Abstract
We explored the influence of body mass index (BMI) on long-term outcomes in patients with esophageal squamous cell carcinoma (ESCC) who underwent esophagectomy as a primary treatment. BMI is a risk factor for development of esophageal cancer. However, the details of the relationship between BMI and cancer prognosis remains unclear. Patients who underwent esophagectomy as an initial treatment in 2000 to 2009 period were included. The patients were divided into 3 groups according to Asian-specific BMI cut-offs. The associations between BMI and long-term outcomes were explored. This study included 1082 ESCC patients between 2000 and 2009; all the patients underwent esophagectomy. The median overall survival (OS) of the BMI <18.5, 18.5 ≤ BMI <23, and BMI ≥23 kg/m2 groups were 21, 24, and 29.5 months, respectively; they differed significantly (P = 0.005). The 5-year survival rates of the 3 groups were 24.6%, 30.4%, and 35.3%, respectively. Multivariate analysis showed that lower BMI was an independent risk factor for a shorter OS (18.5 ≤ BMI <23 kg/m2 vs. BMI ≥23 kg/m2, hazard ratio [HR] = 1.18; 95% confidence interval [CI] = 1.00–1.40, P = 0.054, BMI <18.5 kg/m2 vs. BMI ≥23 kg/m2, HR = 1.38; 95% CI = 1.09–1.75, P = 0.007). The better OS of the BMI ≥23 kg/m2 patients remained statistically significant in never-smoking patients (P < 0.05). In conclusion, patients with BMIs ≥23 kg/m2 experienced better OS, and multivariate analysis further indicated that BMI ≥23 kg/m2 was an independent predictor of survival. When stratified by smoking status, BMI ≥23 kg/m2 was still a factor in better OS among never smokers.
Keywords: body mass index, esophageal squamous cell carcinoma, esophagectomy, overall survival
1. Introduction
Esophageal cancer (EC) is the eighth most common cancer and the sixth leading cause of cancer-related death worldwide; the incidence thereof differs significantly on a regional basis. China is a high-risk country, with more than half of all worldwide diagnosed cases.[1] Squamous cell carcinoma (SCC) and adenocarcinoma (AC) are the 2 most common histopathological cancer categories worldwide.[2] Esophageal squamous cell carcinoma (ESCC) is less common in the United States and Western Europe, but predominates in China.[3,4] Radical esophagectomy is the primary curative approach. However, even with improvements in detection, surgical techniques, preoperative support, chemotherapy, and radiotherapy, the prognosis remains poor.[5] Accurate prognostic predictors are urgently needed.
Body mass index (BMI) is an inexpensive and convenient indicator, and it is associated with EC. A high BMI is a risk factor for development of gastroesophageal reflux disease (GERD) and esophageal adenocarcinoma (EAC), whereas a low BMI is a risk factor for development of ESCC.[6,7] After adjustment for smoking, the risk of ESCC was reduced by 35% (range: 23%–44%) as the BMI increased by 5 kg/m2.[8] However, the relationship between BMI and prognosis remains unclear. Some studies have found that BMI did not influence the survival of EC patients.[9–11] Others have suggested that a high BMI is an independent prognostic factor for EC.[12,13] However, it is possible that high BMI patients are diagnosed at a less-severe stage than others.[14]
Most of these studies have been performed in the West, where EAC is more common, as are patients with higher BMIs. In most previous Asian studies, samples were small and ESCC was not the sole pathological type. Moreover, the studied patients often underwent preoperative chemotherapy and/or radiotherapy, which can affect appetite and BMI. Thus, we explored the relationship between BMI and overall survival (OS) in a large cohort of Asian ESCC patients.
2. Methods
2.1. Patients
We excluded patients who had received neoadjuvant chemotherapy and/or radiotherapy, who had histories of malignancy, EC patients who did not have ESCC, and cases of R1/R2 resection. In total, 1082 ESCC patients who underwent radical esophagectomies as primary treatments between 2000 and 2009 were included. On admission, sex, birth date, smoking history, drinking history, comorbidities (hypertension, diabetes, arrhythmia, coronary artery disease, pulmonary disease, digestive system disease), height, and weight were recorded, and the BMI was calculated as weight (kilograms) divided by the square of height (meter). The BMI of Asians is relatively lower than that of Westerners; in our study, only 9.0% of patients had BMIs ≥25 kg/m2, which is much lower than the figures from Western studies.[15,16] Thus, we used Asian-specific BMI cut-offs and merged the obese and overweight groups, as in a previous Asian study[12]: underweight (<18.5 kg/m2), normal weight (18.5–22.9 kg/m2), overweight and obese (≥23.0 kg/m2). Information on postoperative pathologies, overall hospital stays, and postoperative hospital stays was collected from medical records. TNM staging used the AJCC Cancer Staging Manual, 7th edition.[17] This study was approved by the Ethics Committee of Zhejiang Cancer Hospital.
2.2. Surgery
All patients underwent preoperative evaluations including an electronic gastroscopy, biopsies, a computed tomography (CT) scan, an endoscopic ultrasonography, an esophageal barium meal, an electrocardiogram, and pulmonary function testing. Only patients who could tolerate surgery underwent radical EC resections. The surgical procedures were chosen by an experienced medical group. Left transthoracic esophagogastrectomy, Ivor Lewis esophagogastrectomy, or McKeown esophagogastrectomy was selected, depending on tumor location and lesion length. A 2- or 3-field lymphadenectomy was performed. To ensure that the tumor margins were negative, each incision was made over 5 cm distant from the tumor. The gastric pull-up technique was used to reconstruct the digestive tract.
2.3. Follow-up
All patients were followed every 3 months in the first year, every 6 months in the second and third years, and annually thereafter. At each follow-up, a medical history was taken; the patient underwent a physical examination, a blood test was performed, and an endoscopy and a CT scan were scheduled if indicated. The endpoint was death from any cause. The last follow-up date was January 15, 2015. Follow-up time is defined as that elapsing from the day of surgery to death or the last follow-up visit. The median follow-up time was 90 months.
2.4. Statistical analysis
All statistical analyses were performed with the SPSS software (ver. 22.0 for the Mac; SPSS Inc, Chicago, IL). GraphPad Prism (ver. 6.0c) was used to draw graphs. Lengths of lesions among 3 groups were compared via 1-way analysis of variance. Categorical variables were compared with the χ2 test or Fisher exact test. The numbers of lymph nodes harvested, the days of overall hospital stay, and the days of postoperative hospital stay were compared using the independent samples Kruskal–Wallis test. Survival curves were drawn using the Kaplan–Meier method and compared among groups with the log-rank test. Univariate and multivariate Cox proportional hazards models were used to identify prognostic factors; a forward stepwise likelihood ratio test was employed to eliminate covariates with P values >0.10. The P values <0.05 were considered to indicate statistical significance. All P values were 2-tailed.
3. Results
3.1. Patient characteristics
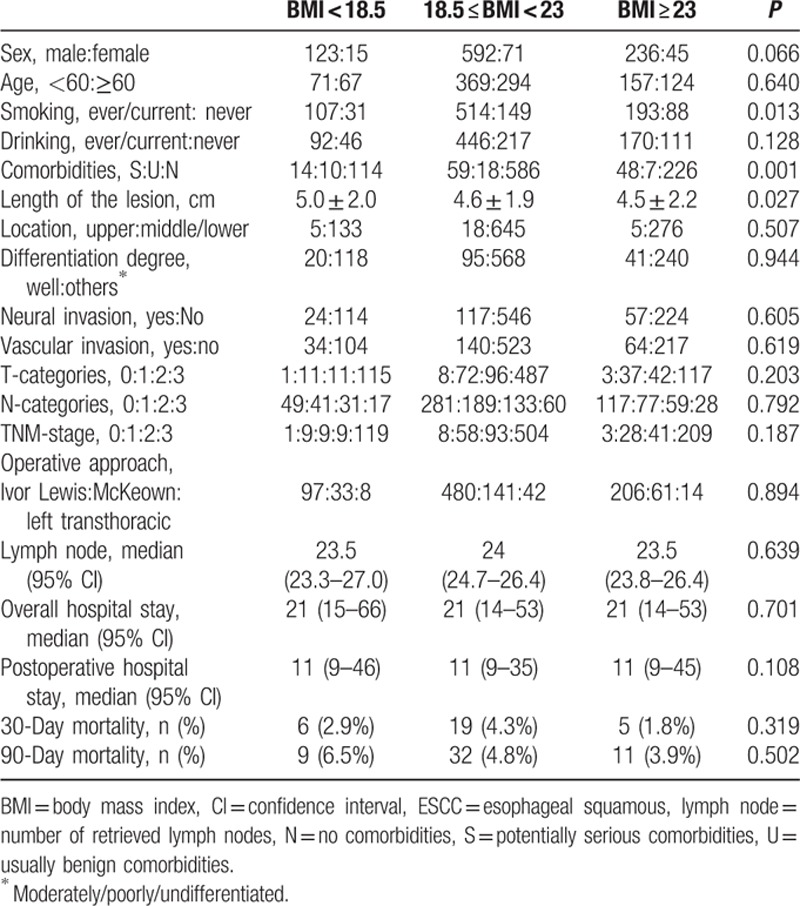
We included 1082 patients in this retrospective study, and their baseline clinicopathological characteristics are summarized in Table 1. Those with BMI ≥23 kg/m2 were more likely to have never smoked (P = 0.013), to have potentially serious comorbidities (P = 0.001), and to have shorter lesions (P = 0.027). None of sex, age, drinking history, tumor location, the extent of tumor differentiation, neural invasion status, vascular invasion status, T-stage, N-stage, TNM-stage, overall or postoperative hospital stay, 30- or 90-day mortality, or lymph node harvest showed a significant difference among the groups (all P > 0.05).
Table 1.
Baseline clinicopathological characteristics of 1082 ESCC patients.
3.2. Long-term outcomes
The median OS of the BMI <18.5 kg/m2 group, 18.5 ≤ BMI <23 kg/m2 group, and BMI ≥23 kg/m2 groups were 21, 24, and 29.5 months, respectively; these differed significantly (P = 0.005; Fig. 1). The 5-year survival rates of the 3 groups were 24.6%, 30.4%, and 35.3%, respectively.
Figure 1.
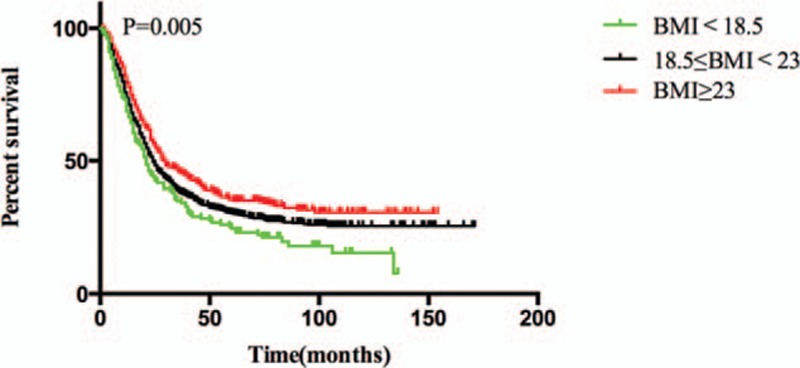
Overall survival among BMI <18.5 kg/m2, 18.5 ≤ BMI <23 kg/m2 and BMI ≥23 kg/m2 of all patients.
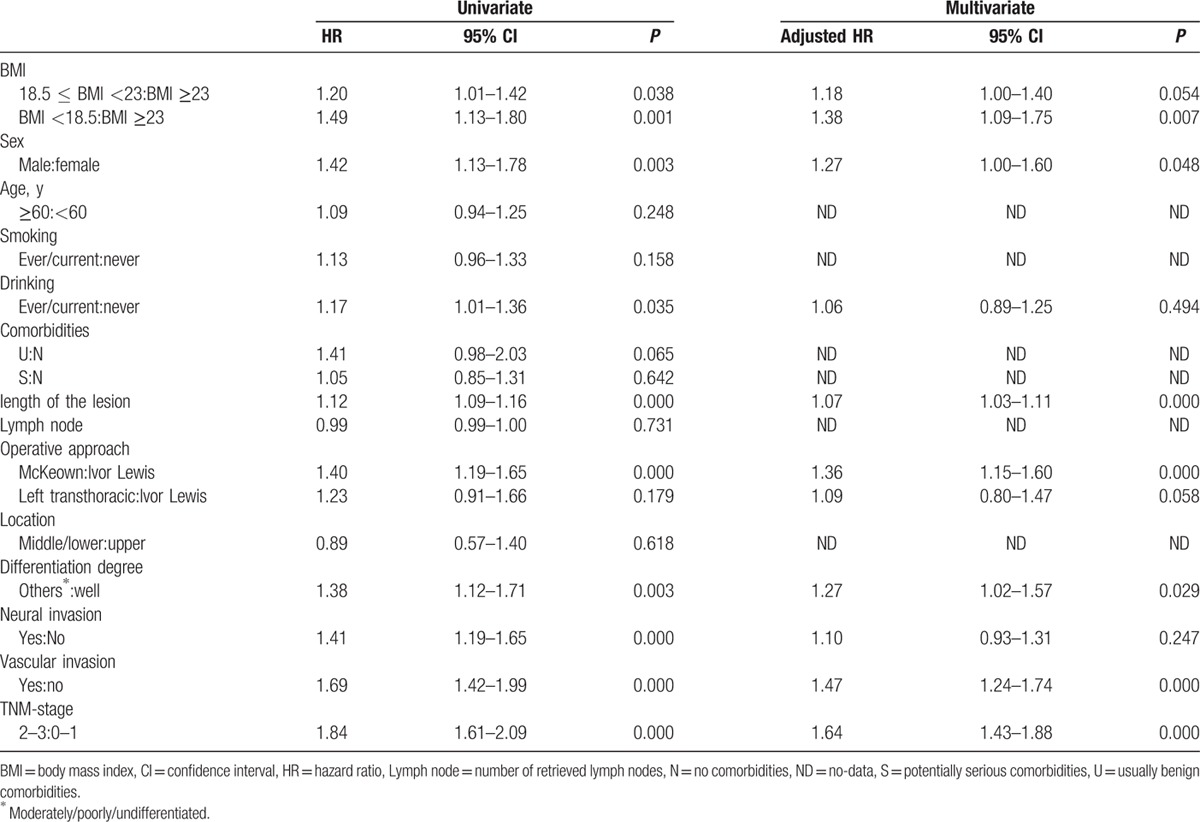
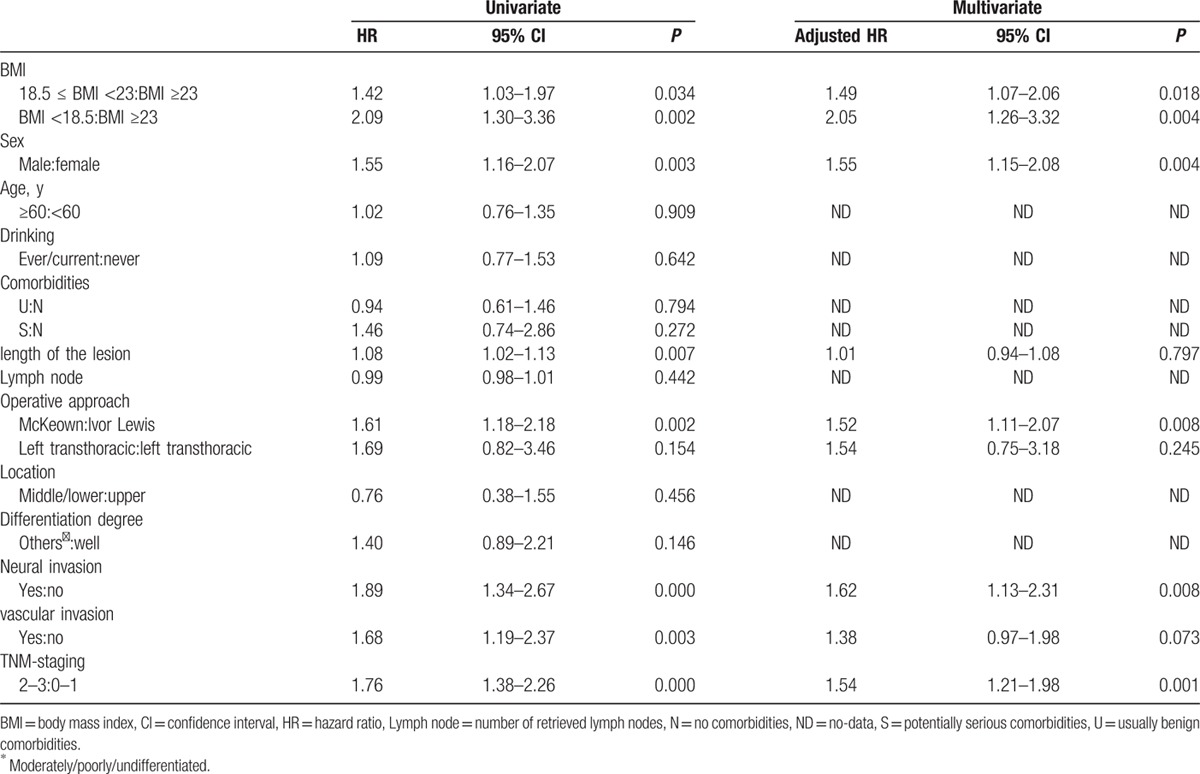
The results of the univariate and multivariate analyses are shown in Table 2. Upon univariate analysis, a lower BMI, male sex, ever/current drinking, longer lesion, operative approach, moderately/poorly/undifferentiated differentiation, neural invasion, vascular invasion, and an advanced TNM stage were risk factors for poor OS. Multivariate analysis confirmed that a lower BMI (using BMI ≥23 kg/m2 as the reference) was an independent risk factor for poor OS. This was true of the 18.5 ≤ BMI <23 kg/m2 group (hazard ratio [HR] = 1.18; 95% confidence interval [CI] = 1.00–1.40, P = 0.054) and the BMI <18.5 kg/m2 group (HR = 1.38; 95% CI = 1.09–1.75, P = 0.007). Also, male sex, a longer lesion, operative approach, vascular invasion, moderately/poorly/undifferentiated differentiation, and an advanced TNM stage were all independent risk factors for poor OS.
Table 2.
Univariate and multivariate analyses of factors related to overall survival in all patients.
3.3. Further analysis
In the present study, the proportion of smokers in the BMI ≥23 kg/m2 group was very low. Cigarette smoking has been shown to influence the effect of BMI on mortality in several types of cancer.[18] Thus, we re-analyzed the data after stratifying it by smoking status. Among ever or current smokers, the OS of the 3 BMI groups showed no difference (P = 0.132). In contrast, among never smokers, the median OS of the BMI <18.5, 18.5 ≤ BMI <23 and BMI ≥23 kg/m2 groups were 16, 26, and 45.5 months, respectively; these differed significantly (P = 0.016). The 5-year survival rates for the 3 groups were 20.7%, 31.3%, and 39.8%, respectively (Fig. 2). On univariate analysis, a lower BMI, male sex, a longer lesion, operative approach, neural invasion, vascular invasion, and an advanced TNM stage were risk factors for poor OS. Multivariate analysis confirmed that a lower BMI (using BMI ≥23 kg/m2 as the reference) was an independent risk factor for poor OS. This was true of the 18.5 ≤ BMI <23 kg/m2 (HR = 1.49; 95% CI = 1.07–2.06, P = 0.018) and the BMI <18.5 kg/m2 (HR = 2.05; 95% CI = 1.26–3.32, P = 0.004) groups. Male sex, operative approach, neural invasion, and an advanced TNM stage were also independent risk factors for poor OS (Table 3).
Figure 2.
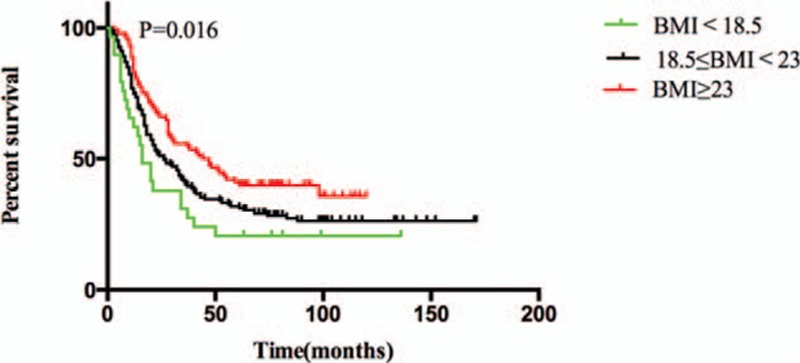
Overall survival among BMI <18.5 kg/m2, 18.5 ≤ BMI <23 kg/m2 and BMI ≥23 kg/m2 of never smoked patients.
Table 3.
Univariate and multivariate analyses of factors related overall survival (never smoked).
4. Discussion
Our study indicated that patients with a BMI ≥23 kg/m2 experienced better OS, and that this parameter was an independent predictor of survival. When stratified by smoking status, BMI ≥23 kg/m2 remained a factor in better OS among never smokers. Most previous studies have been performed in developed counties, where EAC is the major histopathological type of EC and more patients were overweight or obese. The BMI cutoff values were inconsistent and much higher than Asian-specific values. Two recent large-scale studies have been performed[12,19] using Asian-specific cut-offs, but ESCC was not the sole histopathological type. Indeed, to date, few studies have explored how BMI might influence the long-term survival of ESCC patients. In the present large-scale study, we divided patients into 3 BMI groups using Asian-specific cutoffs, and all patients had histopathologically confirmed ESCC.
The impact of BMI on survival of EC patients was inconsistent in previous studies. In our study, patients with higher BMI experienced better OS, and this parameter was an independent factor. One meta-analysis found that obese patients enjoyed significantly better long-term survival than did nonobese patients[20]; moreover, a recent large-scale cohort study using the Asian-specific BMI cut-offs found that a higher BMI significantly improved OS, and this remained significant in a subgroup analysis.[12] However, other studies have shown that high BMI had no significant effect, or even an adverse impact, on survival. Ren et al[21] argued that BMI was not an independent prognostic factor, and that patients with high BMI had longer disease-specific survival (DSS) than did normal and underweight patients among weight loss groups. Cheng et al argued that a high BMI appeared to reduce disease-free survival; however, fewer lymph nodes were removed in the high BMI group than in the other groups, which may explain the poor survival in the former group. Additionally, that study was not large-scale and the BMI cutoff values were not routine.[22] In the present large-scale study, the extent of lymph node harvesting did not differ among the 3 groups and we used the standardized Asian-specific BMI cut-offs. One recent study found that the superior OS of high-BMI patients might be attributable to the fact that such patients had less-severe pathological stages.[19] However, the TNM stage distributions among our 3 groups did not differ. Thus, the results of earlier studies may be attributable to the use of different BMI cut-offs and variations in cancer histopathology. Cigarette smoking has been shown to influence the effect of BMI on mortality in several types of cancer.[18,23] We stratified the data by smoking status to assess the true prognostic impact of BMI, and found that never smokers seemed to more accurately represent this impact. The association of higher BMI and longer OS was observed in never smokers; this result suggests that smoking is responsible for the survival difference.
It remains unclear why high-BMI patients enjoy better OS, although some hypotheses have been suggested, including that the resection margins of high-BMI patients may more often be tumor-free after esophagectomy. Also, nutritional deficiency may be associated with poor survival.[24] Finally, in China, higher-BMI patients tend to be wealthier and thus better able to afford medical treatment at the time of recurrence. Molecular biological mechanisms should also be examined in the further studies.
4.1. Strengths and limitations
Strengths of our study include the large number of patients, the unique histopathology, the use of Asian-specific BMI cutoff values, smoking status stratification to more accurately estimate the prognostic impact of BMI, the performance of radical R0 resection esophagectomy in all patients, and the exclusion of those who received neoadjuvant therapy. Limitations include the retrospective nature of the study, a lack of information on whether BMI changed after therapy, and the absence of data on postoperative adjuvant therapy and nutritional state. Further studies designed to address such limitations are required.
In conclusion, patients with a BMI ≥23 kg/m2 experienced better OS, and multivariate analysis further indicated that a BMI ≥23 kg/m2 was an independent predictor of survival. When stratified by smoking status, BMI ≥23 kg/m2 remained a factor in better OS among never smokers. Large cohort studies using consistent cut-offs are needed, and the mechanisms whereby BMI influences survival require exploration.
Acknowledgments
The authors appreciate the support of Department of Thoracic surgery, Zhejiang Cancer Hospital.
Footnotes
Abbreviations: AC = adenocarcinoma, BMI = Body mass index, CI = confidence interval, CT = computed tomography, DSS = disease-specific survival, EAC = esophageal adenocarcinoma, EC = esophageal cancer, ESCC = esophageal squamous cell carcinoma, GERD = gastroesophageal reflux disease, HR = hazard ratio, N = no comorbidities, ND = no-data, OS = overall survival, S = potentially serious comorbidities, SCC = squamous cell carcinoma, U = usually benign comorbidities.
The authors report no conflict of interest.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Daly JM, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg 2000; 190:562–572.discussion 572-563. [DOI] [PubMed] [Google Scholar]
- 3.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst 2008; 100:1184–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trivers KF, Sabatino SA, Stewart SL. Trends in esophageal cancer incidence by histology, United States, 1998-2003. Int J CancerV 123 2008; 1422–1428. [DOI] [PubMed] [Google Scholar]
- 5.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366:2074–2084. [DOI] [PubMed] [Google Scholar]
- 6.Smith M, Zhou M, Whitlock G, et al. Esophageal cancer and body mass index: results from a prospective study of 220,000 men in China and a meta-analysis of published studies. International journal of cancer. J Int Cancer 2008; 122:1604–1610. [DOI] [PubMed] [Google Scholar]
- 7.Pohl H, Wrobel K, Bojarski C, et al. Risk factors in the development of esophageal adenocarcinoma. Am J Gastroenterol 2013; 108:200–207. [DOI] [PubMed] [Google Scholar]
- 8.Lahmann PH, Pandeya N, Webb PM, et al. Body mass index, long-term weight change, and esophageal squamous cell carcinoma: is the inverse association modified by smoking status? Cancer 2012; 118:1901–1909. [DOI] [PubMed] [Google Scholar]
- 9.Grotenhuis BA, Wijnhoven BP, Hotte GJ, et al. Prognostic value of body mass index on short-term and long-term outcome after resection of esophageal cancer. World J Surg 2010; 34:2621–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blom RL, Lagarde SM, Klinkenbijl JH, et al. A high body mass index in esophageal cancer patients does not influence postoperative outcome or long-term survival. Ann Surg Oncol 2012; 19:766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong L, Zhang H, Zhao Q, et al. Relation of excess body weight and survival in patients with esophageal adenocarcinoma: a meta-analysis. Dis Esophagus 2013; 26:623–627. [DOI] [PubMed] [Google Scholar]
- 12.Zhang SS, Yang H, Luo KJ, et al. The impact of body mass index on complication and survival in resected oesophageal cancer: a clinical-based cohort and meta-analysis. Br J Cancer 2013; 109:2894–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe M, Ishimoto T, Baba Y, et al. Prognostic impact of body mass index in patients with squamous cell carcinoma of the esophagus. Ann Surg Oncol 2013; 20:3984–3991. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi Y, Correa AM, Hofstetter WL, et al. Patients with high body mass index tend to have lower stage of esophageal carcinoma at diagnosis. Dis Esophagus 2012; 25:614–622. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi Y, Correa AM, Hofstetter WL, et al. The influence of high body mass index on the prognosis of patients with esophageal cancer after surgery as primary therapy. Cancer 2010; 116:5619–5627. [DOI] [PubMed] [Google Scholar]
- 16.Scarpa M, Cagol M, Bettini S, et al. Overweight patients operated on for cancer of the esophagus survive longer than normal-weight patients. J Gastrointest Surg 2013; 17:218–227. [DOI] [PubMed] [Google Scholar]
- 17.Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010; 17:1721–1724. [DOI] [PubMed] [Google Scholar]
- 18.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med 2010; 363:2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miao L, Chen H, Xiang J, et al. A high body mass index in esophageal cancer patients is not associated with adverse outcomes following esophagectomy. J Cancer Res Clin Oncol 2015; 141:941–950. [DOI] [PubMed] [Google Scholar]
- 20.Kayani B, Okabayashi K, Ashrafian H, et al. Does obesity affect outcomes in patients undergoing esophagectomy for cancer? A meta-analysis. World J Surg 2012; 36:1785–1795. [DOI] [PubMed] [Google Scholar]
- 21.Ren C, Cai XY, Qiu MZ, et al. Impact of body mass index on survival of esophageal squamous carcinoma patients in southern China. J Thorac Dis 2015; 7:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng Y, Wang N, Wang K, et al. Prognostic value of body mass index for patients undergoing esophagectomy for esophageal squamous cell carcinoma. Jpn J Clin Oncol 2013; 43:146–153. [DOI] [PubMed] [Google Scholar]
- 23.Prospective Studies C, Whitlock G, Lewington S, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009; 373:1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan TM, Tang D, Stratton KL, et al. Preoperative nutritional status is an important predictor of survival in patients undergoing surgery for renal cell carcinoma. Euro Urol 2011; 59:923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]


