Abstract
This study proposed to establish a correlation between the risk score for child obesity and anthropometric, genetic, and bioimpedance characteristics in mothers and newborns, and to assess the discriminant ability for anthropometric parameters to classify over-fatness (defined by bioimpedance body fatness %) in pregnant women.
We performed a cross-sectional study on 388 couples (mother and father) and their newborns admitted in a Tertiary Hospital from Romania. The measured parameters for mothers and their newborns were risk percentage for child obesity, anthropometric characteristics (mid-upper arm circumference [MUAC], tricipital skinfold thickness [TST] of mother and newborn), genetic polymorphisms (human peroxisome proliferator-activated receptor γ [PPARγ2] 34 C > G and transforming growth factor-beta 1 [TGF-β1] 869 T > C gene polymorphisms in both mothers and newborns), and mother's bioimpedance characteristics (fat mass [FM] %).
The obesity risk score according to standard predictable Northern Finland Birth Cohort equation was in our study 4.07%. We found a monotone positive significant correlation between the newborn's risk of childhood obesity and the mother's TST (P = 0.01), as well as a tendency toward statistical significance concerning correlation with mother's MUAC (P = 0.053), without any correlations with the mothers’ bioimpedance parameters and also a positive correlation between the newborn's risk of childhood obesity and the newborn's anthropometrical characteristics like body mass index (BMI), MUAC, and TST (P < 0.001). We observed that the calculated newborn's risk percentage for child obesity was greater for the variant allele of the TGF-β1 869 T > C polymorphism and also for the wild-type C allele of the PPARγ2 34 C > G gene polymorphism. Our study indicated that the best predictors for over-fatness are BMI and MUAC (P = 0.01 < 0.02 and P = 0.019 < 0.02, respectively).
Keywords: bioimpedance gene polymorphisms, mothers, newborns, newborn's risk percentage for child obesity, over-fatness
1. Introduction
Childhood overweight and obesity are major public health problems due to their impact on afterward morbidity and mortality.[1] The incidence of obesity in children increased lately in Europe, even in school-aged children and younger.[2] Obesity is an important entity in pediatric pathology due to both the increased risk of cardiovascular, renal, metabolic complications and the social integration problems as well as psychological ones.[3,4] In addition, prevention and nutritional measures are not adequate, beginning from the moment of birth and then during childhood and adolescence, therefore being in need of major improvements.[5] Thus, according to International Obesity Force Task, the incidence of child obesity increased with 0.8% at the beginning of the 1990s and with over 2% in the 2000s.[6] In Romania, studies estimated a prevalence of overweight between 12.84% and 24%,[7,8] and an incidence of obesity between 5.75%[9,10] and 29%.[7] Overweight and obesity in fertile women represent also an important health problems in both developed and developing countries, varying between 16.8% and 28%.[11–13] This has a great impact on the woman's weight gain during pregnancy and on the newborn's birth weight and children's weight.[2] Assessment of the obesity risk is very important in order to apply early prevention measures and to prevent the complications of obesity in the child, and afterward in the adult period.[14] In cross-sectional studies, it was observed that obesity, genetic factors, low socio-economic status, accelerated weight gain during childhood, sitting in front of a TV, or computer were determining factors for obesity,[14–18] while in longitudinal studies, it was noticed that parental overweight and obesity represented the determining factors.[19,20]
Thus, even since the 1980s, there was the intent to calculate a predictability score for developing obesity. Fuentes's study noticed that the children's obesity risk in adolescence is influenced by the life style, parents’ educational degree,[21] while the study of Plachta-Danielzik et al[22] proved that parents’ obesity, smoking, and reduced physical activity determined overweight in boys and girls between 5 and 16 years. In Finland, 2 big studies called Northern Finland Birth Cohort 1966 and 1986 (NFBC1986) wanted to explore the long-term effect of the genetic and environmental factors on morbidity, on the children's development in the fetal period, and then their influence during childhood, adolescence, and maturity.[23] As a result of these very big cohorts, different authors evaluated diverse aspects of the children's development. Morandi et al[14] analyzed the lifetime NFBC1986 on a number of 4032 children in order to point out predictive factors for overweight/obesity in children, certain parameters among the traditional risk factors (parental body mass index [BMI], gestational weigh gain [GWG] of mothers, behavior, and social indicators) and the genetic score, which included 44 obesity predisposing single-nucleotide polymorphisms (SNPs). The study underlined that the parental BMI was the most important predictor factor in determining children's obesity (P < 0.001 for maternal obesity and P = 0.042 for paternal one), birth weight, maternal GWG, mother's profession, and smoking being independent predictors for obesity, while the genetic factors had a more reduced prediction not globally influencing the standard risk factors.[14] Pirkola et al[24] showed that maternal overweight before pregnancy is a risk factor for abdominal obesity at the age of 16 years. In a meta-analysis which comprised 10 studies on 47,661 participants, Druet et al[2] showed that the risk score, which included the infants’ weight associated with maternal BMI and gender, allowed the obesity risk in children. One study showed that high birth weight is a risk factor for obesity,[11] while other study observed that obesity in early pregnancy doubles the obesity risk in children.[25]
Transforming growth factor-beta (TGF-β) is a multifunctional cytokine involved in the regulation of cellular growth, produced by multiple tissues, including adipose tissue.[26,27] In obese people, both PAI-1 and TGF-β[28] are increased in the visceral and subcutaneous adipose tissues. The correlations between TGF-β and obesity are contradictory. Some studies pointed out that the reduction of TGF-β1, antilipogenesis factor, plays an important role in the pathogenesis of obesity,[29–31] while other studies underlined that the level of TGF-β1 from adipose tissue is increased in the presence of insulin.[32] On the other hand, Yener et al showed lower levels of TGF-β in obese children without being correlated with lipid or insulin resistance,[33] while another study was also pointing out an indirect proportional relation between serum TGF-β levels, BMI, and waist circumference, respectively.[34] Wong et al[35] proved that the TGF-β1 869T > C (Leu10Pro) gene polymorphism is associated with type 2 diabetes mellitus, pointing out a correlation between the C allele of the TGF-β1 869T > C gene polymorphism and serum TGF-β levels.
Human peroxisome proliferator-activated receptor γ (PPAR γ) has also an important role in regulating the lipid and carbohydrates homeostasis, in differentiating adipocytes and depositing the fatty acids, in oncogenesis, and also in inflammation.[4,36,37] The PPARγ2 Pro12Ala gene polymorphism, also known as PPARγ2 34 C > G polymorphisms, was proven to be associated with insulin resistance and obesity, type 2 diabetes, and ischemic stroke.[36,38] The association of the variant allele of PPARγ2 34 C > G with obesity was not constant in all studies. Thus, Luan et al[39] underlined in their study that a reduced intake of polyunsaturated fatty acids versus saturated fatty acids is associated with an increased BMI in carriers of the variant allele. In addition, a direct proportional relationship was pointed out between trans fatty acids and type 2 diabetes mellitus in carriers of the Ala[40] allele, which explains the possible role of PPARγ2 34 C > G polymorphism in the regulation of body weight.[41] Studies on obese children proved that the risk of adiposity is higher in Pro allele homozygotes 34 C > G gene polymorphism, being sex-specific and age-dependent, and related to dietary fat intake,[38,42] while Bhatt et al[36] showed that the variant allele of the PPARγ2 34 C > G polymorphism is associated with obesity and insulin resistance in the Indian population.
On the basis of the above-mentioned facts, we considered the following objectives: The primary objective of our study was to investigate whether the newborns risk percentage for childhood obesity was correlated with the anthropometric, genetic, and bioimpedance characteristics of both mothers and their newborns. We tested the following hypotheses: mid-upper arm circumference (MUAC) and tricipital skinfold thickness (TST) in mothers after delivery and in newborns were correlated with the risk score of obesity; there was a difference concerning the values of risk percentage for obesity between patients (mother and newborn, respectively) with variant TNF-β1 869 T > C or variant PPARγ2 34 C > G versus normal; and fat-free mass percent in mothers and their newborns was correlated with the risk percentage for obesity. Our secondary objective was to assess the discriminant ability of MUAC, BMI, and TST to classify over-fatness defined by fatness % as bioimpedance parameter in pregnant women.
2. Materials and methods
2.1. Study sample
A cross-sectional study was performed on a representative sample of 388 mothers and their newborns, evaluated in a Tertiary Hospital from Romania in an Obstetrics Gynecology Clinic between April 2015 and February 2016.
We included in the study all mothers with the age above 18 years and who had only 1 fetus. We excluded from the study: mothers and newborns who presented intrauterine growth retardation due to congenital malformations, chronic disease, intrauterine infections (C-reactive protein > 5 mg/L), patients without complete clinical, paraclinical, anthropometric, and genetic evaluation, or those who refused to sign the informed consent. All patients included in the study signed the informed consent on their behalf and their newborns’ before inclusion in the study and the research was approved by the Ethics Committee of the University of Medicine and Pharmacy of Tîrgu Mureş (No. 32, March 16, 2015), being also performed in compliance with the principles of the Helsinki declaration.
2.2. Variables of interest
The newborn's risk percentage for child obesity defined by Morandi et al[14] was considered an estimator for predicting childhood obesity at birth, being composed of traditional risk factors, according to the ones used in the Finish cohort,[43] being constituted from parental BMI, number of household members, maternal professional category, gestational smoking, and birth weight.
2.2.1. BMI, MUAC, TST, FM
Mother and newborn measurements were performed by a single trained person and included: weight (kg), height (cm), MUAC, and TST. We evaluated body weight with a daily calibrated scale, with ±10 g error. Also, height was measured with a pedometer, calibrated daily, and was evaluated by standard deviation (SD) (0.1 cm error). In order to measure MUAC, we assessed the arm circumference with a tape measure calibrated at the midpoint between shoulder and elbow tips, and TST was measured in the posterior upper arm using a thickness caliper. BMI was calculated by dividing weight (kg) by standing height squared (m2) and according to Control Disease Center; women with a BMI between 25.0 and 29.9 were considered overweight and obese if BMI was 30.0 or higher.[44]
Bioelectrical impedance analysis (BIA) was effectuated according to the recommendations of the manufacturer's guidelines at a frequency of 50 kHz, using a Tanita BC-420 MA body composition analyzer (Tanita Corp, Tokyo, Japan). Weight was recorded automatically with a 0.5 kg adjustment for the weight of clothes, while height, sex, and age were manually introduced. Prior to the measurement the patients emptied their bladder. The measurement procedure was performed with the patients standing bare feet on the analyzer. BIA assesses the difference in impedance determined by the difference of electric potential between fat and lean tissue. The Tanita Analyzer estimates fat mass (FM).
Over-fatness was a variable defined using a cut-off value of 33% for FM (%).[45,46]
2.2.2. Genetic variables
All genotyping investigations in mothers and newborns were performed on genomic DNA isolated from fresh peripheral blood collected on EDTA, using the Clean-Spin column technology available with the Quick-gDNA MiniPrep Kit (ZymoResearch, Irvine, CA). The TGF-β1 869 T > C gene polymorphism was evaluated by ARMS-PCR (amplification refractory mutation system–polymerase chain reaction) method, as previously described[47] and the PPARγ2 34 C > G gene polymorphism was investigated by PCR-RFLP (restriction fragment length polymorphism) assay, as previously described by Oh et al.[48]
2.3. Statistical analysis
Normally distributed quantitative variables were described using mean ± SD while median and interquartile interval [25th percentile; 75th percentile] were used for variables with deviations from the Gaussian low. Qualitative variables were presented as absolute and relative frequencies. The Mann–Whitney U test for nonparametric data was performed to investigate differences between 2 groups concerning the newborn's risk of childhood obesity while Kruskal–Wallis test was used for comparisons between multiple groups. In order to identify the source of differences, the Kruskal–Wallis test was followed by a post hoc analysis with Dunn test adjusted for multiple testing. A chi-squared test was used to evaluate the Hardy–Weinberg equilibrium.
To assess the size and significance of correlations between the risk for childhood obesity and different mother and newborn parameters, the Spearman correlation coefficient was used.
Univariate logistic regression was performed to test and quantify the individual effect of studied predictors on over-fatness. The effect size was described by odds ratio and their associated 95% confidence interval. In order to establish the performance of MUAC, TST, and BMI in classifying over-fat pregnant women, the receiver operating characteristic (ROC) analysis was used. Over-fatness in pregnant women was defined as FM % >33%. The area under the ROC curve (AUROC) determined using a nonparametric approach was considered an index of predictors’ performance, an estimated value of 0.5 having no ability in predicting the over-fatness, whereas an AUROC of 1.0 had a perfect discriminant ability. For multiple ROC curves, a higher estimated AUROC indicated a better performance of the predictor. The ROC curves for the 3 predictors were then tested for significance using the De Long test adjusted for multiple comparisons.
Considering the coordinates of the ROC curves, we also determined the optimal cut-point for each predictor, as the point that gave a fair weight between specificity and sensitivity. The predictive ability analysis was followed by point estimation and 95% confidence intervals for sensitivity, specificity, positive predictive value, negative predictive values, false positive rate, false negative rate, positive likelihood ratio (LR+), negative LR (LR−), and accuracy. Statistical significance was reached when the estimated level of significance P was <0.05. Statistical analysis was performed using the R software, version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Descriptive statistics of anthropometric treasures
The mean age of mothers included in our study was 29.04 ± 5.41 years. The GWG of the mothers included in the study was 15.05 ± 5.80 kg with a range from 2 to 38 kg, with a BMI before pregnancy of 22.47 ± 3.52 kg/m2 varying between 14.00 and 38.00 kg/m2. At the end of the gestational period, mothers’ BMI was 28.10 ± 3.90 kg/m2. The mean weight of the fathers was 81.68 ± 12.59 kg (ranging from 55 to 150 kg) and their BMI was 26.55 ± 3.81 kg/m2 (ranging from 18.51 to 47.92 kg/m2).
The mean height of the mothers included in the study was 163.56 ± 0.06 cm (ranging from 145 to 180 cm), while for the fathers the mean value of height was 175.43 ± 7.38 (ranging from 150 to 197 cm). Regarding the mothers’ educational degree, 44.80% had superior studies, 31.20% had ≤13 grades, 11.30% had high school studies, 9% had postsecondary studies, and 3.60% were without studies; 65.20% of them were employed, 18.00% were smokers, 0.80% were diabetics and only 4.40% had gestational arterial hypertension (AHT), 47.40% were at their first pregnancy, and 67.30% delivered vaginally (Table 1).
Table 1.
Descriptive statistics of anthropometric characteristics in mothers and their newborns.
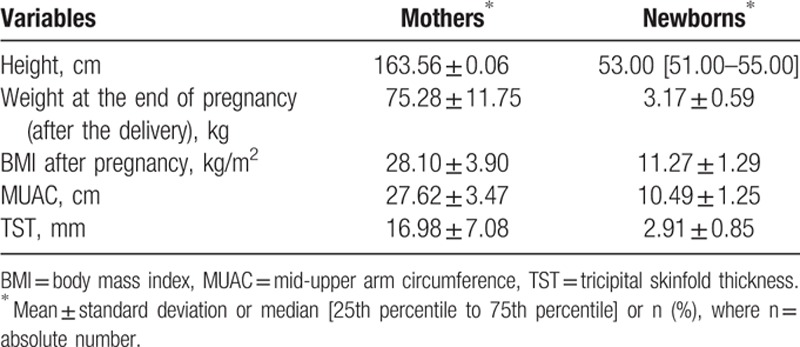
The median length of gestation was 39.00 weeks (25% percentile to 75% percentile: 38.00–40.00) while the majority of mothers had a mean FM equal to 28.74 ± 6.01% with a maximum value of 44%.
Among the newborns included in the study, 77.80% were born at term, 12.40% were preterm babies, and 9.80% were postmature ones. The newborns’ BMI was 11.27 ± 1.29 (ranging from 2.90 to 14.37 kg/m2), with a birth weight of 3167.00 ± 593.80 g, a TST of 2.91 ± 0.85 mm and an MUAC of 10.49 ± 1.25 cm.
The median obesity risk score according to standard predictable NFBC parameters (mother's BMI, father's BMI, number of household members, maternal professional category, gestational smoking, and birth weight) was 4.07% (25% percentile to 75% percentile: 1.57–8.96) with a range from 0.14% to 78.36%.
3.2. Descriptive statistics of genetic characteristics
For the TGF-β1 869 T > C polymorphism, the variant genotype was found in 87.40% of mothers, while the variant PPARγ2 34 C > G genotype was present in 22.40% of cases (Table 2).
Table 2.
Descriptive statistics of genetic characteristics in mothers and their newborns.
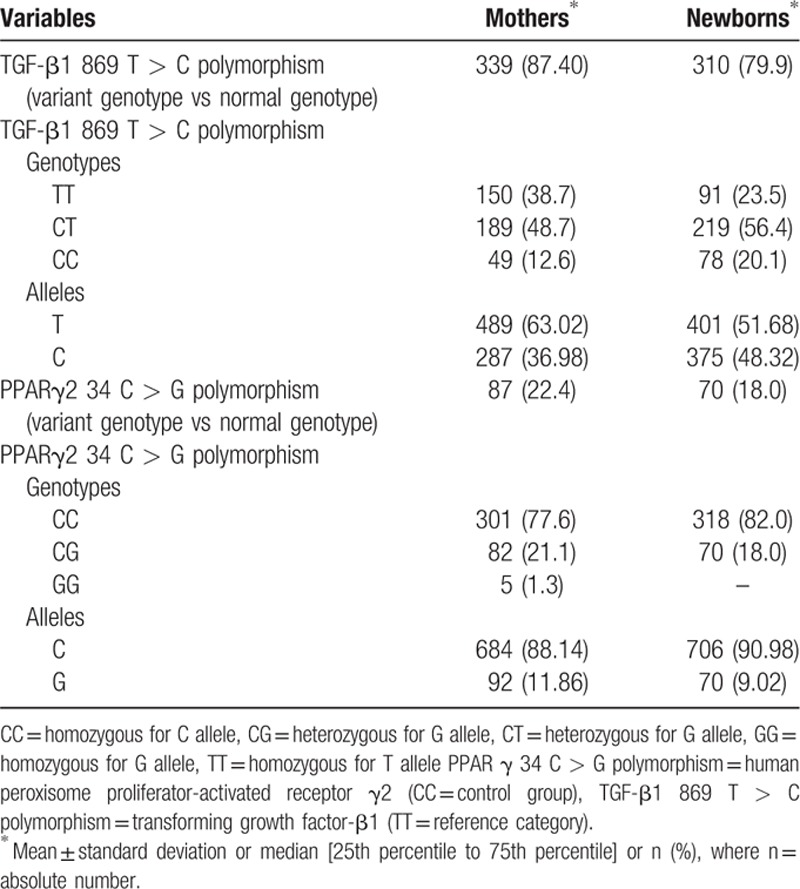
For the TGF-β1 869 T > C polymorphism in newborns, we found the variant genotype in 79.90% of cases, while for the PPARγ2 34 C > G gene polymorphism the variant genotype was observed in 18% of cases (Table 2).
3.3. Main results
3.3.1. Anthropometric parameters
We assessed the correlations between the risk for child's obesity and different maternal parameters (Table 3).
Table 3.
Spearman correlation coefficients (ρ) with the newborn's risk for child obesity and the associated level of significance (P).
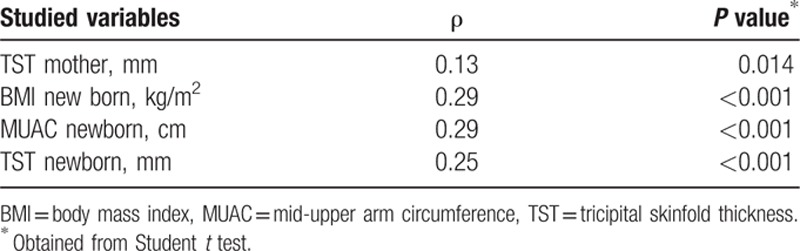
We did not identify a statistical correlation between the fetal risk for obesity and the mother's weight gain (Spearman correlation coefficient ρ = −0.04, P = 0.47), and neither with gestational weeks (Spearman correlation coefficient ρ = 0.10, P = 0.85). Nonetheless, we found a monotone positive correlation, statistically significant, between the child's risk for obesity and mother's TST (Spearman correlation coefficient ρ = 0.13, P = 0.01, Table 3), with a tendency toward correlation between the child's risk for obesity and mother's MUAC (Spearman correlation coefficient ρ = 0.098, P = 0.053). We did not obtain significant statistical correlations between the bioimpedance parameters (FM) in mothers and the child's risk for obesity. We found a monotone positive statistically significant correlation between the newborn's risk for obesity and the newborn's anthropometrical parameters (Table 3): BMI (Spearman correlation coefficient ρ = 0.29, P < 0.001), MUAC (Spearman correlation coefficient ρ = 0.29, P < 0.001), and TST (Spearman correlation coefficient ρ = 0.25, P < 0.001).
We identified an association between the risk for obesity and the newborn's status (Kruskall–Wallis test, P < 0.001); from the post hoc analysis resulted that the source of these correlations was due to the differences between the risks for obesity in preterm newborns and those born at term, and in postmature compared with preterm ones, respectively (Dunn test, P adjusted < 0.001).
3.3.2. Genetics polymorphisms
In our study, we found a statistically significant association between the risk percentage for child obesity and the PPARγ2 34 C > G gene polymorphism in newborns (Kruskal–Wallis test, χ2 = 4.04, P = 0.045). We remarked higher values of risk score for child obesity in carriers of the homozygous CC (wild-type group) than in the heterozygous newborns. No significant association was found related to the PPARγ2 34 C > G polymorphism in mothers (Kruskal–Wallis test, χ2 = 2.13, P = 0.345), although we noticed that mothers with homozygous CC genotype gave birth to the newborns with higher values of risk for child obesity, compared to the mothers with heterozygous and variant homozygous genotype.
Concerning the TGF-β1 869 T > C polymorphism, we did not identify any association with the risk percentage of child obesity neither in mothers (Kruskal–Wallis test, χ2 = 0.31, P = 0.855) nor in newborns (Kruskal–Wallis test, χ2 = 1.50, P = 0.473), but we noticed that the obesity risk values were greater in newborns with heterozygous or variant homozygous genotype than wild-type homozygous.
3.4. Discriminant ability of BMI end-pregnancy, MUAC, and TSF to predict the over-fatness
The results of the univariate logistic regression analysis pointed out that the BMI at the end of the pregnancy, MUAC and TST were predictors for over-fatness (P < 0.001) (Table 4).
Table 4.
Individual effects of possible predictors on over-fatness: results from univariate logistic regression.
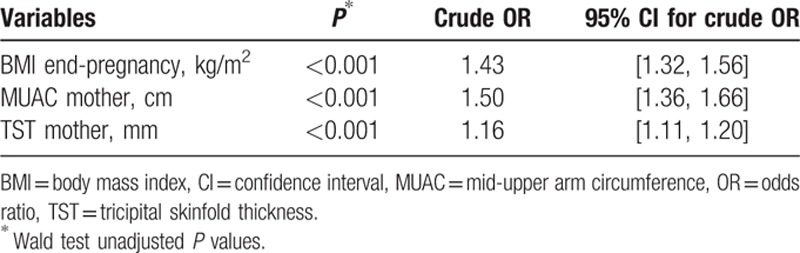
The AUROC values for BMI and MUAC versus over-fatness denoted a good predictive ability: 0.82 (0.78, 0.86) and 0.81 (0.77, 0.86) while TST had an estimated AUROC of 0.75 (0.70, 0.80).
According to the DeLong test, there was no significant difference concerning the discriminant ability of BMI and MUAC (P = 0.83 > 0.02). Based on the ROC graphs (Fig. 1) and the DeLong test, TST was the worst predictor of over-fatness related to BMI and MUAC (P = 0.01 < 0.02 and P = 0.019 < 0.02, respectively).
Figure 1.
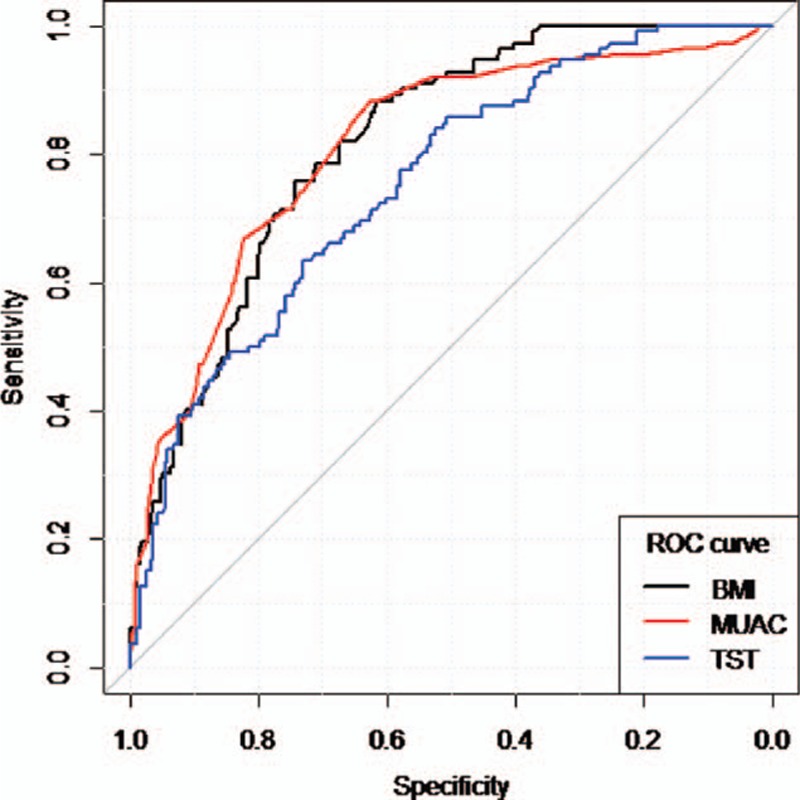
ROC curves for BMI, MUAC, and TST. BMI = body mass index, MUAC = mid-upper arm circumference, ROC = receiver operating characteristic, TST = tricipital skinfold thickness.
Sensitivity and specificity were good for the optimal cut-off value of MUAC (88.4% and 62.3%, respectively), BMI (74.3% and 75.90%, respectively), and TST (72.8% and 63.4%, respectively). The proportion of pregnant women correctly classified using the associated cut-off values of MUAC (69.9%), BMI (74.7%), and TST (70.10%) was acceptable (Table 5).
Table 5.
Indicators of predictive performance for MUAC, BMI, and TST.
4. Discussions
4.1. Correlation between newborn's risk for child obesity and genetic polymorphisms, major findings obtained in our study
Some studies proved that TGF-β1 is a cytokine produced by many tissues, including the adipose tissue.[27] There are some studies, which observed a decreased level of TGF-β during the fat tolerance test in healthy men[31] and an increased level in obese women[49]; also Alessi and Rosmond emphasized an increase of TGF-β1 gene polymorphism with BMI and abdominal tissue in case of morbid obesity.[50,51] Even though there are not any studies performed on children, Kanra et al[28] wanted to establish the relation between the 509 C > T, 915 G > C, and 869 T > C of TGF-β1 gene polymorphisms and obesity-related metabolic disorders, but he did not identify any correlation.
Yamada's study pointed out the correlation between the C allele of the TGF-β1 869 T > C gene polymorphism and serum TGF-β levels.[52] The study of Long proved that both apolipoprotein E and TGF-β1 genes are associated with obesity. For the TGF-β1 869 T > C gene, correlations between the lean mass and SNP5, and the percentage of FM and SNP1 were identified.[26] Correlations of lean mass with haplotype C + C and T + C, and with the G allele of SNP of the TGF-β1 869 T > C gene polymorphism[26] were also encountered. In our study, the TGF-β1 869 T > C variant genotype was present in 87.40% of mothers and in 79.90% of newborns. We also identified in our study that newborns with heterozygous or variant homozygous genotype (TC + CC) have a higher obesity risk, similar to the study of Mao, who showed that the CC genotype of the TGF-β1 869 T > C gene is associated with type 2 diabetes mellitus, thus representing a risk factor,[53] and also with Rodrigues’ study who pointed out that the CC genotype in patients with BMI under 25 kg/m2 and TC genotype of the TGF-β1 869 T > C polymorphism in patients with BMI of 30 kg/m2 are associated with type 2 diabetes mellitus.[54]
The role of PPARγ is well known in the genesis of adiposity and in the regulation of lipid metabolism.[37,38] Even though the PPARγ2 34 C > G gene polymorphism has proven to be associated with obesity, insulin resistance, and type 2 diabetes mellitus,[36,38] the studies performed until now are still contradictory. Therefore, some studies underline that the variant allele of PPARγ2 34 C > G is associated with an increased risk for obesity depending only on the fat dietary intake,[39] with increased risk for type 2 diabetes mellitus,[40] and with a higher risk of adiposity and insulin resistance, respectively.[36] Comparatively, Dedoussis et al[38] showed that adiposity is correlated with PPARγ2 34 C > G polymorphism depending on the sex and age, and Gupta et al[55] pointed out a predominance of the variant allele of the PPAR γ 34 C>G polymorphism in patients with nonalcoholic fatty liver disease. Other studies underlined that thin patients’ carriers of the variant gene have a diminished expression of this gene in their visceral tissue.[41] Mato et al[56] did not obtain any correlation between the polymorphism of this gene and obesity, and type 2 diabetes mellitus, respectively.
In our study, we observed higher values of risk score for child obesity in homozygous wild-type genotype of the PPARγ2 34 C > G polymorphism, so we can say that the presence of the variant allele can have a protective role in determining the obesity in newborns, similar data with those obtained by Scaglioni et al[42] who noticed that the children carrying the variant allele of this polymorphism are protected against the metabolic complications of obesity. Also, we noticed that the homozygous mothers for the C allele had newborns with higher values of risk for child obesity than mothers with heterozygous or variant homozygous genotype. For the PPARγ2 34 C > G polymorphism, the variant genotype was found in 22.40% of mothers and in 18% of newborns.
Therefore, we observed that the genetic factors influenced the determination of obesity in our population, results similar to those reported by Yu et al[11] and Whitaker et al[57] besides other authors.[58–60]
4.2. Correlation between newborn's risk for child obesity and anthropometric characteristics
In time, several studies tried to establish the risk of developing the obesity phenotype, based especially on the traditional risk factors in newborns. Thus, in Finland, on a cohort of over 120,000 pregnant women, the long-term effects of the genetic and environmental factors on fetal development were explored and afterward the manner in which these factors influenced the development during childhood and adolescence.[23] As a result of this cohort, Morandi et al,[14] analyzing the traditional parameters such as the genitor's BMI, GWG of the mother, and other behavioral and social parameters, noticed that these ones are more useful as predictors for severe obesity than for the medium one, the factors being stable until early adulthood. This study proved that parents’ BMI increased the accuracy for the estimation of obesity risk. In addition, Morandi et al[14] underlined that the parents’ BMI, birth weight (big or on the contrary low—the catch-up phenomenon), maternal GWG, number of household members, mother's profession, or obesity represent independent predictor factors (P < 0.001). Also, we observed an association between the risk for obesity and the newborn's status: the difference in risk for obesity in preterm newborns versus term newborns, and also postmature newborns versus preterm ones. There are other studies which sustained the relation between a single factor and obesity in adolescents or adults. Thus, Whitaker et al[57] underlined that parental obesity doubles or more the risk of adult obesity, even though at the age of 10 years the studied children were not obese. In exchange, Plachta-Danielzik et al[22] proved that parents’ obesity or smoking, and also low physical activity were determinant factors in overweight boys, while the parental ones increase the obesity risk in girls.
In our study, maternal GWG was 15.05 ± 5.80 kg, with a BMI at the end of pregnancy of 28.10 ± 3.90 kg/m2 and a paternal BMI of 26.55 ± 3.81 kg/m2; 44.80% of mothers had superior studies, 18% of mothers were smokers, and 4.4% had gestational AHT. Though, these factors, in our study, did not represent independent predictors in determining obesity, when associated they determined a standard predictable obesity risk NFBC of 4.07% (ranging from 1.57% to 8.96%). Morandi et al[14] sustained the validation of this traditional risk score on 2537 children, when he pointed out that the BMI was the most important predictor for obesity, the genetic score (by adding a 44 obesity predisposing SNPs selected according to the genome-wide significant level of association to obesity) having a weak prediction without globally influencing the standard risk factors.
Even though early determination of the child's obesity risk was proven to be important, it is also very important to focus on therapeutic measures such as breastfeeding, alimentation on request, adequate diversification around the age of 6 months, normal size portions, avoidance of prolonged TV or computer activity, avoidance of sweets, and beverage intake.[61] In addition, it is very important to assess the BMI at the moment of adiposity rebound, which seem to be a very good predictor for child and adult obesity. The parents should be aware of these facts, as the previously mentioned parameters cannot be taken under consideration when calculating the neonatal risk score.[62]
Discriminant accuracy is another important parameter as a predictive tool. In the study of Morandi et al, it was noticed that the accuracy of the calculated risk depending on traditional factors was excellent for obesity during childhood (AUROC = 0.85),[14] good for the prediction of overweight/obesity persistence in adolescents (AUROC = 0.75–0.78) and satisfying for the clinical manifestations of obesity in teenagers.[14,63]
Plachta-Danielzik et al[22] proved in his study a decrease of obesity by decreasing parental obesity, and Fuentes et al[21] noticed that children with at least 1 parent with increased BMI had a risk of developing obesity in adolescence, risk that could be influenced by modifications in their life style.
Even though the study of Butte et al[64] underlined the correlation between fat-free mass and FM and birth weight and Larciprete et al[65] observed correlations between total body water and GWG; in our study, we did not identify correlations between fetal risk for obesity and mother's GWG or mother's bioimpedance parameters.
In our study, we encountered correlations between the fetal risk for obesity and mother's TST (P = 0.01) and a tendency toward correlation with the mother's MUAC (P = 0.053). We found in exchange a monotone significant correlation between the fetal risk for obesity at birth and the newborn's anthropometric parameters (BMI, MUAC, and TST).
Pirkola et al[24] and Graversen et al[66] underlined, as we also observed in our study, the fact that maternal obesity clearly represents a risk factor for child obesity at birth and at the age of 16 years. Yu et al[11] and Druet et al[2] proved a relation between birth weight and the risk for obesity, and also the association of the latter one with maternal BMI.
Another previous study by the same authors underlined that GWG may be an important predicting factor for the afterward weight of the fetus, correlated with bioimpedance parameters (total body water, muscle mass, bone mass, and FM) in mothers and also with anthropometric parameters.[67] The authors also emphasized that the variant genotype of the IL-6-572C > G polymorphism was a protection factors against the development of mother obesity.[67]
4.3. Assessment of the discriminant ability of MUAC, BMI, and TST to classify over-fatness
According to Ode et al, BMI should be used carefully when classifying obesity in athletic or nonathletic persons, due to the fact that it does not represent a very accurate predictor for over-fatness according to the ROC curves.[68] In a study performed by Ge et al,[69] it was proven that waist-hip ratio and waist-height ratio were the best predictors for obesity and subclinical atherosclerosis. In exchange, Okereke et al[70] pointed out in their study on 578 pregnant women that MUAC and calf circumference had a sensitivity and specificity of more than 70% for the identification of obesity. In addition, they underlined that MUAC has the best accuracy in predicting obesity (sensitivity of 76% and specificity of 91%).[70] Sagun et al,[71] in a study performed on 387 obese persons from Turkey, of whom 340 were females, showed that MUAC (P < 0.01) was correlated with metabolic syndrome and bioimpedance measurements (over-fatness), and with hip circumference and waist-to-hip ratio, respectively. He underlined that a nonconventional parameter, but easily assessable, namely MUAC, can be a very good predictor for visceral FM (AUROC being 0.63).[71] Craig et al[46] in a study performed in Great Britain on 978 participants (481 children with the age between 5 and 9 years and 497 teenagers with the age between 10 and 14 years) noticed that MUAC is a very good predictor for the assessment of overweight (defined by BMI) and over-fatness (defined by bioelectrical bioimpedance) in children and teenagers. Sensitivity, specificity, and AUC were similar to other studies.[20] In addition, McCarthy et al[72] defined over-fatness as very high levels of body fatness. Higgins et al[45] defined as persons with increased risk of obesity and cardiovascular complications individuals with more than 33% body fat. Based on the ROC curves, Craig et al[46] underlined the fact that the cut-off value of MUAC was the best predictor for overweight.
In our study performed on mothers who gave birth, according to a univariate logistic regression analysis, we noticed that BMI, MUAC, and TST were predictors for over-fatness (P < 0.001). According to AUROC curves, BMI and MUAC were good predictors for over-fatness (0.82 [0.78, 0.86] and 0.81 [0.77, 0.86], respectively), TST being the worst predictor. Similar to the studies of Sagun et al,[71] Okereke et al,[70] Craig et al,[46] we found in our study that sensitivity and specificity were good for the optimal cut-off value of MUAC (88.4% and 62.3%, respectively), with more increased values than for BMI or TST. Therefore, also in our study, MUAC together with BMI are good predictors for over-fatness.
However, certain limitations of this study should be stated. We did not follow the newborns on long term, and thus we do not have a clear image regarding the manner in which risk factors influence the later development of obesity during childhood. A prosecution and a longitudinal re-evaluation of the newborns included in the study at the different stages of life is imposed, when we will also take under consideration other risk factors, such as nutritional intake on different diet principles, eating habits, and other environmental, genetic factors, etc. Another limitation of the study is a result of the fact that we had a limitation in performing the bioimpedance measurements in newborns, limitation provided by the Tanita machine, which could not evaluate the children under the age of 5 years. We did not evaluate food intake on different classes of diet principles (carbohydrates, lipids, proteins) neither in mothers nor in their infants, thus we were not able to establish a correlation between obesity and the energetic intake. The complications of obesity such as insulin resistance, cardiovascular or metabolic outcomes and the degree of social insertion among obese children in collectivities were other lacks that need to be taken under consideration in time. Taking into account the multifactorial etiology of obesity we consider that PPARγ2 and TGF-β1 gene polymorphisms need to be assed in the family members and to be extended to other geographic areas.
Among the advantages of this study, we mention that the statistical parameters’ precision of estimation was very high due to adequate sample size. Also, measurements were taken by a single person who accurately measured all the anthropometric and bioimpedance parameters according to a well-established protocol. All clinically assessed parameters, laboratory parameters, and genetic tests were performed in both mothers and their newborns. This research was, nevertheless, the first performed on mothers and their newborns with regard to the neonatal risk in predicting obesity. As we do not have data in the literature about the association between risk factors, anthropometric and bioimpedance parameters in mothers and their newborns as well as TGF-β1 869 T > C and PPARγ2 34 C > G polymorphisms, gene polymorphisms, we consider that this was a pilot study that needs to be expanded to a larger population. To our knowledge, this was the first study of this kind in Romania and Europe, the study that took under consideration multiple factors that can be involved in determining obesity in newborns. Anyway, the study must be continued and the newborns will have to be evaluated in different, well-established, stages of life in order to observe in which manner these factors influence the children's long-term nutritional status.
5. Conclusions
In our study, we found that the newborn's risk percentage for child obesity was higher for the variant T allele of the TGF-β1 869 T > C gene polymorphism and also for the wild-type C allele of the PPARγ2 34 C > G gene polymorphism.
The obesity risk according to standard predictable parameters (parental BMI, birth weight behavior, and social indicators) was 4.07%. Mother's TST and MUAC were correlated to the child's risk score for obesity, while maternal bioimpedance parameters showed no correlation. The newborn's anthropometrical parameters (BMI, MUAC, and TST) were also correlated with the obesity risk. Maternal BMI and MUAC were the best predictors of over-fatness. Our study pointed out that the most important factors correlated with obesity risk percentage, further longitudinal studies being necessary in this respect, if parameters such as MUAC and TST are considered predictors for child obesity. We can conclude with some clinical implications meaning that genetic polymorphisms and anthropometric measurements like BMI, MUAC, and TST could be useful parameters for diagnosing the obesity in children.
Footnotes
Abbreviations: AHT = arterial hypertension, BIA = bioelectrical impedance analysis , BMI = body mass index, FM = fat mass, GWG = gestational weigh gain, MUAC = mid-upper arm circumference, NFBC = Northern Finland Birth Cohort, PPAR γ = human peroxisome proliferator-activated receptor γ, ROC = receiver operating characteristic, SD = standard deviation, SNP = single-nucleotide polymorphism, TGF-β1 = transforming growth factor-beta 1, TST = tricipital skinfold thickness.
CM and MI contributed equally to this article.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Bj⊘rge T, Engeland A, Tverdal A, et al. Body mass index in adolescence in relation to cause-specific mortality: a follow-up of 230,000 Norwegian adolescents. Am J Epidemiol 2008; 168:30–37.doi:10.1093/aje/kwn096. [DOI] [PubMed] [Google Scholar]
- 2.Druet C, Stettler N, Sharp S, et al. Prediction of childhood obesity by infancy weight gain: an individual-level meta-analysis. Paediatr Perinat Epidemiol 2012; 26:19–26.doi:10.1111/j.1365-3016.2011.01213.x. [DOI] [PubMed] [Google Scholar]
- 3.Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ 2012; 31:219–230.doi:10.1016/j.jhealeco.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Dedoussis GVZ, Kapiri A, Samara A, et al. Expression of inflammatory molecules and associations with BMI in children. Eur J Clin Invest 2010; 40:388–392.doi:10.1111/j.1365-2362.2010.02277.x. [DOI] [PubMed] [Google Scholar]
- 5.Wojcicki JM, Heyman MB. Let's move—childhood obesity prevention from pregnancy and infancy onward. N Engl J Med 2010; 362:1457–1459.doi:10.1056/NEJMp1001857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.eu_platform_2013frep_en.pdf. http://ec.europa.eu/health/nutrition_physical_activity/docs/eu_platform_2013frep_en.pdf Accessed April 5, 2016. [Google Scholar]
- 7.Valean C, Tatar S, Nanulescu M, et al. Prevalence of obesity and overweight among school children in Cluj-Napoca. Acta Endocrinol (Copenh) 2009; 5:213–219. [Google Scholar]
- 8.Mocanu V. Prevalence of overweight and obesity in urban elementary school children in northeastern Romania: its relationship with socioeconomic status and associated dietary and lifestyle factors. BioMed Res Int 2013; 2013:537451.doi:10.1155/2013/537451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coşoveanu S, Bulucea D. Study on the relationship between lifestyle and obesity in kindergarden and primary school children. Acta Medica Marisiensis 2010; 56:322–324. [Google Scholar]
- 10.Coşoveanu S, Bulucea D. Obesity and overweight in children—epidemiology and etiopathogeny. Curr Health Sci J 2011; 37:101–105. [Google Scholar]
- 11.Yu Z, Han S, Zhu J, et al. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS ONE 2013; 8:e61627.doi:10.1371/journal.pone.0061627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heslehurst N, Simpson H, Ells LJ, et al. The impact of maternal BMI status on pregnancy outcomes with immediate short-term obstetric resource implications: a meta-analysis. Obes Rev Off J Int Assoc Study Obes 2008; 9:635–683.doi:10.1111/j.1467-789X.2008.00511.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim SY, Dietz PM, England L, et al. Trends in pre-pregnancy obesity in nine states, 1993–2003. Obes (Silver Spring Md) 2007; 15:986–993.doi:10.1038/oby.2007.621. [DOI] [PubMed] [Google Scholar]
- 14.Morandi A, Meyre D, Lobbens S, et al. Estimation of newborn risk for child or adolescent obesity: lessons from longitudinal birth cohorts. PLoS ONE 2012; 7:e49919.doi:10.1371/journal.pone.0049919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ino T. Maternal smoking during pregnancy and offspring obesity: meta-analysis. Pediatr Int Off J Jpn Pediatr Soc 2010; 52:94–99.doi:10.1111/j.1442-200X.2009.02883.x. [DOI] [PubMed] [Google Scholar]
- 16.Dalziel B, Gosby AK, Richman RM, et al. Association of the TNF-alpha-308G/A promoter polymorphism with insulin resistance in obesity. Obes Res 2002; 10:401–407.doi:10.1038/oby.2002.55. [DOI] [PubMed] [Google Scholar]
- 17.Gortmaker SL, Must A, Sobol AM, et al. Television viewing as a cause of increasing obesity among children in the United States, 1986–1990. Arch Pediatr Adolesc Med 1996; 150:356–362. [DOI] [PubMed] [Google Scholar]
- 18.Janssen I, Katzmarzyk PT, Boyce WF, et al. Comparison of overweight and obesity prevalence in school-aged youth from 34 countries and their relationships with physical activity and dietary patterns. Obes Rev Off J Int Assoc Study Obes 2005; 6:123–132.doi:10.1111/j.1467-789X.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- 19.Dubois L, Girard M. Early determinants of overweight at 4.5 years in a population-based longitudinal study. Int J Obes 2006; 30:610–617.doi:10.1038/sj.ijo.0803141. [DOI] [PubMed] [Google Scholar]
- 20.Reilly JJ, Armstrong J, Dorosty AR, et al. Early life risk factors for obesity in childhood: cohort study. BMJ 2005; 330:1357.doi:10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuentes RM, Notkola I-L, Shemeikka S, et al. Tracking of body mass index during childhood: a 15-year prospective population-based family study in eastern Finland. Int J Obes Relat Metab Disord J Int Assoc Study Obes 2003; 27:716–721.doi:10.1038/sj.ijo.0802271. [DOI] [PubMed] [Google Scholar]
- 22.Plachta-Danielzik S, Landsberg B, Johannsen M, et al. Determinants of the prevalence and incidence of overweight in children and adolescents. Public Health Nutr 2010; 13:1870–1881.doi:10.1017/S1368980010000583. [DOI] [PubMed] [Google Scholar]
- 23.Oulun yliopisto. http://kelo.oulu.fi/NFBC/index.html Accessed April 9, 2016. [Google Scholar]
- 24.Pirkola J, Pouta A, Bloigu A, et al. Risks of overweight and abdominal obesity at age 16 years associated with prenatal exposures to maternal prepregnancy overweight and gestational diabetes mellitus. Diabetes Care 2010; 33:1115–1121.doi:10.2337/dc09-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics 2004; 114:e29–e36. [DOI] [PubMed] [Google Scholar]
- 26.Long J-R, Liu P-Y, Liu Y-J, et al. APOE and TGF-beta1 genes are associated with obesity phenotypes. J Med Genet 2003; 40:918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Sherbini SM, Shahen SM, Mosaad YM, et al. Gene polymorphism of transforming growth factor-β1 in Egyptian patients with type 2 diabetes and diabetic nephropathy. Acta Biochim Biophys Sin 2013; 45:330–338.doi:10.1093/abbs/gmt003. [DOI] [PubMed] [Google Scholar]
- 28.Kanra AR, Tulgar-Kinik S, Verdi H, et al. Transforming growth factor-beta1 (509 C/T, 915 G/C, 869 T/C) polymorphisms are not related to obesity in Turkish children. Turk J Pediatr 2011; 53:645–650. [PubMed] [Google Scholar]
- 29.Corica F, Allegra A, Buemi M, et al. Reduced plasma concentrations of transforming growth factor beta1 (TGF-beta1) in obese women. Int J Obes Relat Metab Disord J Int Assoc Study Obes 1997; 21:704–707. [DOI] [PubMed] [Google Scholar]
- 30.Bastelica D, Mavri A, Verdierl M, et al. Relationships between fibrinolytic and inflammatory parameters in human adipose tissue: strong contribution of TNFalpha receptors to PAI-1 levels. Thromb Haemost 2002; 88:481–487.doi:10.1267/THRO88030481. [PubMed] [Google Scholar]
- 31.Byrne CD, Wareham NJ, Martensz ND, et al. Increased PAI activity and PAI-1 antigen occurring with an oral fat load: associations with PAI-1 genotype and plasma active TGF-beta levels. Atherosclerosis 1998; 140:45–53. [DOI] [PubMed] [Google Scholar]
- 32.Fain JN, Tichansky DS, Madan AK. Transforming growth factor beta1 release by human adipose tissue is enhanced in obesity. Metabolism 2005; 54:1546–1551.doi:10.1016/j.metabol.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 33.Yener S, Demir T, Akinci B, et al. Transforming growth factor-beta 1 levels in women with prior history of gestational diabetes mellitus. Diabetes Res Clin Pract 2007; 76:193–198.doi:10.1016/j.diabres.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 34.Kinik ST, Ozbek N, Yuce M, et al. PAI-1 gene 4G/5G polymorphism, cytokine levels and their relations with metabolic parameters in obese children. Thromb Haemost 2008; 99:352–356.doi:10.1160/TH07-06-0395. [DOI] [PubMed] [Google Scholar]
- 35.Wong TYH, Poon P, Chow KM, et al. Association of transforming growth factor-beta (TGF-beta) T869C (Leu 10Pro) gene polymorphisms with type 2 diabetic nephropathy in Chinese. Kidney Int 2003; 63:1831–1835.doi:10.1046/j.1523-1755.2003.00919.x. [DOI] [PubMed] [Google Scholar]
- 36.Bhatt SP, Misra A, Sharma M, et al. Ala/Ala genotype of Pro12Ala polymorphism in the peroxisome proliferator-activated receptor-β2 gene is associated with obesity and insulin resistance in Asian Indians. Diabetes Technol Ther 2012; 14:828–834.doi:10.1089/dia.2011.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janani C, Ranjitha Kumari BD. PPAR gamma gene—a review. Diabetes Metab Syndr 2015; 9:46–50.doi:10.1016/j.dsx.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 38.Dedoussis GV, Manios Y, Kourlaba G, et al. An age-dependent diet-modified effect of the PPARγ Pro12Ala polymorphism in children. Metabolism 2011; 60:467–473.doi:10.1016/j.metabol.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Luan J, Browne PO, Harding AH, et al. Evidence for gene-nutrient interaction at the PPARgamma locus. Diabetes 2001; 50:686–689. [DOI] [PubMed] [Google Scholar]
- 40.Pisabarro RE, Sanguinetti C, Stoll M, et al. High incidence of type 2 diabetes in peroxisome proliferator-activated receptor gamma2 Pro12Ala carriers exposed to a high chronic intake of trans fatty acids and saturated fatty acids. Diabetes Care 2004; 27:2251–2252. [DOI] [PubMed] [Google Scholar]
- 41.Memisoglu A, Hu FB, Hankinson SE, et al. Interaction between a peroxisome proliferator-activated receptor gamma gene polymorphism and dietary fat intake in relation to body mass. Hum Mol Genet 2003; 12:2923–2929.doi:10.1093/hmg/ddg318. [DOI] [PubMed] [Google Scholar]
- 42.Scaglioni S, Verduci E, Salvioni M, et al. PPAR-gamma2 Pro12Ala variant, insulin resistance and plasma long-chain polyunsaturated fatty acids in childhood obesity. Pediatr Res 2006; 60:485–489.doi:10.1203/01.pdr.0000238259.41560.00. [DOI] [PubMed] [Google Scholar]
- 43.NFBC1986 WEIGHT Calculator. Scribd. https://www.scribd.com/doc/303871455/NFBC1986-WEIGHT-Calculator Accessed May 2, 2016. [Google Scholar]
- 44.Defining Adult Overweight Obesity |Overweight & Obesity|CDC. http://www.cdc.gov/obesity/adult/defining.html Accessed April 27, 2016. [Google Scholar]
- 45.Higgins PB, Gower BA, Hunter GR, et al. Defining health-related obesity in prepubertal children. Obes Res 2001; 9:233–240.doi:10.1038/oby.2001.27. [DOI] [PubMed] [Google Scholar]
- 46.Craig E, Bland R, Ndirangu J, et al. Use of mid-upper arm circumference for determining overweight and overfatness in children and adolescents. Arch Dis Child 2014; 99:763–766.doi:10.1136/archdischild-2013-305137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daneshmandi S, Pourfathollah AA, Pourpak Z, et al. Cytokine gene polymorphism and asthma susceptibility, progress and control level. Mol Biol Rep 2012; 39:1845–1853.doi:10.1007/s11033-011-0927-7. [DOI] [PubMed] [Google Scholar]
- 48.Oh EY, Min KM, Chung JH, et al. Significance of Pro12Ala mutation in peroxisome proliferator-activated receptor-gamma2 in Korean diabetic and obese subjects. J Clin Endocrinol Metab 2000; 85:1801–1804.doi:10.1210/jcem.85.5.6499. [DOI] [PubMed] [Google Scholar]
- 49.Romano M, Guagnano MT, Pacini G, et al. Association of inflammation markers with impaired insulin sensitivity and coagulative activation in obese healthy women. J Clin Endocrinol Metab 2003; 88:5321–5326.doi:10.1210/jc.2003-030508. [DOI] [PubMed] [Google Scholar]
- 50.Alessi MC, Bastelica D, Morange P, et al. Plasminogen activator inhibitor 1, transforming growth factor-beta1, and BMI are closely associated in human adipose tissue during morbid obesity. Diabetes 2000; 49:1374–1380. [DOI] [PubMed] [Google Scholar]
- 51.Rosmond R, Chagnon M, Bouchard C, et al. Increased abdominal obesity, insulin and glucose levels in nondiabetic subjects with a T29C polymorphism of the transforming growth factor-beta1 gene. Horm Res 2003; 59:191–194. [DOI] [PubMed] [Google Scholar]
- 52.Yamada Y, Miyauchi A, Goto J, et al. Association of a polymorphism of the transforming growth factor-beta1 gene with genetic susceptibility to osteoporosis in postmenopausal Japanese women. J Bone Miner Res Off J Am Soc Bone Miner Res 1998; 13:1569–1576.doi:10.1359/jbmr.1998.13.10.1569. [DOI] [PubMed] [Google Scholar]
- 53.Mao S, Zhang J, Zhao M, et al. Association of transforming growth factor-β1 polymorphisms with the risk of diabetes mellitus. Int J Clin Exp Med 2015; 8:21886–21892. [PMC free article] [PubMed] [Google Scholar]
- 54.Rodrigues KF, Pietrani NT, Sandrim VC, et al. Association of a large panel of cytokine gene polymorphisms with complications and comorbidities in type 2 diabetes patients. J Diabetes Res 2015; 2015:605965.doi:10.1155/2015/605965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta AC, Chaudhory AK, Sukriti, et al. Peroxisome proliferators-activated receptor β2 Pro12Ala variant is associated with body mass index in non-alcoholic fatty liver disease patients. Hepatol Int 2010; 5:575–580.doi:10.1007/s12072-010-9225-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mato EPM, Pokam-Fosso PE, Atogho-Tiedeu B, et al. The Pro12Ala polymorphism in the PPAR-β2 gene is not associated to obesity and type 2 diabetes mellitus in a Cameroonian population. BMC Obes 2016; 3:26.doi:10.1186/s40608-016-0104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whitaker RC, Wright JA, Pepe MS, et al. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med 1997; 337:869–873.doi:10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 58.Maio GR, Haddock GG, Jarman HL. Social psychological factors in tackling obesity. Obes Rev Off J Int Assoc Study Obes 2007; 8 suppl 1:123–125.doi:10.1111/j.1467-789X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 59.van Vliet-Ostaptchouk JV, Hofker MH, van der Schouw YT, et al. Genetic variation in the hypothalamic pathways and its role on obesity. Obes Rev Off J Int Assoc Study Obes 2009; 10:593–609.doi:10.1111/j.1467-789X.2009.00597.x. [DOI] [PubMed] [Google Scholar]
- 60.Vámosi M, Heitmann BL, Kyvik KO. The relation between an adverse psychological and social environment in childhood and the development of adult obesity: a systematic literature review. Obes Rev Off J Int Assoc Study Obes 2010; 11:177–184.doi:10.1111/j.1467-789X.2009.00645.x. [DOI] [PubMed] [Google Scholar]
- 61.Dietary Guidelines for Americans 2005. http://health.gov/Dietaryguidelines/dga2005/document/default.htm Accessed May 2, 2016. [Google Scholar]
- 62.Belsky DW, Moffitt TE, Houts R, et al. Polygenic risk, rapid childhood growth, and the development of obesity: evidence from a 4-decade longitudinal study. Arch Pediatr Adolesc Med 2012; 166:515–521.doi:10.1001/archpediatrics.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hosmer DW, Jr, Lemeshow S. Applied Logistic Regression. John Wiley & Sons; 2004. http://books.google.com/books?hl=en&lr=&id=Po0RLQ7USIMC&oi=fnd&pg=PR5&dq=info:XWnvWrtbFK0J:scholar.google.com&ots=DoaVlf0rGX&sig=FQqaXpDYRPx6U-TV3ePEM0Ka2kU Accessed May 2, 2016. [Google Scholar]
- 64.Butte NF, Ellis KJ, Wong WW, et al. Composition of gestational weight gain impacts maternal fat retention and infant birth weight. Am J Obstet Gynecol 2003; 189:1423–1432. [DOI] [PubMed] [Google Scholar]
- 65.Larciprete G, Valensise H, Vasapollo B, et al. Maternal body composition at term gestation and birth weight: is there a link? Acta Diabetol 2003; 40 suppl 1:S222–S224.doi:10.1007/s00592-003-0071-5. [DOI] [PubMed] [Google Scholar]
- 66.Graversen L, S⊘rensen TIA, Gerds TA, et al. Prediction of adolescent and adult adiposity outcomes from early life anthropometrics. Obes (Silver Spring Md) 2015; 23:162–169.doi:10.1002/oby.20921. [DOI] [PubMed] [Google Scholar]
- 67.Mărginean C, Mărginean CO, Bănescu C, et al. The impact of demographic, genetic and bioimpedance factors on gestational weight gain and birth weight in a Romanian population. Medicine (Baltimore) 2016; 95.(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ode JJ, Pivarnik JM, Reeves MJ, et al. Body mass index as a predictor of percent fat in college athletes and nonathletes. Med Sci Sports Exerc 2007; 39:403–409.doi:10.1249/01.mss.0000247008.19127.3e. [DOI] [PubMed] [Google Scholar]
- 69.Ge W, Parvez F, Wu F, et al. Association between anthropometric measures of obesity and subclinical atherosclerosis in Bangladesh. Atherosclerosis 2014; 232:234–241.doi:10.1016/j.atherosclerosis.2013.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okereke CE, Anyaehie UB, Dim CC, et al. Evaluation of some anthropometric indices for the diagnosis of obesity in pregnancy in Nigeria: a cross-sectional study. Afr Health Sci 2013; 13:1034–1040.doi:10.4314/ahs.v13i4.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sagun G, Oguz A, Karagoz E, et al. Application of alternative anthropometric measurements to predict metabolic syndrome. Clin São Paulo Braz 2014; 69:347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCarthy HD, Cole TJ, Fry T, et al. Body fat reference curves for children. Int J Obes 2006; 30:598–602.doi:10.1038/sj.ijo.0803232. [DOI] [PubMed] [Google Scholar]


