Abstract
Shunt infection is a morbid complication of cerebrospinal fluid (CSF) shunting. The catheters with a hydrophilic surface may impede bacterial adherence and thereby reduce catheter-related CSF infection.
A retrospective study compared the occurrence of CSF infection related to use of either standard silastic catheters or hydrogel-coated ventricular catheters (Bioglide, Medtronic). The enrolment was available to neurosurgery patients undergoing shunt surgery from October 2012 to 2015 in two centers. The follow-up period was more than months.
A total of 78 patients were included in the study. In 33 patients 35-cm hydrogel-coated ventricular peritoneum shunts (VPS) were used, and in remaining 45 patients 35-cm standard silastic VPS catheters were used. Infection occurred in 14 (17.9%) patients, including definite VPS-related CSF infection in 6 patients (7.7%) and probable infection in remaining 8 patients (10.3%). There was a significant difference found in patients with total infection between the two groups [RR (95% CI); 0.200 (0.050–0.803), P = 0.014]. Analysis of Kaplan–Meier curve estimates indicated significant statistical difference between the two catheter types in duration (log rank = 4.204, P < 0.05). Significant statistical differences were also found in the subgroups including previous CSF infection within 1 month (log rank = 4.391, P = 0.04), conversion of external ventricular drains to shunt (Log Rank = 4.520, P = 0.03), and hospital stay >1 month (log rank = 5.252, P = 0.02). There was no difference found between the two groups of the patients with other infections within 1 month. The follow-up period was of 36 months.
The hydrogel-coated catheter is a safe and related to lower infection rates for high-risk patients who underwent shunt surgery.
Keywords: hydrocephalus, hydrogel-coated ventricular catheters, infection, ventricular peritoneum shunt
1. Introduction
Shunt infection is a morbid complication of cerebrospinal fluid (CSF) shunting. The different incidences of infection after ventricle peritoneal shunt have been reported by many studies. According to Eager,[1] the infection rate is from 1% to 39%. McGirt et al[2] and Kulkarni et al[3] have also reported the rate as 3% to 15% and 5% to 27%, respectively. Shunt infection may induce epileptic seizure, decrease intellectual performance, and cause the failure of operation.[4,5] Thus, the secondary operation is needed and the mortality rate of patients will be doubled compared with that of the regular patients.[6–8]
Treatment usually requires antibiotic administration and repeated surgery which will extend the hospitalization period, and increase the economic burden and the suffering of patients. Although a lot of ways have been used to prevent infection, including improving the surgical technique and optimizing the timing of surgery, the high infection rate of shunt surgery is still a hard issue to address.[9–10] Therefore, the prospective study on the post shunts infection prevention is crucial.
Because of having bacteria resistance and low infection rate (Bioglide, Medtronic, Wuhan, Hubei), hydrogel-coated ventricular peritoneum shunts (VPSs) have been adopted in our hospital for several years. Strong hydrophilic properties are utilized on the surface which can guarantee a thin aqueous surface coat after dipping in liquid. This “water shield” can effectively prevent bacterial and blood clot adherence in comparison with regular VPS catheters.[11] We report a retrospective clinical study to compare the VPS-related CSF infections between standard catheters and hydrogel-coated catheters (HCCs).
2. Methods
We retrospectively reviewed the records from patients undergoing CSF shunt insertion at the department of neurosurgery (Anhui Provincial Hospital and Tongji Hospital) from October 2012 to 2015. Patient demographics, clinical presentation, CSF shunting history, radiological studies, laboratory values, operative variables, and shunt type were reviewed in each case. The inclusion criteria were new shunts and first shunts for the patient. The high-risk group was defined as previous CSF infection within 1 month, conversion of external ventricular drains (EVDs) to shunt, hospital stay >1 month (both ICU and Neurosurgical ward), and other infections within 1 month.[19] The exclusion criteria were the VPS procedure combined with other surgery, patients <3 years age or > 80 years age. The study was approved by the Ethical Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, China.
Although the group status was not a random control study, the antibiotic regiment, catheter handling, and surgical procedure were not different between the two groups. All patients had their heads shaven and were disinfected routinely. Prophylactic intravenous antibiotics (cefoperazone 1.5g q12h) were used from at a dose of 2/day 30 min pre-operation to 3 days post-operation regardless of the different groups.
Both diagnosis and insertion technique were comprehensively logged by the operating neurosurgeon. The VPS was not exposed to any antibiotics before use. After its insertion, the CSF was sampled immediately for bacterial culture and sensitivity. The CSF was also to be sampled for test through the VPS taps if any clinical symptom occurs (fever, neurological function disorders, headache, bellyache, and other unfavorable conditions). Through the test, VPS-related bacterial infection, which usually could be detected through clinical symptom and bacteria light (<100 colony forming units/0.1 mL) growth in a single CSF sample (or bacterial culture positive), can be traced and treated in time. Two sample confirmations were considered as positive and the bacterial culture of the CSF which sampled at surgery were all negative.
Two CSF samples were sent for bacteria cultures at the same time. Independent t tests and χ2-square analysis were used to compare the differences of composition used in the two groups.
The confidence level was set at 0.05 using two-sided tests. Group infection rates were compared using Kaplan–Meier curve estimates and log rank tests.
3. Result
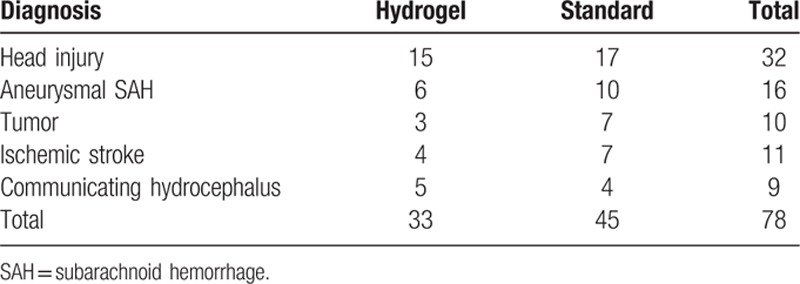
A total of 78 shunt procedures were performed in the high-risk hydrocephalus patients. Of these, in 33 patient 35-cm hydrogel-coated VPSs (Bioglide, Medtronic) were used, and in remaining 45 patients 35-cm standard silastic VPS catheters were used. The comparison of VPS-related infections was eventually based on 78 patients (33 in the hydrogel-coated VPS catheters group and 45 in the silicone catheters group). The follow-up period was 36 months. The man to female ratio was 62%, and the mean patient age was 45 ± 19 years. The standard and HCC groups had similar presenting diagnoses (Table 1), including head injury, subarachnoid hemorrhage ischemic stroke, communicating hydrocephalus, and brain tumor. No relationship was found between the frequency of CSF infection and diagnosis presentation or other characteristics’ presentation.
Table 1.
Diagnosis of qualified subjects.
Infection occurred in 14 (17.9%) patients, including 11 (24.4%) patients in the standard catheters group and 3 (9.1%) in the hydrogel-coated ventricular catheters group (Table 2). There was a significant difference of the total infection patients between the two groups [RR (95% CI); 0.200 (0.050–0.803), P = 0.014]. Analysis of Kaplan–Meier curve estimates indicated significant statistical difference between the two catheter types in duration to onset of definite infection (log rank = 4.204, P < 0.5) (Fig. 1). Most cases were cured by antibiotic therapy. The catheters were removed in 5/11 patients in the standard catheters group and 1/3 patients in the hydrogel-coated ventricular catheters group.
Table 2.
Patients of high-risk infection subgroups (the four high-risk groups overlap each other).
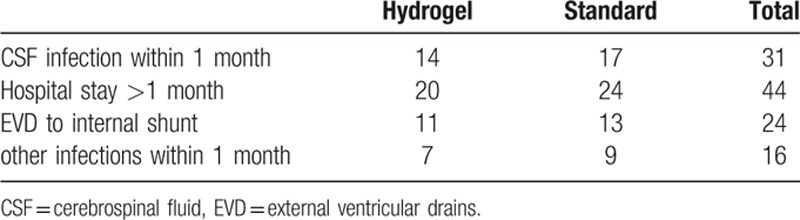
Figure 1.
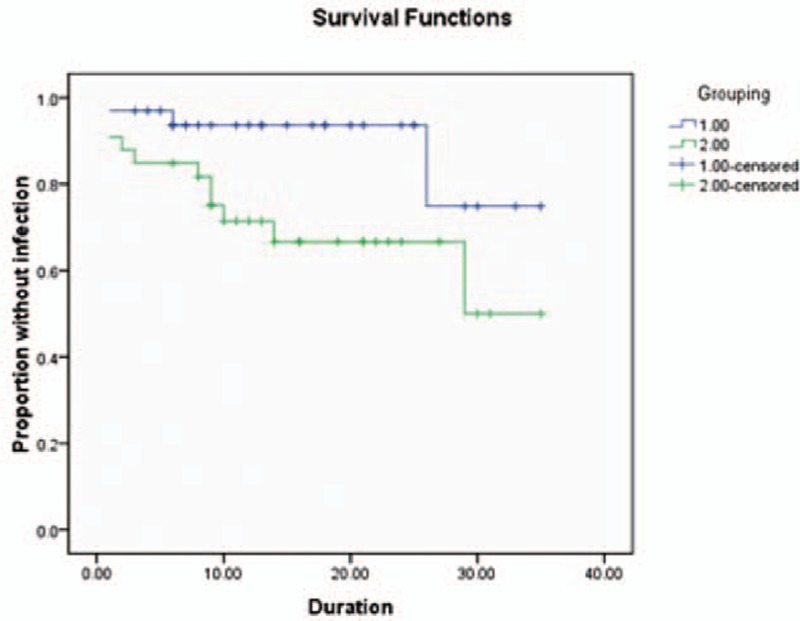
Graph demonstrating Kaplan–Meier cumulative curves for proportion of patients of VPS related CSF infection in the high-risk patients (log rank = 4.204, P < 0.05). CSF = cerebrospinal fluid, VPS = ventricular peritoneum shunt.
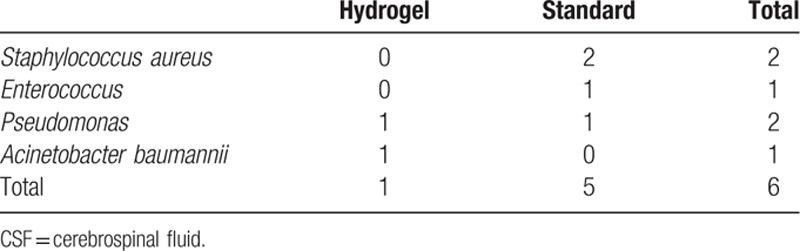
Specific infectious organisms and observed occurrence are listed in Table 3. According to our results, Staphylococcus aureus caused infection in 2 (2.6%) patients in our studies. The other gram-positive agents included Enterococcus (1, 1.2%). Pseudomonas species were the most common gram-negative organism, causing infection in 2 (2.6%) patients. Besides, Acinetobacter baumannii also caused infection in 1 (1.2%) patients.
Table 3.
Organism occurrences in CSF infections.
The four high-risk groups actually overlap each other; yet, the numbers of four groups of two catheter types are similar, respectively. Infection occurs in 7/31 patients with CSF infection within 1 month, including 1/14 in the hydrogel-coated ventricular catheters group and 6/17 in the standard catheters group [RR (95% CI); 0 (1.19–9.09), P = 0.022]. Kaplan–Meier curve estimates indicated significant statistical difference between the two catheter types in duration (log rank = 4.391, P = 0.04) (Fig. 2). Infection occurs in 10/44 patients with hospital stay >1 month. It showed a significant reduction in the hydrogel-coated ventricular catheters group (2/20) compared with the standard catheters group (8/24) [RR (95% CI); 5.143 (0.940–28.141), P = 0.041]. Kaplan–Meier curve estimates showed significant statistical difference between the two catheter types in duration (log rank = 4.520, P = 0.03) (Fig. 3). Owing to the conversion of EVDs to shunt group, infection occurred in 1/11 patients in the coated ventricular catheters group and 5/13 in the standard catheters group. No significant difference were found between the two groups [RR (95% CI); 0.111 (0.010–1.236), P = 0.065]. But Kaplan–Meier curve estimates indicated significant statistical difference between the two catheter types in the duration to onset of definite infection (log rank = 5.252, P = 0.02) (Fig. 4). And for patients, who have other infections within 1 month, no infection occurred in 7 patients of the coated ventricular catheters group and infection occurred in 2/9 patients in the standard catheters group [RR (95% CI); 0.333 (0.022–5.027), P = 0.367]. No differences were found in t test or Kaplan–Meier curve between the two catheter types in the duration to onset of definite infection (log rank = 1.536, P = 0.12) (Fig. 5).
Figure 2.
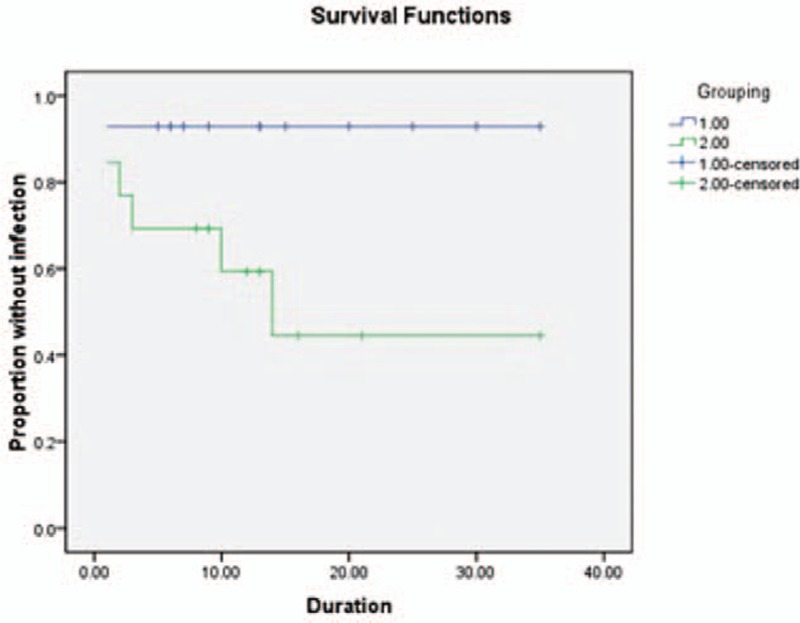
Graph demonstrating Kaplan–Meier cumulative curves for proportion of patients of VPS-related CSF infection in the subgroups of previous CSF infection within 1 month (log rank = 4.391, P = 0.04). CSF = cerebrospinal fluid, VPS = ventricular peritoneum shunt.
Figure 3.
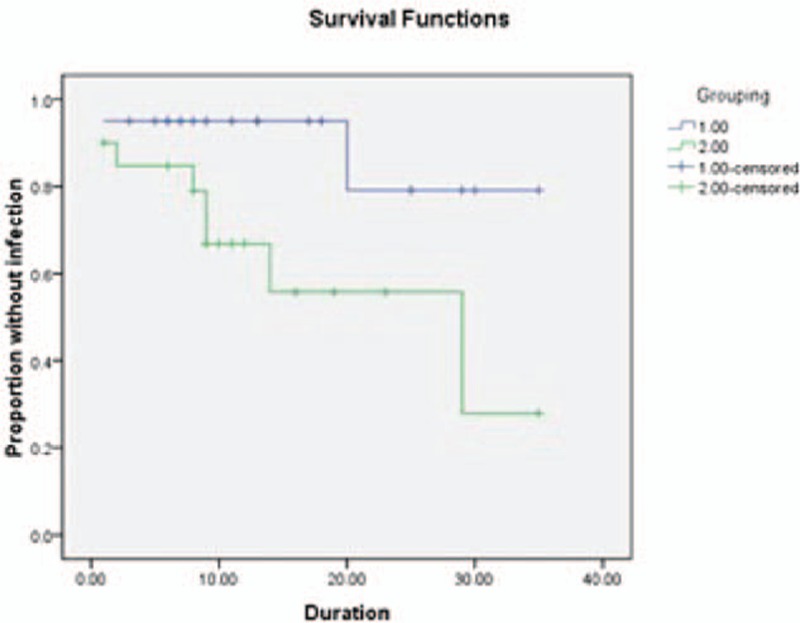
Graph demonstrating Kaplan–Meier cumulative curves for proportion of patients of VPS-related CSF infection in the subgroups conversion of external ventricular drains to shunt (log rank = 4.520, P = 0.03). CSF = cerebrospinal fluid, VPS = ventricular peritoneum shunt.
Figure 4.
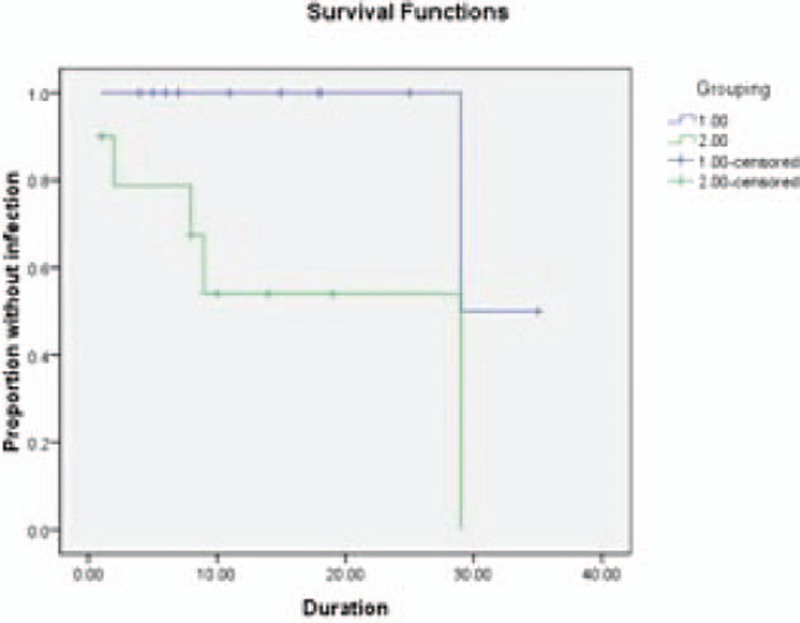
Graph demonstrating Kaplan–Meier cumulative curves for proportion of patients of VPS-related CSF infection in the subgroups hospital stay >1 month (log rank = 5.252, P = 0.02). CSF = cerebrospinal fluid, VPS = ventricular peritoneum shunt.
Figure 5.
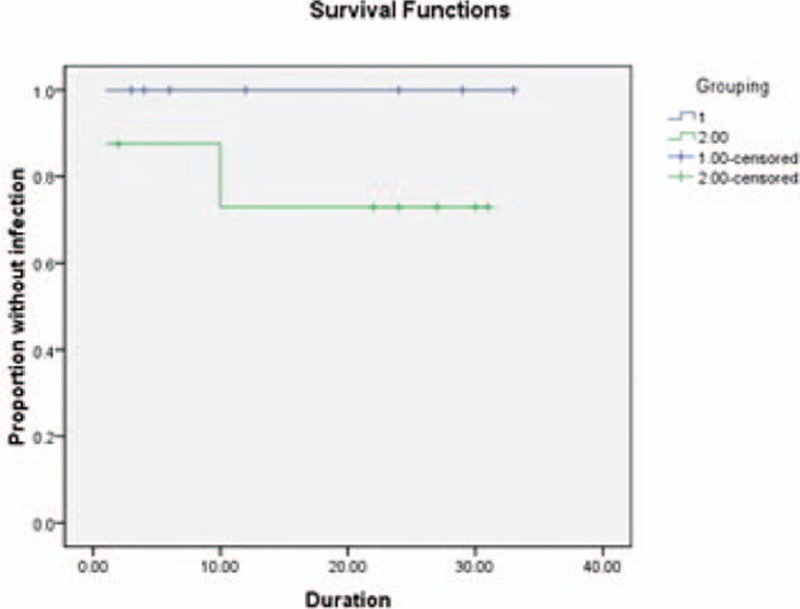
Graph demonstrating Kaplan–Meier cumulative curves for proportion of patients of VPS-related CSF infection in the subgroups of other infections within 1 month (log rank = 1.536, P = 0.12). CSF = cerebrospinal fluid, VPS = ventricular peritoneum shunt.
4. Discussion
Post-operation infections remain the most common and serious problems for ventricular peritoneum shunting. Our study has found a significant incidence of VPS-related CSF infections with coincidence of previous researches.[30–33] According to our experience, the infection rates in normal patients are much lower but can still cause serious consequences if infection occurs. Because the catheter is not the organic part of the body, the infection is more likely to occur compared with regular surgery. However, the infection will be the cause of failure of operations or even death of patients.[12,13]
For shunt surgery, the infection rate depends on many different factors, namely body weight, length of preoperative hospitalization, present ongoing infection, history of previous shunt infection, and the history of multiple shunt revisions.[14–16] Improvements in surgical technique and pre-operative antibiotic therapy have not consistently decreased the incidence of shunt infection. The HCCs have been shown to impair bacterial adherence and surface growth.[17,18] The protective role has been reported in EVD,[19] but the catheters were placed in the ventricular system only for days or weeks. For VPS placement, the anti-infection effect for long time has not been reported.
The bacterial contamination of the catheter at the time of its insertion is considered to be the main cause of VPS-related infections. Staphylococcus aureus was the most common infectious gram-positive bacterium in VPS, which caused infection in 2 patients (4.4%) in the standard catheter group. However, no gram-positive bacterium infection had been found in the HCCs group. Though it lacks statistic difference, we believe that the HCCs can reduce bacterial adherence in the operation, thereby reducing the possibility of gram-positive bacterium infection according to our experience. Yet, it still need further research to prove.
Hydrocephalus can be attributed to various factors, including CSF infection,[20,21] primary meningitis, and the post-surgery infection of a series of neurosurgical diseases. According to Arunodaya's study,[22] the post-neurosurgery nosocomial wound infection rate is 6.9%. Among them, the reoccurrence of infection is the thorniest problem which is not rarely seen even in patient after the VPS implantation. Such patients, who have developed CSF infection within 1 month post-surgery, are recognized and subgrouped as the high risk of shunt infection group. According to our result, HCCs can significantly reduce the infection rate and prolonged the shunt survival duration.
A prolonged hospital stay has been suggested as a correlation factor with low nosocomial infection rates of all types. Yang and Li[23] report an average interval of only 20 days for the detection of post-neurosurgical nosocomial brain infections after prolonged hospital stays. Nosocomial infection rates increase to as high as 30% for patients with extended hospital stays,[24,25] and the infection rate reached 38% in our study. Once infections occurred, patients may have a high tendency to be more antibiotic-resistant and difficult to manage, especially for those in neurosurgical ICU. The advantage of HCCs can also benefit patients in such cases.
EVDs are widely used to relieve high intracranial pressure. However, it also needs to expose the ventricular system to external skin flora for days or even to weeks. Such long exposure can result in infections CSF and raise the infection risk of subsequent shunt infection.[26] Previous research has reported the infection incidence to be 13%.[27] As expected, our research observed a high incidence (28.6%) of infection in this subgroup whereas HCCs can significantly reduce the infection rate. If the infection occurs, a new shunt system is needed. Yet, both the removal of the infected shunt and the new shunt system replacement can increase the catheter-related infection rate. Renier et al[28] found that 17.5% of such shunt replacements developed new infections compared with their overall study rate of 7.9%.
As it is well known, hydrocephalus is usually combined with various neurological dysfunctions, such as coma, hemiplegia, and intelligence quotient reduction. With losing self-living ability, the patients are always bedridden, accompanying various persistent lung infection or urinary tract infection. We conduct combined with other infection as a risk-factor of CSF infection and HCCs will show a preventive effect. Bur the result showed no significant difference between the two groups.
To prevent catheter-related infection, various methods have been adopted such as antibiotic prophylaxis and shunt devices soaked with antiseptics or antibiotics. The effectiveness of prophylactic intravenous antibiotics for infectious prevention remains to be controversial. Several studies have found that it may contribute to a reduced infection rate[29–32] whereas others have reported little benefits.[33–35] According to our assumption, the infecting bacteria enter the intracranial system at the point of catheter insertion and develop at a favorable catheter spot with little immunological intervention within the central nerve system. Nevertheless, the shunt devices can absorb antibiotics during the presoaking period and lower the shunt infection rate which is supported by various recent reports.[36–38] Besides, Parker et al[39] has reported that usage of antibiotic-soaked shunt devices have reduced the catheter-related infection incidence which are effective instruments to prevent pre-operative bacteria colonization of shunt components.
Because of the commercial reason, the HCCs were only applied in the high-risk group rather than in all the patients who underwent VPS surgery. Its effect in the normal risk group and the relationship between the normal and high-risk groups is still confused. However, we believe that the complication prevention will save money and suffering of the patients. Besides, our sample is relatively small and more multicenter-controlled studies are needed in the future.
Recently, a meta-analysis has been published which evaluated antibiotic-impregnated catheters (AICs), silver-coated catheters (SCCs), and HCCs. The result shows that AICs and SCCs reduce the risk for infection in patients undergoing CSF shunting. But lack of date to analysis the effects of HCCs (only 2).[40] Although a retrospective study has some limitation, the trend is clear in our study. It provides some research basis for the use of HCCs to reduce shunt infection especially for patients at a high risk of infection. According to our results, patients within the aforementioned high-risk groups display a higher incidence of infection. Therefore, before VPS surgery, neurosurgeons should evaluate the necessity and timing carefully. HCCs can significantly reduce the infection rate, which is a more safe and efficient choice for the patients at increased risk for shunt infection.
Footnotes
Abbreviations: AICs = antibiotic-impregnated catheters, CSF = cerebrospinal fluid, EVD = external ventricular drain, HCCs = hydrogel-coated catheters, SCCs = silver-coated catheters, VPS = ventricular peritoneum shunt.
The authors have no conflicts of interest to disclose.
References
- 1.Enger PØ, Svendsen F, Wester K. CSF shunt infections in children: experiences from a population-based study. Acta Neurochir 2003; 145:243–248. [DOI] [PubMed] [Google Scholar]
- 2.McGirt MJ1, Woodworth G, Thomas G, et al. Cerebrospinal fluid shunt placement for pseudotumor cerebri-associated intractable headache: predictors of treatment response and an analysis of long-term outcomes. J Neurosurg 2004; 101:627–632. [DOI] [PubMed] [Google Scholar]
- 3.Kulkarni AV1, Drake JM, Lamberti-Pasculli M. Cerebrospinal fluid shunt infection: a prospective study of risk factors. J Neurosurg 2001; 94:195–201. [DOI] [PubMed] [Google Scholar]
- 4.Ammirati M, Raimondi AJ. Cerebrospinal fluid shunt infections in children. A study on the relationship between the etiology of hydrocephalus, age at the time of shunt placement, and infection rate. Childs Nerv Syst 1987; 3:106–109. [DOI] [PubMed] [Google Scholar]
- 5.Choux M, Genitori L, Lang D, et al. Shunt implantation: reducing the incidence of shunt infection. J Neurosurg 1992; 77:875–880. [DOI] [PubMed] [Google Scholar]
- 6.Chadduck W, Adametz J. Incidence of seizures in patients with myelomeningocele: a multifactorial analysis. Surg Neurol 1988; 30:281–285. [DOI] [PubMed] [Google Scholar]
- 7.Shurtleff DB, Foltz EL, Loeser JD. Hydrocephalus. A definition of its progression and relationship to intellectual function, diagnosis, and complications. Am J Dis Child 1973; 125:688–693. [DOI] [PubMed] [Google Scholar]
- 8.Walters BC, Hoffman HJ, Hendrick EB, et al. Cerebrospinal fluid shunt infection. Influences on initial management and subsequent outcome. J Neurosurg 1984; 60:1014–1021. [DOI] [PubMed] [Google Scholar]
- 9.Sciubba DM, Stuart RM, McGirt MJ, et al. Effect of antibiotic-impregnated shunt catheters in decreasing the incidence of shunt infection in the treatment of hydrocephalus. J Neurosurg 2005; 03:131–136. [DOI] [PubMed] [Google Scholar]
- 10.Bruinsma N, Stobberingh EE, Herpers MJ, et al. Ubcutaneous ventricular catheter reservoir and ventriculoperitoneal drain-related infections in preterm infants and young children. Clin Microbiol Infect 2000; 6:202–206. [DOI] [PubMed] [Google Scholar]
- 11.Pearce RS, West LR, Rodeheaver GT, et al. Evaluation of a new hydrogel coating for drainage tubes. Am J Surg 1984; 148:687–691. [DOI] [PubMed] [Google Scholar]
- 12.Caldarelli M, Di Rocco C, La Marca F. Shunt complications in the first postoperative year in children with meningomyelocele. Child's Nerv Syst 1996; 12:748–754. [DOI] [PubMed] [Google Scholar]
- 13.Sgouros S, Malluci C, Walsh AR, et al. Long-term complications of hydrocephalus. Pediatr Neurosurg 1995; 23:127–132. [DOI] [PubMed] [Google Scholar]
- 14.Ratilal B, Costa J, Sampaio C. Antibiotic prophylaxis for surgical introduction of intracranial ventricular shunts: a systematic review. J Neurosurg Pediatr 2008; 1:48–56. [DOI] [PubMed] [Google Scholar]
- 15.Biyani N1, Grisaru-Soen G, Steinbok P, et al. Prophylactic antibiotics in pediatric shunt surgery. Childs Nerv Syst 2006; 22:1465–1471. [DOI] [PubMed] [Google Scholar]
- 16.Bruinsma N. Subcutaneous ventricular catheter reservoir and ventriculoperitoneal drain-related infections in preterm infants and young children. Clin Microbiol Infect 2000; 6:202–206. [DOI] [PubMed] [Google Scholar]
- 17.Choksey MS, Malik IA. Zero tolerance to shunt infections: can it be achieved? J Neurol Neurosurg Psychiatry 2004; 75:87–91. [PMC free article] [PubMed] [Google Scholar]
- 18.Cook AD, Sagers RD, Pitt WG. Bacterial adhesion to poly(HEMA)-based hydrogels. J Biomed Mater Res 1993; 27:119–126. [DOI] [PubMed] [Google Scholar]
- 19.Parker SL1, Attenello FJ, Sciubba DM, et al. Comparison of shunt infection incidence in high-risk subgroups receiving antibiotic-impregnated versus standard shunts. Childs Nerv Syst 2009; 25:77–83. [DOI] [PubMed] [Google Scholar]
- 20.Clark WC, Metcalf JC, Jr, Muhlbauer MS, et al. Mycobacterium tuberculosis meningitis: a report of twelve cases and a literature review. Neurosurgery 1986; 18:604–610. [DOI] [PubMed] [Google Scholar]
- 21.Gelabert M, Castro-Gago M. Hydrocephalus and tuberculous meningitis in children. Report on 26 cases. Childs Nerv Syst 1988; 4:268–270. [DOI] [PubMed] [Google Scholar]
- 22.Arunodaya GR. Infections in neurology and neurosurgery intensive care units. Neurol India 2001; 49 suppl 1:S51–59. [PubMed] [Google Scholar]
- 23.Yang ZM, Li YS. The risk factors of nosocomial infection in severe craniocerebral trauma. Chin J Traumatol 2003; 6:28–31. [PubMed] [Google Scholar]
- 24.Sablotzki A, Ebel H, Muhling J, et al. Dysregulation of immune response following neurosurgical operations. Acta Anaesthesiol Scand 2000; 44:82–87. [DOI] [PubMed] [Google Scholar]
- 25.Tasota FJ, Fisher EM, Coulson CF, et al. Protecting ICU patients from nosocomial infections: practical measures for favorable outcomes. Crit Care Nurse 1998; 18:54–65.quiz 66-57. [PubMed] [Google Scholar]
- 26.Kestle JR, Garton HJ, Whitehead WE, et al. Management of shunt infections: a multicenter pilot study. J Neurosurg 2006; 105:177–181. [DOI] [PubMed] [Google Scholar]
- 27.Hayhurst C, Cooke R, Williams D, et al. The impact of antibiotic-impregnated catheters on shunt infection in children and neonates. Childs Nerv Syst 2008; 24:557–562. [DOI] [PubMed] [Google Scholar]
- 28.Renier D, Lacombe J, Pierre-Kahn A, et al. Factors causing acute shunt infection. Computer analysis of 1174 operations. J Neurosurg 1984; 61:1072–1078. [DOI] [PubMed] [Google Scholar]
- 29.Stenager E, Gerner-Smidt P, Kock-Jensen C. Ventriculostomy related infections—an epidemiological study. Acta Neurochir 1986; 83:20–23. [DOI] [PubMed] [Google Scholar]
- 30.Winfield JA, Rosenthal P, Kanter RK, et al. Duration of intracranial pressure monitoring does not predict daily risk of infectious complications. Neurosurgery 1993; 33:424–430. [DOI] [PubMed] [Google Scholar]
- 31.Wyler AR, Kelly WA. Use of antibiotics with external ventriculostomies. J Neurosurg 1972; 37:185–187. [DOI] [PubMed] [Google Scholar]
- 32.Mayhall CG, Archer NH, Lamb VA, et al. Ventriculostomy-related infections. A prospective epidemiologic study. N Engl J Med 1984; 310:553–559. [DOI] [PubMed] [Google Scholar]
- 33.Kaufmann AM, Lye T, Redekop G, et al. Infection rates in standard vs. hydrogel coated ventricular catheters. Can J Neurol Sci 2004; 31:506–510. [DOI] [PubMed] [Google Scholar]
- 34.Aucoin PJ, Kotilainen HR, Gantz NM, et al. Intracranial pressure monitors. Epidemiologic study of risk factors and infections. Am J Med 1986; 80:369–376. [DOI] [PubMed] [Google Scholar]
- 35.Blomstedt GC. Results of trimethoprim-sulfamethoxazole prophylaxis in ventriculostomy and shunting procedures. A double-blind randomized trial. J Neurosurg 1985; 62:694–697. [DOI] [PubMed] [Google Scholar]
- 36.Darouiche RO, Raad II, Heard SO, et al. Catheter Study Group. A comparison of two antimicrobial-impregnated central venous catheters. N Engl J Med 1999; 340:1–8. [DOI] [PubMed] [Google Scholar]
- 37.Marik PE, Abraham G, Careau P, et al. The ex vivo antimicrobial activity and colonization rate of two antimicrobial-bonded central venous catheters. Crit Care Med 1999; 27:1128–1131. [DOI] [PubMed] [Google Scholar]
- 38.Raad I, Darouiche R, Dupuis J, et al. Central venous catheters coated with minocycline and rifampin for the prevention of catheter-related colonization and bloodstream infections. A randomized, double-blind trial. The Texas Medical Center Catheter Study Group. Ann Intern Med 1997; 127:267–274. [DOI] [PubMed] [Google Scholar]
- 39.Parker SL, Attenello FJ, Sciubba DM, et al. A syst comparison of shunt infection incidence in high-risk subgroups receiving antibiotic-impregnated versus standard shunts. Childs Nerv Syst 2009; 25:77–83. [DOI] [PubMed] [Google Scholar]
- 40.Konstantelias AA1, Vardakas KZ, Polyzos KA, et al. Antimicrobial-impregnated and -coated shunt catheters for prevention of infections in patients with hydrocephalus: a systematic review and meta-analysis. J Neurosurg 2015; 122:1096–1112. [DOI] [PubMed] [Google Scholar]


