Abstract
The ability of the European Neuroendocrine Tumor Society (ENETS) system, 2010 World Health Organization (WHO) grading system, and American Joint Committee on Cancer (AJCC) staging system to predict survival after gastric neuroendocrine tumor (NET) resection has not yet been validated.
We retrospectively evaluated 175 gastric NETs from 1996 to 2014. WHO grade 3 (G3) patients (n = 66) had a lower survival rate than grade 1 (G1) (n = 39) or grade 2 (G2) (n = 13) patients, with similar high survival rates for G1 and G2 patients. G3 patients had a lower survival rate than mixed-type patients (n = 57). Using the AJCC classification, most of the G1/2 NETs (86.6%) were confined to T1/T2, N0 tumor, and stage I/IIa, but the survival rate was not well distributed. In contrast, G3/mixed tumors were well distributed in terms of T, N, stage, and survival. Using the ENETS classification, 64.6% of the tumors were T2 and only 8.6% were T3. In addition, 49.7% were stage IIIb and only 1.9% was IIa, with poor survival distribution.
Our findings strongly suggested that the WHO and ENETS classification systems have shown a low prognostic value. The AJCC TNM system showed a low prognostic value for well-differentiated NETs (G1 or G2). In contrast, the AJCC TNM system had a high prognostic value for G3 or mixed tumors.
Keywords: AJCC TNM system, ENETS TNM system, neuroendocrine tumors, stomach, WHO classification
1. Introduction
In 2000, the World Health Organization (WHO) classified neuroendocrine neoplasms (NENs) into 4 categories according to its behavior: well-differentiated NENs with benign behavior, well-differentiated NENs with uncertain malignant behavior, well-differentiated neuroendocrine carcinomas (NECs), and poorly differentiated NECs.[1–4] Several reports have agreed that this classification had a prognostic relevance.[5–11]
In 2006, the European Neuroendocrine Tumor Society (ENETS) proposed a new grading system for gastroenteropancreatic neuroendocrine tumors (NETs),[12–14] based on the Ki-67 index (grade 1, ≤2%; grade 2, 3%–20%; and grade 3, >20%), and a new TNM staging system was also proposed in the same year. The new grading system was accepted by the WHO in 2010.[15] In addition, the WHO 2010 defined a new category, mixed adenoneuroendocrine carcinomas (MANECs), which shows neuroendocrine cells (exceeding 30% of all tumor cells) mixed with nonendocrine components (usually adenocarcinoma).
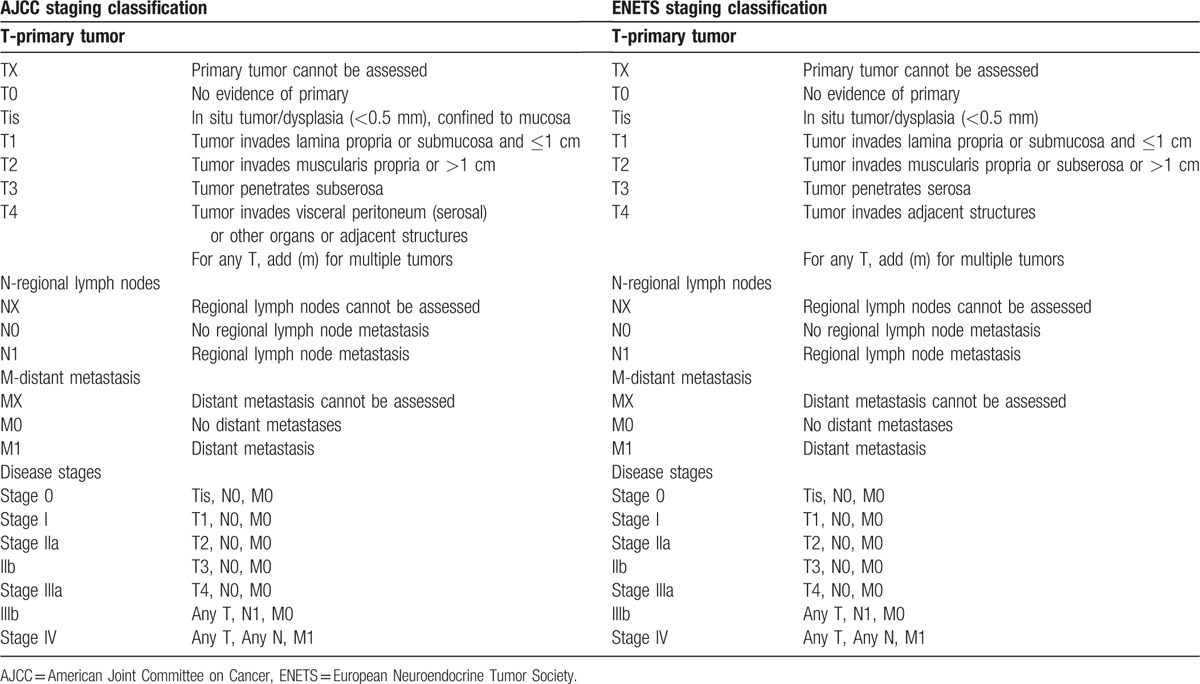
The American Joint Committee on Cancer (AJCC) published a similar TNM staging system (7th edition) in 2010.[16] The AJCC TNM staging system differs from the ENETS staging system in several ways. The ENETS TNM system classifies all NETs in a single system, whereas the AJCC 7th edition for gastric NETs applies only to carcinoid tumors and atypical carcinoid tumors (well-differentiated NETs), which correspond to WHO grade 1 (G1) and grade 2 (G2). The AJCC recommends that high-grade NECs (WHO grade 3 [G3]) and mixed glandular/well-differentiated NETs be classified according to the AJCC TNM staging for gastric cancer. In addition, the ENETS classifies NETs with tumor invasion into the muscularis propria or subserosa or >1 cm as T2, whereas the AJCC classifies NETs with invasion into the subserosa as T3 (Table 1).
Table 1.
AJCC 7th edition and ENETS staging classifications for gastric neuroendocrine tumors.
Unfortunately, the ability of the WHO, ENETS, and AJCC staging to predict survival after surgical resection has not yet been validated. Therefore, in the present study, we evaluated the prognostic accuracy of 3 classifications in terms of relapse-free survival (RFS) and overall survival (OS) after resection of nonmetastatic gastric NETs.
2. Methods
A total of 183 patients with primary gastric NETs were treated at Asan Medical Center in Seoul, Korea, between 1996 and 2014. Eight patients were excluded from our analysis because they were lost to follow-up or had incomplete data. We selected the remaining 175 patients who did not have distant metastasis at the time of diagnosis. All of the 175 patients underwent endoscopic or surgical R0 resection. Thus, 175 specimens were reviewed by a pathologist and reclassified according to the WHO 2010 classification. Mixed type was defined as NET mixed with adenocarcinoma. We classified the 175 gastric NETs using the AJCC 7th edition and the ENETS TNM staging systems (Table 1).[13,16] According to the AJCC 7th edition, the TNM staging system for gastric NETs was applied to WHO G1 and G2 tumors, and the TNM staging system for gastric carcinoma was applied to WHO G3 and mixed-type tumors.
We evaluated the clinicopathologic characteristics and prognosis for each stage after classifying the patients by the 3 different systems: the WHO, AJCC, and ENETS TNM classification systems. We also evaluated the factors influencing prognosis, including the 3 classification systems. Therefore, the aim of this study was to compare the 2010 WHO classification and the AJCC and ENETS staging systems for their prognostic power.
Numeric data are expressed as the mean with standard deviations. Risk factors were analyzed using the χ2 test (univariate analysis) or logistic regression model (multivariate analysis). Survival data were analyzed using the Kaplan-Meier method with log-rank test (univariate analysis) or Cox proportional hazards regression (multivariate analysis). All statistical data were analyzed using SPSS 21.0 (SPSS Inc, Chicago, IL). A P value of 0.05 was considered statistically significant. This study received approval from the Institutional Review Board of Asan Medical Center.
3. Results
3.1. Basic clinicopathologic characteristics of the study patients
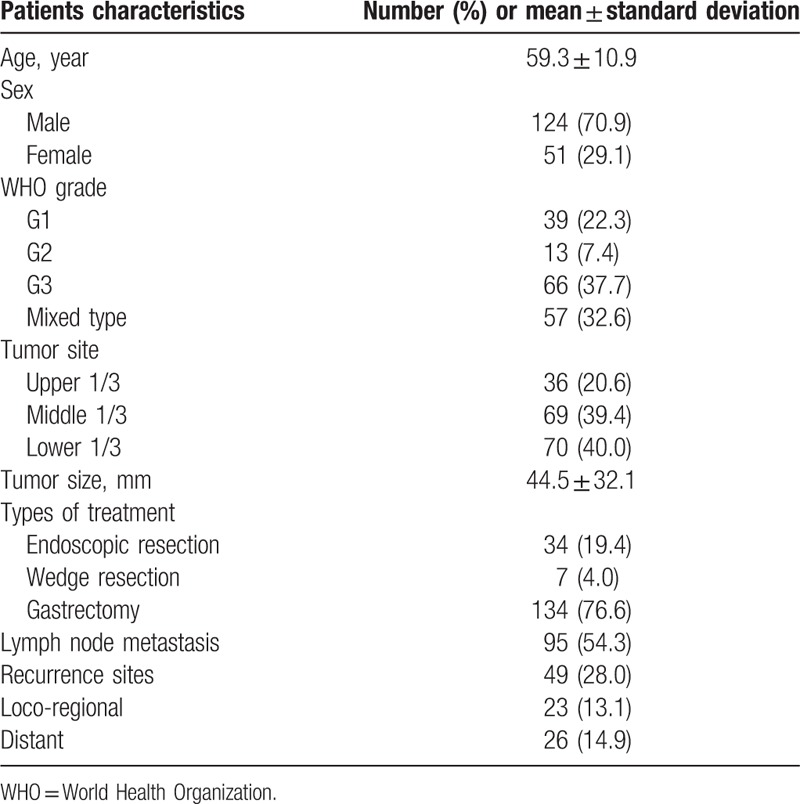
The median follow-up duration was 48.8 months (range 0.3–198.3 months). The clinicopathologic characteristics are shown in Table 2. Of the 175 patients, 124 (70.9%) were male and 51 (29.1%) were female. In total, 39 tumors (22.3%) were classified as G1, 13 (7.4%) as G2, 66 (37.7%) as G3, and 57 (32.6%) as mixed type. Gastrectomy was performed in 76.6% of these cases. Forty-nine patients (28.0%) had tumor recurrence, and 10 of the 49 occurred in the remnant stomach.
Table 2.
Clinicopathologic characteristics.
3.2. WHO subgroup analysis: G1 to G3 and mixed type
Of the 39 G1 patients, 6 (15.4%) experienced tumor recurrence; all 6 recurrences occurred in the remnant stomach. Of the 13 G2 patients, 3 (23.1%) experienced recurrence. Only 1 (G2, recurred in liver) of the 9 G1 and G2 tumor recurrences died of disease progression. The other 8 patients with recurrences in the remnant stomach were all alive at the time of analysis. However, 27 of the 66 G3 patients (40.9%) experienced tumor recurrence and 22 of the 27 (81.5%) died of disease progression. Of the 57 mixed-type patients, 13 (22.8%) experienced tumor recurrence and 12 of the 13 (92.3%) died of disease progression. The Kaplan-Meier survival curves are shown in Figure 1. Patients with G3 had the lowest OS and patients with mixed type had a lower OS than G1 or G2 (P < 0.05). The OS of G1 and G2 was similarly high (P > 0.05).
Figure 1.
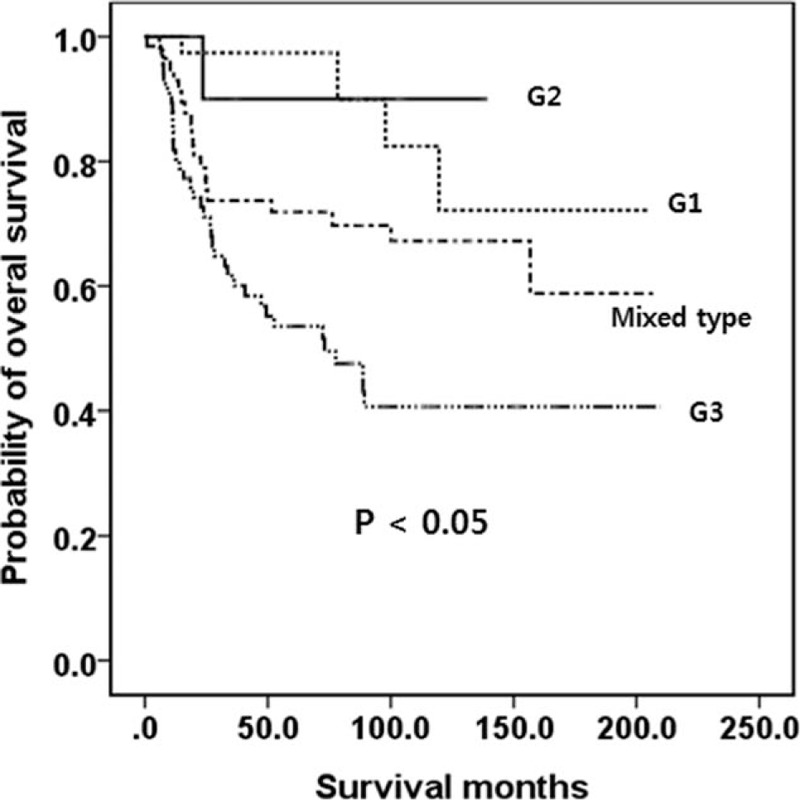
Kaplan-Meier survival curves of OS in patients with WHO G1 to G3 and mixed-type tumors. Patients with a G3 lesion had a lower OS than patients with G1, G2, or mixed-type tumors. Mixed type had a lower OS than G1 or G2. The OS was similar between G1 and G2. G = grade, OS = overall survival, WHO = World Health Organization.
3.3. AJCC and ENETS TNM stages
The definitions of the AJCC and ENETS TNM classifications are shown in Table 1.[13,16] As described above, the AJCC recommends that G1/G2 gastric NETs be classified with the TNM classification for NETs (Table 1), whereas G3 and mixed type are recommended to be classified with the TNM classification for gastric carcinoma. The AJCC and ENETS TNM classifications of our patients are shown in Table 3. Most of the benign/well-differentiated NETs (WHO G1 and G2) were classified as T1/T2, N0 tumor, and stage I/IIa. The OS for patients in each stage classified by the AJCC TNM staging system for gastric NETs (WHO G1 or G2) was not well distributed (P > 0.05; Fig. 2). Only 6 belonged to stage III and only 1 (1.9%, stage IIa) died of disease progression. The TNM staging of the AJCC for gastric carcinoma (G3 or mixed type) showed that tumors were well distributed in the T and N stages and the overall stage. The OS for patients in each stage classified by the AJCC TNM staging for gastric carcinoma (WHO G3 or mixed type) was well distributed (P < 0.05; Fig. 3).
Table 3.
AJCC 7th edition and ENETS staging classifications for gastric neuroendocrine tumors.
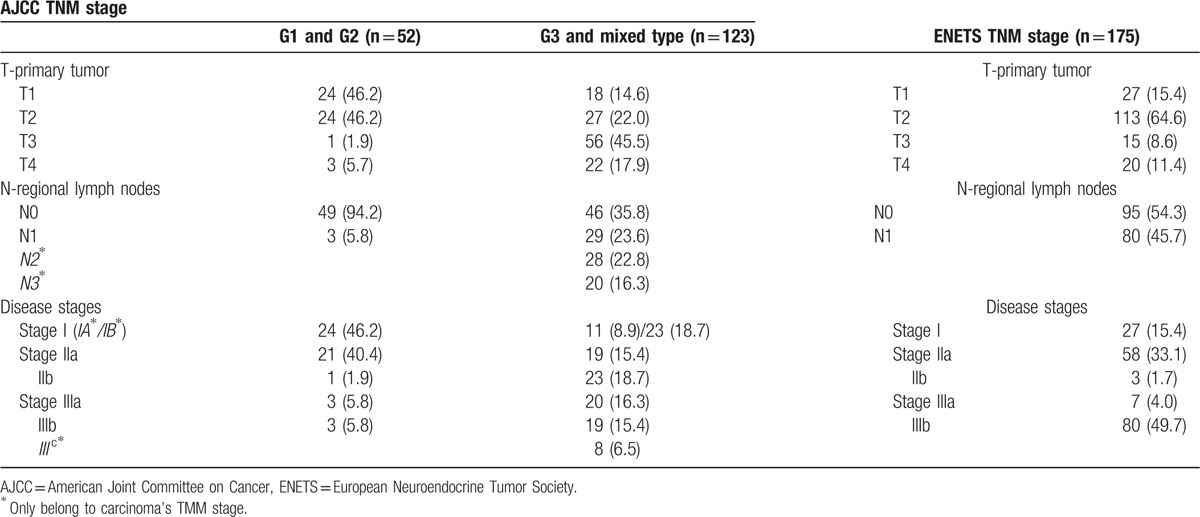
Figure 2.
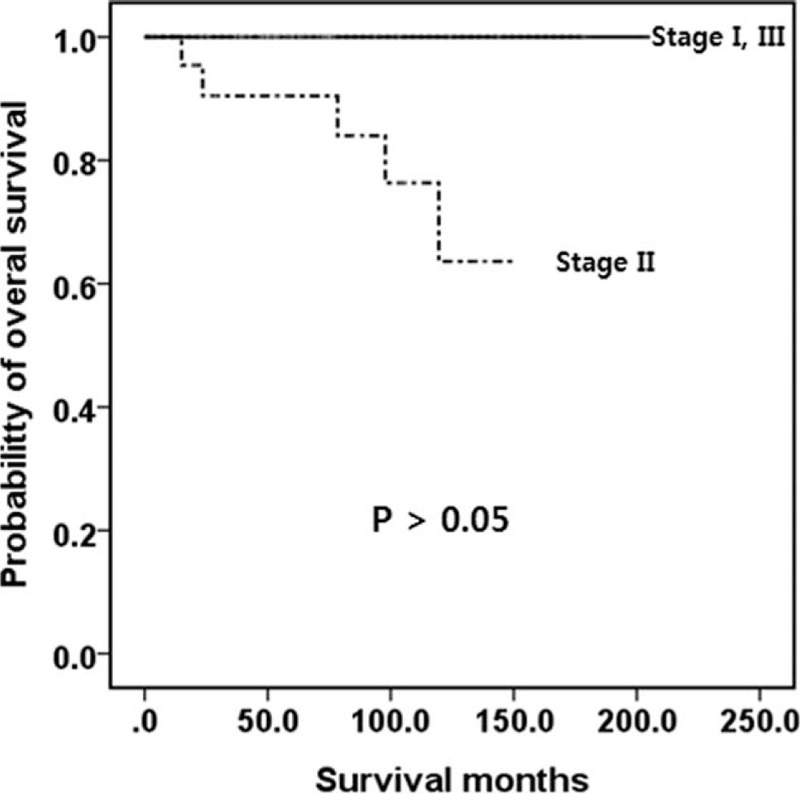
Kaplan-Meier survival curves of OS for patients with a well-differentiated neuroendocrine tumor/carcinoma (WHO G1 or G2) according to the AJCC 7th edition. No significant differences in OS outcomes were observed. AJCC = American Joint Committee on Cancer, G = grade, OS = overall survival, WHO = World Health Organization.
Figure 3.
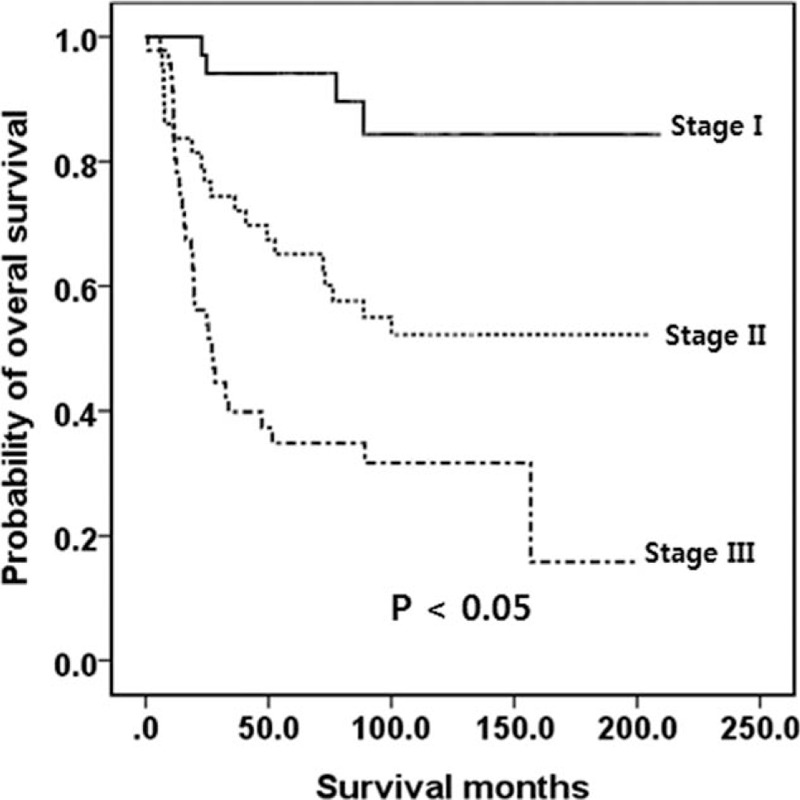
Kaplan-Meier survival curves of OS in patients with poorly differentiated neuroendocrine carcinoma (WHO G3)/mixed-type tumors according to the AJCC 7th edition. A significant difference was observed. AJCC = American Joint Committee on Cancer, G = grade, OS = overall survival, WHO = World Health Organization.
In contrast, 64.6% of the tumors were T2 and only 8.6% were T3 when tumors were classified by the ENETS staging system (Table 3). In addition, 49.7% belonged to stage IIIb, only 1.7% belonged to IIb, and 4.0% belonged to IIIa (Table 3). Thus, OS was not well distributed. Survival analysis was performed specifically for G1 and G2 tumors classified by the ENETS staging system, and the OS was not well distributed (Fig. 4). However, when G3 and mixed-type tumors were staged by the ENETS TNM, the stages were well distributed (Fig. 5).
Figure 4.
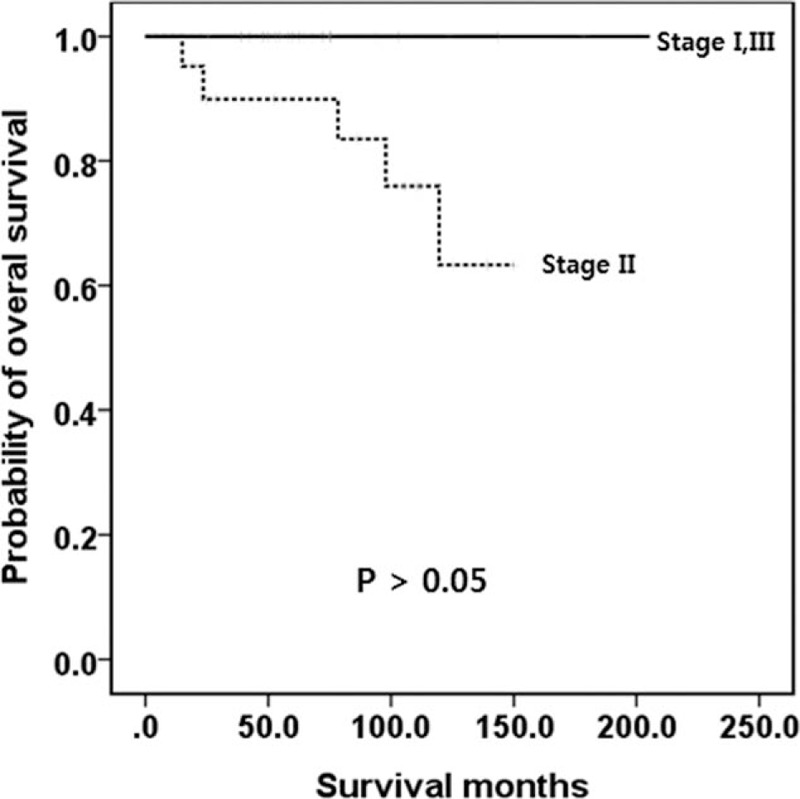
Kaplan-Meier survival curves of OS in patients with well-differentiated neuroendocrine tumor/carcinoma (WHO G1 or G2) according to the ENETS staging system. No significant difference was observed. ENETS = European Neuroendocrine Tumor Society, G = grade, OS = overall survival, WHO = World Health Organization.
Figure 5.
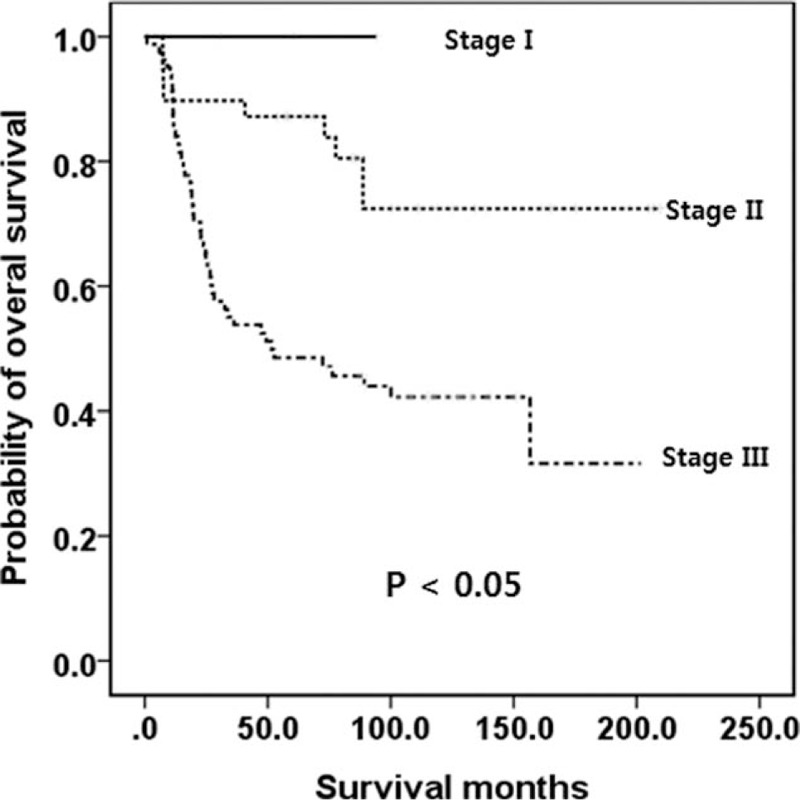
Kaplan-Meier survival curves of OS in patients with poorly differentiated neuroendocrine carcinoma (WHO G3)/mixed type according to the ENETS staging system. A significant difference was observed. ENETS = European Neuroendocrine Tumor Society, G = grade, OS = overall survival, WHO = World Health Organization.
3.4. Factors influencing prognosis
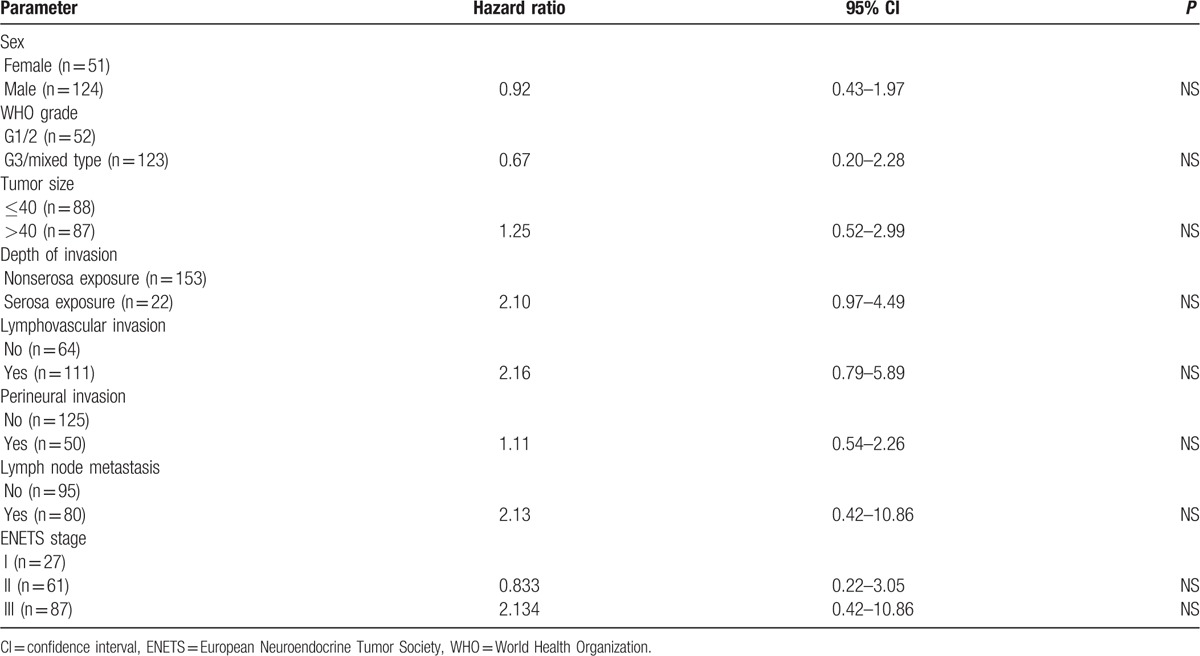
The factors affecting survival are shown in Table 4. When univariate analysis of prognostic factors was performed using the Kaplan-Meier method with log-rank test, sex, WHO grade, tumor size, depth of invasion, lymphovascular invasion, perineural invasion, and lymph node metastasis were found to be prognostic risk factors affecting survival. Lymph node metastasis and depth of invasion were independent prognostic factors in Cox proportional hazards analysis (P < 0.05). The WHO grade was not an independent prognostic risk factor (P > 0.05). The analysis of G3 and mixed-type tumors using the Cox proportional hazards model is shown in Table 5. The AJCC stage and WHO grade were independent prognostic factors (P < 0.05). The AJCC stage was a more important factor (hazard ratios, 3.58 and 5.45) than the WHO grade (hazard ratio, 2.15). The ENETS stage was not an independent prognostic factor by multivariate analysis (Table 6).
Table 4.
Multivariate analysis of prognostic factors in all NETs.
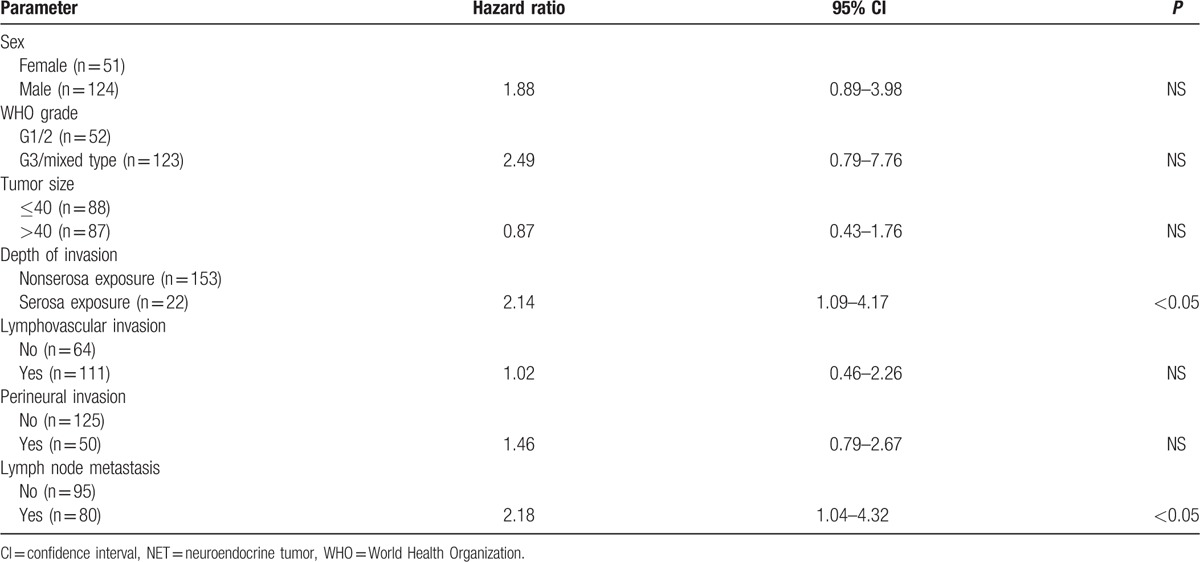
Table 5.
Multivariate analysis of prognostic factors in WHO grade 3/mixed type in the AJCC system.
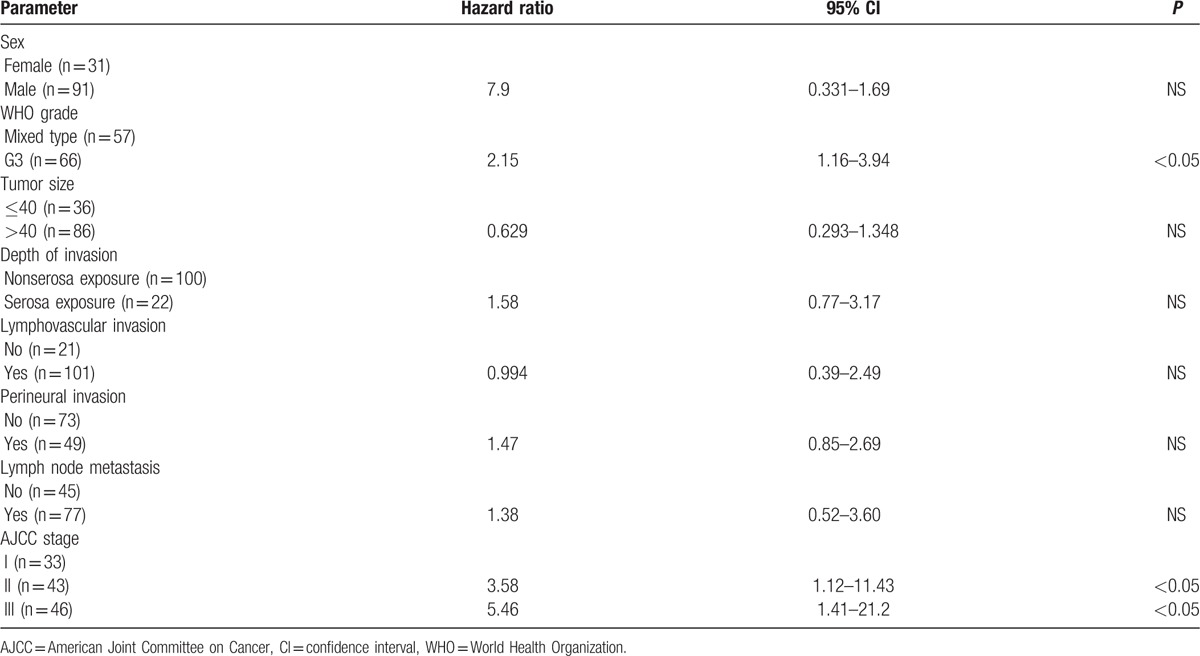
Table 6.
Multivariate analysis of prognostic factors based on the ENETS system.
4. Discussion
The 2010 WHO classification was provided to establish a novel, quantifiable, and reproducible standard for neuroendocrine cancers. However, in our present study, we found that the 2010 WHO grading system was not a powerful predictor of prognosis. Of the 39 G1 patients in our current study series, 6 (15.4%) experienced tumor recurrence, with all 6 occurring in the remnant stomach. Of the 13 G2 patients we examined, 3 (23.1%) experienced recurrence, with 2 of the 3 recurrences (66.7%) occurring in the remnant stomach. None of the 8 G1 or G2 patients with recurrences in the remnant stomach died of disease progression. In contrast, 40 of the 66 G3 or 57 mixed-type tumors (32.5%) experienced recurrence, and only 2 of the 40 recurrences (0.5%) occurred in the remnant stomach. This large difference in the recurrence rate of the remnant stomach and the fact that death did not occur in our G1 or G2 patients with recurred tumor in the remnant stomach might explain the lack of a difference in RFS between WHO subgroups. One could also argue that the endoscopic/surgical approach was insufficient. This point should be evaluated in a future study.
Many attempts have been made to uniformly treat gut endocrine tumors; however, no structured therapeutic approach has been developed due to the rarity of the disease. The North American Neuroendocrine Tumor Society, the National Comprehensive Cancer Network, and ENETs recommended treatment guideline.[2–4] These guidelines were both based on Rindi's three type[17] and tumor size not on the WHO 2010 classification.[18] In our study, of the 39 G1 patients, 6 (15.4%) experienced tumor recurrence; all 6 recurrences occurred in the remnant stomach. Of the 13 G2 patients, 3 (23.1%) experienced recurrence. Only 1 (G2, recurred in liver) of the 9 G1 and G2 tumor recurrences died of disease progression. All of patients with recurrence in remnant stomach were treated with repeat endoscopic or laparoscopic or surgical resection. Also, none of G1 patients died of disease progression and only 1 (with liver metastasis) of G2 patients died of disease progression. In addition, there was no statistical difference in survival (Fig. 1). From these results, we considered that WHO 2010 classification had a limitation of prognostic power for G1 and G2. We reported previously that among G1 or G2 patients with ≤1-cm-sized lesions, there were no cases of lymphovascular, perineural, mucosal, or submucosal invasion, or any cases of lymph node metastasis. These patients could be treated with endoscopic resection or minimally invasive surgery without node dissection.[19]
Mitosis and Ki-67 index, indicators of proliferative activity, are significant prognostic parameters in NETs.[20] The limitations and advantages of the 2010 WHO and ENETS grading systems for gastroenteric NETs, based on the Ki-67 index/mitosis, have been described in several studies.[21–23] The 2010 WHO classification and the ENETS system were found to be valid instruments for gastroenteric NETs in terms of prognostic assessment, with the TNM-based staging system seems to be the best available choice for clinicians, both alone and in association with other classifications.[21] Özaslan et al[24] reported that the mean survival duration decreased in parallel with increased Ki-67 and stage of the disease. The relative risk of death increased to approximately 6- and 12-fold for G2 NET and G3 NET, respectively, compared with G1 NET. Also, in a study carried out by Scarpa et al,[25] decreased survival rates were reported with increased Ki-67 values and higher disease stages. Pape et al[10] reported that the relative risk of death increased to approximately 4- and 30-fold for G2 NET an G3 NET, respectively, compared with G1 NET. However, these studies were investigated all gastroenteric NETs, not confined to gastric NETs. In contrast, in a study on 38 gastroenteric NETs by Alexiev et al,[26] no significant correlation was found between the Ki-67 index and tumor grade and metastatic behavior. Kawahara et al[27] again did not find the Ki-67 index to be associated with malignant behavior in their study of 41 cases. Volante et al[28] reported that mitosis, Ki-67 index, and grade were not associated with poor clinical outcome and in their study on 13 NETs located in the appendix. Rindi et al[23] reported that information on neuroendocrine cancer as classified by the WHO 2010 was forthcoming, revealing the limits and advantages of the system. Endo et al[22] reported the prognosis of 22 patients with gastric NETs according to the 2010 WHO classification, concluding that the prognoses of G1 and G2 were good. In our study, though sample size is relatively small, there was no statistical survival difference between G1 and G2.
The AJCC offers a TNM classification of well-differentiated NETs/carcinoma of the stomach that differs in a number of aspects from the ENETS TNM system.[16] It does not apply to high-grade (large and small cells) NECs or mixed-type tumors and does not exactly follow the ENETS classification (Table 1). No data exist justifying the use of different staging parameters in gastric NETs. In our present study, we found that the AJCC TNM system for gastric NETs was not actually suitable for well-differentiated NETs (G1 or G2) of the stomach because 86.4% of the tumors were classified as T1/T2, 92.4% as N0, and 86.4% as stage I/IIa (Table 3, Fig. 2). Only 1 tumor (1.9%) in our present series belonged to IIb, and this patient was the only one to die of disease progression. In addition, the ENETS TNM system was limited in distribution because 64.6% of the tumors were classified as T2 and 49.7% were classified as stage IIIb because N1 tumors (any T) belong to IIIb in this system (Table 3, Fig. 4).
Our study had several limitations of note. First, this was a retrospective study with inherent bias. In addition, because NETs of the stomach are very rare, the statistical significance of our findings was limited by the small number of patients. Our data were also skewed by the big difference in patient numbers between tumor subgroups. For example, 86.4% of the G1 or G2 tumors were classified as AJCC I/IIa, and 64.6% and 49.7% of the tumors were classified as ENETS T2 and ENETS IIIb, respectively. Recently, the WHO 2010 system defined MANECs as lesions that contain 30% of either component. However, we could not exactly determine the proportion of either component. Therefore, we defined NETs with any portion of adenocarcinoma as mixed type.
In conclusion, the WHO and ENETS systems were shown to have a low prognostic value. The AJCC TNM system also showed a low prognostic value for well-differentiated NETs (G1 or G2). In contrast, the AJCC TNM system had a high prognostic value for G3 or mixed tumors.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, ENETS = European Neuroendocrine Tumor Society, MANEC = mixed adenoneuroendocrine carcinoma, NEC = neuroendocrine carcinoma, NEN = neuroendocrine neoplasm, NET = neuroendocrine tumor, OS = overall survival, WHO = World Health Organization.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Hamilton SR, Aaltonen LA. IARC Press, Pathology and Genetics of Tumours of the Digestive System. Lyon:2000. [Google Scholar]
- 2.Kloppel G, Couvelard A, Perren A, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: towards a standardized approach to the diagnosis of gastroenteropancreatic neuroendocrine tumors and their prognostic stratification. Neuroendocrinology 2009; 90:162–166. [DOI] [PubMed] [Google Scholar]
- 3.Kulke MH, Anthony LB, Bushnell DL, et al. NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas 2010; 39:735–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strosberg JR, Coppola D, Klimstra DS, et al. The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas 2010; 39:799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekeblad S, Skogseid B, Dunder K, et al. Prognostic factors and survival in 324 patients with pancreatic endocrine tumor treated at a single institution. Clin Cancer Res 2008; 14:7798–7803. [DOI] [PubMed] [Google Scholar]
- 6.Fischer L, Kleeff J, Esposito I, et al. Clinical outcome and long-term survival in 118 consecutive patients with neuroendocrine tumours of the pancreas. Br J Surg 2008; 95:627–635. [DOI] [PubMed] [Google Scholar]
- 7.Kloppel G, Rindi G, Anlauf M, et al. Site-specific biology and pathology of gastroenteropancreatic neuroendocrine tumors. Virchows Arch 2007; 451 suppl 1:S9–S27. [DOI] [PubMed] [Google Scholar]
- 8.Lepage C, Rachet B, Coleman MP. Survival from malignant digestive endocrine tumors in England and Wales: a population-based study. Gastroenterology 2007; 132:899–904. [DOI] [PubMed] [Google Scholar]
- 9.Oberg K, Castellano D. Current knowledge on diagnosis and staging of neuroendocrine tumors. Cancer Metastasis Rev 2011; 30 suppl 1:3–7. [DOI] [PubMed] [Google Scholar]
- 10.Pape UF, Jann H, Muller-Nordhorn J, et al. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer 2008; 113:256–265. [DOI] [PubMed] [Google Scholar]
- 11.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008; 26:3063–3072. [DOI] [PubMed] [Google Scholar]
- 12.Plockinger U, Rindi G, Arnold R, et al. Guidelines for the diagnosis and treatment of neuroendocrine gastrointestinal tumours. Neuroendocrinology 2004; 80:394–424. [DOI] [PubMed] [Google Scholar]
- 13.Rindi G, Kloppel G, Alhman H, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch 2006; 449:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kloppel G, Rindi G, Anlauf M, et al. Site-specific biology and pathology of gastroenteropancreatic neuroendocrine tumors. Virchows Arch 2007; 451 suppl 1:S9–S27. [DOI] [PubMed] [Google Scholar]
- 15.Bosman FT, Carneiro F, Hruban RH, et al. WHO Classification of Tumours of the Digestive System. IARC Press, 4th edLyon:2010. [Google Scholar]
- 16.Edge S, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. Springer-Verlag, 7th edNew York:2010. [Google Scholar]
- 17.Rindi G, Luinetti O, Cornaggia M, et al. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology 1993; 104:994–1006. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton SR, Aaltonen LA. IARC Press, WHO Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. Lyon:2010. [Google Scholar]
- 19.Kim BS, Park YS, Yook JH, et al. Differing clinical courses and prognoses in patients with gastric neuroendocrine tumors based on the 2010-WHO classification scheme. Medicine (Baltimore) 2015; 94:e1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foltyn W, Zajecki W, Marek B, et al. The value of the Ki-67 proliferation marker as a prognostic factor in gastroenteropancreatic neuroendocrine tumours. Endokrynol Pol 2012; 63:362–366. [PubMed] [Google Scholar]
- 21.Dolcetta-Capuzzo A, Villa V, Albarello L, et al. Gastroenteric neuroendocrine neoplasms classification: comparison of prognostic models. Cancer 2013; 119:36–44. [DOI] [PubMed] [Google Scholar]
- 22.Endo S, Dousei T, Yoshikawa Y, et al. Gastric neuroendocrine tumors in our institutions according to the WHO 2010 classification. Int Surg 2012; 97:335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rindi G, Petrone G, Inzani F. The 2010 WHO classification of digestive neuroendocrine neoplasms: a critical appraisal four years after its introduction. Endocr Pathol 2014; 25:186–192. [DOI] [PubMed] [Google Scholar]
- 24.Özaslan E, Demir S, Karaca H, et al. Evaluation of the concordance between the stage of the disease and Ki-67 proliferation index in gastroenteropancreatic neuroendocrine tumors. Eur J Gastroenterol Hepatol 2016; Mar 3 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 25.Scarpa A, Mantovani W, Capelli P, et al. Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol 2010; 23:824–833. [DOI] [PubMed] [Google Scholar]
- 26.Alexiev BA, Drachenberg CB, Papadimitriou JC. Endocrine tumors of the gastrointestinal tract and pancreas: grading, tumor size and proliferation index do not predict malignant behavior. Diagn Pathol 2007; 2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawahara M, Kammori M, Kanauchi H, et al. Immunohistochemical prognostic indicators of gastrointestinal carcinoid tumours. Eur J Surg Oncol 2002; 28:140–146. [DOI] [PubMed] [Google Scholar]
- 28.Volante M, Daniele L, Asioli S, et al. Tumor staging but not grading is associated with adverse clinical outcome in neuroendocrine tumors of the appendix: a retrospective clinical pathologic analysis of 138 cases. Am J Surg Pathol 2013; 37:606–612. [DOI] [PubMed] [Google Scholar]


