Abstract
Local recurrence frequently occurs in patients with pancreatic cancer after intended curative resections. However, no treatment strategies have been established for isolated local recurrence. Several series have demonstrated a survival benefit for reoperation in selected pancreatic recurrence cases. This study compares the difference in overall survival (OS) between surgery and nonsurgery groups in recurrent pancreatic cancer.
All patients from 1990 to 2014 with recurrent pancreatic cancer who underwent curative resections were investigated and retrospectively reviewed. Clinicopathological features and OS were compared.
A total of 332 patients were recruited in this series. The majority had histologically pancreatic adenocarcinoma (289 patients, 87.0%). Fourteen of 332 patients (4.2%) with recurrent pancreatic cancer received subsequent resection. Most of these patients underwent curative surgery (R0 resection, 13 patients, 92.9%), and only 1 patient (7.1%) had microscopic residual tumor (R1 resection). Disease-free survival (DFS), OS, and postrecurrence survival (PRS) were all significantly longer in the surgery group (DFS 10.6 vs 6.1 months, P = 0.044; OS 57.8 vs 14.0 months, P < 0.001; PRS 14.1 vs 6.0 months, P < 0.001). The median survival times were comparable in patients with recurrent pancreatic adenocarcinoma who received surgery and those who did not (DFS 10.6 vs 6.1 months, P = 0.226; OS 23.7 vs 14.0 months, P = 0.074; PRS 8.9 vs 5.8 months, P = 0.183). However, the OS and PRS were superior in the patients who did not display adenocarcinoma histologically but underwent operation for recurrence (OS 97.2 vs 16.9 months, P = 0.016; PRS 65.7 vs 6.9 months, P = 0.010). Notably, DFS levels were similar (16.0 vs 7.0 months, P = 0.265).
Surgery can feasibly and safely provide survival benefits in selective recurrent pancreatic cancer. In patients who are histologically negative for adenocarcinoma, survival is prolonged when the operation is performed with R0 resection. Patients with isolated recurrent pancreatic adenocarcinoma need multidisciplinary therapy. In addition to operation, chemoradiotherapy and intraoperative radiotherapy may also be considered; their roles should be further investigated.
Keywords: locoregional recurrence, metastatectomy, recurrent pancreatic cancer, reoperation
1. Introduction
The annual incidence rate of pancreatic cancer is 1.1% to 1.2% in the United States.[1] However, pancreatic cancer remains the 4th leading cause of cancer-related death. In Taiwan, pancreatic cancer was the 8th leading cause of cancer-related death in 2014, which was 8.1%, and increased from 5.1%, the 10th leading cause of cancer-related death in 2004.[2]
In the era of multidisciplinary approaches for the treatment of various types of cancer, complete surgical resection remains the gold standard of primary pancreatic malignancy. However, approximately 10% to 20% of patients are able to undergo resection.[3] A majority of patients develop local recurrence following primary surgical resection, which may contribute to this poor prognosis; 86% of patients have evidence of locoregional recurrence despite complete primary surgical resection.[3–5] Approximately 80% of surgically resected pancreatic cancers recur within 5 years of resection, and >60% of patients develop recurrences within 2 years.[6]
Although the recurrence rates of pancreatic cancer are high, no unanimous effective strategies have been established for these patients. Multiple disciplines have been advocated, such as surgery, chemotherapy, radiation therapy, and intraoperative radiotherapy (IORT). Isolated local recurrence differs from peritoneal dissemination recurrence, and emerging studies have demonstrated that isolated local recurrence may be treated using repetitive resection in selected patients. However, very few cases have been published.[7–13] Notably, the role of reoperation in isolated recurrent pancreatic cancer remains uncertain. This study investigates the role of surgery in patients with recurrent pancreatic cancer. We also compare the survival benefit of surgery for recurrent pancreatic adenocarcinoma or nonadenocarcinoma.
2. Materials and methods
2.1. Study design
Between 1990 and 2014, we prospectively collected patients with recurrent pancreatic cancer who underwent curative resections at Chang Gung Memorial Hospital, Linkou, Taiwan. The Chang Gung Medical Foundation Institutional Review Board has approved this study. All patients with recurrent pancreatic cancer underwent initial curative operations, and the patients with recurrence were selected for a second operation. Based on whether a selected second operation was performed, patients were classified into a surgery group, which was defined as those who underwent initial surgery followed by a second operation for recurrence, and a nonsurgery group, which was defined as those who underwent an initial operation only and palliative treatment for recurrence. Clinicopathological features, including age; sex; initial tumor, node, metastasis (TMN) stage; type of the initial and second operations; resection margin; adjuvant chemotherapy; recurrent site; and primary histopathology were compared between these 2 groups. According to imaging studies during regular follow-up after previous surgery, tumor evidence was defined as progressive soft tissue growth at specific predilection sites. Histopathologic confirmation was not routinely conducted for a diagnosis of tumor recurrence. Patients with suspected local recurrence without evidence of carcinomatosis or multiple distant metastases and those with acceptable performance status were selected for reoperation. Evidence of recurrence or metastasis was diagnosed with computed tomography with contrast. Only 1 patient was confirmed as liver metastasis by means of needle biopsy.
In reoperation, the operative, postoperative, and pathological records were reviewed to assess perioperative outcomes. The length of hospital stay, perioperative morbidity and mortality, resection status of the second operation, and pathology type were recorded. The morbidity and mortality were classified according to Dindo–Clavien definitions. The primary goal of the second surgery was to achieve complete microscopic resection (R0), and resection status was defined as R0 resection based on the histopathological report. Those who achieved complete macroscopic resection but had residual microscopic lesions were categorized as R1 resection, and those with residual macroscopic resection were categorized as R2 resection.
Disease-free survival (DFS) was defined as the duration between the first (in the reoperation group) operation or the only operation (in the nonsurgery group) and the documented progression of residual disease or recurrent disease during regular follow-up. Postrecurrence survival (PRS) was defined as the interval between documented recurrence and death from any cause or the most recent follow-up. Overall survival (OS) was defined as the duration between the date of the first operation to the date of death from any cause or to the date of the most recent follow-up.
2.2. Statistics
Parameters in the groups were compared using Student t test for numerical parameters and the χ2 test or Fisher exact test for categorical parameters. The survival rates were calculated using the Kaplan–Meier method, and a survival analysis was performed with the log-rank test. A statistical analysis was conducted using the Statistical Package for the Social Sciences (SPSS, Inc., Chicago, IL) for Windows Version 13. A 2-sided P value < 0.05 was defined as statistically significant.
3. Results
A total of 332 patients were recruited for this series. The majority of patients were histologically positive for pancreatic adenocarcinoma (289 patients, 87.0%). Fourteen of the 332 patients (4.2%) with recurrent pancreatic cancer received subsequent resection. Based on primary TMN stage, the patients who underwent conservative treatment during recurrence were initially more advanced (stage IIB, 28.6% vs 61.6%, P < 0.001). The majority of patients in both groups who underwent pancreaticoduodenostomy during the initial operation achieved complete microscopic resection (R0 resection, 92.9% vs 70.8%, P = 0.179). However, 42 (13.2%) of the 318 patients who received conservative treatment during recurrence could not achieve tumor-free status but had a grossly visible tumor (R2 resection) in the primary operation. In contrast, no patients had R2 resection during the initial operation in the surgery group. The clinicopathological features are summarized in Table 1. Table 2 compared the parameters in advance.
Table 1.
Clinicopathological features between the surgical and nonsurgical groups’ patients.
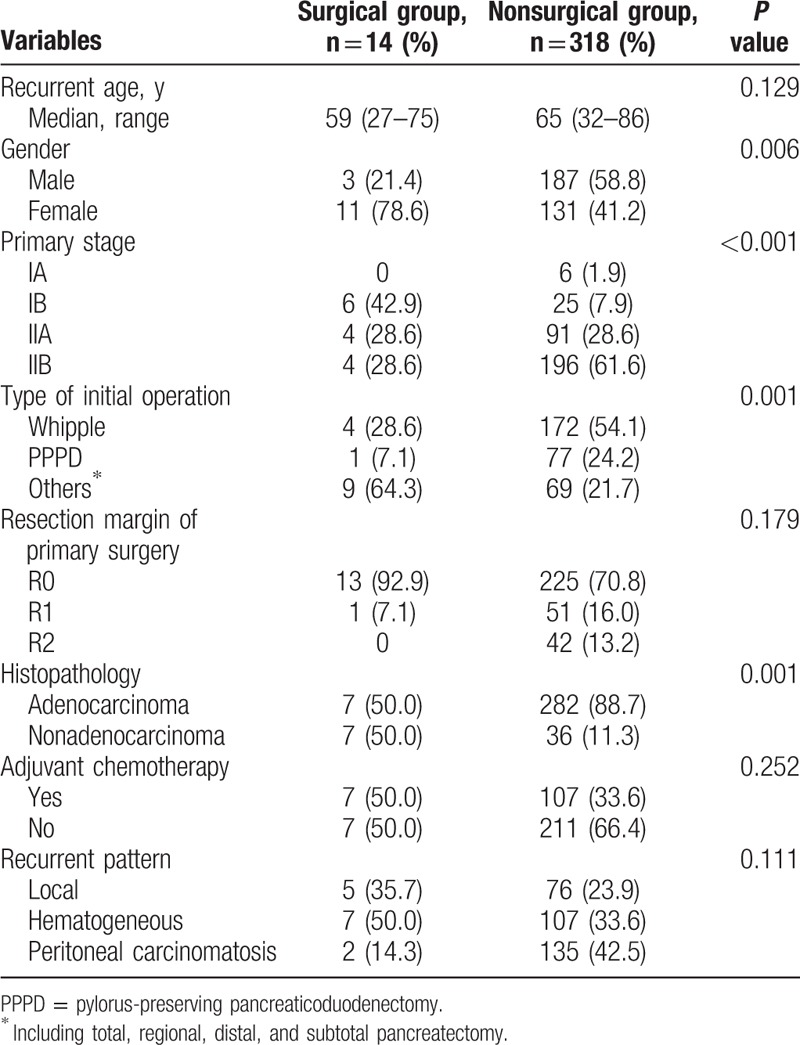
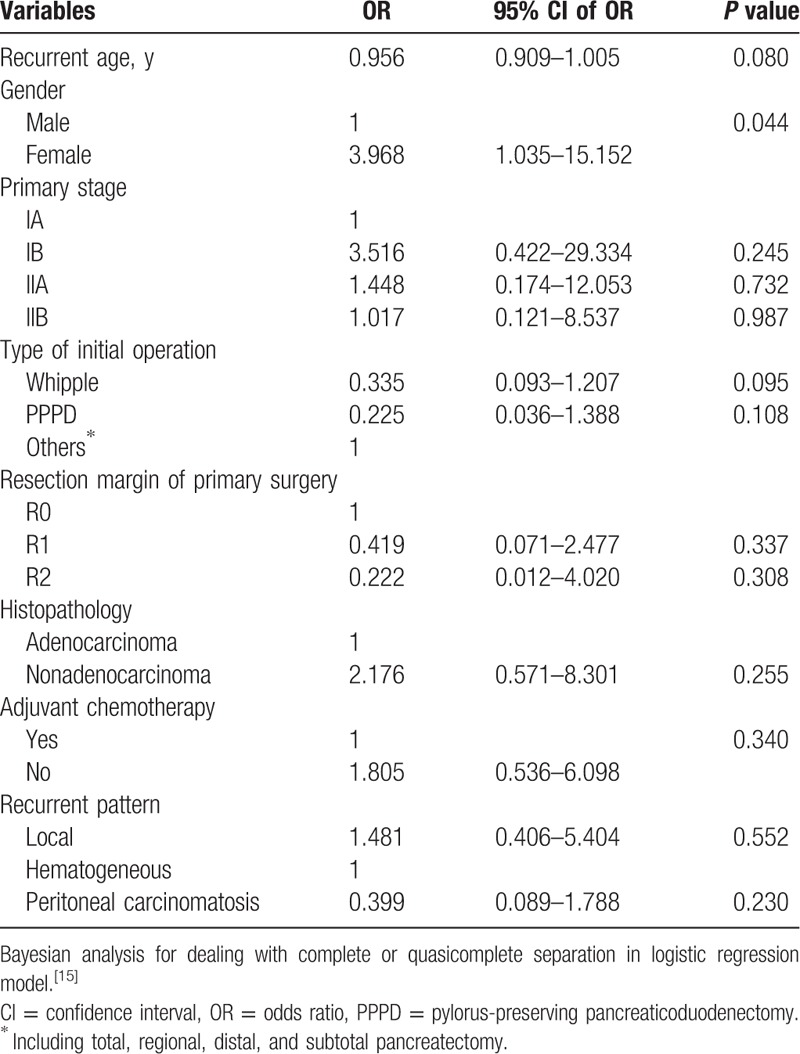
Table 2.
Bayesian analysis for a logistic regression model using surgical group as the dependent variable in recurrent pancreatic cancer.
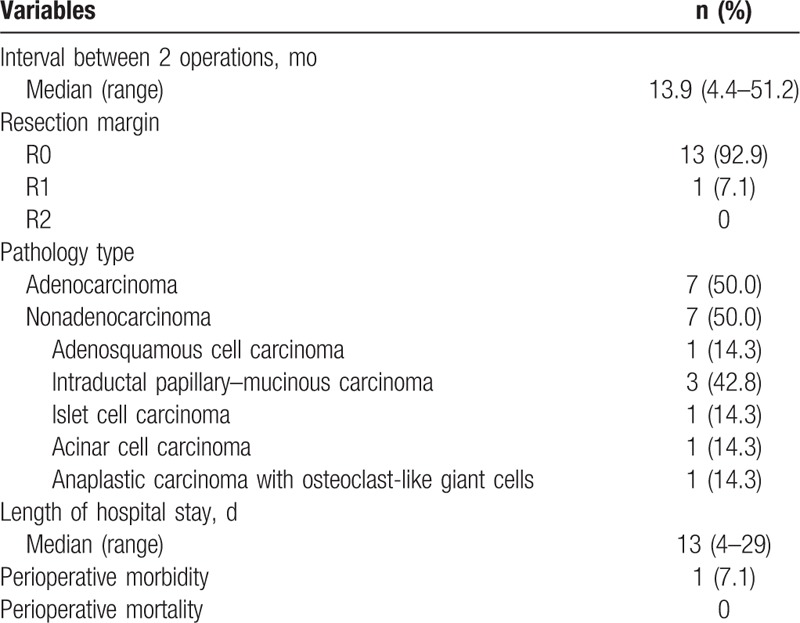
The operative method and perioperative outcomes for the second operation performed for recurrence are presented in Tables 3 and 4, respectively. The interval between the operations was 13.9 months (range 4.4–51.2 months). Among these 14 patients, 7 (50%) had adenocarcinoma; the remaining 7 (50%) did not. Most of these 14 patients underwent curative surgery (R0 resection, 13 patients, 92.9%), and only 1 patient (7.1%) had microscopic residual tumor (R1 resection). The median hospital stay was 13 days (4–29 days), and only 1 patient had surgical morbidity (wound infection). No surgical mortality was observed.
Table 3.
Operative method of reoperation in the surgical group (n = 14).
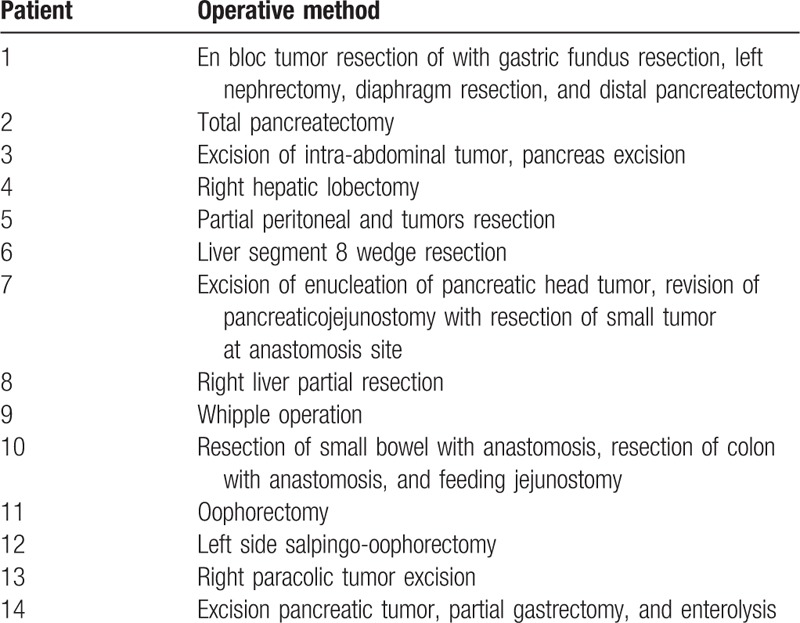
Table 4.
Perioperative outcomes of the surgical group (n = 14).
Oncological outcomes between the surgery and nonsurgery groups were analyzed and are provided in Table 3. The estimated DFS was significantly longer in the surgery group (10.6 vs 6.1 months, P = 0.044, Fig. 1A). The median PRS and OS were also significantly longer in the surgery group (PRS 14.1 vs 6.0 months, P < 0.001, Fig. 1B; OS 57.8 vs 14.0 months, P < 0.001, Fig. 1C). The 3-year DFS rate (21.4% vs 5.0%), recurrence-free survival rate (47.6% vs 4.4%), and OS rate (59.3% vs 17.0%) were statistically longer than in the nonoperation group.
Figure 1.
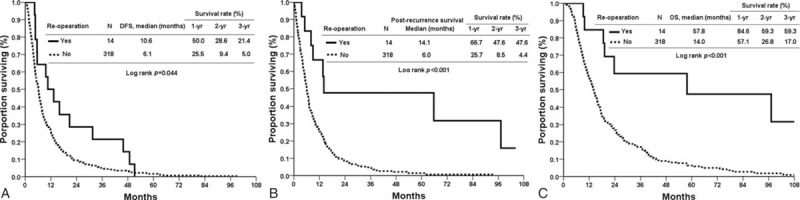
(A) Disease-free survival of the surgical and nonsurgical groups. (B) Postrecurrent-free survival of the surgical and nonsurgical groups. (C) Overall survival of the surgical and nonsurgical groups.
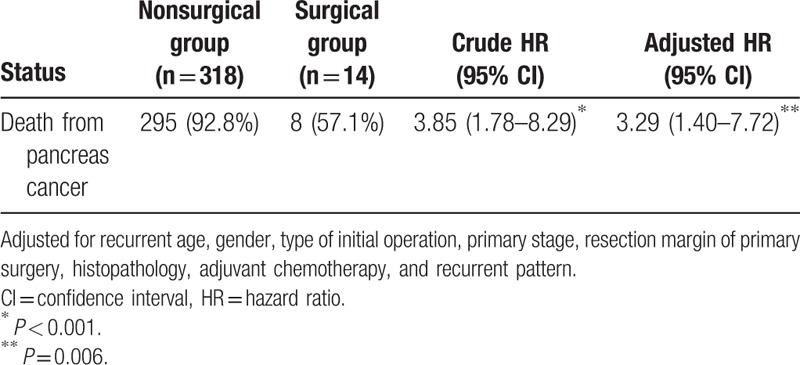
We also assessed the oncological outcomes of reoperation according to histologic features DFS, PRS, and OS were comparable in patients with recurrent pancreatic adenocarcinoma who received surgery and those who did not (DFS 10.6 vs 6.1 months, P = 0.226, Fig. 2A; PRS 8.9 vs 5.8 months, P = 0.183, Fig. 2B; OS 23.7 vs 14.0 months, P = 0.074, Fig. 2C). However, OS and PRS were superior in the patients who were not histologically positive for adenocarcinoma but underwent operation for recurrence (PRS 65.7 vs 6.9 months, P = 0.010, Fig. 3B; OS 97.2 vs 16.9 months, P = 0.016, Fig. 3C). DFS rates were similar (16.0 vs 7.0 months, P = 0.265, Fig. 3A). Table 5 shows the adjusted hazard ratio of the surgical and nonsurgical groups, which took recurrent age, gender, the type of initial operation, primary stage, resection margin of primary surgery, histopathology, adjuvant chemotherapy, and recurrent pattern into account. The adjust hazard ration was 3.29 for reoperation.
Figure 2.
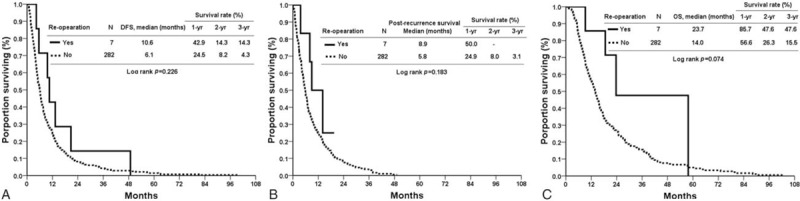
(A) Disease-free survival of the surgical and nonsurgical groups with adenocarcinoma histologically. (B) Postrecurrence-free survival of the surgical and nonsurgical groups with adenocarcinoma histologically. (C) Overall survival of the surgical and nonsurgical groups with adenocarcinoma histologically.
Figure 3.
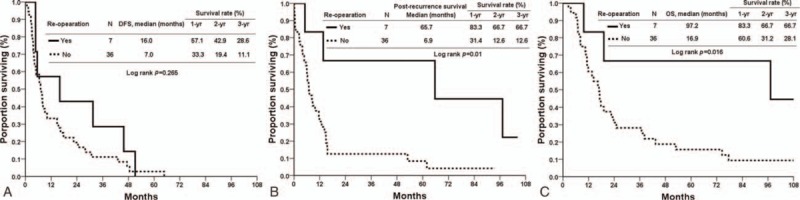
(A) Disease-free survival of the surgical and nonsurgical groups with nonadenocarcinoma histologically. (B) Postrecurrence-free survival of the surgical and nonsurgical groups with nonadenocarcinoma histologically. (C) Overall survival of the surgical and nonsurgical groups with nonadenocarcinoma histologically.
Table 5.
Adjusted hazard ratio of the surgical and nonsurgical groups.
4. Discussion
No consensus has been achieved regarding the standard treatment for recurrent pancreatic cancer. Numerous approaches have been advocated. Additionally, pancreatic cancer recurrence tends to be multifocal and aggressive; thus, resection is rarely indicated. However, emerging data have demonstrated a survival benefit for reoperation in selected cases. Strobel et al[13] presented a significantly longer median survival after resection (26.0 months) than exploration without resection (10.8 months, P = 0.0104) in 41 of 55 patients with recurrent pancreatic cancer. R0 resection was achieved in 18 of these 41 patients and resulted in a median survival of 30.5 months. Thomas et al[12] also demonstrated a longer median survival of 17 months in 15 of 30 patients with isolated local recurrent pancreatic cancer. Remarkably, reoperation in our cohort significantly prolonged DFS, OS, and PRS in patients with recurrent pancreatic cancer (DFS, 10.6 vs 6.1 months, P = 0.044; OS 57.8 vs 14.0 months, P < 0.001; PRS 14.1 vs 6.0 months, P < 0.001). In patients who are not locally advanced or had metastatic pancreatic cancer, operation appears to be most effective therapy to reduce tumor burden; this finding may have contributed to our result.
Our study further clarifies the benefit of operation in recurring adenocarcinoma or nonadenocarcinoma. In contrast with previous studies, we found that reoperation leads to survival benefits only in recurrent pancreatic cancer that is not histologically adenocarcinoma. In these patients, recurrence-free survival improved from 6.9 to 65.7 months (P = 0.010), and OS was significantly prolonged from 16.9 to 97.2 months (P = 0.016). Additionally, operation can be safely performed with minimal morbidity and no mortality and is accompanied by a short hospital stay. Our cohort includes 3 patients with intraductal papillary mucinous carcinoma, 1 patient with adenosquamous cell carcinoma, 1 patient with islet cell carcinoma, 1 patient with acinar cell carcinoma, and 1 patient with anaplastic carcinoma with osteoclast-like giant cells. In contrast, most previous studies primarily included patients with recurrent ductal adenocarcinoma, and oncological outcomes improved in patients with re-resection. However, only half of the patients who underwent operation for recurrence were diagnosed with adenocarcinoma in our study, and the median OS was comparable between the surgical and nonsurgical groups (OS 23.7 vs 14.0 months, respectively, P = 0.074), so were the median DFS (10.6 vs 6.1 months, P = 0.226) and PRS (8.9 vs 5.8 months, P = 0.183). Kleeff et al[9] reported that 15 of 30 patients with recurrent pancreatic adenocarcinoma demonstrated a tendency toward an increased median survival. This phenomenon occurred in a group of patients undergoing resection compared with a bypass and exploration group (although without statistical significance). This finding is compatible with our result. Consequently, multiple strategies should be adopted in these patients. Habermehl et al[14] used chemoradiotherapy as an intended curative therapy in patients with complete remission or after achieving a re-resection for recurrent pancreatic cancer. Strobel et al[13] found that patients with resection of isolated local recurrence receiving IORT combined with percutaneous chemoradiation tended to have superior survival to that of patients without IORT, and patients with unresectable isolated local recurrence had a significantly longer median survival with IORT.
Strict selection is warranted in patients with recurrence; thus, selection bias, heterogeneity, and the lack of control group influence our results. Thus, we cannot conclude that reoperation prolongs survival. However, a significantly longer survival benefit was demonstrated in the surgery group, especially in those without adenocarcinoma. Additionally, a less advanced disease initially with as complete as possible resection in primary surgery may indicate a secondary operation candidate. This phenomenon leads to a better prognosis during recurrence and thus a more favorable prognosis.
Survival improves with re-resection in selected recurrent adenocarcinoma; however, several limitations should be noted. Notably, our study had a small sample size of patients in individual subgroups. Only 7 patients with adenocarcinoma and 7 with nonadenocarcinomal disease were recruited. In the nonadenocarcinoma group, each individual histology subtype had substantially fewer patients. Obviously, the data regarding patients with nonadenocarcinoma were very difficult to interpret due to the small numbers and the heterogeneity of this cohort. However, a survival benefit was demonstrated despite the small sample size, and a similar result is anticipated in a larger group of patients. Second, this study was a retrospective, nonrandomized study. Group characteristics and medical records were as complete as possible. However, selection bias could not be prevented, and the data may not have been comprehensively collected. Third, there were no standardized criteria, and the diagnosis of recurrent disease had no pathological proof in some patients. Radiological evidence was the only foundation of diagnosis in most patients. Fourth, the indication for reoperation for selective recurrent pancreatic cancer has not been standardized nor well established and reoperation was performed by multiple surgeons, so selection bias is inevitably. Finally, more patients had histopathology of adenocarcinoma, in the nonsurgery group, which might partially impacted the survival that led to poorer prognosis in this group.
This limitation may have affected the survival of those initially managed with palliative therapy but who are now surgery candidates.
5. Conclusion
According to our series, surgery can provide feasible and safe survival benefits in selected patients with recurrent pancreatic cancer. In patients who are histologically indicated as nonadenocarcinomal, survival is prolonged when operation is performed with R0 resection. Those with isolated recurrent pancreatic adenocarcinoma require multidisciplinary therapy. Chemoradiotherapy and intraoperative radiotherapy may also be considered in addition to surgery; these modalities warrant further investigation.
Footnotes
Abbreviations: DFS = disease-free survival, IORT = intraoperative radiotherapy, OS = overall survival, PRS = postrecurrence-free survival.
IRB number: 104-4010C.
This work was supported by the Chang Gung Medical Research Program, Taiwan.
The authors have no conflicts of interest to disclose.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5–29. [DOI] [PubMed] [Google Scholar]
- 2.Ministry of Health and Welfare, Taiwan. [Google Scholar]
- 3.Boone BA, Zeh HJ, Mock BK, et al. Resection of isolated local and metastatic recurrence in periampullary adenocarcinoma. HPB (Oxford) 2014; 16:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sperti C, Pasquali C, Piccoli A, et al. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg 1997; 21:195–200. [DOI] [PubMed] [Google Scholar]
- 5.Westerdahl J, Andren-Sandberg A, Ihse I. Recurrence of exocrine pancreatic cancer—local or hepatic? Hepatogastroenterology 1993; 40:384–387. [PubMed] [Google Scholar]
- 6.Wangjam T, Zhang Z, Zhou XC, et al. Resected pancreatic ductal adenocarcinomas with recurrence limited in lung have a significantly better prognosis than those with other recurrence patterns. Oncotarget 2015; 6:36903–36910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyazaki M, Yoshitomi H, Shimizu H, et al. Repeat pancreatectomy for pancreatic ductal cancer recurrence in the remnant pancreas after initial pancreatectomy: is it worthwhile? Surgery 2014; 155:58–66. [DOI] [PubMed] [Google Scholar]
- 8.Müller MW, Friess H, Kleef J, et al. Is there still a role for total pancreatectomy? Ann Surg 2007; 246:966–974. [DOI] [PubMed] [Google Scholar]
- 9.Kleeff J, Reiser C, Hinz U, et al. Surgery for recurrent pancreatic ductal adenocarcinoma. Ann Surg 2007; 245:566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miura F, Takada T, Amano H, et al. Repeated pancreatectomy after pancreatoduodenectomy. J Gastrointest Surg 2007; 11:179–186. [DOI] [PubMed] [Google Scholar]
- 11.Lavu H, Nowcid LJ, Klinge MJ, et al. Reoperative completion pancreatectomy for suspected malignant disease of the pancreas. J Surg Res 2011; 170:89–95. [DOI] [PubMed] [Google Scholar]
- 12.Thomas RM, Truty MJ, Nogueras-Gonzalez GM, et al. Selective reoperation for locally recurrent or metastatic pancreatic ductal adenocarcinoma following primary pancreatic resection. J Gastrointest Surg 2012; 16:1696–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strobel O, Hartwig W, Hackert T, et al. Re-resection for isolated local recurrence of pancreatic cancer is feasible, safe, and associated with encouraging survival. Ann Surg Oncol 2013; 20:964–972. [DOI] [PubMed] [Google Scholar]
- 14.Habermehl D, Brecht IC, Bergmann F, et al. Chemoradiation in patients with isolated recurrent pancreatic cancer—therapeutical efficacy and probability of re-resection. Radiat Oncol 2013; 8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gelman A, Jakulin A, Pittau MG, et al. A weakly informative default prior distribution for logistic & other regression models. Ann Appl Stat 2008; 2:1360–1383. [Google Scholar]


