Abstract
An elevated preoperative aspartate aminotransferase (AST) to platelet ratio index (APRI) is reported to be a prognostic factor for patients with hepatocellular carcinoma (HCC) after treatment. However, delta APRI (ΔAPRI), which represents the change from preoperative to postoperative APRI, has received little attention. The present study was designed to evaluate the prognostic value of ΔAPRI in patients with small HCC after liver resection.
A retrospective cohort study analyzing 244 patients with small HCC who had undergone liver resection was conducted. Medical data were retrieved from our prospectively maintained database. Patients were divided into 2 groups according to ΔAPRI as follows: group A (ΔAPRI ≥0.02) and group B (ΔAPRI <0.02). The association of demographic and clinical data, overall survival (OS), and recurrence-free survival (RFS) were statistically compared in the 2 groups, and a multivariate analysis was used to identify prognostic factors.
The 1, 3, and 5-year OS of patients in group A were 94.2%, 79.5%, and 62.3%, respectively, and 95.1%, 87.9%, and 84.6%, respectively, for patients in group B (P = 0.001). The corresponding 1, 3, and 5-year RFS was 69.0%, 44.7 %, and 28.1%, and 77.4%, 57.0%, and 54.2% for patients in the 2 groups, respectively (P = 0.009). The results of a multivariate analysis indicated that ΔAPRI was an independent prognostic factor for both OS (P = 0.001, hazard ratio 3.115, 95% confidence interval 1.642–5.912) and RFS (P = 0.006, hazard ratio 1.689, 95% confidence interval 1.163–2.452).
A positive ΔAPRI after liver resection predicts decreased OS and RFS in patients with small HCC.
Keywords: aspartate aminotransferase to platelet ratio index, hepatocellular carcinoma, liver resection, Milan criteria, prognostic factor
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most lethal malignancy and the third most frequent cause of cancer-related death worldwide.[1,2] Due to a high prevalence of hepatitis B virus (HBV), 50% of diagnosed HCC cases worldwide are located in China, making HCC a significant burden for this country.[3] Liver resection remains the standard of care for patients with small HCC who meet the Milan criteria.[4,5] Despite sophisticated advances in early diagnosis and surgical techniques, overall survival (OS) and recurrence-free survival (RFS) remain dissatisfactory in HCC patients.[6,7] However, prognostic indicators of HCC have not been completely elucidated. Established prognostic factors include liver function reserve, tumor size and number, vascular invasion, and the systemic inflammation response.[8–12]
Previous studies have suggested that liver inflammation and hepatic fibrosis are critical factors in the development of HCC,[13] and are considered prognostic indicators of ineffective liver regeneration,[14] risk factors for postoperative hepatic failure,[15] and prognostic factors for HCC.[16] Although liver biopsy remains the gold standard for assessing the degree of active hepatitis and hepatic fibrosis, the clinical use of the procedure is limited due to its high cost and risk of complications.[17] Recently, the aspartate aminotransferase (AST) to platelet ratio index (APRI)—a parameter that can be determined using noninvasive assays—was identified as a biomarker of hepatic fibrosis and cirrhosis.[18] Subsequent studies validated its value in predicting HCC prognosis after hepatic resection[19] and radiofrequency ablation (RFA).[20] These recent studies focused on preoperative APRI; however, there are few studies evaluating ΔAPRI, which represents the change from preoperative to postoperative APRI, in patients who have received hepatic resection.
The present study evaluated the predictive value of ΔAPRI in patients with small HCC who underwent hepatic resection.
2. Methods
Between February 2007 and March 2013, 346 patients newly diagnosed with small HCC who met the Milan Criteria underwent liver resection in the Department of Liver Surgery & Liver Transplantation Center of West China Hospital. The present study was approved by the Ethics Committee of West China Hospital, Sichuan University.
Patients were preoperatively diagnosed with HCC if 2 types of imaging examinations revealed features indicative of HCC, or if 1 imaging examination revealed signs of HCC and the patient had an α-fetoprotein (AFP) level greater than 400 ng/mL. The diagnosis of HCC was also confirmed in a postoperative histopathology examination. The degree of liver fibrosis or liver cirrhosis was also determined in postoperative histopathology examination in accordance with the Ishak scoring system.[21] The demographic data, oncological data, hematological tests, liver function tests, HBV markers, and follow-up data were retrieved from our prospectively maintained database.
In the present study, our inclusion criteria were as follows: primary small HCC meeting Milan criteria (solitary tumor <5 cm in diameter or ≤3 nodules ≤3 cm in diameter) and no extrahepatic metastasis; adequate reserve liver function (Child–Pugh grade A); appropriate renal function (serum creatinine <124 mmol/ L; and underwent liver resection as initial treatment.
Exclusion criteria included the following: recurrent HCC; simultaneous splenectomy and liver resection; loss to follow-up within 3 months after liver resection; and poor data integrity.
2.1. Definition of APRI
All preoperative liver function tests, including AST and platelet counts, were administered 2 days before the operation. The APRI was calculated as the following ratio: ([AST/upper limit of normal value]/platelet counts [109/L]) × 100, with the AST upper limit of normal value defined as 40 IU/L. Postoperative APRI was assessed at the first follow-up visit at the outpatient department 1 month after the operation. ΔAPRI was calculated by subtracting preoperative APRI from the postoperative APRI minus preoperative APRI.
2.2. Follow-up visit
All of the 244 patients were regularly followed up during the first, third, and sixth month immediately after the operation, every 3 months during the following 3 years, and every 6 months in subsequent years.
Physical examination, blood cell and differential counts, AFP level, liver function test, HBV markers and HBV-DNA (if the patient was diagnosed with an HBV infection), and radiology examination (in select cases) were included in the follow-up examinations. Tumor recurrence was suspected if there was an increase in serum AFP level (>20 ng/mL) or if a new lesion was detected by surveillance imaging during the examination. The last follow-up occurred at the end of April 2014.
2.3. Statistical analysis
SPSS software version 21.0 (SPSS Company, Chicago, IL) and R software 3.1.1 (The R foundation for statistical computing, Vienna, Austria) were used to conduct statistical analyses. Time-dependent receiver-operating characteristic (ROC) curve was used to establish the cut-off value of ΔAPRI in predicting mortality. An independent-sample t test was used to compare continuous variables. Categorical data were compared using the chi-square test or Fisher exact test. Cumulative recurrence rates and overall survival rates were estimated using the Kaplan–Meier method, and compared using a log-rank test. Variables with statistical significance (P < 0.05) or close to statistical significance (P < 0.1) according to univariate analysis underwent multivariate analysis using Cox forward stepwise logistic regression model; variables with P > 0.1 were removed before multivariate analysis. Calculated P values were 2-sided, and P < 0.05 was considered statistically significant.
3. Results
Based on our inclusion and exclusion criteria, a total of 102 patients were excluded from the present study. Among the excluded patients, 20 presented with recurrent HCC, 11 had Child–Pugh grade B, 12 had received previous therapy (including RFA or transhepatic arterial chemotherapy and embolization [TACE]), 18 were lost to follow-up within the first 3 months after the liver resection, 16 underwent simultaneous splenectomy and liver resection, and data for 25 patients were of poor integrity. Ultimately, 244 patients with small HCC that had undergone liver resection and that met our criteria were included in this retrospective analysis. The patients included 31 (12.7%) females and 213 (87.3%) males, and the mean age of the patients was 50 years (range 21–78 years). A total of 208 patients (85.2%) presented with 1 nodule, and 36 (14.8%) patients presented with 2 or 3 nodules. A total of 84 patients (34.4%) presented with a nodule ≤3 cm in diameter, and 160 (65.6%) presented with a nodule 3 to 5 cm in diameter. After a median follow-up period of 36.3 months (range from 3 to 85.9), 118 (48.4%) patients experienced disease recurrence, and 42 (17.2%) patients had died.
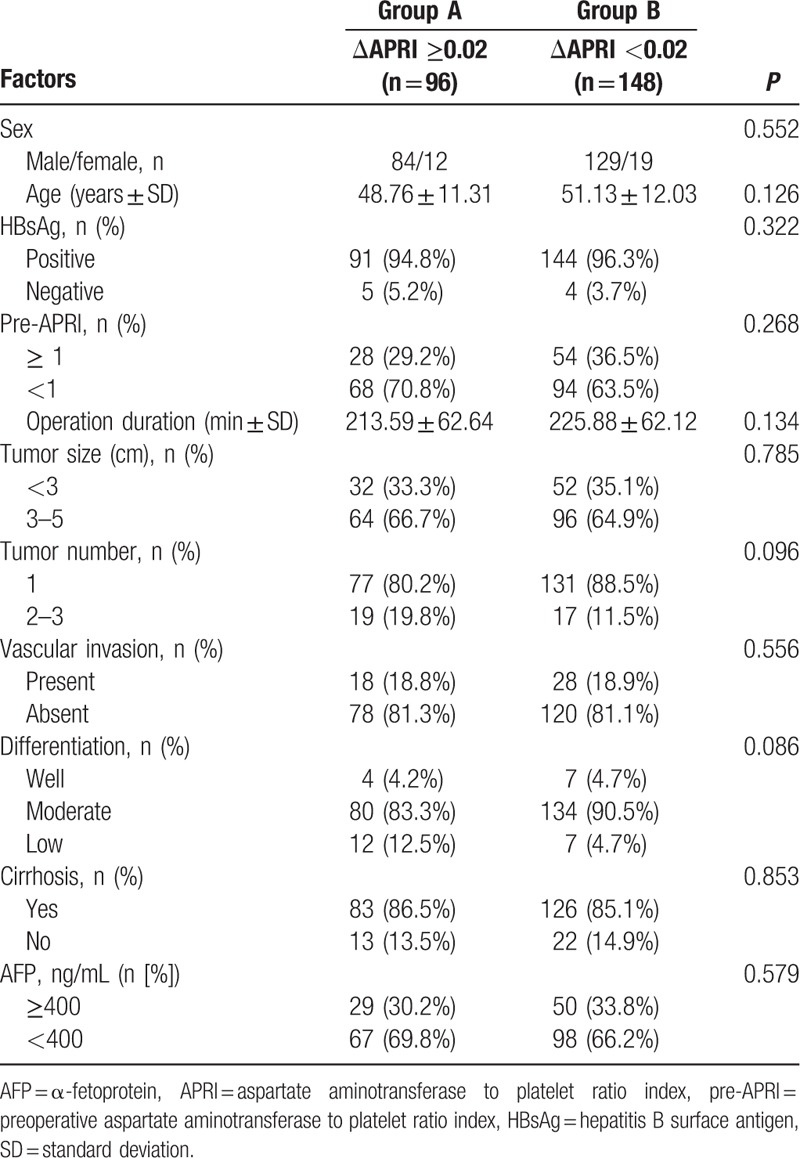
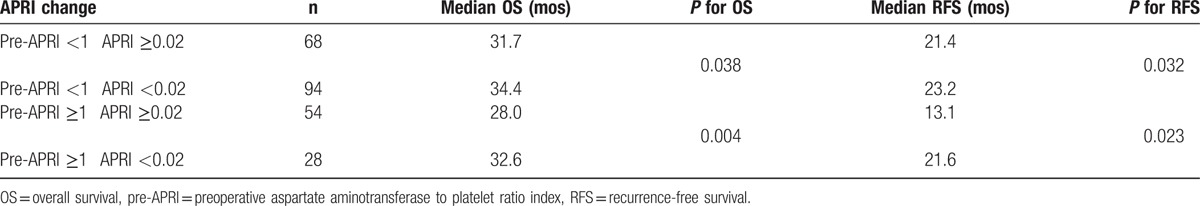
An optimal cut-off value of 1.0 corresponded to the maximum joint sensitivity and specificity on the ROC plot for preoperative APRI (Fig. 1). There were 82 (33.6%) patients with preoperative APRI ≥1 and 162 (66.4%) with preoperative APRI <1. Figure 2A–E shows the ROC curve of ΔAPRI for the prediction of mortality at 1, 2, 3, 4, and 5 years, respectively, after the start of follow-up using time-dependent ROC analysis. The area under curves (AUCs) at 1, 2, 3, 4, and 5 years were 0.37, 0.55, 0.53, 0.57, and 0.61, respectively. Also, the cut-off value was set at 0.02. Then patients were divided into 2 groups: group A (ΔAPRI ≥0.02, n = 96) and group B (ΔAPRI < 0.02, n = 148), according to the time-dependent ROC analysis. The clinicopathological characteristics of the 2 groups are described in Table 1. There were no significant differences in the baseline characteristics between the 2 groups.
Figure 1.
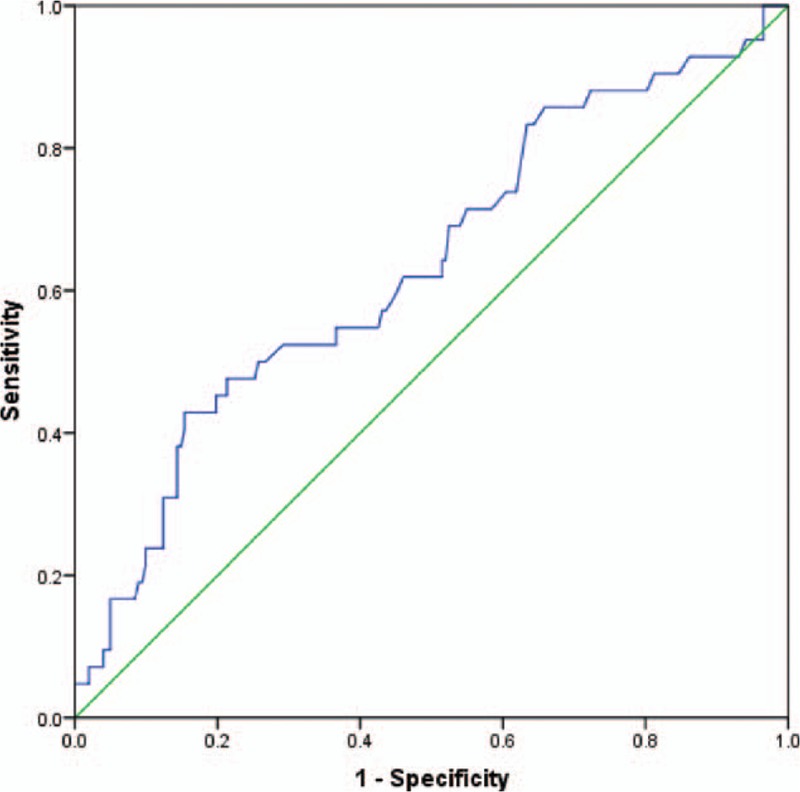
The ROC curve of preoperative APRI predicting mortality showing the cut-off value of 1.015 (sensitivity = 0.524, specificity = 0.708, P = 0.006). APRI = aspartate aminotransferase to platelet ratio index, ROC = receiver-operating characteristic.
Figure 2.
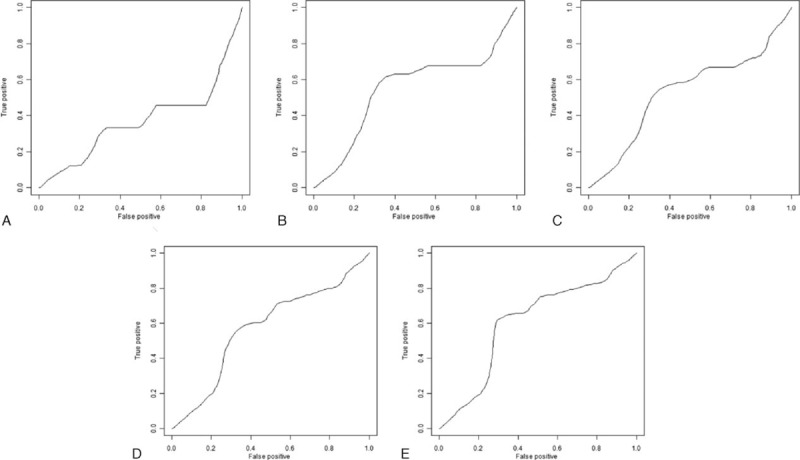
Time-dependent ROC curves of ΔAPRI for small HCC survival after the start of follow-up. A, 1-year: the AUC was 0.37, cut-off point was 0.08. B, 2-year: the AUC was 0.55, cut-off point was 0.02. C, 3-year: the AUC was 0.53, cut-off point was 0.02. D, 4-year: the AUC was 0.57, cut-off point was 0.02. E, 5-year: the AUC was 0.61, cut-off point was 0.02. APRI = aspartate aminotransferase to platelet ratio index, HCC = hepatocellular carcinoma, ROC = receiver-operating characteristic.
Table 1.
The clinicopathological characteristics of patients with respect to ΔAPRI.
3.1. Impact of APRI on overall survival
The cumulative 1, 3, and 5-year OS rates among all of the patients in the study were 95.3%, 84.3%, and 72.7%, respectively. The 1, 3, and 5-year OS rates were 92.8%, 75.7%, and 63.6%, respectively, for patients with preoperative APRI ≥1, and 97.4%, 90.0%, and 78.0%, respectively, for patients with preoperative APRI <1 (log-rank test, P = 0.010) (Fig. 3).
Figure 3.
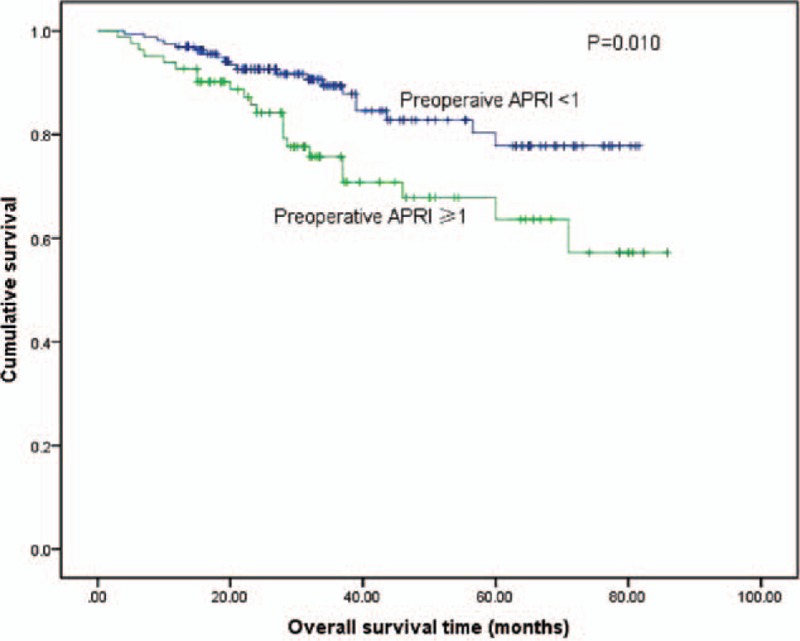
Relationship between pre-APRI and overall survival in patients with small HCC after liver resection. Patients with a pre-APRI ≥1 were associated with a significant reduction in overall survival rate compared with patients with a pre-APRI <1 (log-rank test, P = 0.002). Pre-APRI = preoperative aspartate aminotransferase to platelet ratio index, HCC = hepatocellular carcinoma.
With respect to the ΔAPRI, 1, 3, and 5-year OS rates were 95.1%, 87.9%, and 84.6%, respectively, for patients withΔAPRI <0.02, and 94.2%, 79.5%, and 62.3%, respectively, for patients with ΔAPRI ≥0.02 (log-rank test, P = 0.001; Fig. 4). In the subgroup analysis of the 162 patients with preoperative APRI <1, the 1, 3, and 5-year OS rates for patients with ΔAPRI <0.02were 98.3%, 92.0%, and 88.7%, respectively, and 95.6%, 86.2%, and 68.6%, respectively, for patients with ΔAPRI ≥0.02 (log-rank test, P = 0.038; Table 2). Similarly, for the 82 patients with preoperative APRI ≥1, the 1, 3, and 5-year OS rates for patients with ΔAPRI <0.02 were 94.8%, 81.8%, and 78.2%, respectively, and 89.5%, 64.7%, and 36.9%, respectively, for patients with ΔAPRI ≥ 0.02 (log-rank test, P = 0.004; Table 2).
Figure 4.
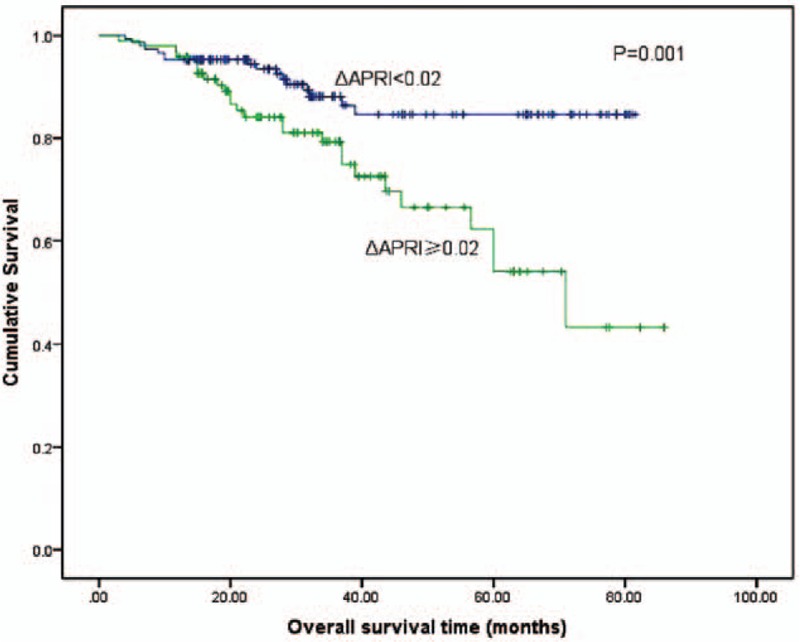
Relationship between ΔAPRI and overall survival of patients with small HCC after liver resection. Patients with ΔAPRI ≥0.02 were associated with a significant reduction in overall survival rate compared with patients with a ΔAPRI <0.02 (log-rank test, P = 0.001). ΔAPRI = postoperative aspartate aminotransferase to platelet ratio index change, HCC = hepatocellular carcinoma.
Table 2.
Analysis for subgroup patients with different preoperative APRI and ΔAPRI after liver resection.
3.2. Impact of APRI on recurrence-free survival
The cumulative 1, 3, and 5-year RFS rates among all the patients were 74.1%, 53.4%, and 44.3%, respectively. The 1, 3, and 5-year RFS rates were 78.2%, 58.3%, and 46.8%, respectively, for patients with preoperative APRI <1, and 66.2%, 40.7%, and 38.4%, respectively, for patients with preoperative APRI ≥1 (log-rank test, P = 0.014) (Fig. 5).
Figure 5.
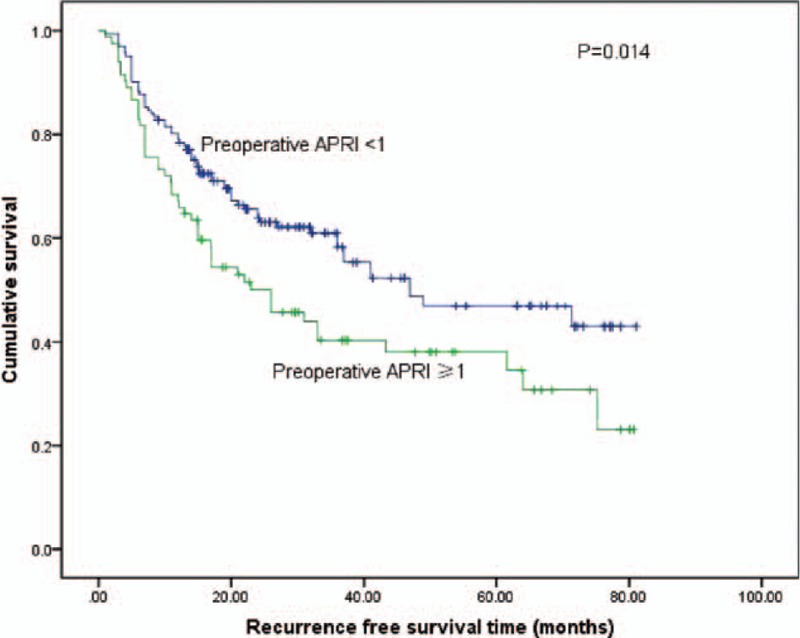
Relationship between pre-APRI and recurrence-free survival of patients with small HCC after liver resection. Patients with a pre-APRI ≥1 were associated with a significant reduction in recurrence-free survival compared with patients with a pre-APRI <1 (log-rank test, P = 0.007). Pre-APRI = preoperative aspartate aminotransferase to platelet ratio index, HCC = hepatocellular carcinoma.
With respect to ΔAPRI, the 1, 3, and 5-year RFS rates were 77.4%, 57.0%, and 54.2%, respectively, for patients with ΔAPRI <0.02, and 69.0%, 44.7%, and 28.9%, respectively, for patients with ΔAPRI ≥ 0.02 (log-rank test, P = 0.009; Fig. 6). In the subgroup analysis of the 162 patients with preoperative APRI <1, the 1, 3, and 5-year RFS rates for patients with ΔAPRI <0.02 were 81.8%, 65.0%, and 61.9%, respectively, and 76.8%, 56.2%, and 24.2%, respectively, for patients with ΔAPRI ≥0.02 (log-rank test, P = 0.032; Table 2). Similarly, in the subgroup analysis of the 82 patients with preoperative APRI ≥1, the 1, 3, and 5-year RFS rates were 73.8%, 45.7%, and 42.4%, respectively, for patients with ΔAPRI <0.02, and 50.4%, 31.6%, and 21.4% for patients with ΔAPRI ≥0.02 (log-rank test, P = 0.023; Table 2).
Figure 6.
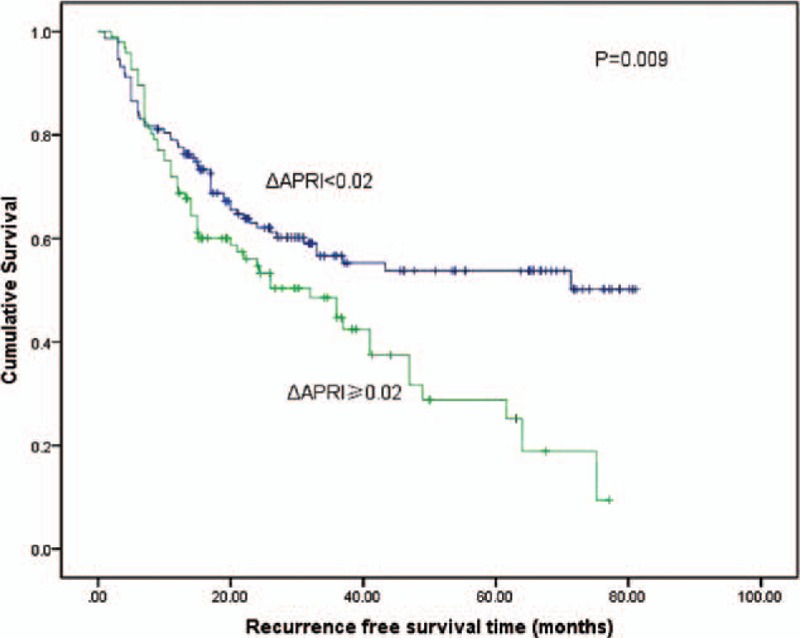
Relationship between ΔAPRI and recurrence-free survival of patients with small HCC after liver resection. Patients with increased ΔAPRI ≥0.02 were associated with a significant reduction in recurrence-free survival compared with patients with a ΔAPRI <0.02 (log-rank test, P = 0.009). ΔAPRI = postoperative aspartate aminotransferase to platelet ratio index change, HCC = hepatocellular carcinoma.
3.3. Risk factors for prognosis of small HCC after liver resection
As shown in Table 3, the univariate analysis revealed that vascular invasion, preoperative APRI ≥1, and ΔAPRI ≥0.02 were all significantly associated with poor OS. Similarly, vascular invasion, the female sex, tumor number ≥2, liver cirrhosis, AFP level ≥400 ng/mL, preoperative APRI ≥1, and ΔAPRI ≥0.02 were associated with poor RFS (Table 4).
Table 3.
Univariate and multivariate analysis of prognostic factors of OS in patients with small hepatocellular carcinoma after liver resection.
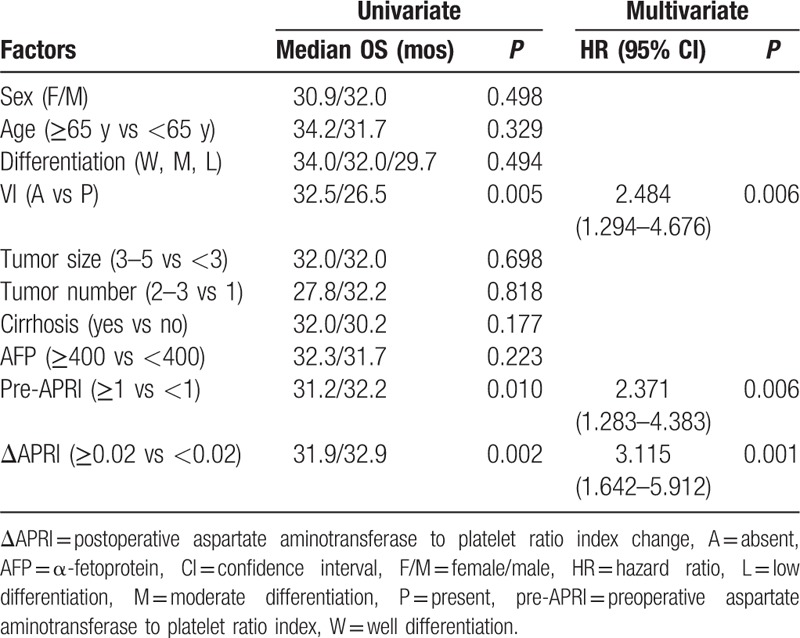
Table 4.
Univariate and multivariate analysis of prognostic factors of RFS in patients with small hepatocellular carcinoma after liver resection.
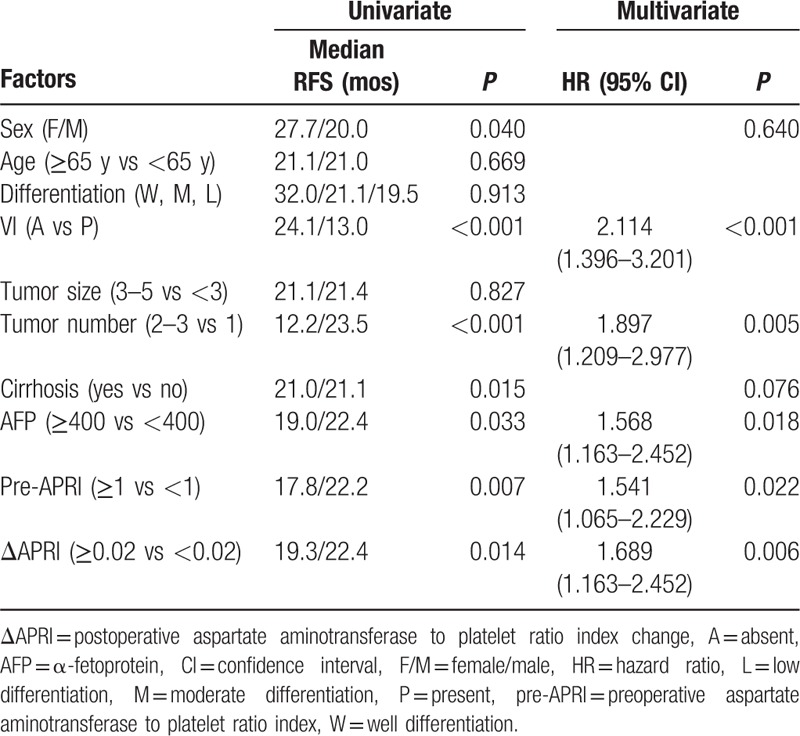
The multivariate analysis confirmed that vascular invasion (P = 0.006, hazard ratio [HR] 2.484, 95% confidence interval [CI] 1.294–4.676), preoperative APRI ≥1 (P = 0.006, HR 2.371, 95% CI 1.283–4.383), and ΔAPRI ≥0.02 (P = 0.001, HR 3.115, 95% CI 1.642–5.912) were independent risk factors for poor OS, and that vascular invasion (P < 0.001, HR 2.114, 95% CI 1.396–3.201), AFP level ≥400 ng/mL (P = 0.018, HR 1.568, 95% CI 1.163–2.452), tumor number ≥2 (P = 0.005, HR 1.897, 95% CI 1.209–2.977), preoperative APRI ≥1 (P = 0.022, HR 1.541, 95% CI 1.065–2.229), and ΔAPRI ≥0.02 (P = 0.006, HR 1.689, 95% CI 1.163–2.452) were independent prognostic indicators of RFS in patients with small HCC that had undergone liver resection.
4. Discussion
Aspartate aminotransferase to platelet ratio index was initially used to evaluate the staging of liver fibrosis and cirrhosis in patients with chronic hepatitis C.[22] In subsequent years, the application of APRI has been extended to the evaluation of liver function reserves and the assessment of the prognosis of patients with chronic hepatitis.[23] Kao et al[20] suggested that APRI was associated with OS and disease recurrence after RFA in patients with HCC. However, these studies focused on pretreatment APRI, whereas the significance of the differences between preoperative and postoperative APRI has been given little attention.
The results of the present study suggest that vascular invasion, AFP level ≥400 ng/mL, tumor number ≥2, preoperative APRI ≥1, and ΔAPRI >0.02 are prognostic indicators in patients with small HCC that have undergone liver resection. Notably, preoperative APRI ≥1 and ΔAPRI ≥0.02 are independent risk factors of both OS and RFS. Furthermore, even in the subgroup analysis of patients with preoperative APRI <1, a poorer prognosis was observed in patients with ΔAPRI ≥0.02 compared with patients with ΔAPRI <0.02. These findings indicate that ΔAPRI might represent a reliable and stable prognostic factor in patients with small HCC and that preoperative APRI and ΔAPRI might have valuable applications in clinical practice for determining postoperative treatment in patients with small HCC. Preoperative APRI might be used to stratify patients before surgery, and ΔAPRI might be an indicator of early treatment efficacy and survival.
Recently, Hung et al[24] reported that preoperative APRI can serve as a surrogate measure of liver function reserves and hepatic fibrosis, and as a predictor of survival in patients with small HCC after liver resection. They found that preoperative APRI exhibits a highly discriminative ability to stage liver fibrosis and that preoperative APRI >0.47 predicted poor OS and RFS. Similarly, preoperative APRI >1 was demonstrated to be an independent risk factor for tumor recurrence and poor OS in patients with small HCC after RFA.[20] Shen et al[19] reported that disease-free survival and OS rates of patients with preoperative APRI <0.62 were significantly better compared with patients with a relatively high APRI. The results of our study are consistent with some of their findings; we found that patients with a preoperative APRI <1 had a more favorable prognosis after liver resection compared with those with a preoperative APRI ≥1. However, the cut-off value of preoperative APRI in this study differed from that used in the studies by Hung et al and Shen et al, indicating that a higher preoperative APRI is associated with a poor prognosis in patients with HCC. Unfortunately, it is not possible to establish a universal cut-off value, because different patient populations from different medical centers inevitably use different cut-off values. In the present study, we focused our attention on ΔAPRI, which we calculated by subtracting preoperative from postoperative APRI. ΔAPRI is a value that reflected the dynamic change between the preoperative and postoperative periods. Ultimately, we determined that ΔAPRI was an independent prognosis indicator for patients with small HCC after liver resection.
The significance of APRI in the prognosis of patients with HCC after liver resection is consistent with the established pathogenesis of HCC. APRI was calculated using AST and platelet levels. Multiple studies have reported that increased AST and decreased platelet levels are associated with the progression of liver fibrosis. Decreased platelet levels in patients with severe fibrosis and cirrhosis decrease the production of thrombopoietin by hepatocytes. This phenomenon reduces platelet production[25] and increases the sequestration and destruction of platelets in enlarged spleens due to fibrosis and portal hypertension.[26] Furthermore, advanced liver disease is associated with mitochondrial injury, a feature that can substantially increase the release of AST.[27] Moreover, fibrosis and cirrhosis can reduce the sinusoidal clearance of AST,[28] which, in turn, increases APRI. Theoretically, APRI is expected to decrease after liver resection, because a number of patients who receive this procedure are prescribed antiviral therapy. Antiviral therapy might ameliorate liver inflammation, improve liver functional reserves in patients with chronic hepatitis,[29] and improve the prognosis of patients with HCC.[30] Therefore, we hypothesized that the absence of a decrease in APRI after the operation, despite the administration of antiviral therapy, is indicative of reduced liver function reserves and a low functional potential of the remnant liver livers, features that are associated with disease recurrence.
We acknowledge the limitations of our study. The present study was a retrospective cohort study conducted at a single medical center, and the study population was comprised primarily of HBV-related HCC cases that only have been internally validated. Therefore, multicenter and potentially prospective studies are needed to verify the prognostic value of ΔAPRI in HCC, and the potential mechanism underlying this association.
In conclusion, we demonstrated that increased ΔAPRI was an independent risk factor of OS and RFS in patients with small HCC after liver resection.
Footnotes
Abbreviations: AFP = α-fetoprotein, APRI = aspartate aminotransferase to platelet ratio index, AST = aspartate aminotransferase, HCC = hepatocellular carcinoma, OS = overall survival, RFA = radiofrequency ablation, RFS = recurrence free survival, TACE = transhepatic arterial chemotherapy and embolization.
The authors have no conflicts of interest to disclose.
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011; 365:1118–1127. [DOI] [PubMed] [Google Scholar]
- 3.Venook AP, Papandreou C, Furuse J, et al. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist 2010; 15:5–14. [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334:693–699. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis 2005; 25:181–200. [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005; 42:1208–1236. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 8.Poon RT, Fan ST, Lo CM, et al. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg 1999; 229:216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ercolani G, Grazi GL, Ravaioli M, et al. Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg 2003; 237:536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikai I, Arii S, Kojiro M, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer 2004; 101:796–802. [DOI] [PubMed] [Google Scholar]
- 11.Yeh CN, Chen MFU, Lee WC, et al. Prognostic factors of hepatic resection for hepatocellular carcinoma with cirrhosis: univariate and multivariate analysis. J Surg Oncol 2002; 81:195–202. [DOI] [PubMed] [Google Scholar]
- 12.Peng W, Li C, Zhu WJ, et al. Prognostic value of the platelet to lymphocyte ratio change in liver cancer. J Surg Res 2015; 194:464–470. [DOI] [PubMed] [Google Scholar]
- 13.Liaw YF, Chu CM. Hepatitis B virus infection. Lancet 2009; 373:582–592. [DOI] [PubMed] [Google Scholar]
- 14.Nagasue N, Yukaya H, Ogawa Y, et al. Human liver regeneration after major hepatic resection. A study of normal liver and livers with chronic hepatitis and cirrhosis. Ann Surg 1987; 206:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farges O, Malassagne B, Flejou JF, et al. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg 1999; 229:210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu JC, Huang YH, Chau GY, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol 2009; 51:890–897. [DOI] [PubMed] [Google Scholar]
- 17.Rockey DC, Bissell DM. Noninvasive measures of liver fibrosis. Hepatology 2006; 43:S113. [DOI] [PubMed] [Google Scholar]
- 18.Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology 2007; 46:912–921. [DOI] [PubMed] [Google Scholar]
- 19.Shen SL, Fu SJ, Chen B, et al. Preoperative aspartate aminotransferase to platelet ratio is an independent prognostic factor for hepatitis B-induced hepatocellular carcinoma after hepatic resection. Ann Surg Oncol 2014; 21:3802–3809. [DOI] [PubMed] [Google Scholar]
- 20.Kao WY, Chiou YY, Hung HH, et al. Risk factors for long-term prognosis in hepatocellular carcinoma after radiofrequency ablation therapy: the clinical implication of aspartate aminotransferase-platelet ratio index. Eur J Gastroenterol Hepatol 2011; 23:528–536. [DOI] [PubMed] [Google Scholar]
- 21.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22:696–699. [DOI] [PubMed] [Google Scholar]
- 22.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38:518–526. [DOI] [PubMed] [Google Scholar]
- 23.Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology 2011; 53:726–736. [DOI] [PubMed] [Google Scholar]
- 24.Hung HH, Su CW, Lai CR, et al. Fibrosis and AST to platelet ratio index predict post-operative prognosis for solitary small hepatitis B-related hepatocellular carcinoma. Hepatol Int 2010; 4:691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawasaki T, Takeshita A, Souda K, et al. Serum thrombopoietin levels in patients with chronic hepatitis and liver cirrhosis. Am J Gastroenterol 1999; 94:1918–1922. [DOI] [PubMed] [Google Scholar]
- 26.Aster R. Pooling of platelets in the spleen: role in the pathogenesis of “hypersplenic” thrombocytopenia. J Clin Invest 1996; 45:645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okuda M, Li K, Beard MR, et al. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology 2002; 122:366–375. [DOI] [PubMed] [Google Scholar]
- 28.Kamimoto Y, Horiuchi S, Tanase S, et al. Plasma clearance of intravenously injected aspartate aminotransferase isozymes: evidence for preferential uptake by sinusoidal liver cells. Hapatology 1985; 5:367–375. [DOI] [PubMed] [Google Scholar]
- 29.Liaw YF, Leung N, Kao JH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int 2008; 2:263–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breitenstein S, Dimitroulis D, Petrowsky H, et al. Systematic review and meta-analysis of interferon after curative treatment of hepatocellular carcinoma in patients with viral hepatitis. Br J Surg 2009; 96:975–981. [DOI] [PubMed] [Google Scholar]


