences in levels of income and urbanization between cancer cases and controls were statistically significant (P < 0.001). Individuals with endometrial cancer were significantly more likely to have comorbid type 2 DM, hypertension, hypercholesterolemia, and obesity (P < 0.0001). Compared with controls, the case population was more likely to be prescribed estrogen, progesterone/estrogen, aspirin, and statins (P < 0.0001).
Table 1.
Demographic data, medical diseases, and drugs used of cases and controls.
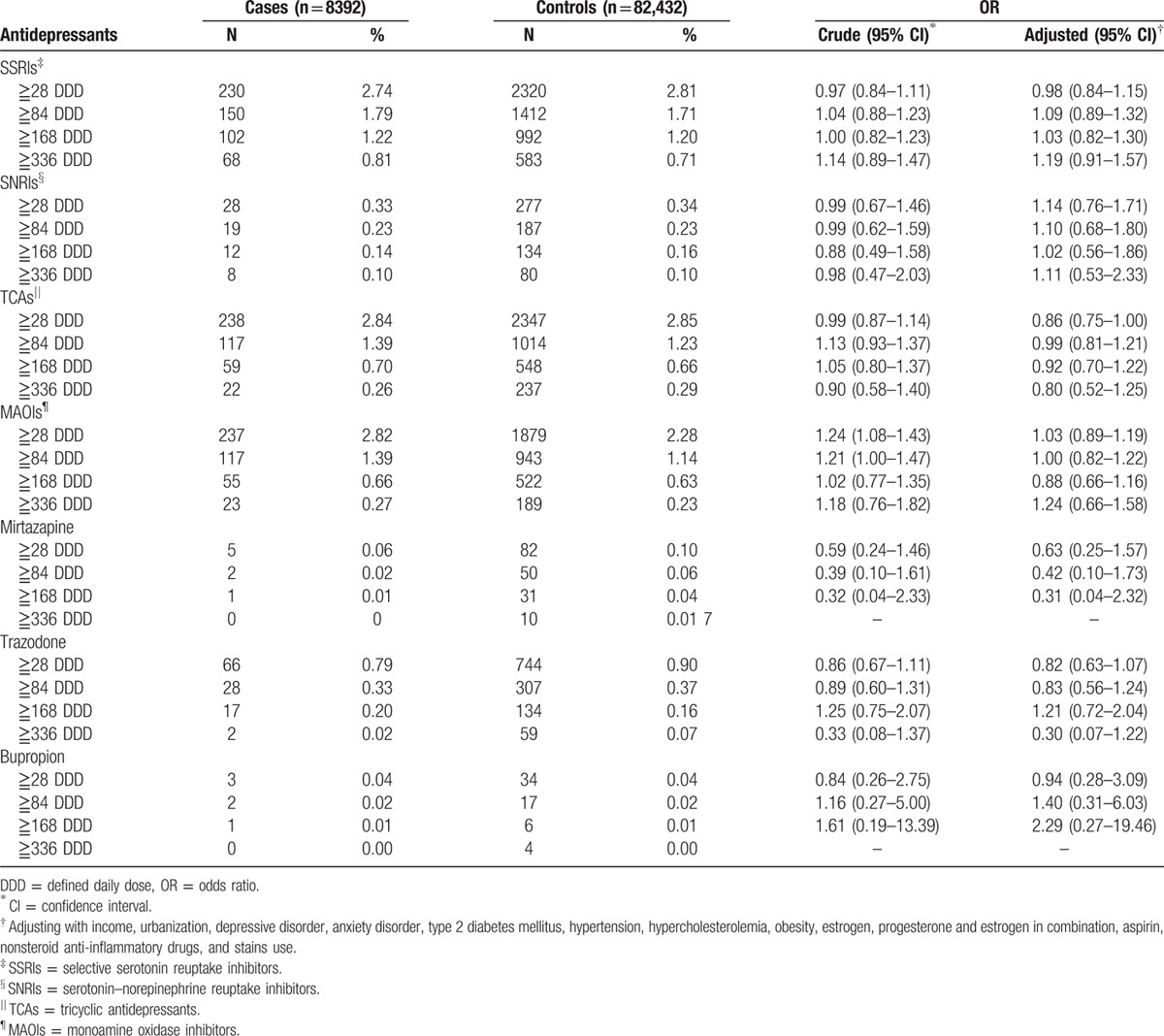
The main findings are presented in Table 2. Among individuals prescribed antidepressants, a higher percentage of individuals were prescribed SSRIs, TCAs, and MAOIs, in comparison with trazodone. A much lower percentage had been prescribed SNRIs, mirtazapine and bupropion at least 1 year before the index date. The adjusted OR of cumulative SSRI exposure ≧28 DDD was 0.98 (95% CI, 0.84–1.15); SNRI exposure ≧28 DDD was 1.14 (95% CI, 0.76–1.71). No differences in antidepressant exposure of any cumulative dose were observed between the case and control populations (the adjusted ORs of cumulative SSRI exposure ≧336 DDD were 1.19 [95% CI, 0.91–1.57]; SNRI exposure ≧336 DDD was 1.11 [95% CI, 0.53–2.33]). The exposure time effect was similar to the dosage effect (table S2, Supplemental content).
Table 2.
Associations of antidepressants use and endometrial cancer risk.
4. Discussion
To the best of our knowledge, this is the first population-based study to explore the association between mechanistically dissimilar antidepressants, including novel antidepressants like SNRIs, and endometrial cancer. The results of this study indicate that there is no association between antidepressant exposure and incidence of endometrial cancer. Our results remained unchanged after adjusting for confounding factors such as comorbid psychiatric diseases, comorbid physical diseases, use of estrogen, progesterone/estrogen, aspirin, NSAIDs, and statins.
The primary result of our study is consistent with the findings reported by Fortuny et al[13] who reported no association between SSRI use (i.e., paroxetine and fluoxetine) and the risk of endometrial cancer (OR = 0.9; 95% CI, 0.6–1.4). Fortuny et al included 469 endometrial cancer cases from The Estrogen, Diet, Genetics, and Endometrial Cancer Study, a population-based case–control study conducted in Northern New Jersey, United States. It was not reported however if any relationship existed between SSRI total exposure dosage and cancer risk.[13]
Kato et al reported that the use of antidepressants was associated with greater risk for self-reported hormone-related cancers (RR = 1.8; 95% CI, 1.15–2.81). They included 672 incident cases of hormone-related cancer, of which only 20 cases had been prescribed antidepressants at least 4 weeks before enrollment. Within this foregoing sample, 16 developed breast cancer, and 4 developed either ovarian or endometrial cancer. The number of cases with endometrial and/or ovarian cancer was not sufficient for separate analysis.[12]
Several epidemiological studies have investigated the association between antidepressant use and the risk for female hormone-related cancer. Reeves et al[17] conducted a prospective cohort study and concluded that there might be an elevated risk with SSRI use (OR = 1.16; 95% CI, 0.96–1.39). However, several other studies have reported a null association between antidepressant use and the risk for breast cancer.[18,19] Harlow et al[20,21] reported that pharmacological agents that operate through dopaminergic mechanism may increase relative risk for ovarian cancer (OR = 2.9; 95% CI, 1.3–6.4). A recent publication by Wu et al[22] reported that there was no association between the risk for ovarian cancer and use of antidepressants. Studies investigating the association between antidepressant and hormone-related cancers have been inconsistent.
Amerio et al reviewed the US Food and Drug Administration (FDA) preclinical in vivo evidence to compare the carcinogenic risk between drug classes, with a focus on psychotropic drugs. Among antidepressants, 63.6% (7/11) of examined agents were associated with carcinogenicity. Specific agents associated with carcinogenicity were mirtazapine, sertraline, paroxetine, citalopram and escitalopram, duloxetine, and bupropion.[23] Tricyclics can induce hepatic microsomal enzymes capable of enhancing estrogen metabolism, which may lead to increased gonadotropin levels. Endogenous and exogenous estrogens have known to be associated with the risk of endometrial cancer.[24] In our study, we did not identify a moderational effect of pharmacological estrogen on the association between antidepressant and endometrial cancer.
There are several strengths of the study herein. First, the data were driven from national representative population, which reduces selection bias. Second, information on medication use was obtained from prescription claims data, consequently reducing recall bias. Third, confounding factors such as income, and urbanization, medications (such as estrogen, estrogen/progesterone, aspirin, NSAIDs, and statins) prescribed before the index date, and medical conditions (including depressive disorder, anxiety disorder, type 2 DM, hypertension, hypercholesterolemia, and obesity), were adjusted for in the analysis. Finally, we explored the association between all classes of antidepressants, and the cumulative dose effect of antidepressants on the risk for endometrial cancer, which provides further merit to our conclusion that a nonassociation exists.
Notwithstanding the strengths in our study, there were several methodological limitations. Data on smoking and lifestyle were not reported. A meta-analysis identified that cigarette smoking was significantly associated with a reduced risk of endometrial cancer, especially among postmenopausal women.[25] Furberg conducted a prospective study and demonstrated that inactivity and high-energy intake are major risk factors for endometrial cancer.[26] Further study to discover the relationship among antidepressants, lifestyle, and the risk of endometrial cancer is needed.
Antidepressants are widely used in treating symptoms such as depressed mood, anxiety, and pain. Female patients are often prescribed antidepressants for protracted periods of time. The clinical recommendation for multiple-year exposure to antidepressants, in some cases, invites the need for comprehensive characterization of safety concerns related to their exposure. The findings have important implications for clinicians to discuss with female patients about the safety of antidepressants use and endometrial cancer.
5. Conclusion
The results showed that the use of TCA, SSRIs, and novel antidepressants are not associated with the increase risk of endometrial cancer.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, DDD = defined daily dose, DM = diabetes mellitus, MAOIs = monoamine oxidase inhibitors, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, NSAID = nonsteroid anti-inflammatory drugs, OR = odds ratio, RR = relative risk, SNRIs = serotonin norepinephrine reuptake inhibitors, SSRIs = selective serotonin reuptake inhibitors, TCAs = tricyclic antidepressants.
WCC and CFL have contributed equally to the article.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Graziottin A, Serafini A. Depression and the menopause: why antidepressants are not enough? Menopause Int 2009; 15:76–81. [DOI] [PubMed] [Google Scholar]
- 2.Pae CU, Mandelli L, Kim TS, et al. Effectiveness of antidepressant treatments in pre-menopausal versus post-menopausal women: a pilot study on differential effects of sex hormones on antidepressant effects. Biomed Pharmacother 2009; 63:228–235. [DOI] [PubMed] [Google Scholar]
- 3.Bahl S, Cotterchio M, Kreiger N. Use of antidepressant medications and the possible association with breast cancer risk. A review. Psychother Psychosom 2003; 72:185–194. [DOI] [PubMed] [Google Scholar]
- 4.Dalton SO, Johansen C, Mellemkjaer L, et al. Antidepressant medications and risk for cancer. Epidemiology 2000; 11:171–176. [DOI] [PubMed] [Google Scholar]
- 5.Cosgrove L, Shi L, Creasey DE, et al. Antidepressants and breast and ovarian cancer risk: a review of the literature and researchers’ financial associations with industry. PLoS One 2011; 6:e18210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taler M, Gil-Ad I, Lomnitski L, et al. Immunomodulatory effect of selective serotonin reuptake inhibitors (SSRIs) on human T lymphocyte function and gene expression. Eur Neuropsychopharmacol 2007; 17:774–780. [DOI] [PubMed] [Google Scholar]
- 7.Abdul M, Logothetis CJ, Hoosein NM. Growth-inhibitory effects of serotonin uptake inhibitors on human prostate carcinoma cell lines. J Urol 1995; 154:247–250. [PubMed] [Google Scholar]
- 8.Wu CS, Lu ML, Liao YT, et al. Ovarian cancer and antidepressants. Psychooncology 2015; 24:579–584. [DOI] [PubMed] [Google Scholar]
- 9.Ashbury JE, Levesque LE, Beck PA, et al. Selective serotonin reuptake inhibitor (SSRI) antidepressants, prolactin and breast cancer. Front Oncol 2012; 2:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steingart AB, Cotterchio M. Do antidepressants cause, promote, or inhibit cancers? J Clin Epidemiol 1995; 48:1407–1412. [DOI] [PubMed] [Google Scholar]
- 11.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 12.Kato I, Zeleniuch-Jacquotte A, Toniolo PG, et al. Psychotropic medication use and risk of hormone-related cancers: the New York University Women's Health Study. J Public Health Med 2000; 22:155–160. [DOI] [PubMed] [Google Scholar]
- 13.Fortuny J, Sima C, Bayuga S, et al. Risk of endometrial cancer in relation to medical conditions and medication use. Cancer Epidemiol Biomarkers Prev 2009; 18:1448–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welfare MoHa. Overview of National Health Insurance. Taipei, Taiwan: Welfare MoHa; 2013. [Google Scholar]
- 15.Wacholder S, Silverman DT, McLaughlin JK, et al. Selection of controls in case–control studies. III. Design options. Am J Epidemiol 1992; 135:1042–1050. [DOI] [PubMed] [Google Scholar]
- 16.WHO. Collaborating Centre for Drug Statistics Methodology. ATC classification index with DDDs, 2013. (Available from: http://www.whocc.no/atc_ddd_publications/atc_ddd_index/.) [Accessed July 10, 2014]. [Google Scholar]
- 17.Reeves KW, Okereke O, Qian J, et al. Depression and antidepressant use in relation to breast cancer risk in the Nurses Health Study. Cancer Epidemiol Biomarkers Prev 2015; 24:761–762. [Google Scholar]
- 18.Chen VC, Liao YT, Yeh DC, et al. Relationship between antidepressant prescription and breast cancer: a population based study in Taiwan. Psychooncology 2015. [DOI] [PubMed] [Google Scholar]
- 19.Eom CS, Park SM, Cho KH. Use of antidepressants and the risk of breast cancer: a meta-analysis. Breast Cancer Res Treat 2012; 136:635–645. [DOI] [PubMed] [Google Scholar]
- 20.Harlow BL, Cramer DW. Self-reported use of antidepressants or benzodiazepine tranquilizers and risk of epithelial ovarian-cancer – evidence from 2 combined case–control studies (Massachusetts, United States). Cancer Cause Control 1995; 6:130–134. [DOI] [PubMed] [Google Scholar]
- 21.Harlow BL, Cramer DW, Baron JA, et al. Psychotropic medication use and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev 1998; 7:697–702. [PubMed] [Google Scholar]
- 22.Wu CS, Lu ML, Liao YT, et al. Ovarian cancer and antidepressants. Psychooncology 2015; 24:579–584. [DOI] [PubMed] [Google Scholar]
- 23.Amerio A, Galvez JF, Odone A, et al. Carcinogenicity of psychotropic drugs: a systematic review of US Food and Drug Administration-required preclinical in vivo studies. Aust N Z J Psychiatry 2015; 49:686–696. [DOI] [PubMed] [Google Scholar]
- 24.Chubak J, Doherty JA, Cushing-Haugen KL, et al. Endometrial cancer risk in estrogen users after switching to estrogen-progestin therapy. Cancer Cause Control 2007; 18:1001–1007. [DOI] [PubMed] [Google Scholar]
- 25.Zhou B, Yang L, Sun Q, et al. Cigarette smoking and the risk of endometrial cancer: a meta-analysis. Am J Med 2008; 121:501–508.e3. [DOI] [PubMed] [Google Scholar]
- 26.Furberg AS, Thune I. Metabolic abnormalities (hypertension, hyperglycemia and overweight), lifestyle (high energy intake and physical inactivity) and endometrial cancer risk in a Norwegian cohort. Int J Cancer 2003; 104:669–676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


