Abstract
The functional crosstalk between nonalcoholic fatty liver disease (NAFLD) and hypertension has been reported by some literatures; however, in nonhypertensive individuals, there is no article describes the characteristic of NAFLD. In this study, we aimed to determine the strength of the association between NAFLD with normal blood pressure (BP) in nonhypertensive individuals. This cross-sectional study was conducted in the sixth Affiliated Hospital of Wenzhou Medical University, from October 2007 to December 2011. In brief, 24,200 subjects were enrolled to participate in the survey. Among those subjects, there were 5305 enrolled subjects, those with filling the diagnostic criteria for NAFLD (21.9%; 4803 males and 502 females). Nonhypertension was identified in 17,403 (71.9%; 8179 males and 9224 females). The PR% of NAFLD for the systolic blood pressure (SBP) in quartiles 1 to 4 was 10.83, 12.55, 20.38, and 19.97. SBP, diastolic blood pressure (DBP), sex, age, glutamic pyruvic transaminase, glutamic oxaloacetic transaminase, fasting plasma glucose, uric acid, triglyceride, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol are closely associated with the risk for NAFLD. SBP (odds ratio [OR]: 1.092, 95% confidence interval [CI]: 1.030–1.158; P < 0.05) and DBP (OR: 1.157, 95%CI: 1.094–1.223; P < 0.05) were found to be independent risk factors for NAFLD. Our analysis indicates that BP is significantly associated with NAFLD in nonhypertensive individuals; SBP and DBP are found to be independent risk factors for NAFLD.
Keywords: metabolic syndrome, nonalcoholic fatty liver disease, nonhypertension, risk factor
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is a global public health issue with different features to take into account in different parts of the world. It has become the leading cause of liver disorders in developed countries and also become a significant public health concern in developing countries. Data suggest that the increasing prevalence of NAFLD is closely associated with adoption of a sedentary lifestyle and globalization of Western diet, and this trend is particularly likely to be observed in Asia in the coming decades.[1] In recent years, NAFLD has been regarded as a hepatic manifestation of metabolic syndrome (MS).[2] All factors determining or closely related with MS were significantly higher in NAFLD compared to controls, such as weight, body mass index (BMI), waist circumference, hip circumference, hypertension and blood pressure (BP) values, diabetes, uric acid (UA), glucose and triglyceride (TG).[3]
The most commonly MS definition used is the national cholesterol education program adult treatment panel phase III revised recommendations, in which the MS is defined as having 3 or more of the following 5 metabolic abnormalities: fasting serum glucose 100 mg/dL or more, fasting serum TG 150 mg/dL or more, fasting serum high-density lipoprotein cholesterol (HDL-C) less than 40 mg/dL for men and less than 50 mg/dL for women, systolic blood pressure (SBP)/diastolic blood pressure (DBP) 130/85 mm Hg or more, or use of antihypertensive medications, and waist circumference 102 cm or more for men and 88 cm or more for women.[4,5] The Framingham Offspring Study found that in men and women, elevated BP was the most common component of MS, followed by elevated waist circumference and low HDL-C levels.[6] Another study suggested that primary hypertension and the MS are long-term consequences of the arousal of hypothalamic centers, with background factors in the competitive, complex and hectic social.[7] On the other hand, Sesso et al[8] suggested hypertension may be an inflammatory disease. There is evidence to suggest that high-normal levels of C-reactive protein which lead to inclusion of a proinflammatory state as one of the syndrome's components are common in persons with the MS.[9] The present demonstration of an apparent connection between low-grade inflammation and hypertension supports the concept that elevated BP should be listed as one of the components of the MS.[10]
The MS is a clustering of metabolic risk factors, which represent the main cardiovascular risk factors. NAFLD is associated with an increased risk of cardiovascular diseases (CVDs).[3,11] Schillaci et al[12] proposed that in initially untreated men and women with essential hypertension who had no clinically overt CVD at the baseline examination, MS was an independent predictor of subsequent CVD. Using a modified ATP-III definition with BMI in place of waist circumference, the MS was found to predict coronary heart disease in hypercholesterolemic men and in apparently healthy women.[12] Some literatures have reported the relationship between NAFLD and hypertension,[13–15] but few article are available describing the characteristic of NAFLD in nonhypertensive individuals. This study aimed to determine the strength of the association between NAFLD with normal BP in nonhypertensive individuals.
2. Materials and methods
2.1. Study population
A cross-sectional study was conducted among patients who came to the First Affiliated Hospital of Wenzhou Medical University from October 2007 to December 2011 evaluate the relationship between the BP and NAFLD. Subjects meeting the following criteria were excluded: those taking antihypertensive, antidiabetic agents, lipid-lowing agents, or hypouricemic agents; those with alcohol consumption greater than 140 g/wk for men and 70 g/wk for women; and those with a history of other known causes of chronic liver disease, such as viral hepatitis or autoimmune hepatitis, and those using hepatotoxic medications.[16] A total of 24,200 eligible subjects were enrolled in which 5305 met the diagnostic criteria for NAFLD (4803 male and 502 female). The study protocol was approved by the Local Ethics Committee.
2.2. BP measurements
Readings of clinical BP were obtained in the left arm of patients in the sitting position, after 5 minutes of quiet rest, with a mercury sphygmomanometer. A maximum of 3 BP readings were taken on 3 separate occasions at least 2 weeks apart. Systolic and diastolic BP were recorded at the first appearance (phase I) and the disappearance (phase V) of Korotkoff sounds. Baseline BP values were the average of the last 2 of 3 consecutive measurements obtained at intervals of 3 minutes. Patients with a clinical SBP less than 130 mm Hg and/or DBP less than 80 mm Hg were defined as nonhypertensive. PP was the difference between the SBP and DBP. Pulse pressure index (PPI) = pulse pressure (PP)/SBP. BMI (kg/m2), used as an index of body fat, was calculated as weight in kilograms divided by height in meters squared.[17]
2.3. Biochemical analyses
Fasting food samples were obtained from an antecubital vein. All laboratory measurements were performed after at least 12 hours. The values included serum albumin, glutamic pyruvic transaminase (GPT), glutamic oxaloacetic transaminase (GOT), fasting plasma glucose (FPG), urea nitrogen, creatinine, UA, total cholesterol (TC), TG, HDL-C, and low-density lipoprotein cholesterol (LDL-C). All values were measured by an Olympus AU640 autoanalyzer (Olympus, Kobe, Japan) using standard methods.
2.4. Ultrasonography
Hepatic ultrasonographies were examined by experienced radiologists who were blinded to the laboratory and clinical details of the subjects at the time of the procedure. Hepatic ultrasonic examination was performed in all subjects by a trained ultrasonographist who was unaware of the clinical and laboratory data, using a Toshiba Nemio 20 sonography machine (Toshiba, Tokyo, Japan) with a 3.5-MHz probe. The diagnosis of fatty liver was made on the basis of characteristic ultrasonographic features consistent with liver and kidney echo discrepancy, the presence of an increased liver echogenicity or “bright liver,” poor echo penetration into the deep portion of the liver, and vascular blurring either singly or in combination.[17]
2.5. Statistical analyses
Results were expressed as mean ± standard deviations for continuous variables and as frequencies for categorical variables. The Student t test or the Mann–Whitney U test was used to compare continuous data, while categorical variables was performed using the chi-square test.[18] Linear regression analysis was used to compare to determine the relationship between BP level and prevalence of NAFLD and MS. Stepwise multiple regression analysis (backward: Wald; entry: 0.05, removal: 0.10) was used to evaluate the risk factors for NAFLD. P < 0.05 was considered statistically significant. All analyses were performed using the SPSS software package version 11.5 for Windows (SPSS Inc., Chicago, IL).
3. Results
3.1. Characteristics of study subjects
Of the 24,200 enrolled subjects, 5305 filled the diagnostic criteria for NAFLD (21.9%; 4803 males and 502 females). Nonhypertension was identified in 17,403 (71.9%; 8179 males and 9224 females). Furthermore, in these nonhypertension patients, 2651 patients (15.23%; 2383 males and 268 females) were diagnosed as NAFLD. Characteristics of the subjects according to their NAFLD status are presented in Table 1. The NAFLD group was older, and had higher serum albumin, GPT, GOT, FPG, urea nitrogen, creatinine, UA, TC, TG, LDL-C, and lower HDL-C. More male appear to have NAFLD than females. Higher BP levels within normal BP were observed in the subjects with NAFLD than in those without NAFLD (Table 1).
Table 1.
Characteristics of study subjects according to NAFLD status in nonhypertensive individual.

3.2. Association of BP level with prevalence rate of NAFLD and MS
All subjects were classified into quartile by their SBP or DBP levels. The PR% of NAFLD in the subjects with different quartile levels of SBP or DBP was analyzed. For males, SBP quartile 1 (Q1) was less than 108 mm Hg, quartile 2 (Q2) was 108 to 112 mm Hg, quartile 3 (Q3) was 113 to 120 mm Hg, and quartile 4 (Q4) was 121 to 129 mm Hg. For males, DBP Q1 was less than 70 mm Hg, Q2 was 70 to 78 mm Hg, Q3 was 79 to 80 mm Hg, and Q4 was 81 to 89 mm Hg. For females, SBP quartile 1(Q1) was less than 100 mm Hg, quartile 2 (Q2) was 100 to 108 mm Hg, quartile 3 (Q3) was 109 to 115 mm Hg, and quartile 4 (Q4) was 116 to 129 mm Hg. For females, DBP Q1 was less than 66 mm Hg, Q2 was 66 to 70 mm Hg, Q3 was 71 to 78 mm Hg, and Q4 was 79 to 89 mm Hg. As shown in Table 2, the PR% of NAFLD for the SBP in Q1, Q2, Q3, and Q4 was 8.99, 16.63, 20.58, and 16.20, respectively. In Table 4, the PR% of NAFLD for the DBP in Q1, Q2, Q3, and Q4 was 10.83, 12.55, 20.38, and 19.97, respectively. Table 3, Table 5, Figs. 1 and 2 showed that the PR% of MS, all tended to increase with increases in the SBP or DBP level. These results show that the SBP or DBP level in normal range is an important factor for MS.
Table 2.
Associations of SBP level with prevalence rate of NAFLD.
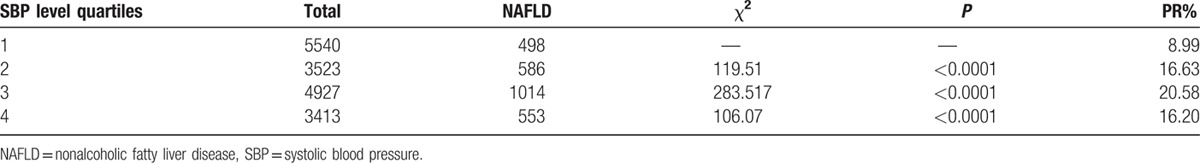
Table 4.
Association of DBP level with prevalence rate of NAFLD.

Table 3.
Association of SBP level with prevalence rate of MS.

Table 5.
Association of DBP level with prevalence rate of MS.
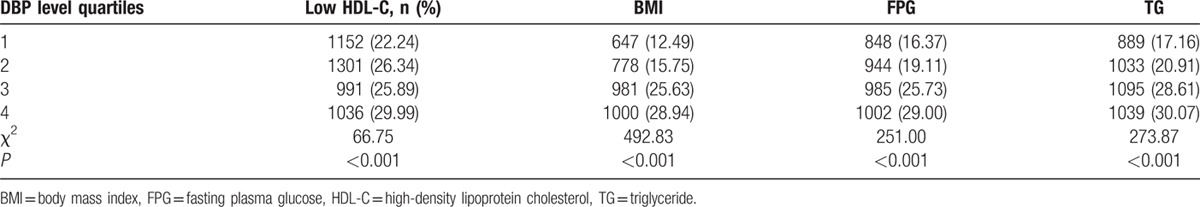
Figure 1.
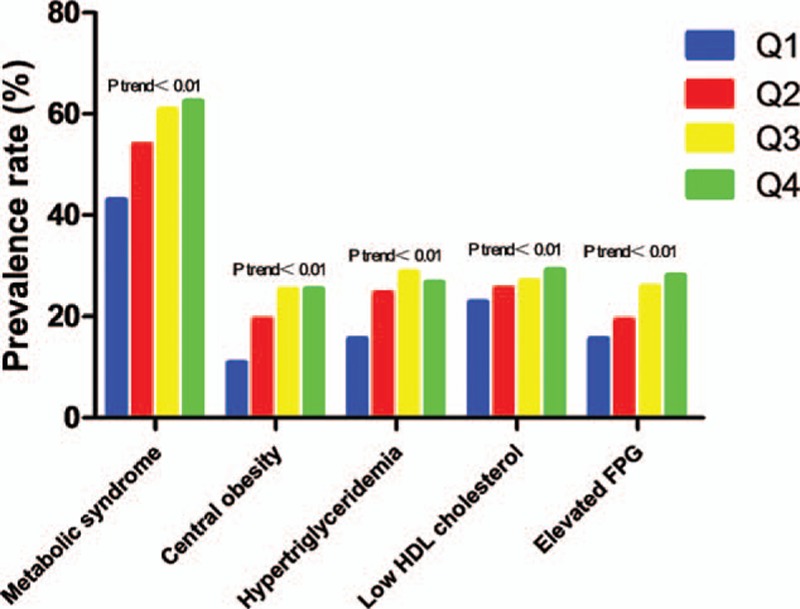
Prevalence rate of MS in the subjects with different quartile levels of SBP. The prevalence rate of MS and its components including low HDL-C, BMI, elevated FPG, and hypertriglyceridemia all showed increasing trends with the increases in SBP levels. BMI = body mass index, FPG = fasting plasma glucose, HDL-C = high-density lipoprotein cholesterol, MS = metabolic syndrome, SBP = systolic blood pressure.
Figure 2.

Prevalence rate of MS in the subjects with different quartile levels of DBP. The prevalence rate of metabolic syndrome and its components including low HDL-C, BMI, elevated FPG, and hypertriglyceridemia all showed increasing trends with the increases in DBP levels. BMI = body mass index, FPG = fasting plasma glucose, HDL-C = high-density lipoprotein cholesterol, MS = metabolic syndrome, DBP = diastolic blood pressure.
3.3. Risk factor analysis for NAFLD
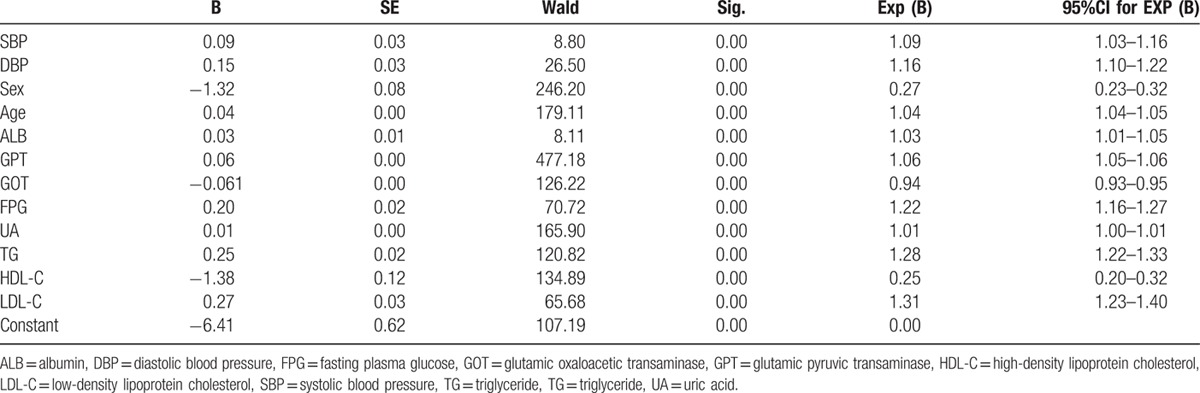
We used stepwise multiple regression analysis to evaluate risk factors for NAFLD. Our results showed that SBP, DBP, sex, age, ALB, GPT, GOT, FPG, UA, TG, HDL-C, and LDL-C are closely associated with the risk for NAFLD. SBP (odds ratio [OR]: 1.092, 95% confidence interval [CI]: 1.030–1.158; P < 0.05) and DBP (OR: 1.157, 95%CI: 1.094–1.223; P < 0.05) were found to be independent risk factors for NAFLD (Table 6).
Table 6.
Risk factors for NAFLD.
4. Discussion
The main findings of this study were as follows: the BP level even in nonhypertensive individuals was significantly associated with NAFLD. The SBP or DBP levels correlated with the PR% of NAFLD. The SBP or DBP levels had a close relationship with MS, such as FPG, UA, TC, TG, and LDL-C. The data also showed that elevated BP level even in nonhypertensive individuals contributed to the risk for NAFLD.
NAFLD may be considered as an additional feature of MS, with specific hepatic insulin resistance. Using the homeostasis model assessment method, Marchesini et al[19] showed that, in a large series of patients, irrespective of BMI, fat distribution, or glucose tolerance, insulin resistance was the laboratory finding most closely associated with the presence of NAFLD. It is possible that a change in body fat during the follow-up period could influence risk of incident hypertension.[20] A study by Bombelli et al[21] showed that an increased in BMI and waist circumference is relationship with an increased adjusted risk of developing hypertension and the rate of change in BMI over the life course increased the risk of incident hypertension in a dose-response fashion, with the highest risk among men with the greatest increase in BMI (hazard ratio, 2.52). Regression analyses showed that elevated insulin resistance mediated the association between hypertension and fatty liver.[22] The present article revealed that the BP level even within normal range also associated with MS.
Early functional and structural changes of the arterial wall have been recently identified both as independent prognostic factors and as a target of treatment in patients with essential hypertension.[23] A higher level of brachial-ankle pulse wave velocity, a measurement of arterial stiffness, was independently associated with the prevalence risk for NAFLD regardless of classical CVD risk factors and other components of MS.[24]
In carotid artery wall thickness and prevalence of carotid plaques in diabetic as well as nondiabetic individuals with NAFLD, cross-sectional observations had documented a marked increase.[25] Data showed that the level of liver steatosis represented the damage degree of vascular and it was also considered as a risk factor for cardiovascular complications, which was regarded as a clinical condition characterized by hemodynamic overload and a complex disease. Further, dyslipidemia, IR, and some other metabolic factors can operate on vascular damage and liver disease in this complex disease.[25] On the other hand, the left ventricular (LV) hypertrophy was a major determinant of diastolic abnormalities in hypertension. In response to LV pressure overload, myocardial tissue damage, myocardial fibrosis, and impairment of the LV function would be caused by increased BP levels.[26] And, in patients with NAFLD compared to the controls, mild abnormalities could be found in the LV structure, including increased left ventricular mass (LVM), left ventricular mass index (LVMI), and LV wall thickness.[27]
The concurrence of insulin resistance and cardiac diastolic dysfunction in hypertensive patients with NAFLD is in agreement with the recent view of a common pathogenetic mechanism for both increased hepatic fat content and heart damage.[28] There are some limitations of this study. First, ultrasonography has limitations in detecting fatty liver content, and it is neither able to quantify fatty liver deposits nor able to detect the level of inflammation or fibrosis. However, the advantages of ultrasonography include its safety, low cost, repeatability, satisfied sensitivity, and specificity. Second, patients with antihypertensive, antidiabetic, and lipid-lowering medications, which may influence the natural characteristics of NAFLD, were excluded from this study. Third, although the PR% of NAFLD for both SBP and DBP in Q4 (highest BP among the groups) were lower than those in Q3, the increasing trend of NAFLD in Q4 is obvious compared with Q1 and Q2. The reason why NAFLD in Q4 lower than those in Q3 might be that the size sample is not large enough to exhibit the phenomenon of NAFLD in Q4 higher than those in Q3.
In conclusion, BP is significantly associated with NAFLD in nonhypertensive individuals, SBP and DBP are found to be independent risk factors for NAFLD.
Footnotes
Abbreviations: BMI = body mass index, BP = blood pressure, CI = confidence interval, CVDs = cardiovascular diseases, DBP = diastolic blood pressure, FPG = fasting plasma glucose, GOT = glutamic oxalacetic transaminase, GPT = glutamic pyruvic transaminase, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, LV = left ventricular, LVM = left ventricular mass, LVMI = left ventricular mass index, MS = metabolic syndrome, NAFLD = nonalcoholic fatty liver disease, NCEP-ATP III = national cholesterol education program adult treatment panel phase III, OR = odds ratio, PP = pulse pressure, PPI = pulse pressure index, SBP = systolic blood pressure, TC = total cholesterol, TG = triglyceride, UA = uric acid.
L-YQ, J-FT, and Y-HD are the first coauthors.
HZ, L-YQ, D-SH, and J-FT planned the article and contributed to data collection, discussing content, writing, and reviewing the article. Y-HD, JP, and X-DC conceived the article, and participated in its design and helping to writing the article.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013; 10:686–690. [DOI] [PubMed] [Google Scholar]
- 2.Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 2001; 50:1844–1850. [DOI] [PubMed] [Google Scholar]
- 3.Salvi P, Ruffini R, Agnoletti D, et al. Increased arterial stiffness in nonalcoholic fatty liver disease: the Cardio-GOOSE study. J Hypertens 2010; 28:1699–1707. [DOI] [PubMed] [Google Scholar]
- 4.National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421. [PubMed] [Google Scholar]
- 5.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 6.Rutter MK, Meigs JB, Sullivan LM, et al. Insulin resistance, the metabolic syndrome, and incident cardiovascular events in the Framingham Offspring Study. Diabetes 2005; 54:3252–3257. [DOI] [PubMed] [Google Scholar]
- 7.Bjorntorp P, Holm G, Rosmond R, et al. Hypertension and the metabolic syndrome: closely related central origin? Blood Press 2000; 9:71–82. [DOI] [PubMed] [Google Scholar]
- 8.Sesso HD, Buring JE, Rifai N, et al. C-reactive protein and the risk of developing hypertension. JAMA 2003; 290:2945–2951. [DOI] [PubMed] [Google Scholar]
- 9.Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001; 285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 10.Grundy SM. Inflammation, hypertension, and the metabolic syndrome. JAMA 2003; 290:3000–3002. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds K, Wildman RP. Update on the metabolic syndrome: hypertension. Curr Hypertens Rep 2009; 11:150–155. [DOI] [PubMed] [Google Scholar]
- 12.Schillaci G, Pirro M, Vaudo G, et al. Prognostic value of the metabolic syndrome in essential hypertension. J Am Coll Cardiol 2004; 43:1817–1822. [DOI] [PubMed] [Google Scholar]
- 13.Catena C, Bernardi S, Sabato N, et al. Ambulatory arterial stiffness indices and non-alcoholic fatty liver disease in essential hypertension. Nutr Metab Cardiovasc Dis 2013; 23:389–393. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Suarez A, Guerrero JM, Elvira-Gonzalez J, et al. Nonalcoholic fatty liver disease is associated with blood pressure in hypertensive and nonhypertensive individuals from the general population with normal levels of alanine aminotransferase. Eur J Gastroenterol Hepatol 2011; 23:1011–1017. [DOI] [PubMed] [Google Scholar]
- 15.Fallo F, Dalla Pozza A, Sonino N, et al. Nonalcoholic fatty liver disease, adiponectin and insulin resistance in dipper and nondipper essential hypertensive patients. J Hypertens 2008; 26:2191–2197. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Xu C, Yu C, et al. Association of serum uric acid level with non-alcoholic fatty liver disease: a cross-sectional study. J Hepatol 2009; 50:1029–1034. [DOI] [PubMed] [Google Scholar]
- 17.Sesti G, Fiorentino TV, Arturi F, et al. Association between noninvasive fibrosis markers and chronic kidney disease among adults with nonalcoholic fatty liver disease. PloS One 2014; 9:e88569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu ZY, Cen C, Shao Z, et al. Association between serum alpha-L-fucosidase and non-alcoholic fatty liver disease: cross-sectional study. World J Gastroenterol 2016; 22:1884–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med 1999; 107:450–455. [DOI] [PubMed] [Google Scholar]
- 20.Sung KC, Wild SH, Byrne CD. Development of new fatty liver, or resolution of existing fatty liver, over five years of follow-up, and risk of incident hypertension. J Hepatol 2014; 60:1040–1045. [DOI] [PubMed] [Google Scholar]
- 21.Bombelli M, Facchetti R, Sega R, et al. Impact of body mass index and waist circumference on the long-term risk of diabetes mellitus, hypertension, and cardiac organ damage. Hypertension 2011; 58:1029–1035. [DOI] [PubMed] [Google Scholar]
- 22.Donati G, Stagni B, Piscaglia F, et al. Increased prevalence of fatty liver in arterial hypertensive patients with normal liver enzymes: role of insulin resistance. Gut 2004; 53:1020–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 24.Lee YJ, Shim JY, Moon BS, et al. The relationship between arterial stiffness and nonalcoholic fatty liver disease. Digest Dis Sci 2012; 57:196–203. [DOI] [PubMed] [Google Scholar]
- 25.Sciacqua A, Perticone M, Miceli S, et al. Endothelial dysfunction and non-alcoholic liver steatosis in hypertensive patients. Nutr Metab Cardiovasc Dis 2011; 21:485–491. [DOI] [PubMed] [Google Scholar]
- 26.Capasso JM, Palackal T, Olivetti G, et al. Left ventricular failure induced by long-term hypertension in rats. Circ Res 1990; 66:1400–1412. [DOI] [PubMed] [Google Scholar]
- 27.Fotbolcu H, Yakar T, Duman D, et al. Impairment of the left ventricular systolic and diastolic function in patients with non-alcoholic fatty liver disease. Cardiol J 2010; 17:457–463. [PubMed] [Google Scholar]
- 28.Bellentani S, Bedogni G, Tiribelli C. Liver and heart: a new link? J Hepatol 2008; 49:300–302. [DOI] [PubMed] [Google Scholar]


