Abstract
The incidence of nontuberculous mycobacterial pulmonary disease (NTMPD) is increasing worldwide. Secondary spontaneous pneumothorax occurs as a complication of underlying lung disease and is associated with higher morbidity, mortality, and recurrence than primary spontaneous pneumothorax. We here investigated the clinical features and long-term outcomes of pneumothorax associated with NTMPD.
We conducted a retrospective study on consecutive adult patients with pneumothorax associated with NTMPD at Fukujuji Hospital and Keio University Hospital from January 1992 to December 2013. We reviewed the medical records of 69 such patients to obtain clinical characteristics, radiological findings, and long-term outcomes, including pneumothorax recurrence and mortality.
The median age of the patients was 68 years; 34 patients were women. The median body mass index was 16.8 kg/m2. Underlying pulmonary diseases mainly included chronic obstructive pulmonary disease and pulmonary tuberculosis. On computed tomography, nodules and bronchiectasis were observed in 46 (98%) and 45 (96%) patients, respectively. Consolidation, pleural thickening, interlobular septal thickening, and cavities were most common, and observed in 40 (85%), 40 (85%), 37 (79%), and 36 (77%) patients, respectively. Regarding pneumothorax treatment outcomes, complete and incomplete lung expansion were observed in 49 patients (71%) and 15 patients (22%), respectively. The survival rate after pneumothorax was 48% at 5 years. By the end of the follow-up, 33 patients had died, and the median survival was 4.4 years with a median follow-up period of 1.7 years. The rate of absence of recurrence after the first pneumothorax was 59% at 3 years. By the end of the follow-up, 18 patients had experienced pneumothorax recurrence. Furthermore, 12/18 patients (66%) with recurrent pneumothorax died during the study period. Twenty-three patients (70%) died because of NTMPD progression. Low body mass index (BMI) was a negative prognostic factor for pneumothorax associated with NTMPD in multivariate analysis (HR 0.79, 95% CI 0.64−0.96; P = 0.018)
Patients with pneumothorax associated with NTMPD have advanced disease, a high rate of pneumothorax recurrence, and poor prognosis, regardless of the pneumothorax treatment used. Further improvements in early diagnosis of NTMPD and appropriate management in both NTMPD and NTMPD-associated pneumothorax are needed.
Keywords: mycobacterium avium complex, nontuberculous mycobacteria, prognosis, recurrent pneumothorax, secondary spontaneous pneumothorax
1. Introduction
The incidence of nontuberculous mycobacterial (NTM) pulmonary disease (NTMPD) has been increasing worldwide.[1,2] In contrast to pulmonary tuberculosis (TB), NTMPD, most commonly caused by the Mycobacterium avium complex (MAC), generally causes chronic, indolent, or slowly progressive disease in immunocompetent patients.[3] Therapy involving multiple antimicrobials against NTMPD is not fully effective and is often limited to established NTMPD; moreover, the disease has a high recurrence rate. NTMPD not only affects health-related quality of life,[4] but is also an important cause of morbidity and mortality.[5,6]
Secondary spontaneous pneumothorax (SSP) occurs as a complication of an underlying pulmonary disease, such as chronic obstructive pulmonary disease (COPD), cystic lung diseases, malignancy, pulmonary infections, and interstitial lung diseases.[7] SSP is associated with higher morbidity, mortality, and recurrence than is primary spontaneous pneumothorax.[8,9] Therefore, SSP associated with NTMPD may also be associated with a high recurrence rate and poor prognosis. However, only a few reports have described pneumothorax associated with NTMPD.[10,11] This study aimed to evaluate the clinical features and long-term outcomes of pneumothorax associated with NTMPD and to clarify the impact of pneumothorax on NTMPD.
2. Patients and methods
2.1. Study design and patient selection
This retrospective observational study included all patients with pneumothorax associated with NTMPD treated at Fukujuji Hospital and Keio University Hospital from January 1992 to December 2013. All patients were diagnosed with NTMPD according to the 2007 American Thoracic Society/Infectious Disease Society of America diagnostic criteria.[3] None of the patients had cystic fibrosis or human immunodeficiency virus. Only the first episode of pneumothorax was included for the analysis. We identified 69 cases of pneumothorax associated with NTMPD at the two hospitals between January 1992 and December 2013. The total number of patients diagnosed with NTMPD was available only from January 2004 to December 2013, at both sites; in this period, we identified 1689 patients with NTMPD, of which 49 (2.9%) had pneumothorax associated with NTMPD. Forty-four of 1453 patients (3.0%), 3 of 74 patients (4.1%), and 2 of 162 patients (1.2%) had pneumothorax associated with infection with MAC, Mycobacterium kansasii, and other species, respectively. The institutional review boards of Fukujuji Hospital and Keio University Hospital approved this study (#15022 and #20080131).
2.2. Data extraction
Patients’ age, sex, body mass index (BMI), smoking status, Charlson comorbidity index (CCI),[12] underlying pulmonary diseases, use of immunosuppressive agents and home oxygen therapy, disease and treatment durations, NTMPD treatment status, bacterial smear and culture results, radiographic features (including computed tomography), and treatment and clinical outcomes for pneumothorax were collected. NTMPD treatment status at the onset of pneumothorax was classified as never treated, previously treated, or presently treated. NTM species was identified by the AccuProbe system (Gen-Probe Inc, San Diego, CA), the COBAS AMPLICOR system (Roche Diagnostic Co., Ltd, Tokyo, Japan), or a DNA−DNA hybridization test (Kyokuto Pharmaceutical Industrial Co., Ltd, Tokyo, Japan), as previously described.[4] For determining clarithromycin susceptibility for MAC, a broth microdilution method (BrothMIC NTM; Kyokuto Pharmaceutical Industrial Co., Ltd, Tokyo, Japan) was used.[13] A minimum inhibitory concentration ≥32 μg was defined as clarithromycin resistance.[14]
Sputum smear or culture results were defined based on results obtained 3 months before and after pneumothorax. After the onset of pneumothorax, all patients were observed until their date of death, the date of their last visit, or the end of the study (December 31, 2015). The patient's status at the end of the follow-up (deceased or alive, pneumothorax recurrence, and cause of death if applicable) was systematically recorded.
2.3. Radiological findings
Two investigators who were blinded to the clinical data evaluated chest radiography and computed tomography (CT) scans. Discrepancies were resolved through a consensus review. The pattern on chest radiography was classified into the following four forms, according to previous reports: nodular/bronchiectatic (NB), fibrocavitary (FC), NB + FC, or unclassified.[6] Pneumothorax severity was classified as mild (above the clavicle), moderate (between mild and severe), or severe (complete or nearly complete lung collapse). The extent and location of lung involvement were evaluated on CT scans.
2.4. Statistical analysis
Statistical analyses were conducted by using JMP v11.0 (SAS Institute Japan Ltd, Tokyo, Japan). Data are presented as median (interquartile range [IQR] or range), or number (%). Univariate analysis was performed by using Fisher's exact test to compare categorical variables and the Mann−Whitney test (between two groups) or the Kruskal−Wallis test (among three groups) to compare continuous variables. All P values were two-tailed; P <0.05 was considered significant.
We estimated survival rates and the rate of pneumothorax recurrence by using the Kaplan−Meier method. To identify the factors related to survival in pneumothorax associated with NTMPD, we first performed univariate Cox regression analysis. Then, multivariate Cox regression analysis was performed using variables strongly associated with (P <0.2) mortality in univariate analysis, in addition to age, sex, BMI, and CCI. CT findings were not included, as only 47 patients in the study had undergone CT.
3. Results
3.1. Patients and clinical characteristics
The patient characteristics are shown in Table 1. The median (IQR) age of the patients was 68 (61–77) years; 34 patients (49%) were women. The median (IQR) BMI was 17 (15–18) kg/m2. Thirty-seven (54%), 26 (38%), and 6 (9%) patients had never smoked, were former smokers, and were present smokers, respectively. The underlying pulmonary diseases mainly included COPD (24 patients, 35%) and history of TB (18 patients, 26%). Ten patients (14%) were first diagnosed with NTMPD because of the onset of pneumothorax; 9 patients had underlying pulmonary disease (5 patients with COPD and a history of TB, 3 patients with COPD, and 2 patients with interstitial lung disease). The median (IQR) disease and treatment duration from diagnosis to pneumothorax were 30 (2–73) and 9 (0–36) months, respectively. Twenty-three patients (33%) had never been treated, 19 (28%) had previously been treated, and 27 (39%) were being treated at the time of the first pneumothorax. Sputum smears and cultures were positive for NTMPD in 40 (58%) and 56 (81%) patients during the 3 months before and after the onset of pneumothorax, respectively. CAM resistance was investigated in 14 patients with MAC-related pulmonary disease, and 5 of the 14 patients had CAM-resistant infections.
Table 1.
Clinical characteristics of 69 nontuberculous mycobacteria patients with pneumothorax on admission.
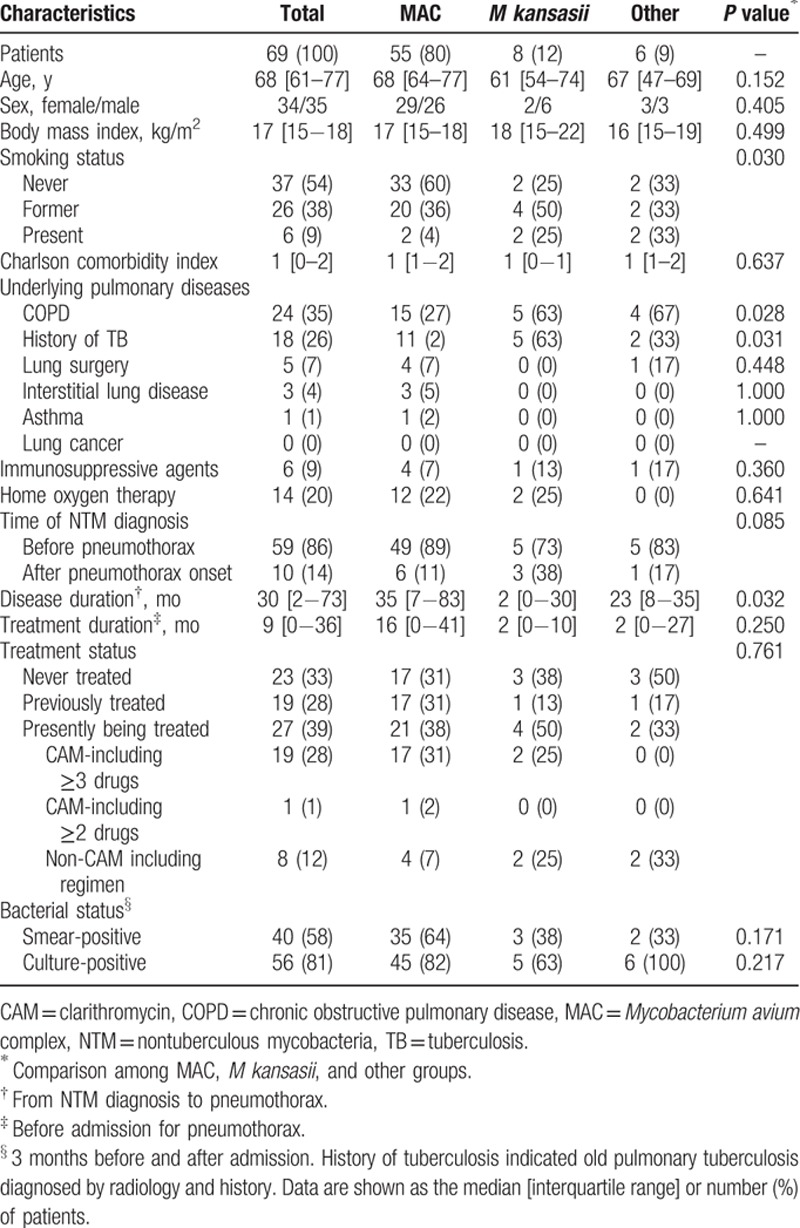
In terms of the bacterial species, MAC was the most commonly isolated bacteria and was detected in 55 patients (80%). The “other” group included 6 patients with Mycobacterium abscessus (4 patients), Mycobacterium fortuitum (1 patient), and Mycobacterium xenopi (1 patient). COPD and a history of TB were significantly more prevalent in the M kansasii and other groups. Disease duration was significantly longer in the MAC group. The remaining parameters were not significantly different among the 3 bacterial species groups.
3.2. Radiological features
The radiological features are shown in Table 2. The radiological patterns included the NB form in 25 patients (36%), the FC form in 12 patients (17%), the NB + FC form in 18 patients (26%), and the unclassified form in 14 patients (20%). The pneumothorax lesion was predominantly on the right side in 44 patients (64%); in most cases, the pneumothorax severity was moderate (42 patients, 61%). CT findings were available for only 47 patients who had undergone a CT scan within 1 year before the onset of pneumothorax. On CT, nodules and bronchiectasis were observed in 46 (98%) and 45 (96%) patients, respectively. The most common other findings were consolidation, pleural thickening, interlobular septal thickening, and cavities, which were present in 40 (85%), 40 (85%), 37 (79%), and 36 (77%) patients, respectively. Representative figures of CT findings (2-mm-thick slices) before and after the onset of pneumothorax are shown in Figs. 1–3. NTMPD lesions were located in almost all lobes. The clinical characteristics of 69 patients with pneumothorax-associated NTMPD who did or did not undergo a CT scan are shown in Table 3. The only parameter that differed between the groups was the percentage of cases that was culture-positive.
Table 2.
The radiological features of 69 patients with PNX-associated NTMPD on admission.
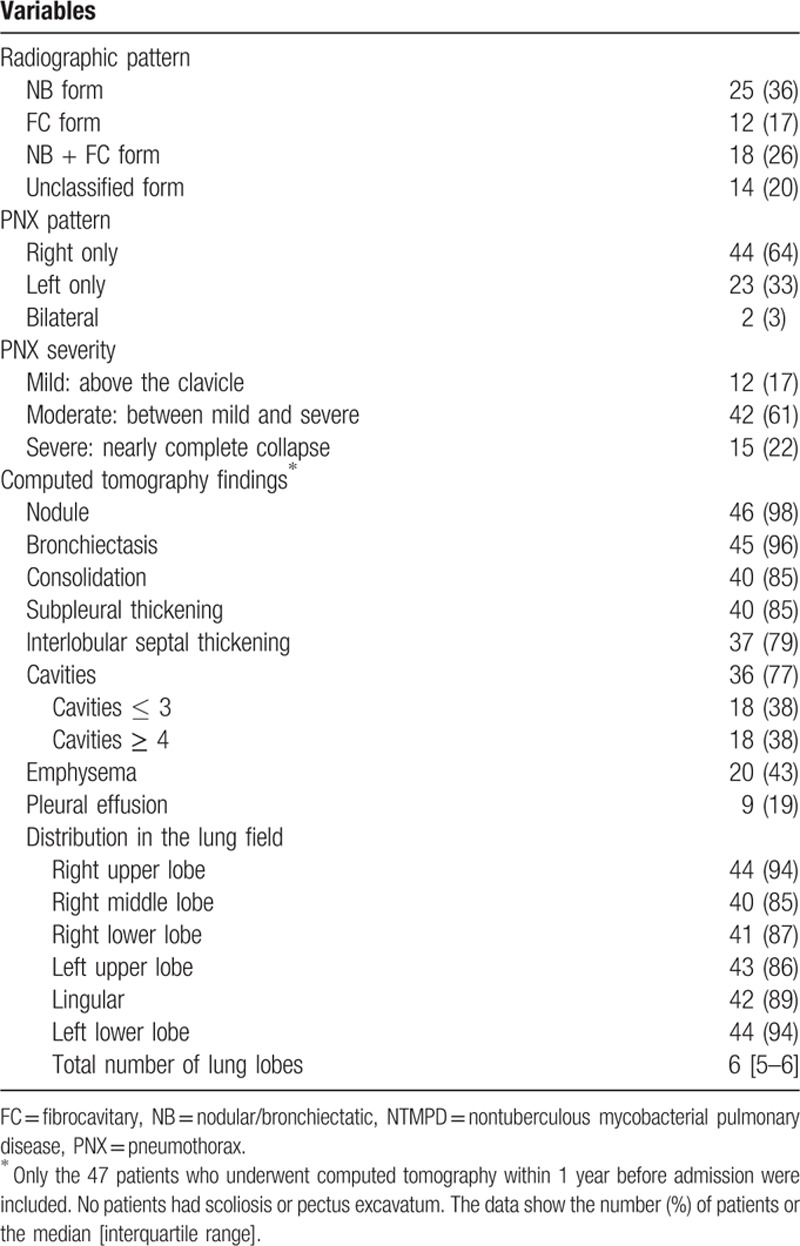
Figure 1.
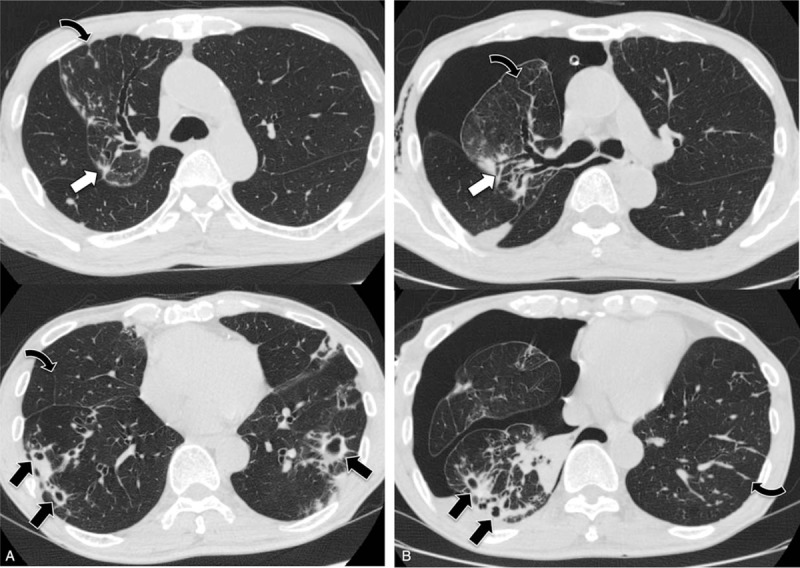
Computed tomography images obtained from a 77-year-old man with Mycobacterium avium complex pulmonary disease before (A) and after (B) the onset of pneumothorax, show nodules involving the pleura (white arrows), interlobular septal thickening (curved arrows), and cavities (black arrows).
Figure 3.
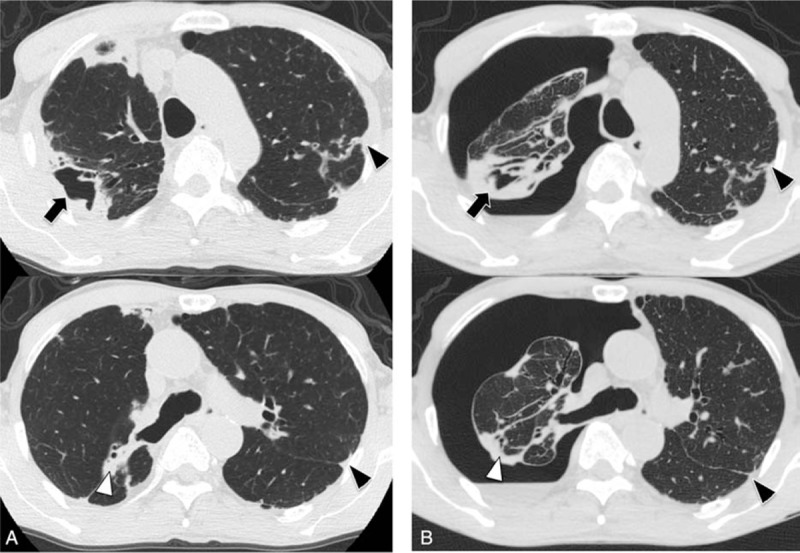
Computed tomography scans obtained from a 68-year-old man with Mycobacterium avium complex pulmonary disease before (A) and after (B) the onset of pneumothorax, show consolidation (white arrowheads), pleural thickening (black arrowheads), and cavities (black arrows).
Table 3.
Clinical characteristics of 69 patients with pneumothorax-associated nontuberculous mycobacterial pulmonary disease who did or did not undergo computed tomography imaging.
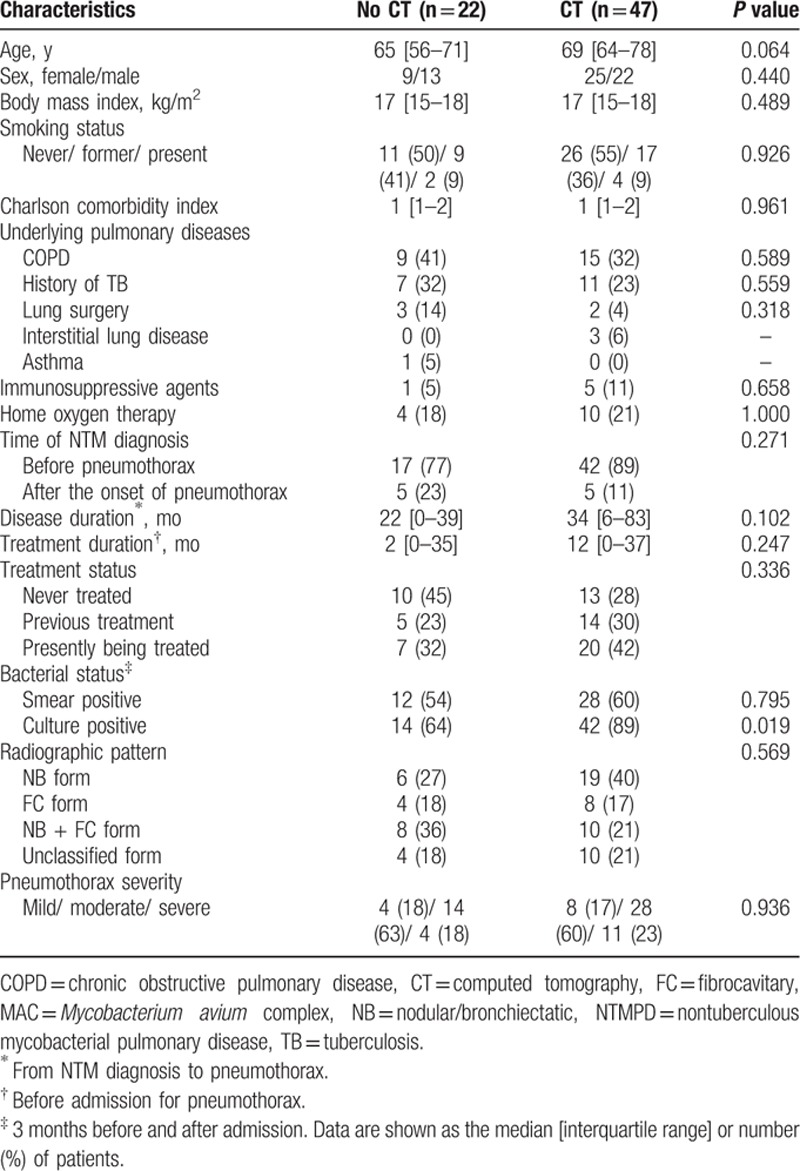
Figure 2.
Computed tomography scans obtained from a 76-year-old woman with Mycobacterium avium complex pulmonary disease before (A) and after (B) the onset of pneumothorax, show consolidation (white arrowheads), pleural thickening (black arrowheads), and cavities (black arrows).
3.3. Pneumothorax treatments and clinical outcomes
The pneumothorax treatments and clinical outcomes are shown in Table 4. Eighteen patients (26%) were only observed and advised to rest. The remaining 51 patients (74%) underwent chest tube drainage with (7 patients, 10%) or without (44 patients, 63%) pleurodesis and with an endobronchial Watanabe spigot (7 patients, 10%); the median (IQR) duration of drainage was 16 (10–34) days. Only 2 patients underwent surgical intervention. Complete and incomplete expansions were observed in 49 patients (71%) and 15 patients (22%), respectively. Sixty-two patients (93%) were treated with multiple antimicrobial agents after the onset of pneumothorax.
Table 4.
Treatment and clinical outcomes of 69 nontuberculous mycobacteria patients with pneumothorax.
3.4. Prognosis and factors related to survival in pneumothorax associated with NTMPD
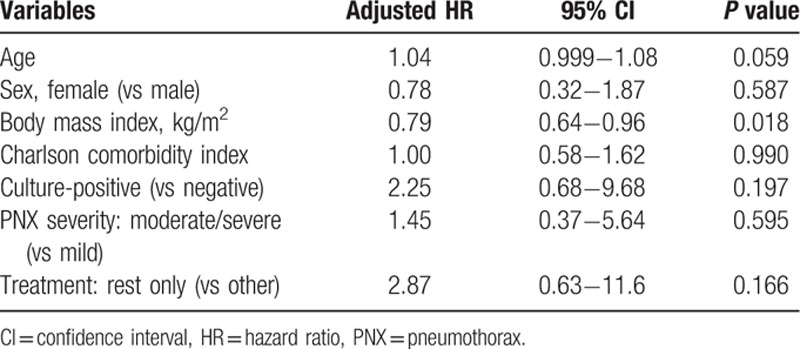
The median follow-up duration among the 69 patients was 1.7 years (range, 0–14.4 years). The survival rate after pneumothorax was 90% at 1 year, 78% at 2 years, 56% at 3 years, 48% at 5 years, and 32% at 8 years (Fig. 4). At the end of follow-up, 33 patients had died, and the median survival was 4.4 years. Three of 5 patients (60%) with CAM-resistant infections and 3 of 9 patients (33%) with CAM-susceptible infections died during the study period. Overall, 23 patients (70%) died because of NTMPD progression. Other causes of death were complications associated with surgery for NTMPD (bronchial stump fistula, 1 patient), other pulmonary diseases (pneumonia, 3 patients; chronic pulmonary aspergillosis, 2 patients; and lung cancer, 1 patient), and nonpulmonary diseases (cerebral infarction, 1 patient; gastrointestinal bleeding, 1 patient; and liver cirrhosis, 1 patient). The results of univariate Cox regression analysis of the factors related to survival are shown in Table 5. The only predictors of poor prognosis were age (HR 1.05, 95% CI 1.01−1.09; P = 0.011) and BMI (HR 0.78, 95% CI 0.65−0.92; P = 0.002). The results of multivariate Cox regression analysis incorporating variables strongly associated (P < 0.2) with mortality in univariate analysis in addition to age, sex, BMI, and CCI are shown in Table 6. A low BMI was the only factor associated with a negative prognostic for pneumothorax associated with NTMPD (HR 0.79, 95% CI 0.64−0.96; P = 0.018).
Figure 4.
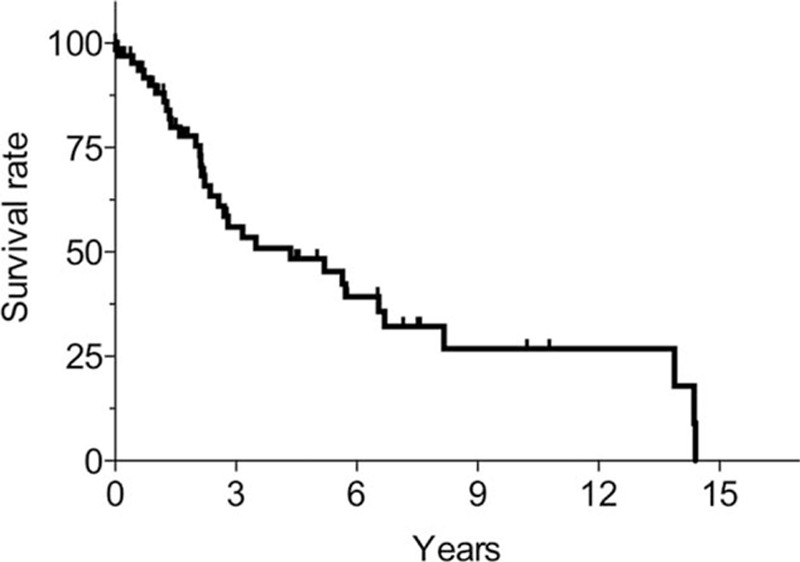
Kaplan−Meier survival curves, from the onset of pneumothorax. The survival rate after pneumothorax was 90% at 1 year, 78% at 2 years, 56% at 3 years, 48% at 5 years, and 32% at 8 years. The median survival was 4.4 years.
Table 5.
Univariate analysis of factors related to survival in pneumothorax associated with nontuberculous mycobacterial pulmonary disease.
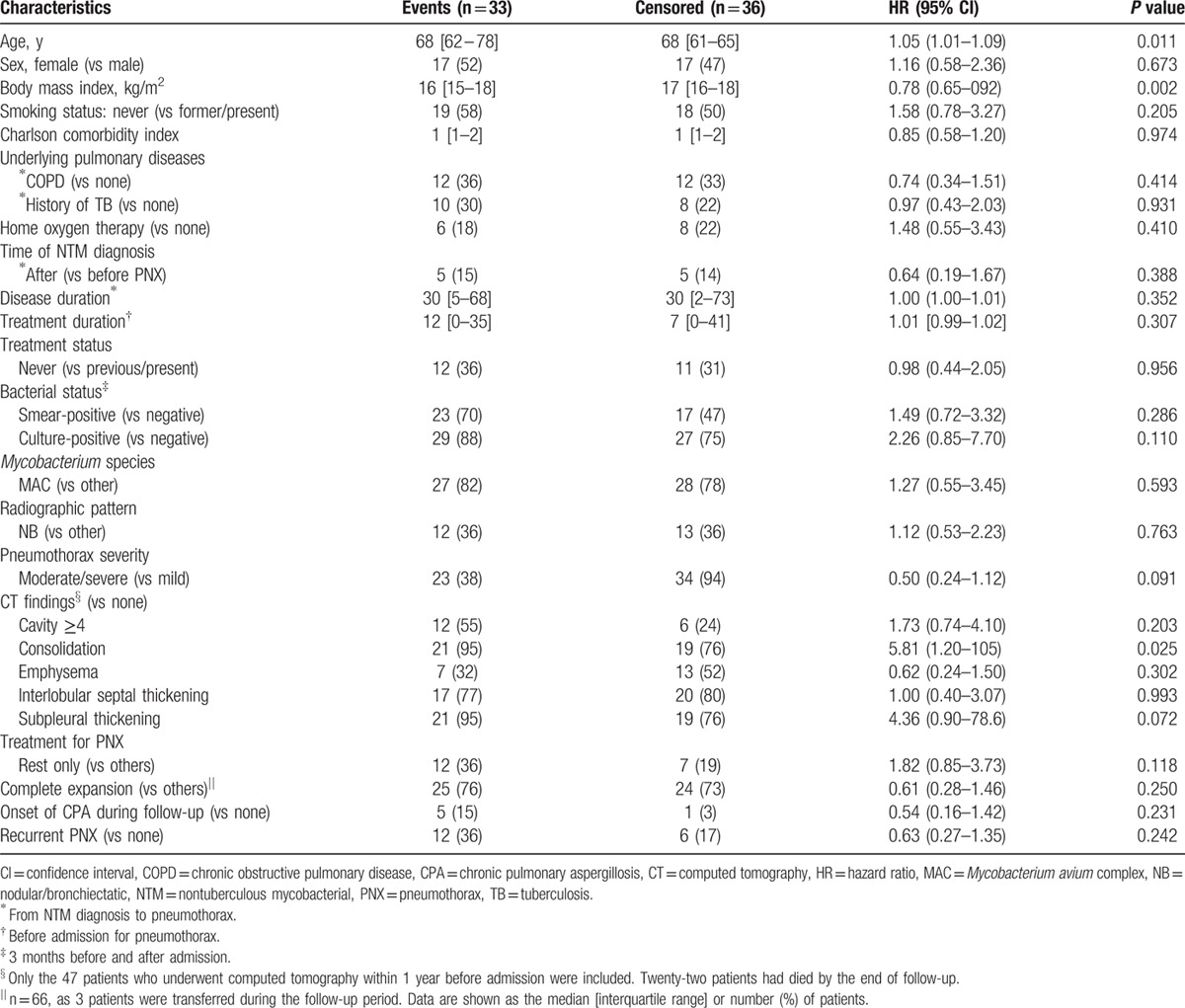
Table 6.
Multivariate analysis of factors related to survival in pneumothorax associated with nontuberculous mycobacterial pulmonary disease.
The rate of pneumothorax recurrence after the first pneumothorax was 20% at 1 year, 32% at 2 years, and 41% at 3 years (Fig. 5). No further pneumothorax recurrence was noted after 3 years. By the end of the follow-up, 18 patients had experienced pneumothorax recurrence. Moreover, 6 patients and 2 patients had experienced recurrence of pneumothorax 2 and 3 times, respectively. Furthermore, 12/18 patients (66%) with recurrent pneumothorax died during the follow-up period.
Figure 5.
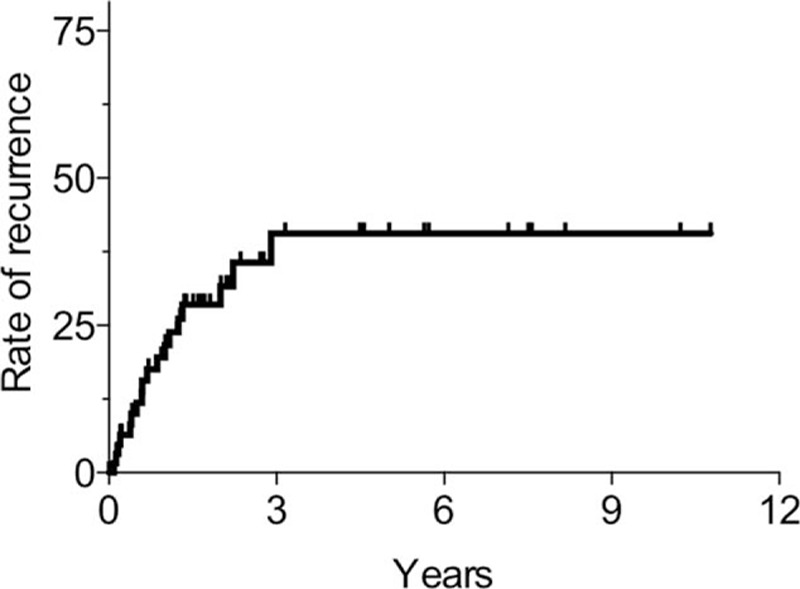
The rate of pneumothorax recurrence in patients, from the diagnosis of pneumothorax, according to the Kaplan−Meier method. The rate of pneumothorax recurrence after the first pneumothorax was 20% at 1 year, 32% at 2 years, and 41% at 3 years.
4. Discussion
To our knowledge, the present study of 69 cases is the largest clinical study of pneumothorax associated with NTMPD to date. This study revealed important findings in patients with NTMPD-associated pneumothorax, including the clinical characteristics and radiological features that indicated an advanced stage of NTMPD with various lesions, a poor prognosis, and a high pneumothorax recurrence rate within 3 years. One recent study of Japanese patients with MAC pulmonary disease, not identified as pneumothorax, showed that the survival rate at 5 years was 76.1%.[6] Another study in Denmark also indicated that the 5-year survival rate of MAC pulmonary disease was 60.3%.[15] Moreover, a study in Finland showed that the median survival in MAC-related and other NTM-related pulmonary disease was 13.0 years and 4.6 years, respectively.[16] In comparison to previous studies, our study showed a high mortality rate, despite mainly including patients with MAC-related pulmonary disease. Therefore, pneumothorax has a high impact on prognosis in patients with NTMPD.
NTMPD, which were mostly because of MAC infection in this study, was classified into 4 main forms.[6,17] The present study showed a higher proportion of the FC and unclassified form, which are associated with a higher mortality than the NB form.[6] Moreover, the proportion of men, former and present smokers, and percentages of patients with COPD and a history of TB were higher in this study than in previous studies.[4,18,19] Smoking is not only a risk factor for primary spontaneous pneumothorax and pneumothorax recurrence,[7] but also affects the development of COPD, especially in patients with NTMPD.[20] Therefore, smoking may also increase pneumothorax in patients with NTMPD. Although the disease and treatment duration and treatment status varied in our study, 9 of 10 patients who were diagnosed with NTMPD with the onset of pneumothorax had underlying pulmonary disease, particularly COPD. Kobashi et al[10] also reported that 3 patients with a history of COPD were diagnosed at the onset of pneumothorax. Underlying pulmonary disease may lead to a delay in the diagnosis of NTMPD and may thereby result in the onset of pneumothorax.
We found several CT findings that correlate with the onset of pneumothorax. The radiological features of NTMPD generally included nodules, consolidation, bronchiectasis, and cavities. In the present study, patients with pneumothorax associated with NTMPD had extended lesions, indicating a more advanced stage of the disease, compared with those reported in previous studies.[17,21,22] Notably, cavities, consolidation, pleural thickening, and interlobular septal thickening were more common in our study, in addition to nodules and bronchiectasis. Cavities and consolidation were reported as important predictors of progression requiring treatment, as well as of a poor prognosis.[21,23] In pneumothorax associated with pulmonary TB, the organism invades the pleura and causes liquefactive necrosis, followed by pleural rupture.[24] In NTMPD, Wittram and Weisbrod[25] reported that 15 of 26 patients demonstrated pleural involvement upon CT. Moreover, Hagiwara et al[11] described pathological evidence of air leakage from MAC lesions. Taken together, these findings suggest that pleural involvement may be strongly associated with the onset of pneumothorax and with a poor prognosis.
We revealed that patients with pneumothorax associated with NTMPD had poor prognoses and high rates of pneumothorax recurrence within 3 years. A poor prognosis and high recurrence were also reported in SSP associated with other respiratory diseases;[26] specifically, pneumothorax with COPD, the main cause of SSP, is reported to have a higher mortality and morbidity, a lower healing rate, and a higher recurrence rate after chest tube drainage.[27,28] Pneumothorax with cystic fibrosis, especially in the elderly, also has a high recurrence rate with a poor prognosis, with a median survival of only 30 months.[29] Pneumothorax with pulmonary fibrosis also has a poor prognosis, with high postoperative mortality, despite surgical intervention.[30,31] However, little is known about the prognostic factors related to long-term survival in specific pulmonary diseases. In patients with NTMPD in general, the FC form of the disease, a low BMI, presence of cavities, advanced age, and male sex are poor prognostic factors.[6,15,17] Our cases had these clinical features associated with a poorer prognosis. Moreover, we revealed that a low BMI is the only prognostic factor in patients with pneumothorax associated with NTMPD. A low BMI is known to be a risk factor not only for NTMPD progression, but also for recurrent spontaneous pneumothorax,[32] and may be associated with a poor prognosis and recurrent pneumothorax. CAM resistance has also been reported to be a poor prognostic factor for MAC pulmonary disease.[33] Although recent studies revealed various mechanisms of resistance in NTM, the relationship between in vitro antibiotic susceptibility testing and treatment outcome in a clinical setting has not been established.[34] Since CAM resistance may have been associated with mortality rate in the present study, further investigations, including microbiological studies, are required.
There are several limitations to our study. First, the study design was retrospective; there may be some confounding factors, and our microbiological data were limited. Second, the timing of the diagnosis or antimicrobial treatment could modify the clinical course of NTMPD. Finally, the choice of pneumothorax treatment was affected by the decision of the pulmonary physician. Prospective enrollment and data collection from a large cohort of patients with NTMPD in a multicenter clinical trial would be ideal, but it would be considerably difficult because of the low rate of pneumothorax complications.
In conclusion, we have here described the clinical characteristics of patients with pneumothorax associated with NTMPD and have shown an advanced disease stage, a high rate of pneumothorax recurrence, and a poor prognosis, regardless of the pneumothorax treatment. Further improvements in early diagnosis of NTMPD and appropriate management in both NTMPD and NTMPD-associated pneumothorax are needed.
Acknowledgments
Authors thank Kazuma Yagi, Shoji Suzuki, and Hiroaki Sugiura for assistance with the radiological analysis. They also thank Kazunari Yamana, Ryozo Yano, Maki Miyamoto, Hiroyuki Kokuto, Masao Okumura, Yuka Sasaki, Takashi Uchiyama, Takashi Yoshiyama, Mikio Saotome, Yuji Shiraishi, Hideo Ogata, Kozo Yoshimori, and Shoji Kudoh for assistance with data collection and patient care.
Footnotes
Abbreviations: COPD = chronic obstructive pulmonary disease, CT = computed tomography, MAC = Mycobacterium avium complex, NTMPD = nontuberculous mycobacterial pulmonary disease, SSP = secondary spontaneous pneumothorax, TB = tuberculosis.
MU and TA have contributed equally to this work.
Authors’ contributions: MU and TA conceived of the study and wrote the first version of the manuscript. KM, HN, SM, TO, NH, AK, and HG supervised the study and made critical revisions for important intellectual content. All the authors approved the final version as submitted to the journal.
The authors have no conflict of interest to disclose.
References
- 1.Mirsaeidi M, Machado RF, Garcia JG, et al. Nontuberculous mycobacterial disease mortality in the United States, 1999-2010: a population-based comparative study. PLoS One 2014; 9:e91879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satta G, McHugh TD, Mountford J, et al. Managing pulmonary nontuberculous mycobacterial infection. time for a patient-centered approach. Ann Am Thorac Soc 2014; 11:117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175:367–416. [DOI] [PubMed] [Google Scholar]
- 4.Asakura T, Funatsu Y, Ishii M, et al. Health-related quality of life is inversely correlated with C-reactive protein and age in Mycobacterium avium complex lung disease: a cross-sectional analysis of 235 patients. Respir Res 2015; 16:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med 2010; 182:970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayashi M, Takayanagi N, Kanauchi T, et al. Prognostic factors of 634 HIV-negative patients with Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2012; 185:575–583. [DOI] [PubMed] [Google Scholar]
- 7.Noppen M. Spontaneous pneumothorax: epidemiology, pathophysiology and cause. Eur Respir Rev 2010; 19:217–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lippert HL, Lund O, Blegvad S, et al. Independent risk factors for cumulative recurrence rate after first spontaneous pneumothorax. Eur Respir J 1991; 4:324–331. [PubMed] [Google Scholar]
- 9.MacDuff A, Arnold A, Harvey J. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010; 65 suppl 2:ii18–ii31. [DOI] [PubMed] [Google Scholar]
- 10.Kobashi Y, Mouri K, Obase Y, et al. Clinical analysis of patients with pulmonary nontuberculous mycobacterial disease complicated by pneumothorax. Intern Med 2013; 52:2511–2515. [DOI] [PubMed] [Google Scholar]
- 11.Hagiwara E, Komatsu S, Nishihira R, et al. Clinical characteristics and prevalence of pneumothorax in patients with pulmonary Mycobacterium avium complex disease. J Infect Chemother 2013; 19:588–592. [DOI] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383. [DOI] [PubMed] [Google Scholar]
- 13.Inagaki T, Yagi T, Ichikawa K, et al. Evaluation of a rapid detection method of clarithromycin resistance genes in Mycobacterium avium complex isolates. J Antimicrob Chemother 2011; 66:722–729. [DOI] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. Susceptibility Testing for Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes: Approved Standard M24-A. Wayne, PA, USA: CLSI; 2011. http://shop.clsi.org/microbiology-documents/M24.html. [PubMed] [Google Scholar]
- 15.Andrejak C, Thomsen VO, Johansen IS, et al. Nontuberculous pulmonary mycobacteriosis in Denmark: incidence and prognostic factors. Am J Respir Crit Care Med 2010; 181:514–521. [DOI] [PubMed] [Google Scholar]
- 16.Kotilainen H, Valtonen V, Tukiainen P, et al. Clinical findings in relation to mortality in non-tuberculous mycobacterial infections: patients with Mycobacterium avium complex have better survival than patients with other mycobacteria. Eur J Clin Microbiol Infect Dis 2015; 34:1909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujita K, Ito Y, Hirai T, et al. Prevalence and risk factors for chronic co-infection in pulmonary Mycobacterium avium complex disease. BMJ Open Respir Res 2014; 1:e000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morimoto K, Iwai K, Uchimura K, et al. A steady increase in nontuberculous mycobacteriosis mortality and estimated prevalence in Japan. Ann Am Thorac Soc 2014; 11:1–8. [DOI] [PubMed] [Google Scholar]
- 19.Ide S, Nakamura S, Yamamoto Y, et al. Epidemiology and clinical features of pulmonary nontuberculous mycobacteriosis in Nagasaki, Japan. PLoS One 2015; 10:e0128304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeh JJ, Wang YC, Sung FC, et al. Nontuberculosis mycobacterium disease is a risk factor for chronic obstructive pulmonary disease: a nationwide cohort study. Lung 2014; 192:403–411. [DOI] [PubMed] [Google Scholar]
- 21.Lee G, Lee KS, Moon JW, et al. Nodular bronchiectatic Mycobacterium avium complex pulmonary disease. Natural course on serial computed tomographic scans. Ann Am Thorac Soc 2013; 10:299–306. [DOI] [PubMed] [Google Scholar]
- 22.Fujita K, Ito Y, Hirai T, et al. Association between polyclonal and mixed mycobacterial Mycobacterium avium complex infection and environmental exposure. Ann Am Thorac Soc 2014; 11:45–53. [DOI] [PubMed] [Google Scholar]
- 23.Shu CC, Lee CH, Hsu CL, et al. Clinical characteristics and prognosis of nontuberculous mycobacterial lung disease with different radiographic patterns. Lung 2011; 189:467–474. [DOI] [PubMed] [Google Scholar]
- 24.Kim HY, Song KS, Goo JM, et al. Thoracic sequelae and complications of tuberculosis. Radiographics 2001; 21:839–858.discussion 859-860. [DOI] [PubMed] [Google Scholar]
- 25.Wittram C, Weisbrod GL. Mycobacterium avium complex lung disease in immunocompetent patients: radiography-CT correlation. Br J Radiol 2002; 75:340–344. [DOI] [PubMed] [Google Scholar]
- 26.Videm V, Pillgram-Larsen J, Ellingsen O, et al. Spontaneous pneumothorax in chronic obstructive pulmonary disease: complications, treatment and recurrences. Eur J Respir Dis 1987; 71:365–371. [PubMed] [Google Scholar]
- 27.Sahn SA, Heffner JE. Spontaneous pneumothorax. N Engl J Med 2000; 342:868–874. [DOI] [PubMed] [Google Scholar]
- 28.Schoenenberger RA, Haefeli WE, Weiss P, et al. Timing of invasive procedures in therapy for primary and secondary spontaneous pneumothorax. Arch Surg 1991; 126:764–766. [DOI] [PubMed] [Google Scholar]
- 29.Spector ML, Stern RC. Pneumothorax in cystic fibrosis: a 26-year experience. Ann Thorac Surg 1989; 47:204–207. [DOI] [PubMed] [Google Scholar]
- 30.Aihara K, Handa T, Nagai S, et al. Efficacy of blood-patch pleurodesis for secondary spontaneous pneumothorax in interstitial lung disease. Intern Med 2011; 50:1157–1162. [DOI] [PubMed] [Google Scholar]
- 31.Nakajima J, Takamoto S, Murakawa T, et al. Outcomes of thoracoscopic management of secondary pneumothorax in patients with COPD and interstitial pulmonary fibrosis. Surg Endosc 2009; 23:1536–1540. [DOI] [PubMed] [Google Scholar]
- 32.Hatzitolios AI, Ntaios G, Sion ML. Both spontaneous pneumothorax and spontaneous pneumomediastinum may constitute a complication in underweight patients. Chest 2008; 134:216–217.author reply 217. [DOI] [PubMed] [Google Scholar]
- 33.Philley JV, Griffith DE. Management of nontuberculous mycobacterial (NTM) lung disease. Semin Respir Crit Care Med 2013; 34:135–142. [DOI] [PubMed] [Google Scholar]
- 34.van Ingen J, Boeree MJ, van Soolingen D, et al. Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug Resist Updat 2012; 15:149–161. [DOI] [PubMed] [Google Scholar]


