Abstract
Background and objective:
Intramedullary nailing is commonly used for treating femoral shaft fractures, one of the most common long bone fractures in adults. The reamed intramedullary nail is considered the standard implant for femoral fractures. This meta-analysis was performed to verify the superiority of reamed intramedullary nailing over unreamed intramedullary nailing in fractures of the femoral shaft in adults. Subgroup analysis of implant failure and secondary procedure was also performed.
Methods:
Electronic literature databases were used to identify relevant publications and included MEDLINE (Ovid interface), EMBASE (Ovid interface), and the Cochrane Central Register of Controlled Trials (CENTRAL; Wiley Online Library). The versions available on January 30, 2016, were utilized. Only human studies, which were designed as randomized controlled clinical trials, were included. Two authors independently evaluated the quality of original research publications and extracted data from the studies that met the criteria.
Results:
Around 8 randomized controlled trials involving 1078 patients were included. Reamed intramedullary nailing was associated with shorter time to consolidation of the fracture (SMD = –0.62, 95% CI = –0.89 to –0.35, P < 0.00001), lower secondary procedure rate (OR = 0.25, 95% CI 0.10–0.62, P = 0.003), lower nonunion rate (OR = 0.14, 95% CI = 0.05–0.40, P < 0.01), and lower delayed-union rate (OR = 0.19, 95% CI = 0.07–0.49, P < 0.01) compared to unreamed intramedullary nailing. The 2 groups showed no significant differences in risk of implant failure (OR = 0.50, 95% CI 0.14–1.74, P = 0.27), mortality risk (OR = 0.94, 95% CI 0.19–4.68, P = 0.94), risk of acute respiratory distress syndrome (ARDS; OR = 1.55, 95% CI 0.36–6.57, P = 0.55), or blood loss (SMD = 0.57, 95% CI = –0.22 to 1.36, P = 0.15).
Conclusion:
Reamed intramedullary nailing is correlated with shorter time to union and lower rates of delayed-union, nonunion, and reoperation. Reamed intramedullary nailing did not increase blood loss or the rates of ARDS, implant failure, and mortality compared to unreamed intramedullary nailing. Therefore, the treatment of femoral fractures using reamed intramedullary nailing is recommended.
Keywords: femoral shaft fracture, meta-analysis, randomized controlled trials, reamed intramedullary nailing, unreamed intramedullary nailing
1. Introduction
Femoral shaft fractures comprise 5% to 6% of long bone fractures in adults, making them one of the most common fractures. Fractures of the femur are very frequently seen in patients who suffered multiple trauma or high-energy injury.[1] The femur is an important weight-bearing bone and the improper treatment of a femoral fracture can result in deformity or dysfunction of the lower limb. Reamed intramedullary nailing (RIN) is considered the standard method of treatment for femoral fractures.[2–5] The advantages of RIN include higher biomechanical stability,[6] rapid fracture healing,[7] and lower frequency of secondary procedure.[7,8] However, several articles questioned whether or not the medullary cavity should be reamed. Opponents of reaming pointed out that reaming can decrease bone blood flow in the diaphysis[9–11] and may cause bone necrosis[12] and emboli.[9,13] Reaming may also increase blood loss[14–16] and operative time.[14,15] This meta-analysis was performed to verify the superiority of reamed intramedullary nailing over unreamed intramedullary nailing in fractures of the femoral shaft in adults. In addition, we performed subgroup analysis of implant failure and secondary procedures. Although this systematic review was in progress, 2 similar meta-analyses were published.[17,18] However, these meta-analyses did not include the articles published between 2011 and January 2016; thus, this review is more comprehensive.
2. Materials and methods
This study was performed with guidance from the Cochrane Handbook for Systematic Reviews of Interventions and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.[19,20] As the present meta-analysis was performed based on previous published studies, ethical approval and patient consent were not required.
2.1. Inclusion and exclusion criteria
The criteria for inclusion were: (i) human studies that were designed as randomized controlled clinical trials, (ii) studies had to perform a comparison of RIN and URIN for the treatment of femoral shaft fractures, (iii) all participants were adults with mature skeletons, and (iv) when there was more than 1 study from the same center using the same protocol, the study with the longest follow-up was used. Exclusion criteria included: (i) pathological fractures, (ii) studies of fractures in animals or children, and (iii) nonrandomized studies, review articles, conference abstracts, biomechanical studies, or case reports.
2.2. Search strategy and study selection
We searched electronic literature databases for all relevant studies published prior to January 30, 2016. The databases used were MEDLINE (Ovid interface), EMBASE (Ovid interface), and the Cochrane Central Register of Controlled Trials (CENTRAL; Wiley Online Library). The search was performed without language restrictions, but was limited to human studies. The search strategies are shown in Table 1.
Table 1.
Search strategy.

The function of “related article” was also used to expand the search. In addition, we also manually searched references from review articles to supplement the electronic database search.
2.3. Data extraction
Two authors (ABL and HMJ) independently extracted the following data: the first author's last name, publication year, country, study follow-up duration, sample size, characteristics of patients, interventions, and post-operative complications. The third author (WJG) checked the agreement between the extracted information. If necessary, the primary authors were contacted to provide additional data.
2.4. Risk of bias assessment
All potential articles were independently assessed for methodological quality by 2 reviewers (ABL and WJZ) and any conflict was resolved by means of discussion with the third independent reviewer (YMZ). The reviewers assessed the risk of bias of included studies according to the Cochrane Handbook for Systematic Reviews of Interventions: random sequence generation; allocation concealment; double blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data addressed; selective reporting; or other bias. The judgments of reviewers of bias were “low risk,” “high risk,” or “unclear risk.”
2.5. Statistical analysis
We used odd ratio (OR) as the effect measure of dichotomous outcomes, with 95% confidence intervals (CI). I2 statistic was used to evaluate the statistical heterogeneity. I2 > 50% was considered significantly statistical heterogeneity.[21] A fixed effects model and 95% confidence intervals (CI) were used, and a random-effect model was considered if there was significant heterogeneity. Statistical analyses were conducted using the RevMan 5.3.5 software (The Nordic Cochrane Center, Denmark). P < 0.05 was considered statistically significant.
3. Results
3.1. Included studies
A total of 810 potentially relevant articles were identified from the databases, and 506 studies were excluded after screening of the title and abstract. A total of 45 full-text articles were assessed for eligibility. Of these, 2 were excluded as not randomized clinical trials; 15 trials were excluded due to the uninteresting outcomes; 19 studies were excluded as reviews articles; and 1 study was excluded because it was a preliminary report.[22] The remaining 8 articles[7,8,14–16,23–25] were included in this meta-analysis. Of these 8 studies, 2 were multicenter, randomized, controlled clinical trials and 6 were from a single investigational site and studied patients with femoral fractures. The selection of articles for inclusion is presented in Fig. 1.
Figure 1.
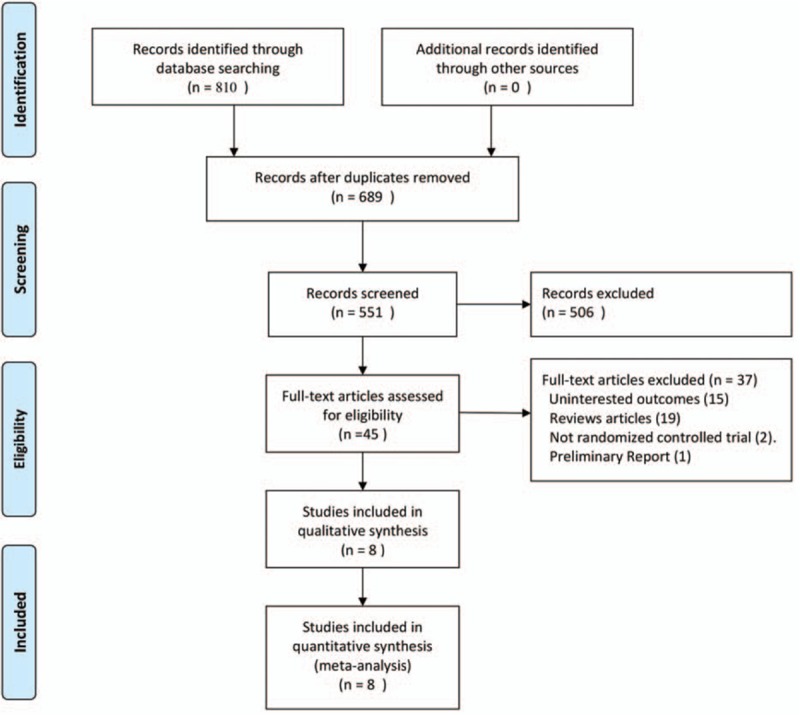
Flowchart of article selection for inclusion.
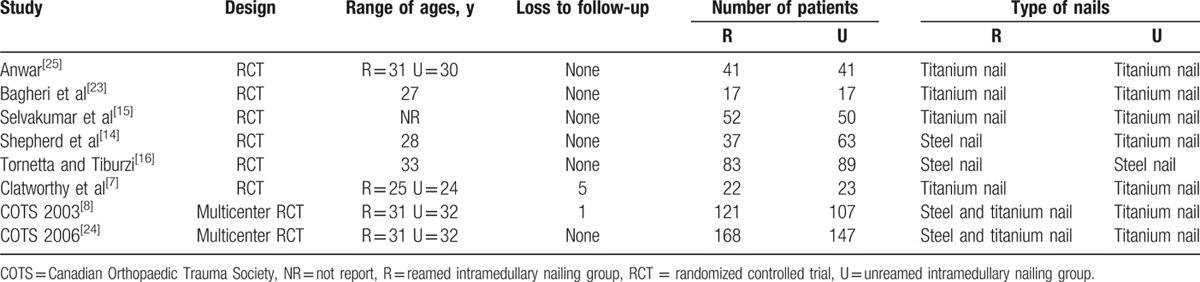
The total number of participants studied was 1078 (541 in the RIN group and 537 in the URIN group) individuals; the studies were performed in various countries with no obvious discrepancies in baseline demographics between the RIN and URIN groups, and the individuals enrolled in all 8 studies were basically homogeneous. A summary of selected studies is shown in Table 2.
Table 2.
Characteristics of the included studies.
3.2. Risk of bias assessment
The risk of bias of included studies is shown in Fig. 2 and summarized in Fig. 3. The randomization technique was mentioned in 4 trials,[8,14,24,25] and information of allocation concealment was not provided for 5 studies.[7,14–16,23–25] Because there was no difference in the postoperative radiological data between the 2 groups; thus, the term “blinding of outcome assessment” was assessed as “low risk” for all 8 studies.
Figure 2.
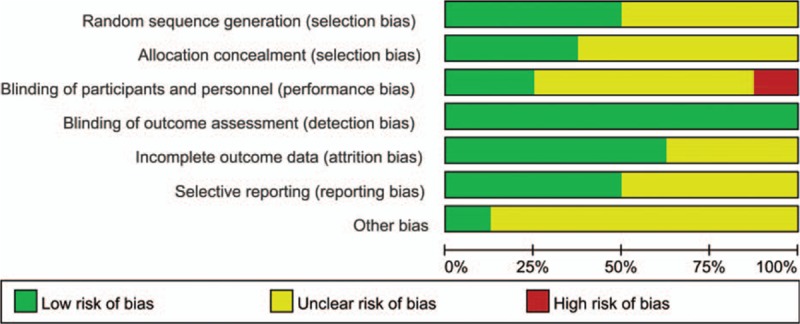
Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies.
Figure 3.
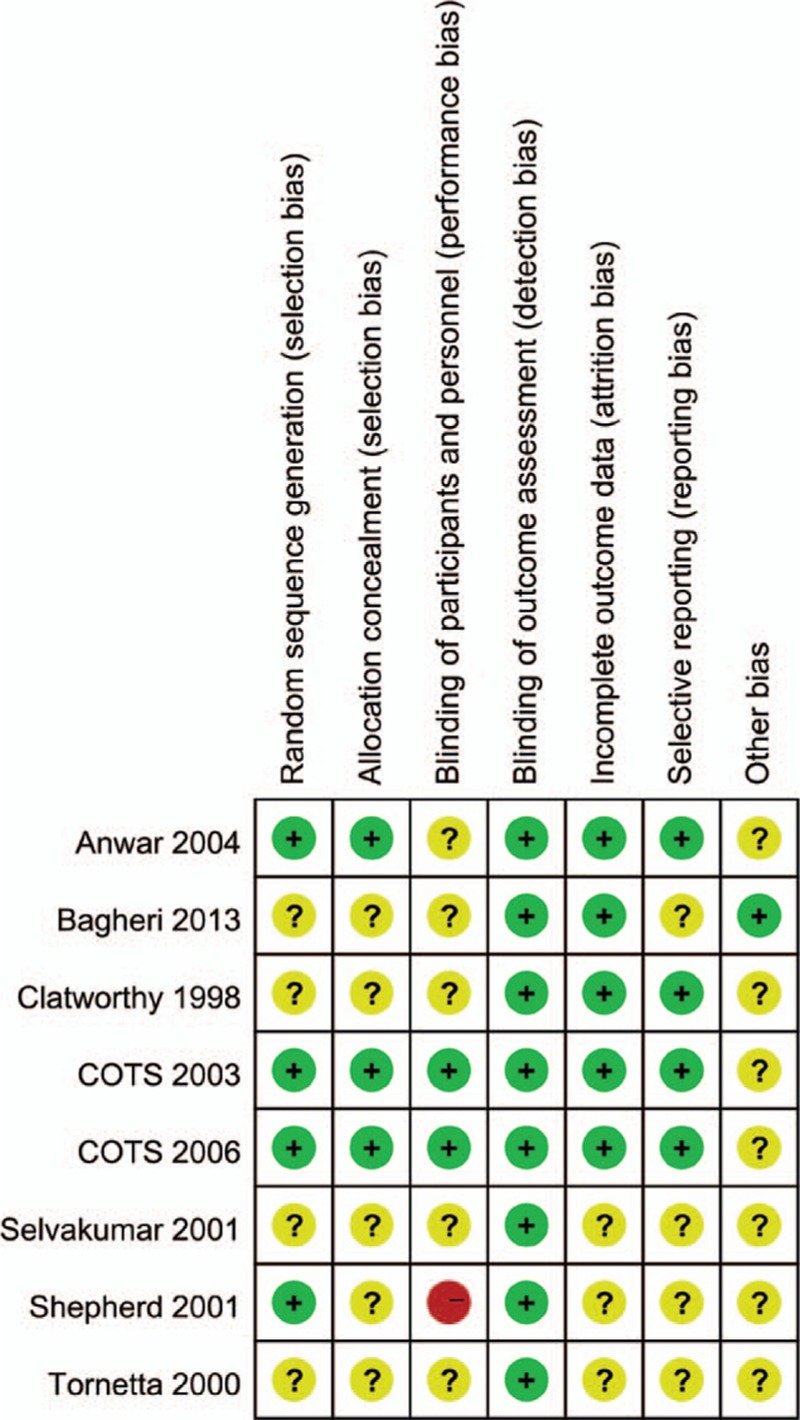
Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
3.3. Clinical outcomes
Due to different definitions of complications for the included studies, we did not perform a meta-analysis of overall complications. Thus, only the major adverse events including the incidence of nonunion, delayed-union, fixation failure, infection, acute respiratory distress syndrome (ARDS), and mortality were embedded into the meta-analysis for evaluation. Five studies[7,14–16,23] reported the incidence of implant failure, with a low frequency in both groups.
The incidences of nonunion (OR = 0.14, 95% CI = 0.05–0.40, P < 0.01, Fig. 4) and delayed-union (OR = 0.19, 95% CI = 0.07–0.49, P < 0.01, Fig. 5) were significantly higher in the URIN group. Reamed intramedullary nailing showed a significantly lower rate of secondary procedures when compared to URIN (OR = 0.25, 95% CI 0.10–0.62, P = 0.003 Fig. 6). The subgroup analysis demonstrated a higher risk of implant exchange (OR = 0.17, 95% CI = 0.04–0.81, P = 0.03, Fig. 6) of patients treated with URIN. However, there were no significant differences between the groups for the risk of bone grafting (OR = 0.15, 95% CI = 0.02–1.27, P = 0.08, Fig. 6) or dynamization (OR = 0.50, 95% CI = 0.13–0.97, P = 0.32, Fig. 6). Our subgroup analysis found no differences in nail failure (OR = 1.05, 95% CI = 0.19–5.87, P = 0.95, Fig. 7) or screw failure (OR = 0.20, 95% CI = 0.02–1.76, P = 0.015, Fig. 7). Additionally, the 2 groups showed no significant differences for risk of ARDS (OR = 1.55, 95% CI = 0.36–6.57, P = 0.55, Fig. 8) or mortality (OR = 0.94, 95% CI = 0.19–4.68, P = 0.94, Fig. 9).
Figure 4.
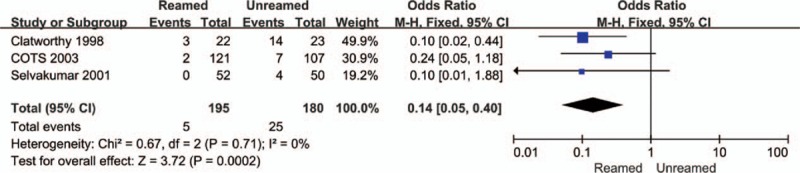
Forest plot showing nonunion rate of reamed intramedullary nailing and unreamed intramedullary nailing.
Figure 5.
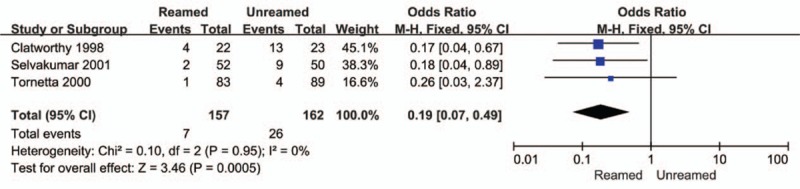
Forest plot showing delayed-union rate of reamed intramedullary nailing and unreamed intramedullary nailing.
Figure 6.
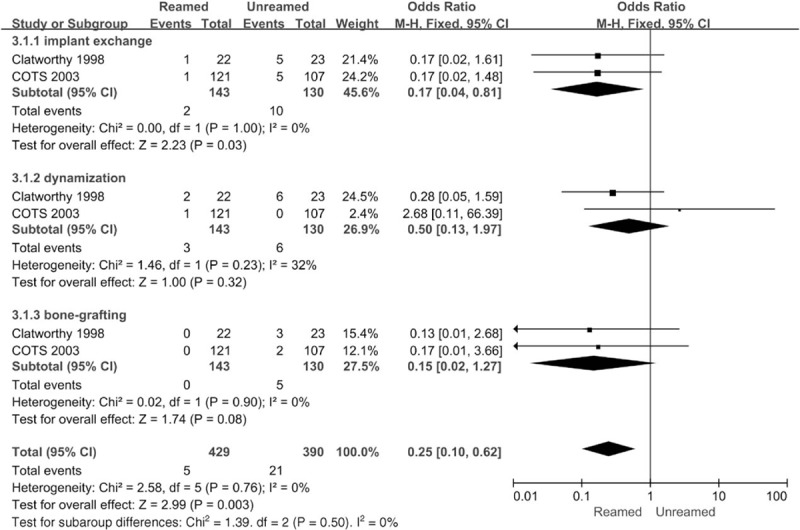
Forest plot showing subgroups analysis of secondary procedure of reamed intramedullary nailing and unreamed intramedullary nailing.
Figure 7.
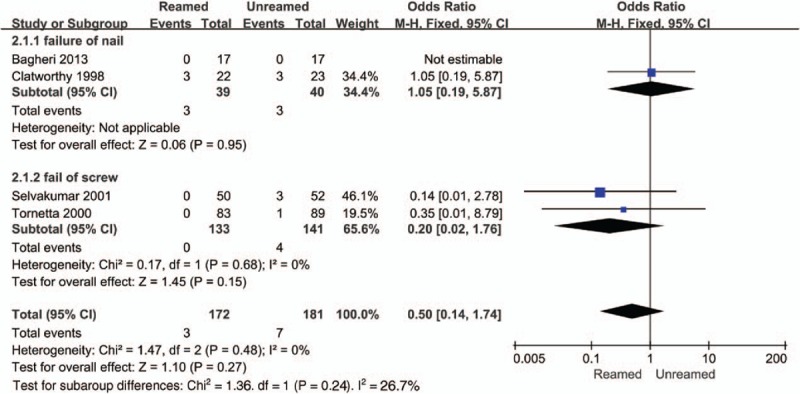
Forest plot showing subgroups analysis of implant failure of reamed intramedullary nailing and unreamed intramedullary nailing.
Figure 8.
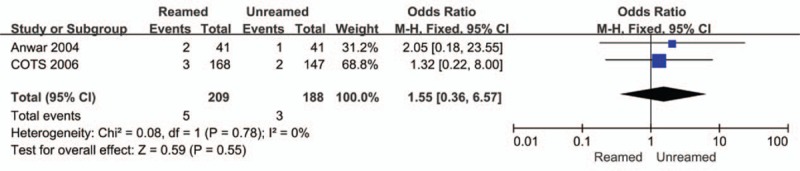
Forest plot showing the ARDS rate of reamed intramedullary nailing and unreamed intramedullary nailing.
Figure 9.
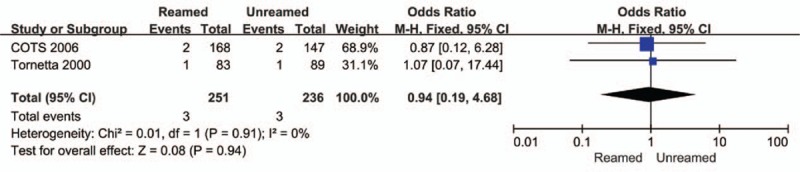
Forest plot showing mortality rate of reamed intramedullary nailing and unreamed intramedullary nailing.
The differences in blood loss between the 2 groups were not significant (SMD = 0.57, 95% CI = –0.22 to 1.36, P = 0.15 Fig. 10). There was obvious statistical heterogeneity in these results (Chi2 = 8.6, I2 = 88%, P = 0.003 Fig. 10). There was also apparent decreased time to union for the RIN group (SMD = –0.62, 95% CI = −0.89 to −0.35, P < 0.00001 Fig. 11).
Figure 10.
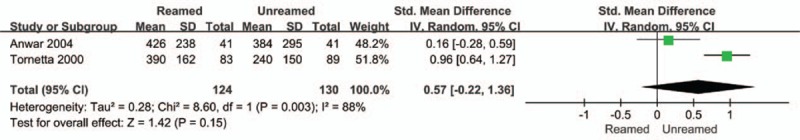
Forest plot showing blood loss of reamed intramedullary nailing and unreamed intramedullary nailing.
Figure 11.
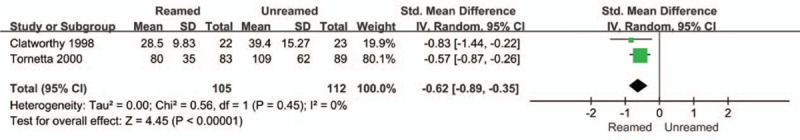
Forest plot showing time to union of reamed intramedullary nailing and unreamed intramedullary nailing.
4. Discussion
The purpose of this study was the evaluation of the relative merits of RIN versus URIN in the treatment of femoral shaft fractures. This analysis indicated that RIN improved the union rate of fractures, decreased the time to union, and decreased the incidence of non-union or delayed-union; furthermore, it did not correlate with increased blood loss or the risks of ARDS, implant failure, and mortality. Although there was no obvious difference in implant failure between the 2 groups, the incidence of secondary procedures (implant exchange) was higher in the URIN group than in the RIN group.
Intramedullary nailing is the standard treatment for fractures of the femoral shaft in adults. Titanium alloy or stainless steel is often used as the material for nails. The biomechanical properties of the materials can affect fracture healing. Titanium alloy has a lower elastic modulus, which is close to the human bone elasticity and is more biocompatible than stainless steel. Therefore, the insertion of a Titanium nail enhances callus formation and shortens time to bone union, resulting in a high healing rate. In addition, compared with unreamed intramedullary nailing, reamed intramedullary nails of larger diameters can be inserted and their fatigue strength or bending stiffness is higher. We noted a shorter time to union for the RIN group and the time to union was shorter in patients in whom a Titanium nail was inserted. However, Trompeter and Newman[26] reported no significant difference in time required for union and failure rates for the 2 materials. Due to the relatively small size of samples in this study, these results should still be interpreted with caution. The differences between titanium or steel nails should be further verified by additional prospective randomized clinical trials with larger sample sizes.
El Maraghy et al[11] reported that reaming might destroy the nutrient artery and decrease bone blood flow in the diaphysis. Based on this, researchers predicted that bone blood supply that was reduced due to reaming damage could influence fracture healing and increase the risk of infection.[27] However, this is not supported our analysis. Instead, we found that fracture union was significantly slower in the URIN group and the incidences of nonunion and delayed-union were significantly lower in the RIN group. The blood supply of long bones was well-characterized by Rhinelander who showed that the medullary arteries supply the inner two-thirds of the cortex, and that the outer third is supplied by the periosteal vasculature via its soft tissue attachments to the bone.[28] When a fracture occurs, the medullary vessels are disrupted, leading to 50% to 70% necrosis of the cortex near the fracture site. Some researchers have speculated that the debris produced by reaming may include osteoblasts[29] and multipotent stem cells[30] placed at the fracture site to act as an autologous bone graft.[31] Reaming may damage the blood supply of the inner cortical bone, but in response, the periosteal blood flow can increase 6-fold, which may stimulate fracture healing.[32]
The treatment methods for delayed-union or nonunion after bone fracture include dynamization, bone grafting, implant exchange, and electrostimulation.[33] Clatworthy et al[7] concluded that fracture stability was an important determinant of rapid union. A larger nail is inserted into the medullary cavity after reaming to improve cortical contact and provide greater stability.[34,35] Grundnes et al[36] reported a tightly fitting nail increased the periosteal reaction. However, it is still unclear if RIN, thought to provide increased mechanical stability, will reduce the need for implant exchange compared to the URIN group. By subgroup analyses, our study found no obvious differences in the risks of bone grafting and dynamization between the 2 groups, but the risk of implant exchange was lower for the RIN group. Our findings indicate RIN may provide greater stability and reduce the risk of implant exchange. In the treatment of femoral fractures, economic costs must also be considered. Secondary procedures are very expensive and are correlated with high rates of complications and mortality. Our study found a high rate of secondary procedures in the URIN groups, which would require higher costs. Therefore, the treatment of femoral fractures using reamed intramedullary nailing is recommended.
From a technical point of view, implant failures include clinical screw or nail failure. Screw failures are more common than nail failures.[37] Screw failure and nail failure correlated with a higher risk of implant failures. However, we observed no obvious differences between RIN and URIN groups for the risk of implant failures. By subgroup analyses, we also found that reamed intramedullary nailing, in contrast with the URIN group, did not increase the incidences of screw failure and nail failure. Consistent with the conclusions of a separate analysis, our results suggest that the time to union, the presence of an open wound, and the configuration of the fracture were the most critical predisposing factors of implant failures. This conclusion should be further verified by additional prospective randomized clinical trials with larger sample sizes.[7]
Various clinical studies have suggested that reaming increased intramedullary pressure of the femur, releasing more bone marrow components and fat emboli into pulmonary circulation compared to treatment without reaming.[38] Potential clinical adverse events include FES, ARDS, multiple organ dysfunction syndrome (MODS), and sudden death. However, the rates of FES and ARDS were low in our analysis. Only 8 cases of ARDS were reported in these studies. No cases of FES were documented in any of the included studies.[7,8,14–16,23–25] We found no obvious differences in ARDS or mortality rate between the 2 groups.
Alho et al[39] concluded that the risk of infective complications was higher in the RIN group when compared to the URIN group. However, some studies found that there was no obvious discrepancy in infection rates between the groups.[40,41] In all included studies, the rate of infective complications was low and only 2 studies reported infective complications.[8,23] In 1 study, there were 4 cases with infection (3 superficial and 1 deep infection).[8,23] However, the number of infections for each group was not reported. Another study described a superficial infection in the RIN group.[8,23] Due to insufficient data, we did not perform meta-analysis of the rate of infection.
Unreamed femoral nailing may have a potential advantage of less blood loss. Less blood loss can reduce the need for transfusion, eliminating complications of blood transfusion and reducing costs.[42,43] The reduced intraoperative blood loss will benefit elderly patients with multimorbidity, as other diseases may take precedence. However, we did not observe significant differences in blood loss for the 2 groups. Due to high heterogeneity, these results should be interpreted with caution. In practice, surgeons usually estimate the blood loss and different assessment methods of intraoperative bleeding were used in different hospitals, such as collection from a plastic bag taped to the surgical drapes, suction drain, or from the weight of swabs. These differences may mask any differences in blood loss for these methods. That could explain the statistic significant difference of heterogeneity.
Most studies compared reamed and unreamed intramedullary nails for closed femoral fractures, but a few studies compared the reamed and unreamed intramedullary nails for treatment of both closed femoral fractures and open femoral fractures. However, any complications that occurred were not distinguished by fracture type, precluding our ability to perform subgroup analysis according to different types of fractures.
Several limitations of this analysis should be noted. First, this article only focused on the rates of nonunion, delayed-union, mortality, implant failure, reoperation, ARDS, blood loss, and the time to union. We did not assess outcomes such as functional results or satisfactory outcomes or time to definitive treatment because these parameters were not always reported or were reported in various forms and not directly comparable. Second, this study did not evaluate quantitative outcome measures such as weight-bearing time, operative time, and hospital stay. Finally, only 8 studies with 1078 participants were included in the review, which might weaken the reliability of this meta-analysis. Despite these limitations, our quantitative evaluation of the rates of complications, blood loss, and the time to union provide an important foundation for surgical treatment decisions.
5. Conclusion
Reamed intramedullary nailing is correlated with shorter time to union and lower rates of delayed-union, nonunion, and reoperation. Reamed intramedullary nailing did not increase blood loss or the rates of ARDS, implant failure, and mortality compared to unreamed intramedullary nailing. Therefore, the treatment of femoral fractures using reamed intramedullary nailing is recommended.
Acknowledgments
The authors thank all the anonymous reviewers and editors for their helpful suggestions on the quality improvement of our paper.
Footnotes
Abbreviations: ARDS = acute respiratory distress syndrome, CI = confidence interval, FES = fat embolism syndrome, OR = odd ratio, RCTs = randomized controlled trials, RIN = Reamed intramedullary nailing, URIN = unreamed intramedullary nailing.
Authorship: ABL, WJZ, and YMZ contributed to the conception and design of the study. XHW, ABL, WJG, HMJ, and YMZ contributed to the analysis and interpretation of data. ABL, WJZ, WJG, and XHW contributed to the drafting of the article. All authors revised the manuscript critically for important intellectual content and gave final approval of the version to be published.
The authors have no funding or conflicts of interest to disclose.
References
- 1.Logters T, Windolf J, Flohe S. Fractures of the shaft of the femur. Unfallchirurg 2009; 112:635–650.quiz 651. [DOI] [PubMed] [Google Scholar]
- 2.Hardy BM, Yoshino O, Quail AW, et al. Influence of the timing of internal fixation of femur fractures during shock resuscitation on remote organ damage. ANZ J Surg 2015; 85:966–971. [DOI] [PubMed] [Google Scholar]
- 3.Botolin S, Mauffrey C, Hammerberg EM, et al. Heterotopic ossification in the reaming tract of a percutaneous antegrade femoral nail: a case report. J Med Case Rep 2013; 7:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solooki S, Mesbahi SA. Complex fractures of the tibia and femur treated with static interlocking intramedullary nail. Iran Red Crescent Med J 2011; 13:178–180. [PMC free article] [PubMed] [Google Scholar]
- 5.Crist BD, Wolinsky PR. Reaming does not add significant time to intramedullary nailing of diaphyseal fractures of the tibia and femur. J Trauma 2009; 67:727–734. [DOI] [PubMed] [Google Scholar]
- 6.Kessler SB, Hallfeldt KK, Perren SM, et al. The effects of reaming and intramedullary nailing on fracture healing. Clin Orthop Rel Res 1986; 212:18–25. [PubMed] [Google Scholar]
- 7.Clatworthy MG, Clark DI, Gray DH, et al. Reamed versus unreamed femoral nails. J Bone Joint Surg B 1998; 80:485–489. [DOI] [PubMed] [Google Scholar]
- 8.Canadian Orthopaedic Trauma Society. Nonunion following intramedullary nailing of the femur with andwithout reaming: results of amulticenter randomized clinical Trial. J Bone Joint Surg A 2003; 85:2093–2096. [PubMed] [Google Scholar]
- 9.Gansslen A, Gosling T, Hildebrand F, et al. Femoral shaft fractures in adults: treatment options and controversies. Acta Chir Orthop Traumatol Cech 2014; 81:108–117. [PubMed] [Google Scholar]
- 10.Trueta J, Cavadias AX. Vascular changes caused by the Kuntscher type of nailing; an experimental study in the rabbit. J Bone Joint Surg Br 1955; 37-b:492–505. [DOI] [PubMed] [Google Scholar]
- 11.ElMaraghy AW, Humeniuk B, Anderson GI, et al. Femoral bone blood flow after reaming and intramedullary canal preparation: a canine study using laser Doppler flowmetry. J Arthroplasty 1999; 14:220–226. [DOI] [PubMed] [Google Scholar]
- 12.Leunig M, Hertel R. Thermal necrosis after tibial reaming for intramedullary nail fixation. A report of three cases. J Bone Joint Surg Br 1996; 78:584–587. [PubMed] [Google Scholar]
- 13.Wenda K, Runkel M, Degreif J, et al. Pathogenesis and clinical relevance of bone marrow embolism in medullary nailing—demonstrated by intraoperative echocardiography. Injury 1993; 24 suppl 3:S73–S81. [DOI] [PubMed] [Google Scholar]
- 14.Shepherd LE, Shean CJ, Gelalis ID, et al. Prospective randomized study of reamed versus unreamed femoral intramedullary nailing: an assessment of procedures. J Orthop Trauma 2001; 15:28–32.discussion 32–23. [DOI] [PubMed] [Google Scholar]
- 15.Selvakumar K, Saw KY, Fathima M. Comparison study between reamed and unreamed nailing of closed femoral fractures. Med J Malays 2001; 56 (suppl D):24–28. [PubMed] [Google Scholar]
- 16.Tornetta P, 3rd, Tiburzi D. Reamed versus nonreamed anterograde femoral nailing. J Orthop Trauma 2000; 14:15–19. [DOI] [PubMed] [Google Scholar]
- 17.Duan X, Li T, Mohammed AQ, et al. Reamed intramedullary nailing versus unreamed intramedullary nailing for shaft fracture of femur: a systematic literature review. Arch Orthop Trauma Surg 2011; 131:1445–1452. [DOI] [PubMed] [Google Scholar]
- 18.Forster MC, Aster AS, Ahmed S. Reaming during anterograde femoral nailing: is it worth it? Injury 2005; 36:445–449. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 20.New York, Higgins JPGS. Cochrane Handbook for Systematic Reviews of Interventions. Vol 5. 2008; Wiley Online Library, [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tornetta P, 3rd, Tiburzi D. The treatment of femoral shaft fractures using intramedullary interlocked nails with and without intramedullary reaming: a preliminary report. J Orthop Trauma 1997; 11:89–92. [DOI] [PubMed] [Google Scholar]
- 23.Bagheri F, Sharifi SR, Mirzadeh NR, et al. Clinical outcome of ream versus unream intramedullary nailing for femoral shaft fractures. Iran Red Crescent Med J 2013; 15:432–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canadian Orthopaedic Trauma Society. Reamed versus unreamed intramedullary nailing of the femur: comparison of the rate of ARDS in multiple injured patients. J Orthop Trauma 2006; 20:384–387. [DOI] [PubMed] [Google Scholar]
- 25.Anwar IA, Battistella FD, Neiman R, et al. Femur fractures and lung complications: a prospective randomized study of reaming. Clin Orthop Rel Res 2004; 422:71–76. [PubMed] [Google Scholar]
- 26.Trompeter A, Newman K. Femoral shaft fractures in adults. Orthop Trauma 2013; 27:322–331. [Google Scholar]
- 27.Klein MP, Rahn BA, Frigg R, et al. Reaming versus non-reaming in medullary nailing: interference with cortical circulation of the canine tibia. Arch Orthop Trauma Surg 1990; 109:314–316. [DOI] [PubMed] [Google Scholar]
- 28.Rhinelander FW. Effects of medullary nailing on the normal blood supply of diaphyseal cortex. Clin Orthop Rel Res 1973; 1998:5–17. [PubMed] [Google Scholar]
- 29.Frolke JP, Nulend JK, Semeins CM, et al. Viable osteoblastic potential of cortical reamings from intramedullary nailing. J Orthop Res 2004; 22:1271–1275. [DOI] [PubMed] [Google Scholar]
- 30.Wenisch S, Trinkaus K, Hild A, et al. Human reaming debris: a source of multipotent stem cells. Bone 2005; 36:74–83. [DOI] [PubMed] [Google Scholar]
- 31.Reynders PA, Broos PL. Healing of closed femoral shaft fractures treated with the AO unreamed femoral nail. A comparative study with the AO reamed femoral nail. Injury 2000; 31:367–371. [DOI] [PubMed] [Google Scholar]
- 32.Reichert IL, McCarthy ID, Hughes SP. The acute vascular response to intramedullary reaming. Microsphere estimation of blood flow in the intact ovine tibia. J Bone Joint Surg Br 1995; 77:490–493. [PubMed] [Google Scholar]
- 33.Zelle BA, Gruen GS, Klatt B, et al. Exchange reamed nailing for aseptic nonunion of the tibia. J Trauma 2004; 57:1053–1059. [DOI] [PubMed] [Google Scholar]
- 34.Chapman MW. The effect of reamed and nonreamed intramedullary nailing on fracture healing. Clinical Orthop Rel Res 1998; 355 (suppl):S230–S238. [DOI] [PubMed] [Google Scholar]
- 35.Fairbank AC, Thomas D, Cunningham B, et al. Stability of reamed and unreamed intramedullary tibial nails: a biomechanical study. Injury 1995; 26:483–485. [DOI] [PubMed] [Google Scholar]
- 36.Grundnes O, Utvag SE, Reikeras O. Effects of graded reaming on fracture healing. Blood flow and healing studied in rat femurs. Acta Orthop Scand 1994; 65:32–36. [DOI] [PubMed] [Google Scholar]
- 37.Forster MC, Bruce AS, Aster AS. Should the tibia be reamed when nailing? Injury 2005; 36:439–444. [DOI] [PubMed] [Google Scholar]
- 38.Kropfl A, Berger U, Neureiter H, et al. Intramedullary pressure and bone marrow fat intravasation in unreamed femoral nailing. J Trauma 1997; 42:946–954. [DOI] [PubMed] [Google Scholar]
- 39.Alho A, Stromsoe K, Ekeland A. Locked intramedullary nailing of femoral shaft fractures. J Trauma 1991; 31:49–59. [DOI] [PubMed] [Google Scholar]
- 40.Orler R, Locher S, Lottenbach M, et al. Retrospective analysis of healing problems after reamed and unreamed nailing of femoral shaft fractures. Unfallchirurg 2002; 105:431–436. [DOI] [PubMed] [Google Scholar]
- 41.Giannoudis PV, Furlong AJ, Macdonald DA, et al. Reamed against unreamed nailing of the femoral diaphysis: a retrospective study of healing time. Injury 1997; 28:15–18. [DOI] [PubMed] [Google Scholar]
- 42.Ponnusamy KE, Kim TJ, Khanuja HS. Perioperative blood transfusions in orthopaedic surgery. J Bone Joint Surg Am 2014; 96:1836–1844. [DOI] [PubMed] [Google Scholar]
- 43.Marik PE. The hazards of blood transfusion. Br J Hosp Med (London, England: 2005) 2009; 70:12–15. [DOI] [PubMed] [Google Scholar]


