Abstract
Talin-1 functions to regulate cell–cell adhesion, and its altered expression was reported to be associated with human carcinogenesis.
A total of 280 tissue specimens from prostate cancer (PCa) patients who underwent radical prostatectomy, 75 cases of benign prostatic hyperplasia (BPH) tissue, and 6 cases of normal prostate tissue specimens were collected for construction of tissue microarray and subsequently subjected to immunohistochemical staining of Talin-1 expression.
Talin-1 expression was significantly higher in PCa than both normal and BPH tissues (P <0.001). Talin-1 expression in PCa tissues was associated with preoperative prostate-specific antigen (PSA) level, Gleason score, tumor stage, lymph node metastasis, positive surgical margin, extracapsular extension and seminal vesicle invasion (all P <0.05). Logistic regression analysis showed that Talin-1 and Gleason score were independent risk factors for lymph node metastasis of PCa (P <0.001). Receiver operating characteristic (ROC) curve indicated that Talin-1 expression (AUC = 0.766) had a better accuracy to predict PCa lymph node metastasis than Gleason score (AUC = 0.697), whereas their combination could further enhance the prediction accuracy (AUC = 0.803). Kaplan–Meier curve analysis showed that increased Talin-1 expression was associated with shortened biochemical-free survival of PCa patients after radical prostatectomy (P <0.001).
These findings suggested that Talin-1 protein was significantly upregulated in PCa tissues compared with that of BPH tissue and Talin-1 expression was an independent predictor for lymph node metastasis and biochemical recurrence of PCa. Further study will investigate the underlying molecular mechanism and the role of Talin-1 in PCa.
Keywords: lymph node metastasis, pathological feature, predict, prostate cancer, Talin-1
1. Introduction
Prostate cancer (PCa) is one of the most commonly diagnosed malignancies in men, ranking as the second worldwide incidence rate in male malignancies.[1] Although most PCa grows and progresses slowly, it contributed to more than a quarter million cancer-related death globally as reported in 2008.[2] The risk factors of PCa are older age, African ethnic, and family history of the disease. For example, approximately 62% of all PCa cases in the United States have been diagnosed in ≥65 years old males.[2] There are several treatment options for PCa , including surgery, radiation therapy, hormone therapy, chemotherapy, or targeted therapy, and treatment selection depends on tumor stages and age of patients.[3] Radical prostatectomy is one of the most effective therapies of PCa; however, it is debatable whether it needs to combine with pelvic lymph node dissection (PLND),[4] because most of studies indicated that PLND was only necessary for moderate- and high-risk PCa patients.[5–7] Like most of other cancers, PCa lymph node metastasis could lead to mortality of the disease; thus, development of biomarkers to predicate tumor metastasis before surgery could help medical oncologists to make an appropriate decision and selection of treatment.[8,9]
Tumor metastasis is a complex of multiple processes in which cancer cells obtain an ability and capacity to leave the primary tumor site via the lymphatic system and/or the bloodstream and then establish a secondary tumor site in other parts of the body.[10] Tumor cells undergo the epithelial–mesenchymal transition (EMT) to gain mobility and invasion capacity by altering cell adhesion and degradation of extracellular matrix,[11] thereby leading to cancer metastasis. At the gene levels, a great number of proteins could participate tumor metastasis and inhibition, such as cell adhesion molecules, proteinases, integrins, and EMT-related proteins.[12] In this study, our research is to focus on Talin-1, a high-molecular-weight cytoskeletal protein. Talin-1 functions to regulate cell–cell adhesion by binding to a variety of adhesion molecules (such as integrins) and actin to induce tumor cytoskeletal remodeling and leading to tumor cell adhesion and migration.[13,14] In PCa, a previous study demonstrated that Talin-1 could promote PCa cell migration and invasion.[15] However, another study showed that Talin-1 was an important factor in mediating PCa bone metastasis.[16] Thus, our present study detected Talin-1 expression in PCa versus benign prostatic hyperplasia (BPH) tissue specimens and then associated Talin-1 expression with clinicopathological characteristics and lymph node metastasis in PCa patients.
2. Materials and methods
2.1. Tissue specimen and data collection
This study was approved by the ethics committee of the First Affiliated Hospital of Fujian Medical University (Fuzhou, Fujian, China). We collected 280 cases of tissue specimens from PCa patients between January 2012 and June 2015 from the First Affiliated Hospital of Fujian Medical University. These patients were diagnosed with PCa according to EAU guidelines[3] and underwent radical prostatectomy plus PLND without any preoperative adjuvant endocrine therapy or radiotherapy (238 patients received standard PLND with a resection range including obturator foramen and external iliac vein peripheral lymph nodes, whereas 42 patients received extended PLND with a peripheral lymph node fat tissue having an upper bound to the common iliac artery bifurcation, a lower bound to the femoral canal, both sides to the pelvic wall, and a back bound to the obturator nerves, blood vessels, and anterior internal vein).[17] Moreover, we also collected 75 cases of BPH tissue specimens randomly and 6 cases of normal prostate tissue specimens as a control from our hospital. Clinical data, including age, prostate-specific antigen (PSA), prostate volume, PSA density (PSAD), body mass index (BMI), tumor stage, numbers of lymph node metastasis, positive surgical margin, extracapsular extension, and seminal vesicle invasion, were collected (Table 1). After radical prostatectomy, patients were followed up regularly for physical examination and serum PSA test at the first month after surgery, then every 3 months for 2 years, and semiannually thereafter. As patients with detectable PSA (>0.1 ng/mL) after surgery usually have persistent cancer, the serum PSA levels of patients >0.1 ng/mL at the first month after surgery were therefore excluded from this study according to previous reports.[18,19] Patients were diagnosed histologically and did not receive any preoperatively adjuvant endocrine therapy or radiotherapy and patients with uncompleted clinical and pathological data were also excluded from this study. In this study, we excluded 95 cases from further analysis of biochemical recurrence. The time of biochemical recurrence was recorded or to the last follow-up date (on May 2016). Patients were followed up for median 22 months (ranged between 4 and 49 months), and 41 of 185 (14.4%) patients had biochemical disease progression and the median time of biochemical recurrence was 13 months (ranged between 4 and 37 months). We did not calculate the time of overall survival because of the shortness of follow-up time.
Table 1.
Clinicopathological parameters of 280 cases of prostate cancer.
2.2. Construction of tissue microarray
Paraffin blocks from both PCa and BPH were retrieved from Pathology Department and sectioned for hematoxylin and eosin (H&E) staining to confirm diagnosis and identify the representative areas for tissue microarray construction. A tissue microarray maker designed by us was used to generate tissue microarrays using two 2 × 2 mm tissue cores of each case. In the end, we obtained 15 tissue microarrays with 5 × 10 tissue cores of each for both PCa and BPH tissues and then sectioned 4-μm-thick tissue sections for immunohistochemical staining of Talin-1 protein.[20,21]
2.3. Immunohistochemistry
The sections were conventionally de-waxed and rehydrated and antigen retrieval was performed using a high-pressure cook in 0.1 M citric acid buffer (pH 5.0; Fuzhou Maixin Biotech. Co., Ltd, Fuzhou, China). After that, tissue microarrays were then incubated with 3% H2O2 for 10 minutes to block endogenous peroxidase activity and subsequently with 20% normal goat serum at the room temperature for 30 minutes. Next, the tissue microarray sections were incubated with a rabbit monoclonal anti-Talin-1 antibody (Cell Signaling Technology, Danvers, MA) at a dilution of 1:400 at 4 °C overnight. On the next day, the sections were washed briefly with phosphate buffered saline (PBS) 3 times and further incubated with a secondary antibody (DAKO Company, CA) at 37 °C for 30 minutes and subsequently with a ChemMate EnVision DetectionKit (DAKO Company, CA). The sections were subjected to color reaction with 3,3′-diaminobenzidine (DAB) solution and counterstained with hematoxylin briefly and mounted with a cover slip. Confirmed positive sections were used as positive control and PBS to replace the primary antibody was used as a negative control. The immunostained tissue microarray sections were reviewed and scored under a light microscope (Olympus, Tokyo, Japan) by two pathologists blindly. Any discrepancy was resolved by their re-reviewing of the sections. A semi-quantitative method was conducted and the total Talin-1 immunostaining score included staining intensity and proportion of positive cells. Staining intensity (I) was recorded as 0, absent; 1, weak; 2, moderate; 3, strong, whereas the proportion (P) of positive cells was recorded as 0, <5%; 1, 5% to 25%; 2, 26% to 50%; 3, 51% to 75%; and 4, >75%. A score for each histological grade (H-score) was determined as the summary of intensity and proportion H-score = Σ (I × P) according to a previous study.[14] The final Talin-1 expression score was calculated using the value of the percent positivity score multiplied by the staining intensity score as “–” (score, 0–1), “+” (score, 2–3), “++” (score, 4–5), and “+++” (score ≥6).[22]
2.4. Statistical methods
SPSS 19.0 statistical software (SPSS, Chicago, IL) was utilized to perform all statistical analyses. Quantitative data were compared using independent samples t test, Mann–Whitney U test, or Kruskal–Wallis test. Qualitative data were compared using independent sample χ2-square test or Fisher exact test. Lymph node metastasis predictors were analyzed using logistic regression analysis and double-indicator combination was analyzed using logistic regression model. Receiver operating characteristic (ROC) curve was used to evaluate the predictive value of each indicator for PCa lymph node metastasis. Kaplan–Meier plots and the log-rank test were performed to assess the association of Talin-1 expression with biochemical recurrence-free survival (BFS). P <0.05 was considered statistically significant.
3. Results
3.1. Differential expression of Talin-1 protein in PCa versus normal and BPH tissue specimens
Immunohistochemical data showed that Talin-1 protein was mainly expressed in the cytoplasm of positive cells (Fig. 1) and Talin-1 expression was significantly higher in PCa than in both normal and BPH tissues. Talin-1 expression was significantly higher in poorly differentiated PCa than in both moderately and well-differentiated PCa. Talin-1 expression was significantly higher in LN(+) PCa than in LN(–) PCa (P <0.05 for all, Tables 1 and 2). However, there was no significant difference in Talin-1expression between normal and BPH tissue specimens (P >0.05, Tables 1 and 2).
Figure 1.
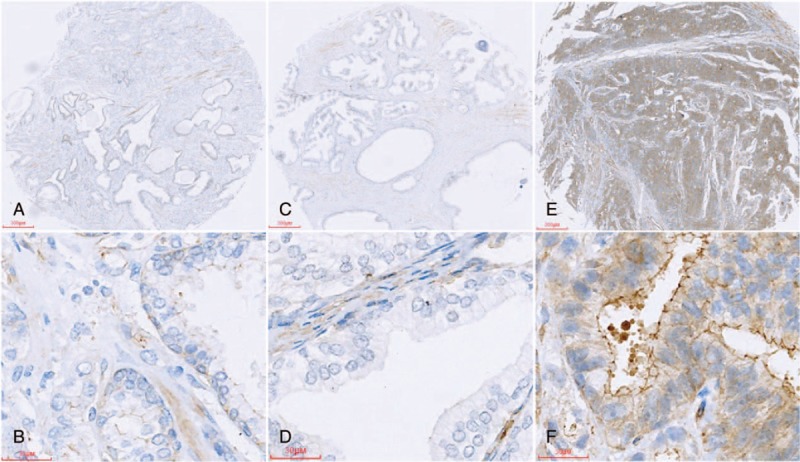
Tissue microarrays containing normal prostate, BPH, and prostate cancer tissues were immunostained with a monoclonal anti-Talin-1 antibody and the data were semiquantitatively analyzed: (A, B) normal prostate; (C,D) BPH; (E,F) prostate cancer. BPH = benign prostatic hyperplasia.
Table 2.
Expression of Talin-1 in normal, BPH, and human prostate cancer.
3.2. Association of Talin-1 expression with clinicopathological data from prostate cancer patients
We then associated the expression of Talin-1 protein with clinicopathological data from PCa patients. We found that high Talin-1 expression was associated with higher PSA levels, Gleason score, tumor stage, lymph node metastasis, positive surgical margin, extracapsular extension, and seminal vesicle invasion (P <0.001; Table 3), whereas Talin-1 expression was not associated with age of patients (P >0.05; Table 3). Talin-1 expression was more commonly observed in poorly differentiated, high-stage, and lymph node-positive PCa tissue specimens (Fig. 2). Upregulated Talin-1 expression was associated with PCa malignant behaviors and lymph node metastasis.
Table 3.
Association of Talin-1 expression with clinicopathological features from prostate cancer patients.

Figure 2.
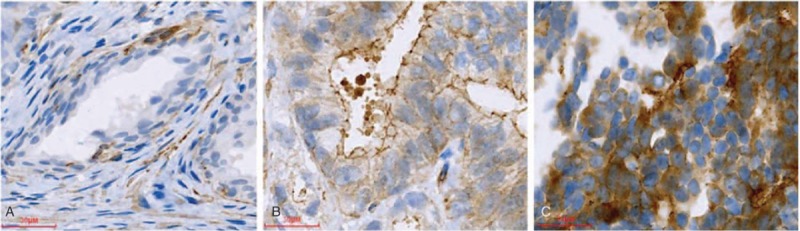
Different expression level of Talin-1 protein in prostate cancer tissues: (A) well differentiated: + (1 × 1 = 1); (B) moderately differentiated: ++ (2 × 2 = 4); (C) poorly differentiated: +++ (3 × 3 = 9).
3.3. Association of clinicopathological factors and Talin-1 expression with pelvic lymph node metastasis of prostate cancer
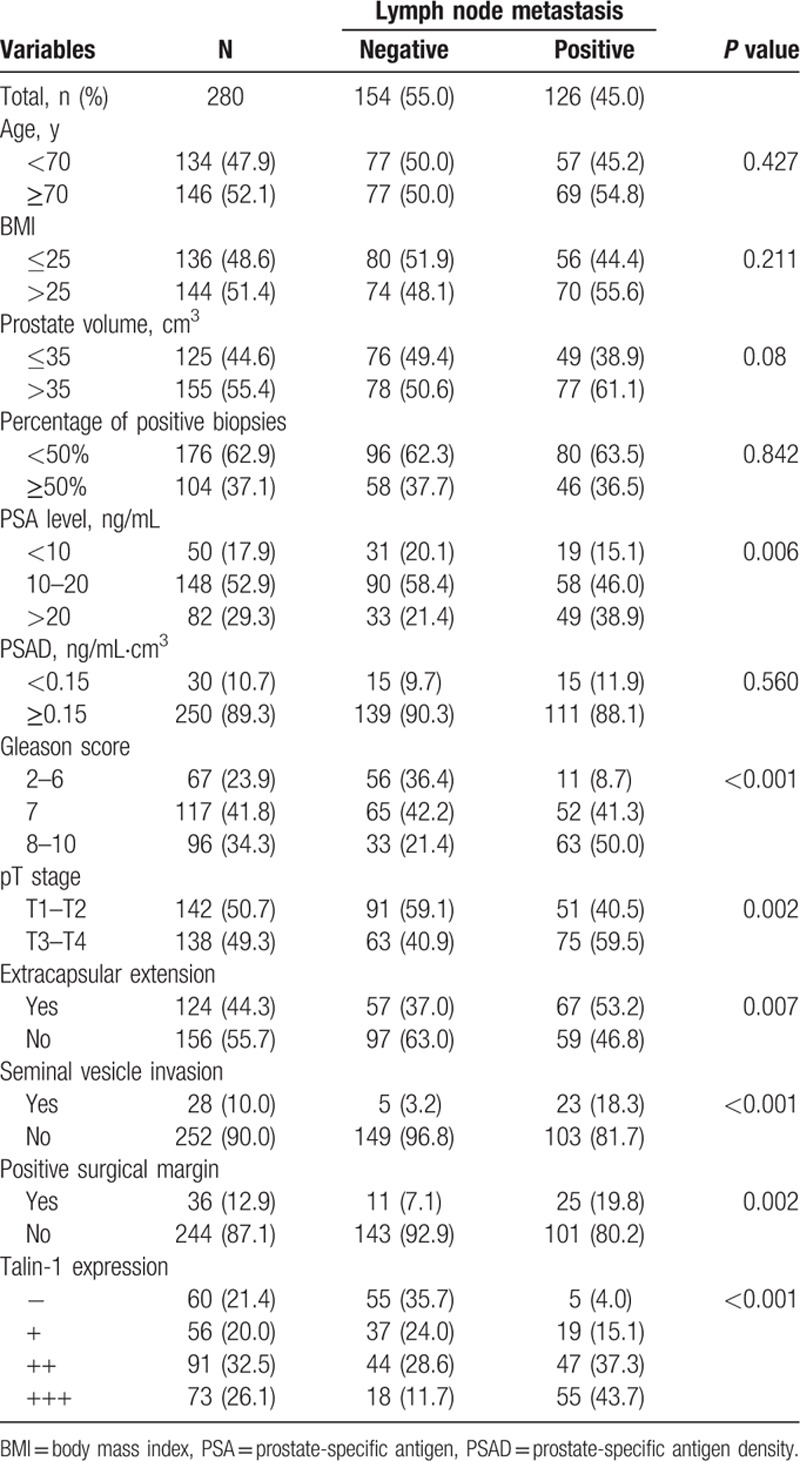
We then performed subgroup analysis to associate clinicopathological factors and Talin-1 expression with pelvic lymph node metastasis of PCa. The results showed that PCa metastasis to pelvic lymph nodes was associated with Talin-1 expression, higher PSA level, PSAD, Gleason score, tumor grade, positive surgery margin, extracapsular extension, and seminal vesicle invasion (all P <0.05; Table 4), but not associated with age and BMI of patients, prostate volume, or percentage of positive prostate needle biopsies (Table 4).
Table 4.
Association of clinicopathological features with lymph node metastasis of prostate cancer.
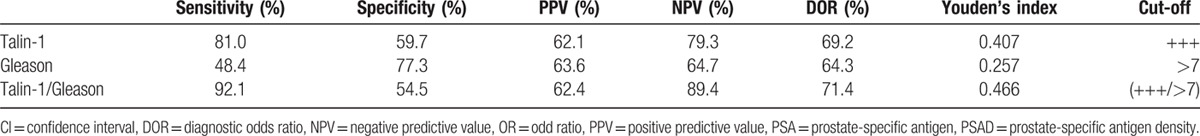
Multivariate logistic regression analysis showed that Gleason score and Talin-1 expression were independent risk factors for PCa lymph node metastasis (P <0.001, Table 5). The ROC curve analysis showed that the area under the curve (AUC) of Talin-1 expression (AUC = 0.766) was much higher than that of Gleason scores (AUC = 0.699), although their combination could further enhance the accuracy in predicting PCa lymph node metastasis (AUC = 0.802) (Fig. 3). Further analysis of their diagnostic sensitivity, specificity, positive predictive value, negative predictive value, and accuracy showed that combination of Talin-1 expression and Gleason score had the highest accuracy in predicting PCa lymph node metastasis (71.4%), whereas Gleason scores were lowest (64.3%) and Talin-1 expression was moderate (69.2%) (Table 6). Combination of Talin-1 with Gleason score (>7) could help us to predict tumor lymph node metastasis.
Table 5.
Multivariable analysis of clinicopathological features for association with lymph node metastasis of prostate cancer.
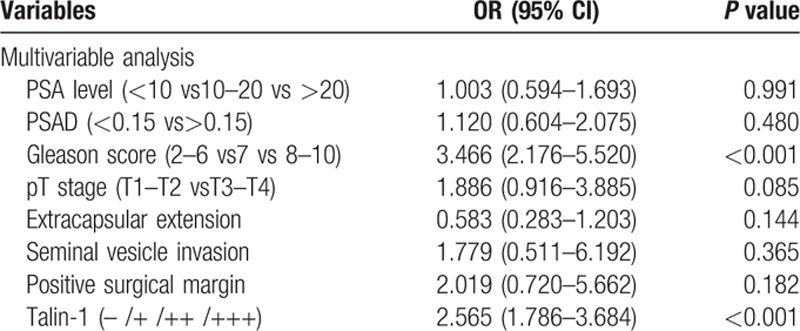
Figure 3.
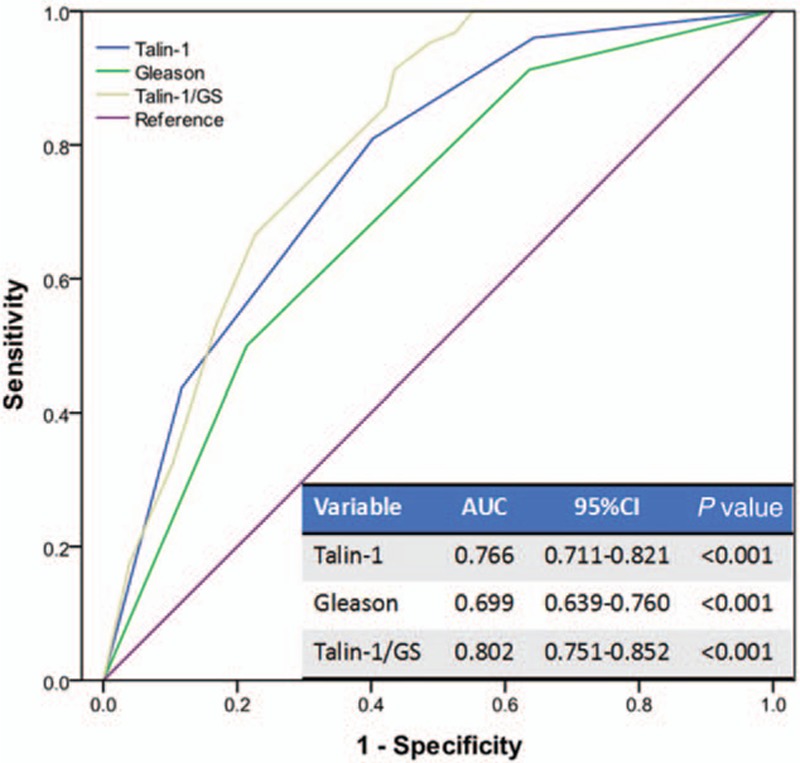
The ROC curves of Talin-1, Gleason score, and their combination in diagnosis of prostate cancer lymph node metastasis. ROC = receiver operating characteristic.
Table 6.
Sensitivity, specificity, PPV, NPV, and accuracy (%) of Talin-1 expression, Gleason score, and their combination in diagnosis of prostate cancer lymph node metastasis.
3.4. Association of Talin-1 expression with biochemical recurrence-free survival
Kaplan–Meier curve analysis showed that increased Talin-1 expression was associated with shortened BFS of PCa patients after radical prostatectomy (P <0.001, Fig. 4).
Figure 4.
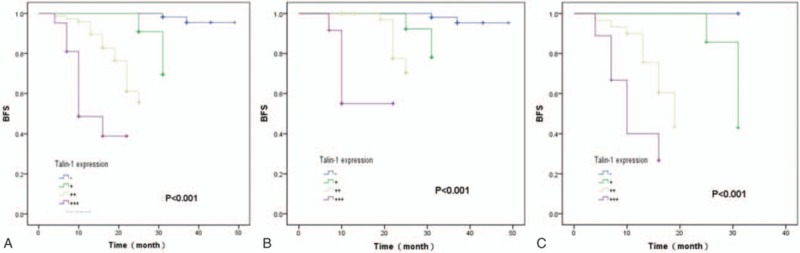
Kaplan–Meier curve analyses of biochemical recurrence-free survival of prostate cancer patients stratified by Talin-1 expression. (A) The total of 185 prostate cancer patients; (B) 134 cases of prostate cancer patients with negative lymph node metastasis; (C) 51 cases of prostate cancer patients with positive lymph node metastasis.
4. Discussion
The 5-year survival rate of PCa patients in the United States could reach to 99% and all patients with PCa may not die from the disease.[1] It is because different individuals with PCa show very different disease progression. For example, in some moderate/high-risk cases, there is no disease progression for a long period of time, whereas lymph node metastasis and even bone metastasis may occur in certain low-risk cases in the early stage.[7,23] To date, the application of PLND to all PCa patients remains controversial and previous studies have suggested that PLND could help medical oncologist to accurately evaluate tumor stage and improve prognosis of patients to certain extent,[24,25] whereas other studies revealed that PLND may not affect prognosis of PCa patients with postoperatively confirmed pT×N0 stage, even if such patients had a relatively high PSA level, pathological grade, or capsule invasion before surgery.[26,27] Moreover, PLND may also involve in different complications, such as deep venous thrombosis of the lower limbs, lymphatic leakage, pelvic infection, and so on, especially for extended PLND.[28] Thus, to date, surgeons in the field have suggested that low-risk PCa may not need PLND and that a standard or extended PLND should be performed in the moderate- and high-risk PCa cases.[29,30] However, it lacks a standard protocol or tumor markers to evaluate or predict the risk of PCa metastasis to the pelvic lymph nodes.
Our present study therefore evaluated Talin-1 expression in 280 PCa versus 75 BPH tissue specimens for association with lymph node metastasis and other clinicopathological data. We found that Talin-1 was highly expressed in PCa tissues compared with BPHs. Talin-1 expression was associated with higher PSA levels, Gleason score, tumor stage and differentiation, lymph node metastasis, positive surgical margin, extracapsular extension, and seminal vesicle invasion, whereas PSA level, PSAD, Gleason score, tumor grade, positive surgery margin, extracapsular extension, and seminal vesicle invasion were all associated with PCa lymph node metastasis. Combination of Talin-1 expression and Gleason score can predicate >70% PCa metastasis to the pelvic lymph node. Further study will identify better biomarkers to predicate lymph node metastasis of PCa.
Indeed, increase in cell proliferation and mobility could promote tumor growth and distant metastasis. The cell migration includes the steps of actin phosphorylation and cytoskeletal remodeling.[31,32] Talin-1 is a focal adhesion protein to mediate integrin interaction with extracellular matrix and Talin-1 can also bind to a variety of cell–cell adhesion molecules (such as integrin) and actin to induce cell cytoskeleton remodeling; thus, increase in Talin-1 expression could result in promotion of tumor cell adhesion and migration. For example, Lai et al[33] showed that high Talin-1 expression in tumor tissues resulted in a poor prognosis of oral squamous cell carcinoma in ex vivo, whereas knockdown of Talin-1 expression inhibited proliferation and invasion ability of oral squamous cell carcinoma cells. The data reported by Xing et al[34] revealed that injection of an anti-Talin-1 antibody or antisense RNA was able to destroy the stress fibers of cellulose cells and inhibit adhesion, extension, and migration of breast cancer cells. In PCa, Zhang et al[15] showed that Talin-1 was overexpressed in PCa tissue specimens and cell lines, whereas microRNA-124 (miR-124) and Talin-1 impaired cellular adhesion and motility through integrins and the focal adhesion kinase/Akt pathway. Their data concluded that miR-124 impairs the adhesion, migration, and invasion of PCa cells by downregulation of Talin-1.[15] Our present data further confirmed these previous studies; however, the underlying molecular mechanisms responsible for Talin-1-asscoaited PCa lymph node metastasis remain to be determined, although a previous study showed that Talin-1 phosphorylation activated β1 integrin as a novel mechanism to promote PCa bone metastasis.[16]
Clinically, PCa metastasis is mainly involved in pelvic lymph nodes and contributes to an unfavorable prognosis of PCa patients.[35] Previous studies have shown that Talin-1 expression associated with lymph node metastasis of oral squamous cell carcinoma and colon cancer.[33,36] In our present study, we found that Talin-1 expression and other clinicopathological factors, such as higher PSA level, PSAD, Gleason score, tumor grade, positive surgery margin, extracapsular extension, and seminal vesicle invasion, were all associated with PCa metastasis to the pelvic lymph nodes. Thus, we speculate that PCa metastasis is a multiple factor-induced phenomenon and further study is needed to understand the underlying molecular mechanism and therefore, to effectively control disease progression, and provide a means to evaluate and predict PCa metastasis.
Xu et al[37] demonstrated that expressions of Talin-1 mRNA and protein were significantly upregulated in nasopharyngeal carcinoma tissues; high expression of Talin-1 was associated with poor prognosis of nasopharyngeal carcinoma patients. Lai et al[33] showed that high Talin-1 expression in tumor tissues is also associated with poor prognosis of oral squamous cell carcinoma. As most patients diagnosed with PCa do have a favorable disease progression-free survival after radical prostatectomy and the follow-up time of our present study was short, we just assessed Talin-1 expression for association with biochemical recurrence of PCa after radical prostatectomy and found that increase in Talin-1 expression was associated with shortened postoperative BFS.
However, our present study does have some limitations. For example, we could include the tumor cell-positive number of lymph nodes versus total numbers of lymph nodes in this study, which could lead to a more accurate determination of association between Talin-1 expression and lymph node metastasis. The reason was simply because surgeons who performed PLND on each patient might not have a consistent technique, leading to inconsistence in the extent of lymph node dissection. Moreover, some patients received a standard PLND, whereas others may have received an extended PLND. In addition, the present study is retrospective and thus was not well controlled. In conclusion, our present data demonstrated that upregulated Talin-1 expression was associated with PCa malignant behaviors and lymph node metastasis. Combination of Talin-1 with Gleason score (>7) could help to predict tumor lymph node metastasis. Talin-1 expression was associated with shortened biochemical-free survival of PCa patients after radical prostatectomy.
Footnotes
Abbreviations: AUC = area under the curve, BFS = biochemical-free survival, BMI = body mass index, BPH = benign prostatic hyperplasia, DAB = 3,3′-diaminobenzidine, DOR = diagnostic odds ratio, EMT = epithelial–mesenchymal transition, H&E = hematoxylin and eosin staining, LN = lymph node, NPV = negative predictive value, PBS = phosphate buffered saline, PCa = prostate cancer, PLND = pelvic lymph node dissection, PPV = positive predictive value, PSA = prostate-specific antigen, PSAD = prostate-specific antigen density, ROC = receiver operating characteristic value.
The authors have no conflicts of interest to disclose.
References
- 1.Attard G, Parker C, Eeles RA, et al. Prostate cancer. Lancet (London, England) 2016; 387:70–82. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich A, Bastian PJ, Bellmunt J, et al. Urology EAo. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 2014; 65:124–137. [DOI] [PubMed] [Google Scholar]
- 4.Bogdanović Sekulić, Firas Abdollah, Giorgio Gandaglia, et al. More extensive pelvic lymph node dissection improves survival in patients with node-positive prostate cancer. Eur Urol 2015; 67:212–219. [DOI] [PubMed] [Google Scholar]
- 5.Thurairaja R, Studer UE, Burkhard FC. Indications, extent, and benefits of pelvic lymph node dissection for patients with bladder and prostate cancer. Oncologist 2009; 14:40–51. [DOI] [PubMed] [Google Scholar]
- 6.Ordon M, Nam RK. Lymph node assessment and lymphadenectomy in prostate cancer. J Surg Oncol 2009; 99:215–224. [DOI] [PubMed] [Google Scholar]
- 7.Mitsuzuka K, Koie T, Narita S, et al. Is pelvic lymph node dissection required at radical prostatectomy for low-risk prostate cancer. Int J Urol 2013; 20:1092–1096. [DOI] [PubMed] [Google Scholar]
- 8.Briganti A, Abdollah F, Nini A, et al. Performance characteristics of computed tomography in detecting lymph node metastases in contemporary patients with prostate cancer treated with extended pelvic lymph node dissection. Eur Urol 2012; 61:1132–1138. [DOI] [PubMed] [Google Scholar]
- 9.Denkçeken T, Canpolat M, Baykara M, et al. Diagnosis of pelvic lymph node metastasis in prostate cancer using single optical fiber probe. Int J Biol Macromol 2015; 90:63–67. [DOI] [PubMed] [Google Scholar]
- 10.Klein CA. Cancer. The metastasis cascade. Science (New York, NY) 2008; 321:1785–1787. [DOI] [PubMed] [Google Scholar]
- 11.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 2008; 14:818–829. [DOI] [PubMed] [Google Scholar]
- 12.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 2013; 13:97–110. [DOI] [PubMed] [Google Scholar]
- 13.Desiniotis A, Kyprianou N. Significance of talin in cancer progression and metastasis. Int Rev Cell Mol Biol 2011; 289:117–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakamoto S, McCann RO, Dhir R, et al. Talin1 promotes tumor invasion and metastasis via focal adhesion signaling and anoikis resistance. Cancer Res 2010; 70:1885–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W, Mao YQ, Wang H, et al. MiR-124 suppresses cell motility and adhesion by targeting talin 1 in prostate cancer cells. Cancer Cell Int 2015; 15:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin JK, Tien PC, Cheng CJ, et al. Talin1 phosphorylation activates β1 integrins: a novel mechanism to promote prostate cancer bone metastasis. Oncogene 2015; 34:1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heidenreich A, Ohlmann CH, Polyakov S. Anatomical extent of pelvic lymphadenectomy in patients undergoing radical prostatectomy. Eur Urol 2007; 52:29–37. [DOI] [PubMed] [Google Scholar]
- 18.Naselli A, Introini C, Andreatta R, et al. Prognostic factors of persistently detectable PSA after radical prostatectomy. Int J Urol 2009; 16:82–86. [DOI] [PubMed] [Google Scholar]
- 19.Ploussard G, Staerman F, Pierrevelcin J, et al. Predictive factors of oncologic outcomes in patients who do not achieve undetectable prostate specific antigen after radical prostatectomy. J Urol 2013; 190:1750–1756. [DOI] [PubMed] [Google Scholar]
- 20.Fedor HL, De Marzo AM. Practical methods for tissue microarray construction. Methods Mol Med 2005; 103:89–101. [DOI] [PubMed] [Google Scholar]
- 21.Richani K, Romero R, Kim YM, et al. Tissue microarray: an effective high-throughput method to study the placenta for clinical and research purposes. J Matern Fetal Neonatal Med 2006; 19:509–515. [DOI] [PubMed] [Google Scholar]
- 22.Ma HQ, Liang XT, Zhao JJ, et al. Decreased expression of Neurensin-2 correlates with poor prognosis in hepatocellular carcinoma. World J Gastroenterol 2009; 15:4844–4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beltran H, Tomlins S, Aparicio A, et al. Aggressive variants of castration-resistant prostate cancer. Clin Cancer Res 2014; 20:2846–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdollah F, Gandaglia G, Suardi N, et al. More extensive pelvic lymph node dissection improves survival in patients with node-positive prostate cancer. Eur Urol 2015; 67:212–219. [DOI] [PubMed] [Google Scholar]
- 25.Moschini M, Briganti A, Murphy CR, et al. Outcomes for patients with clinical lymphadenopathy treated with radical prostatectomy. Eur Urol 2016; 69:193–196. [DOI] [PubMed] [Google Scholar]
- 26.DiMarco DS, Zincke H, Sebo TJ, et al. The extent of lymphadenectomy for pTXNO prostate cancer does not affect prostate cancer outcome in the prostate specific antigen era. J Urol 2005; 173:1121–1125. [DOI] [PubMed] [Google Scholar]
- 27.Murphy AM, Berkman DS, Desai M, et al. The number of negative pelvic lymph nodes removed does not affect the risk of biochemical failure after radical prostatectomy. BJU Int 2010; 105:176–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suardi N, Larcher A, Haese A, et al. Indication for and extension of pelvic lymph node dissection during robot-assisted radical prostatectomy: an analysis of five European institutions. Eur Urol 2014; 66:635–643. [DOI] [PubMed] [Google Scholar]
- 29.Schiavina R, Manferrari F, Garofalo M, et al. The extent of pelvic lymph node dissection correlates with the biochemical recurrence rate in patients with intermediate- and high-risk prostate cancer. BJU Int 2011; 108:1262–1268. [DOI] [PubMed] [Google Scholar]
- 30.Ji J, Yuan H, Wang L, et al. Is the impact of the extent of lymphadenectomy in radical prostatectomy related to the disease risk? A single center prospective study. J Surg Res 2012; 178:779–784. [DOI] [PubMed] [Google Scholar]
- 31.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 2009; 10:445–457. [DOI] [PubMed] [Google Scholar]
- 32.O’Hayre M, Salanga CL, Handel TM, et al. Chemokines and cancer: migration, intracellular signalling and intercellular communication in the microenvironment. Biochem J 2008; 409:635–649. [DOI] [PubMed] [Google Scholar]
- 33.Lai MT, Hua CH, Tsai MH, et al. Talin-1 overexpression defines high risk for aggressive oral squamous cell carcinoma and promotes cancer metastasis. J Pathol 2011; 224:367–376. [DOI] [PubMed] [Google Scholar]
- 34.Xing B, Thuppal S, Jedsadayanmata A, et al. TA205, an anti-talin monoclonal antibody, inhibits integrin–talin interaction. FEBS Lett 2006; 580:2027–2032. [DOI] [PubMed] [Google Scholar]
- 35.Verhagen PC, Schröder FH, Collette L, et al. Does local treatment of the prostate in advanced and/or lymph node metastatic disease improve efficacy of androgen-deprivation therapy? A systematic review. Eur Urol 2010; 58:261–269. [DOI] [PubMed] [Google Scholar]
- 36.Bostanci O, Kemik O, Kemik A, et al. A novel screening test for colon cancer: Talin-1. Eur Rev Med Pharmacol Sci 2014; 18:2533–2537. [PubMed] [Google Scholar]
- 37.Xu YF, Ren XY, Li YQ, et al. High expression of Talin-1 is associated with poor prognosis in patients with nasopharyngeal carcinoma. BMC Cancer 2015; 15:332. [DOI] [PMC free article] [PubMed] [Google Scholar]


