Supplemental Digital Content is available in the text
Keywords: Crohn disease, inflammatory bowel disease, systematic review, thalidomide, ulcerative colitis
Abstract
Background:
Thalidomide is an immunomodulatory drug used in the experimental treatment of refractory Crohn disease and ulcerative colitis. We aimed to review the existing evidence on the efficacy and safety of thalidomide in the treatment of inflammatory bowel diseases.
Methods:
CENTRAL, MEDLINE, LILACS, POPLINE, CINHAL, and Web of Science were searched in March 2016. Manual search included conference and reference lists. All types of studies, except single case reports, were included. Outcomes evaluated were: induction of remission; maintenance of remission; steroid reduction; effect on penetrating Crohn disease; endoscopic remission; adverse events.
Results:
The research strategies retrieved 722 papers. Two randomized controlled trials and 29 uncontrolled studies for a total of 489 patients matched the inclusion criteria. Thalidomide induced a clinical response in 296/427 (69.3%) patients. Clinical remission was achieved in 220/427 (51.5%) cases. Maintenance of remission was reported in 128/160 (80.0%) patients at 6 months and in 96/133 (72.2%) at 12 months. Reduction in steroid dosage was reported in 109/152 (71.7%) patients. Fistulas improved in 49/81 (60.5%) cases and closed in 28/81 (34.6%). Endoscopic improvement was observed in 46/66 (69.7%) and complete mucosal healing in 35/66 (53.0%) patients. Cumulative incidence of total adverse events and of those leading to drug suspension was 75.6 and 19.7/1000 patient-months, respectively. Neurological disturbances accounted for 341/530 (64.3%) adverse events and were the most frequent cause of drug withdrawal.
Conclusion:
Existing evidence suggests that thalidomide may be a valid treatment option for patients with inflammatory bowel diseases refractory to other first- and second-line treatments.
1. Introduction
Thalidomide is a small molecule with immunomodulatory properties. It is currently approved for the treatment of erythema nodosum leprosum, an immunological complication of leprosy[1,2] and multiple myeloma. It has also been used in several other inflammatory diseases of the skin and of the mucosal membranes, such as Behcet disease, oropharingeal ulcers in AIDS, cutaneous lupus, and graft versus host disease.[3]
Two Cochrane reviews explored the efficacy and safety of thalidomide for the induction and maintenance of remission in Crohn disease (CD).[4,5] These reviews, which were published and last updated in 2009, included only studies with a randomized controlled trial (RCT) design, and did not identify at time of publication any paper matching these criteria. More recently another systematic review was published on the subject, but the number of studies included and the type of outcomes reported was limited.[6] In order to evaluate the most recent literature, and in order to explore and report a wide range of outcomes that may be important in clinical practice in guiding decision for treatment of patients with CD or ulcerative colitis (UC), we conducted the present systematic review.
1.1. Objectives
The objective of this review is to synthesize the existing evidence on the efficacy and safety of thalidomide in patients, both adults and in children, with either CD or UC.
2. Methods
2.1. Criteria for considering studies for this review
This review follows the PRISMA standards on reporting on systematic review (see Table, Supplemental Digital Content 1—PRISMA Checklist, which illustrates PRISMA Checklist).[7] Approval of ethics committee was not required because the study consisted in reviewing the existent literature. The following were the inclusion criteria that we used: as study design, all study types, excluding single case reports, were considered; as participants, patients with inflammatory bowel disease (IBD), defined as CD, UC, or indeterminate colitis (IC), of any age; as intervention, thalidomide, any dosage; in case of controlled trials, either placebo or active treatment were accepted as a control intervention; as efficacy outcomes, induction of clinical remission, maintenance of clinical remission, clinical response, steroid reduction, effect on fistulas and perianal disease in patients with Crohn disease, endoscopic remission. As a safety outcome, we included in the review any adverse effects (AE), as defined by the study authors.
When studies allowed and when a considerable number of patients were described (>10 patients), the effects of thalidomide when given in association with biological therapies or other major immunosuppressive treatments were reported separately.
2.2. Search methods for identification of studies
The Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, LILACS, POPLINE, CINHAL, and Web of Science were searched in March 2016 (last search date, March 31st). The following search strategy was used for MEDLINE: (“Thalidomide”[Mesh] OR thalidomide) AND (“Inflammatory Bowel Diseases”[Mesh] OR “Crohn Disease” [Mesh] OR “Colitis, Ulcerative” [Mesh] OR Crohn OR colitis OR “inflammatory bowel”). For LILACS, and CINHAL, we used the combination of the following Keywords: “thalidomide AND Crohn's disease”; “thalidomide AND ulcerative colitis”; “thalidomide AND inflammatory bowel disease.” For POPLINE and Web of Science the search strategy was: “(thalidomide AND [Crohn's disease OR ulcerative colitis OR inflammatory bowel disease]).”
Manual searching included presentations from European Crohn's and Colitis Organisation and from the most recent European Society for Paediatric Gastroenterology Hepatology and Nutrition Congresses, plus reference lists from studies identified.
The above-described searches were performed to retrieve all relevant trials regardless of language, publication status, or study type.
2.3. Data collection and analysis
Two authors (ML and MB) independently evaluated studies for inclusion. The full text of all potentially relevant studies was assessed, except for 1 single study in Chinese that we could not translate. For studies existing only as conference abstract, the authors were contacted.
In cases of duplicate case series, the most recent and complete series was considered as the “primary study”; duplicates presenting additional information available in the primary report were considered as “secondary studies” and they were used, if appropriate, only as a complementary source of data. Similarly, if 2 very similar abstracts were published by the same author within a short time period (<36 months), and if no further information was available from the author, the most recent report was included in the review whereas the one published earlier was considered as a duplicate.
Data were extracted from studies using a predefined data extraction form. To avoid mistakes due to data manipulation, we first collected the data as they were reported and only subsequently we performed data transformations.
Pooled results on the efficacy outcomes were reported for each outcome as the percentage of patients with the outcome, calculated as the rate between the total absolute number of patients with the event, on the total absolute number of patients treated (by intention to treat).
In order to compare safety outcomes, we used the cumulative incidence rate of AE, calculated based on the total number of AE on the total follow-up of patients expressed in months. When mean follow-up time was reported, it was multiplied by the number of patients included in the case series. When only median follow-up time was reported and no other information was available from study authors, this was approximated to the mean follow-up, as this was considered to be the best possible approximation.
Risk of bias was rated for each study by 2 authors independently, using the Cochrane criteria[8] for RCTs. Uncontrolled studies were always rated as moderate or high risk of bias (never at low risk of bias) and were categorized as follows: moderate risk of bias, when the description of both patients, intervention, and outcomes of interest was complete and clear throughout the observation period; high risk of bias, when the description of patients, intervention, and outcomes was incomplete or unclear, or when follow-up was incomplete.
As data could not be pooled in forest plots, findings were reported in tables and text.
3. Results
The process of study selection is reported in Fig. 1. The search strategy retrieved 722 papers. Among these, we identified for inclusion 31 primary studies.[9–39] Five reports were considered duplicate studies[40–44] and used to complement information given in the primary reports.
Figure 1.
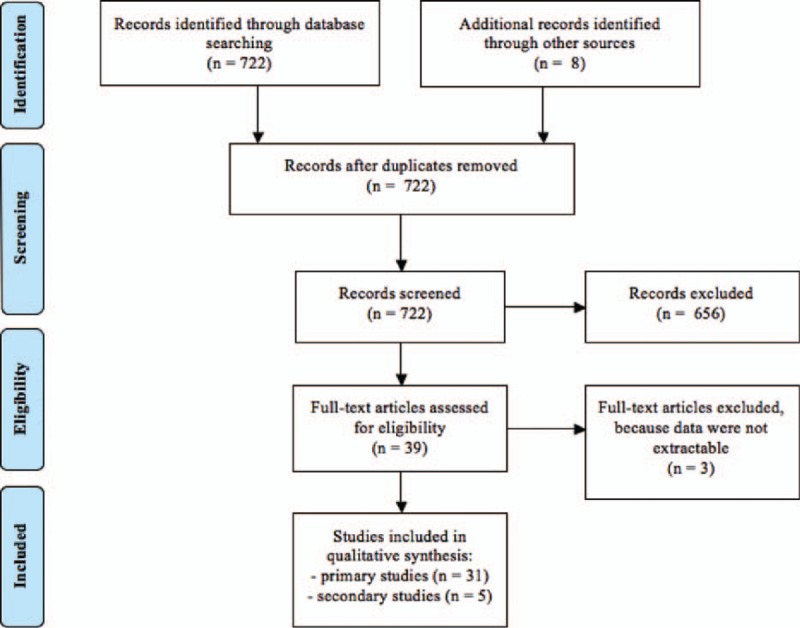
Flow diagram of study selection.
3.1. Characteristics of included studies
Characteristics of primary studies are detailed in Table 1 (see Table, Supplemental Digital Content 2—Table 1, which illustrates the characteristics of the studies included). Except for 2 prospective placebo-controlled randomized trials, all studies were uncontrolled before and after studies (case series). Risk of bias was ranked as low for the primary analysis of the 2 RCTs (8 weeks)[10,15] and moderate to high in all the other studies.
Table 1.
Characteristics of included studies.
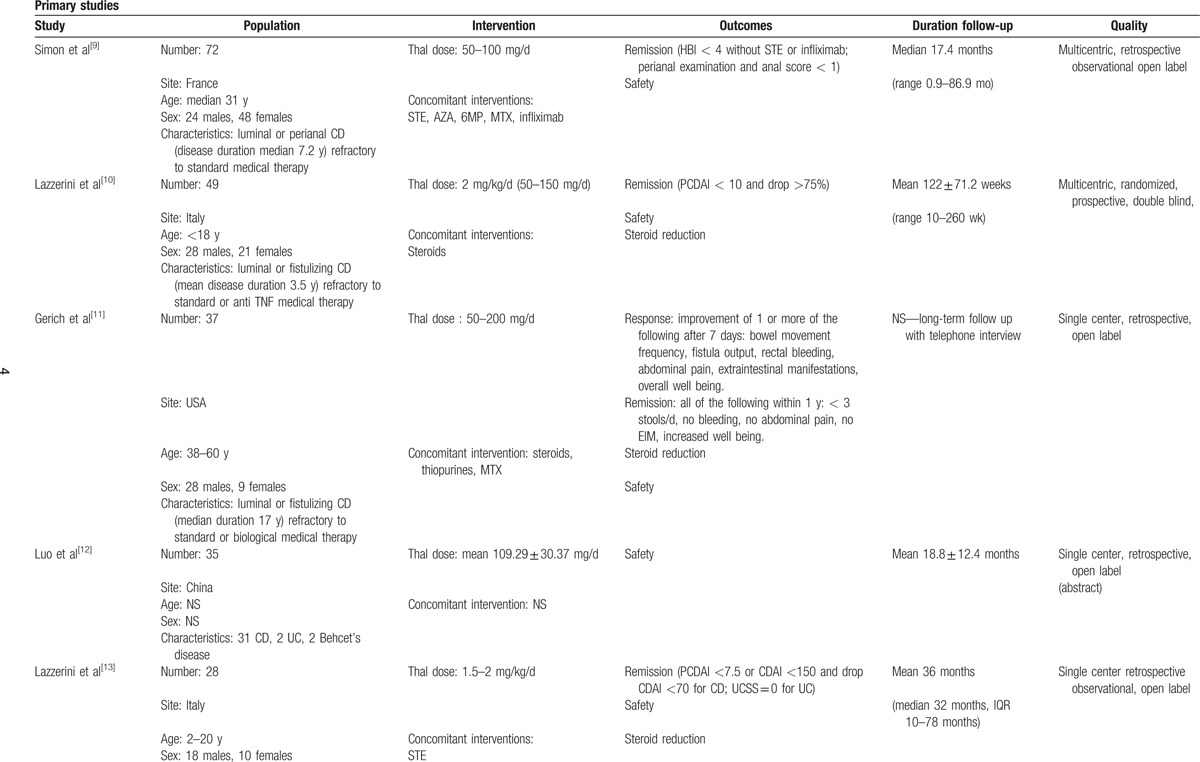
Characteristics of patients are reported in Table 2. Overall, 435/489 (89.0%) patients had CD, whereas only 50 (10.2%) presented with UC (risk ratio, RR 9.7, 95% P < 0.001). The total population included 135 (28.4%) children < 18 years of age (RR 0.5, P < 0.001). With regard to sex, 247/475 (50.5%) patients were males whereas 178/475 (36.4%) were females (RR 1.4 P < 0.001). Patients treated with thalidomide were characterized by being in moderate to severe activity, and by being refractory to standard or biological treatments.
Table 1 (Continued).
Characteristics of included studies.
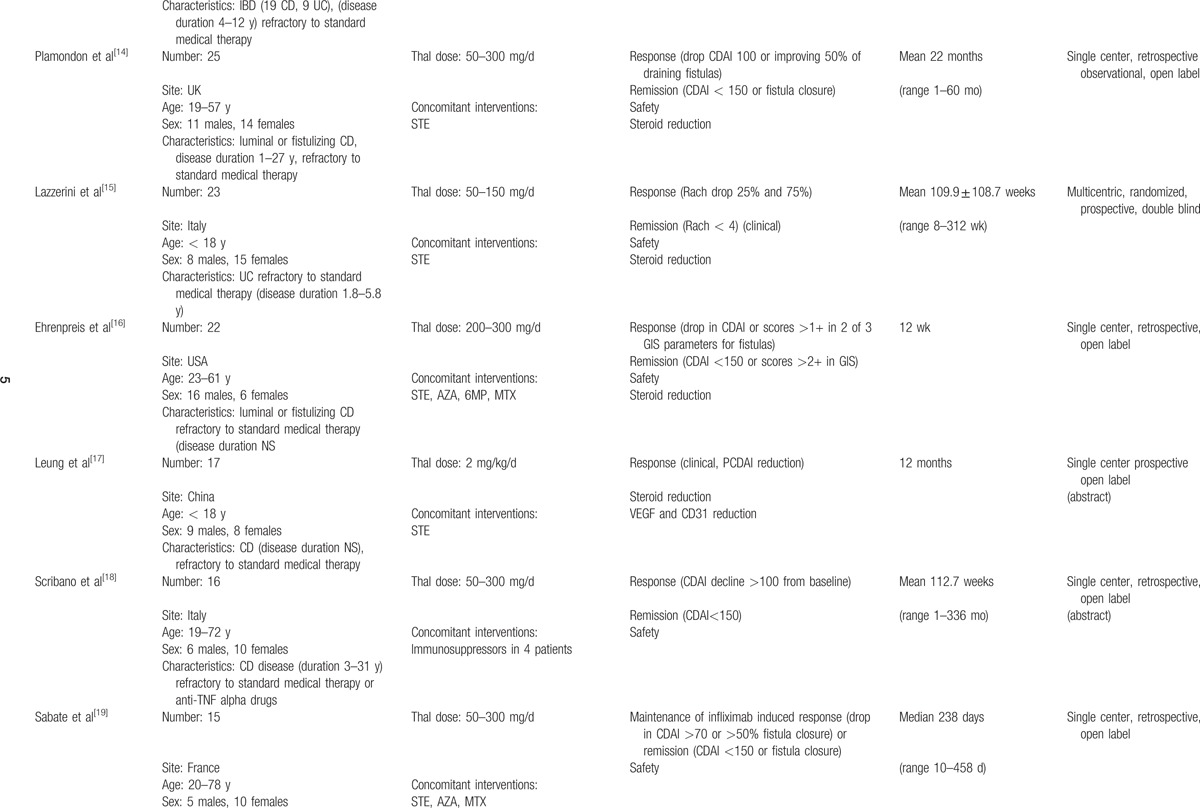
Thalidomide was used at doses ranging from 50 to 400 mg/d in adults and 1.5 to 2.5 mg/kg/d in children.
All patients of 1 study[28] and selected patients in a second study[14] were treated concomitantly with thalidomide and infliximab or adalimumab, whereas in another case series,[20] thalidomide was administered together with cyclophosphamide; findings from these studies were reported separately from findings of studies where thalidomide was not associated with other immunosuppressive drugs.
Studies reported on outcomes of interest as follows: 25 studies (80.6%) reported on induction of clinical remission, generally in the short term (4–16 weeks); 27 (87.1%) reported on clinical response in the short term; 15 (48.4%) reported on the follow up after the induction period (6–24 months); 17 (54.8%) reported on steroid reduction; 8 (25.8%) evaluated endoscopic remission; 10 (32.2%) reported fistulas outcome; 7 (22.6) reported on thalidomide's efficacy after a biological drug failure; 29 (93.5%) reported on AE.
3.2. Effects of thalidomide
3.2.1. Efficacy in inducing clinical response or remission
Twenty-eight studies[9–11,13–19,21–25,27,29,31–39] overall reported on thalidomide's efficacy in inducing clinical response, and of these 25 reported on clinical remission (Table 3). Out of 427 patients included in the analysis, 296 (69.3%) had a clinical response (27 studies) and 220 (51.5%) achieved clinical remission (25 studies). When the effect of thalidomide was evaluated over time, generally this was done at 4, 8, or 12 weeks from the onset of the therapy, and the percentage of patients with a benefit increased at the 8th and 12th week compared to the 4th week.
Table 1 (Continued).
Characteristics of included studies.
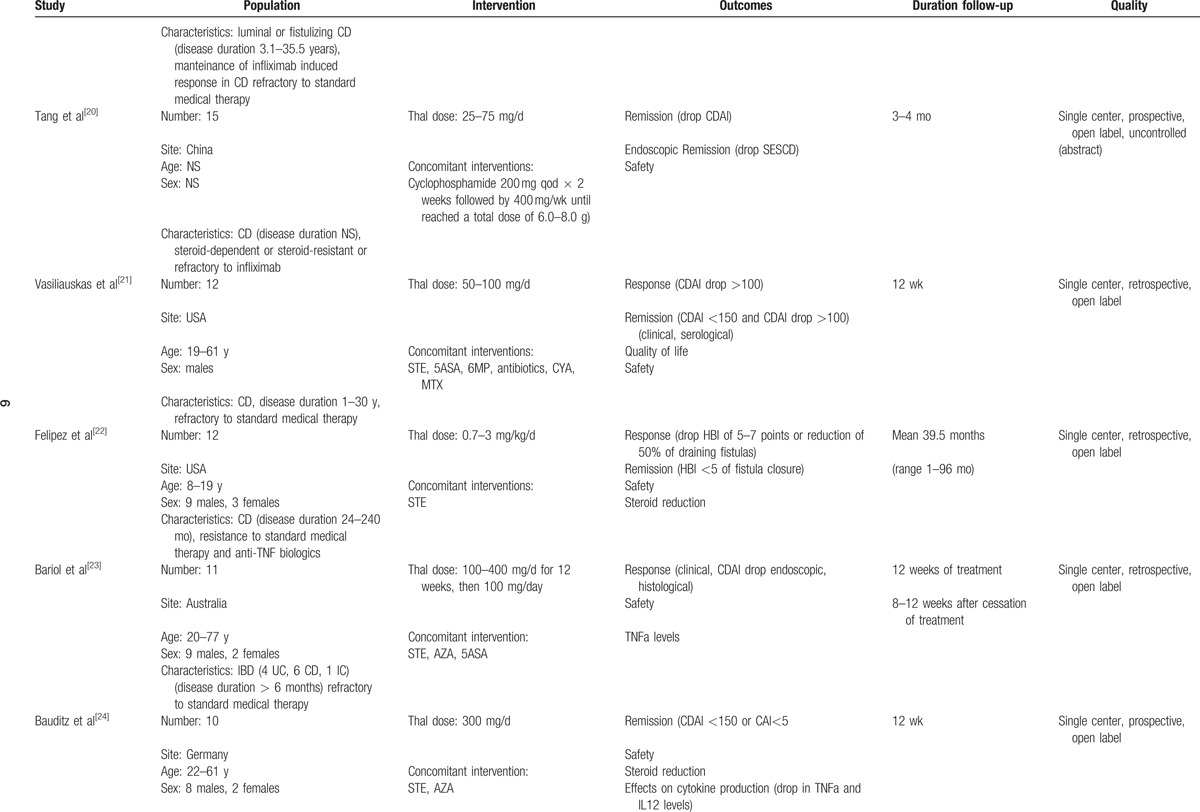
Seven studies[12–15,19,23–25] enrolled patients with UC, but only 2 focused specifically on UC. In a pilot RCT on children with UC 18/23 (78.3%) children treated with thalidomide achieved clinical remission, compared to 2/11 (18.2%) in placebo.[15] In 4 other studies[13,23,24,29] outcomes of patients with UC were as follows: of the 16 patients identified, 9 (56.2%) responded to thalidomide, and 7 (43.7%) achieved remission.
Efficacy of thalidomide in inducing clinical remission or clinical response according to IBD type is reported in Fig. 2.
Figure 2.
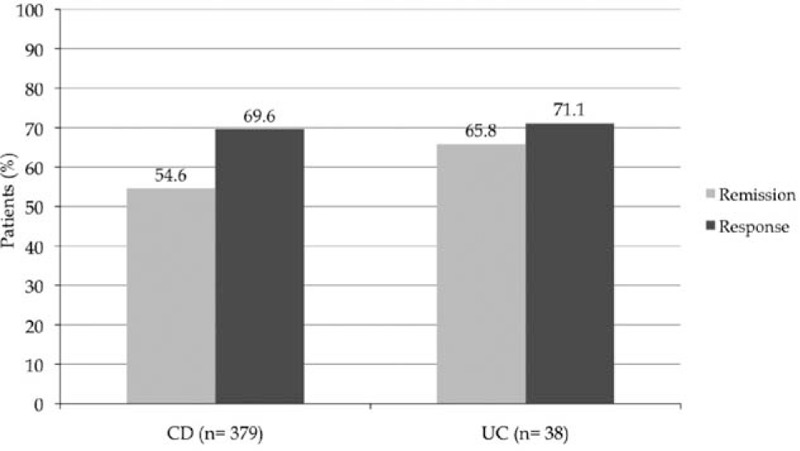
Efficacy in inducing clinical remission or response according to IBD type. In 2 studies,[23,31] the number of patients achieving clinical remission was not specified; for 1 study,[9] the number of responder was considered the same as the patients who achieved remission.
3.2.2. Efficacy in maintaining clinical remission in the long term
Overall, the effect of thalidomide in maintaining clinical remission in the long term was reported in 170 patients (15 studies).[10,13–15,17,22,27,33–36,38–41,43] The number of patients evaluated at each time-point is specified in Table 4. The remission rate over time was as follows: 128/1160 (80.0%) at 6 months (15 studies); 96/133 patients (72.2%) at 12 months (13 studies); and 61/112 patients (54.5%) at 24 months (11 studies).
Table 1 (Continued).
Characteristics of included studies.
Table 4.
Efficacy of in maintaining of clinical remission in the long term.
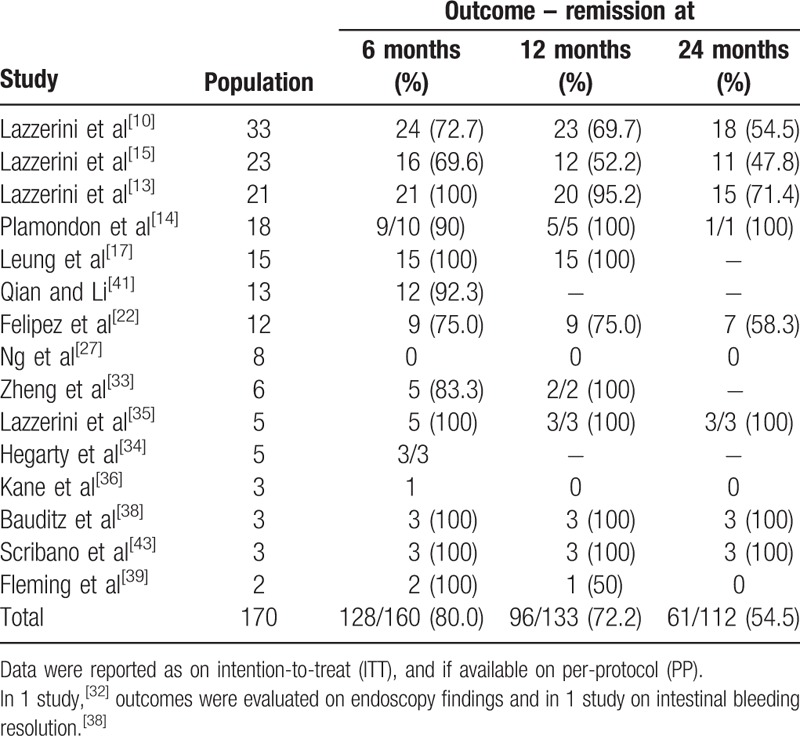
Occurrence of relapses were described after thalidomide tapering or withdrawn in 4 studies, with subsequent recovery of clinical remission following the re-establishment of the full drug treatment.[9,13,34,38]
Twelve papers[10,11,13–15,17,19,24,27,31,36,39] stated the cause of drug discontinuation during the long-term period: 58/68 patients (85.3%) withdrawn thalidomide because of an AE, whereas 10/68 patients (14.7%) discontinued because of a loss of efficacy despite an initial clinical response.
3.2.3. Steroids reduction or suspension
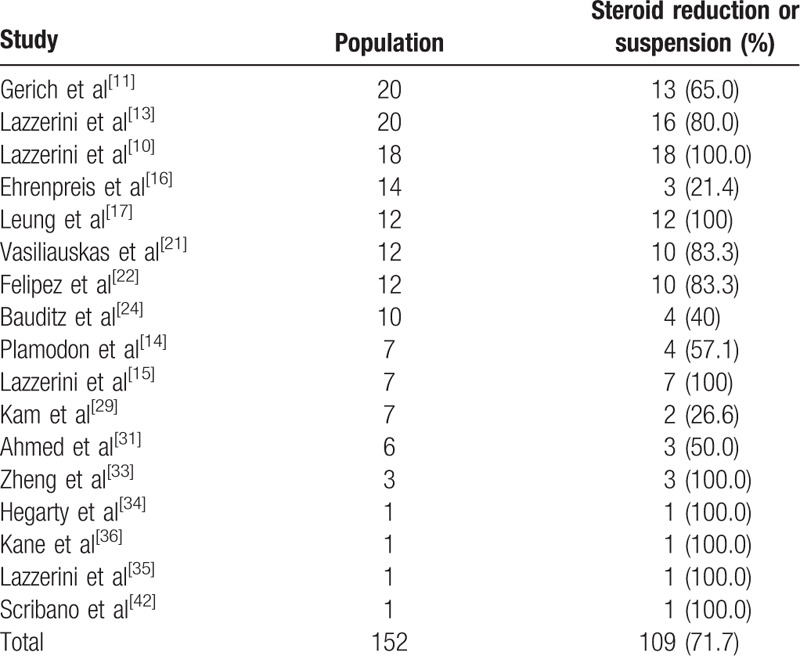
Seventeen studies[10,11,13–17,21,22,24,29,31,33,34–36,42] reported reduction or complete suspension of steroids in 109/152 (71.7%) patients during treatment with thalidomide (Table 5). The time frame for steroid reduction/suspension was generally 12 to 16 weeks from thalidomide start.
Table 1 (Continued).
Characteristics of included studies.
Table 5.
Steroid reduction or suspension.
3.3. Efficacy on fistulizing Crohn's disease
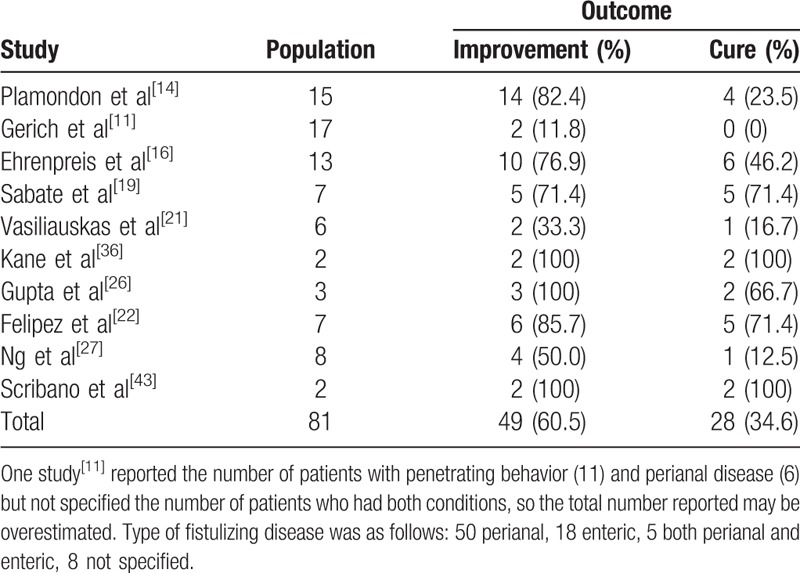
Ten studies (81 patients)[11,14,16,19,21,22,16,27,36,43] reported on the efficacy of thalidomide in patients with fistulizing disease (Table 6): 50 patients had perianal fistulas, 18 had enteric fistulas, 5 had both, whereas in 8 cases the localization of fistulas was not specified. Overall, a clinical improvement was noted in 49/81 (60.5%) patients, whereas a complete healing of the fistula was reported in 28/81 (34.6%) patients.
Table 1 (Continued).
Characteristics of included studies.
Table 6.
Efficacy on fistulizing Crohn's disease.
3.4. Efficacy of thalidomide after or in association with biological therapy
Eight studies[10,15,16,18,22,26,35,36] reported on thalidomide efficacy after failure of either infliximab or adalimumab. Of the 73 patients identified, 47 (64.4%) had a clinical response and 37 (50.7%) achieved clinical remission (Table 7). Two additional studies[14,28] reported the association between thalidomide and infliximab or adalimumab in patients who lost response to the antitumor necrosis factor (TNF) alpha biologic drug: 7/10 (70.0%) had a clinical response, whereas 3/10 (30.0%) achieved clinical remission.
Table 1 (Continued).
Characteristics of included studies.
Table 7.
Efficacy of thalidomide after biological therapy failure.
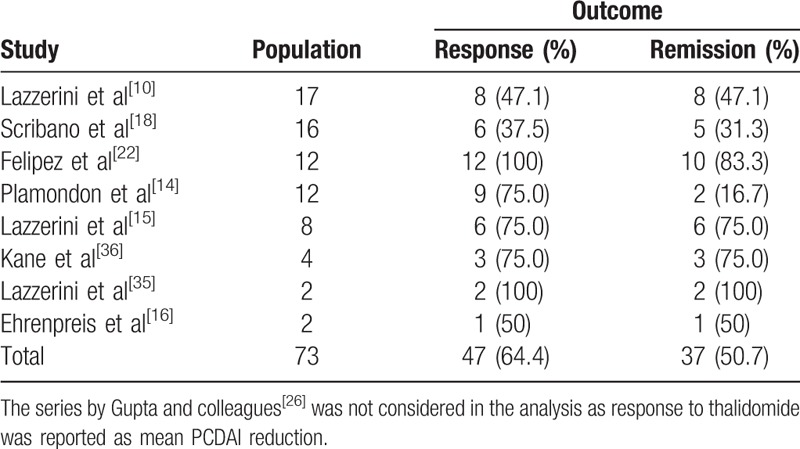
Moreover 1 further study[19] used thalidomide in patients with CD after infliximab as a maintenance therapy. Of the 15 patients enrolled, at the time of starting thalidomide treatment, 5 (33.3%) were still in clinical remission after infliximab, whereas 10 (66.6%) had experienced a relapse; the clinical remission rate during thalidomide treatment, as reported by the authors, was 73%, 73%, and 59% on an intention-to-treat analysis respectively at 3, 6, and 12 months after the last infliximab infusion.
3.5. Thalidomide in association with other immunosuppressive drugs
One study[20] reported the experimental association between cyclophosphamide and thalidomide (25–75 mg/d) for 3 to 4 months in 15 patients refractory to standard therapy or to infliximab. Clinical remission was achieved in 10/15 (66.6%) patients within 2 weeks and in 12/14 (85.7%) patients at week 10. Endoscopic improvement was noted in 12/14 (85.7%) patients; mucosal healing was observed in 4 (33.3%) patients. Five patients (33.3%) had adverse events (3 mild aminotrasferase level elevation, 1 leukemia), but they were supposed to be more closely correlated to cyclophosphamide therapy than to thalidomide.
3.6. Efficacy of thalidomide on endoscopic remission
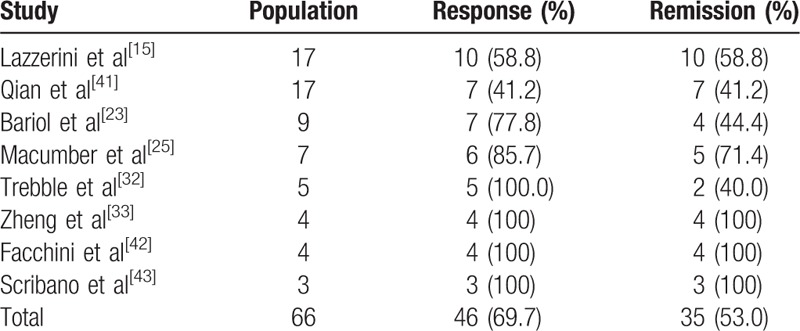
Eight studies reported findings from the endoscopic evaluations[15,23,25,32,33,41,43] on an overall sample of 66 patients. Of these, 46 (69.7%) showed an improvement in their macroscopic appearance with a reduction of mucosal inflammation and 35 (53.0%) had a complete resolution of their mucosal lesions (Table 8). With a separate analysis for the IBD type, 5 studies[28,32,33,41,42] could be used to evaluate mucosal healing in CD and 1 for UC.[15] In the CD subgroup, 23/33 (69.7%) patients had a macroscopic response, whereas 16/33 (48.5%) showed complete endoscopic remission; among UC patients, 10/17 (58.8) had complete mucosal healing.
Table 2.
Characteristics of patients.
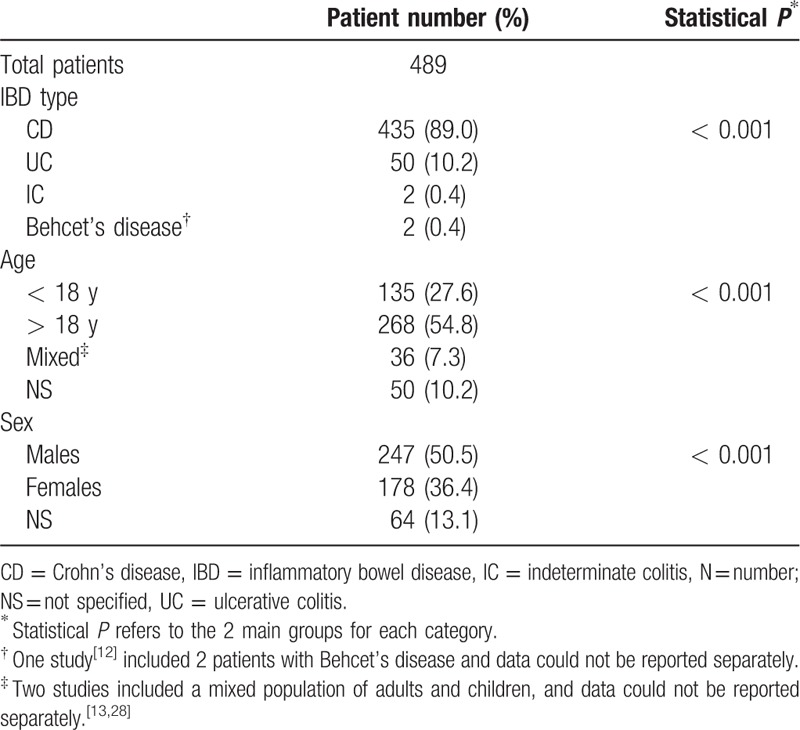
Table 8.
Efficacy of thalidomide on endoscopic remission.
3.7. Safety data
Overall 530 AE were reported in 29 studies. Four studies[13,14,19,21] reported the number of AE but not the exact number of patients involved; due to this uncertainty, the total number of patients who experienced an AE during thalidomide treatment can be estimated in a number ranging between 258 and 338. Table 9 describes the AE and the cumulative AE incidence rate/1000 patient-months; the type and number of AE that required the drug suspension is reported.
Table 3.
Efficacy in inducing clinical response or remission.
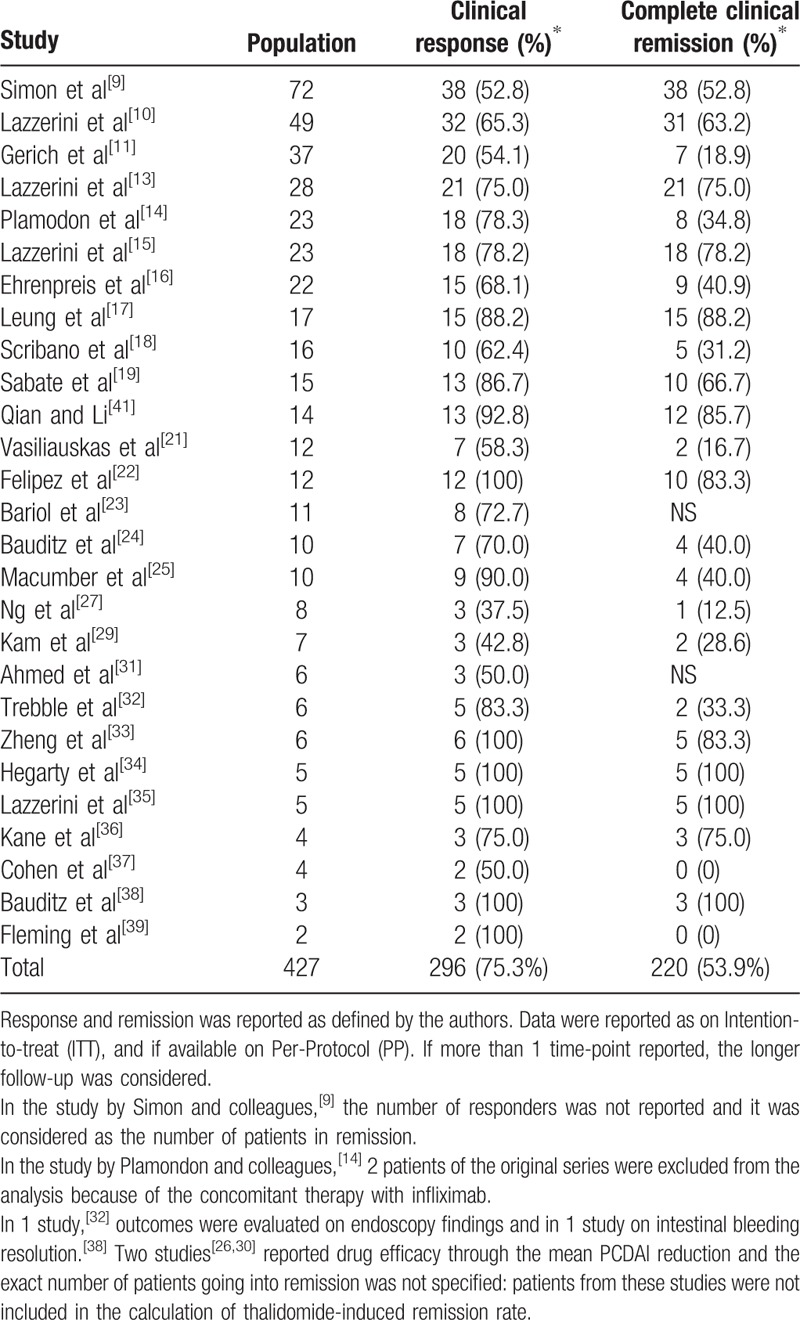
Table 9.
Safety data.
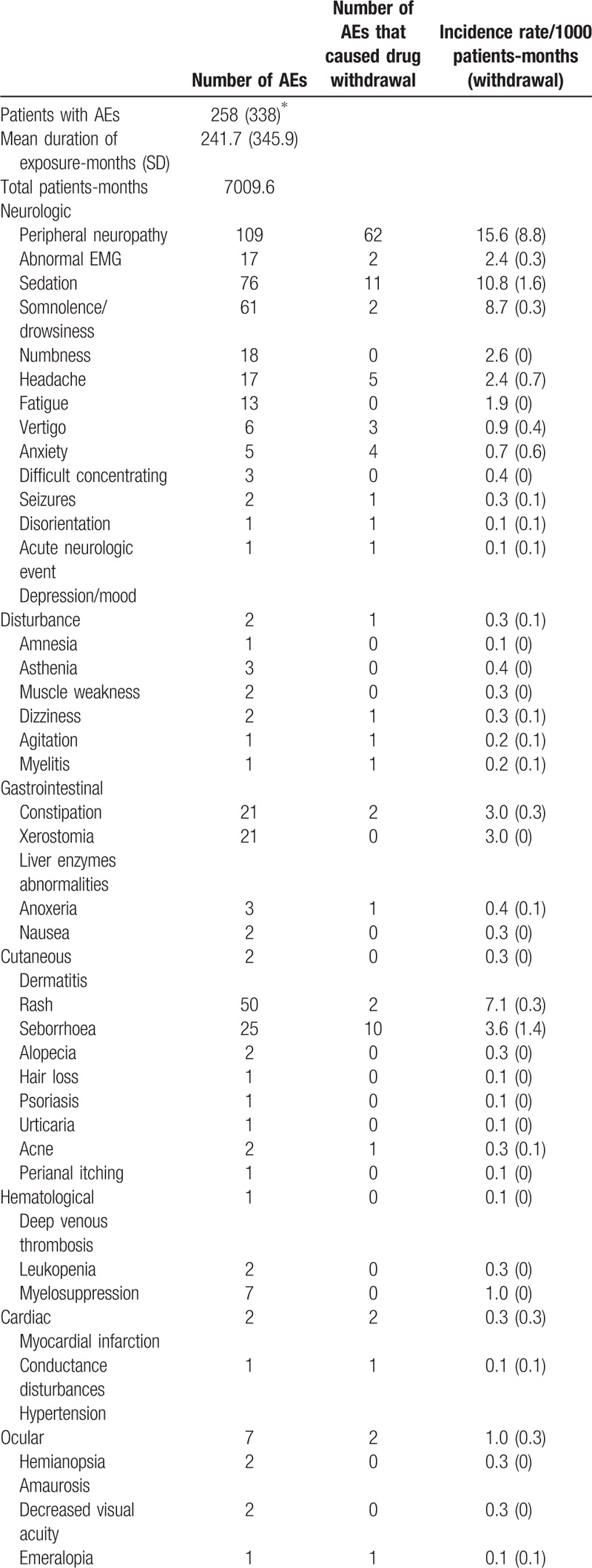
Table 9 (Continued).
Safety data.
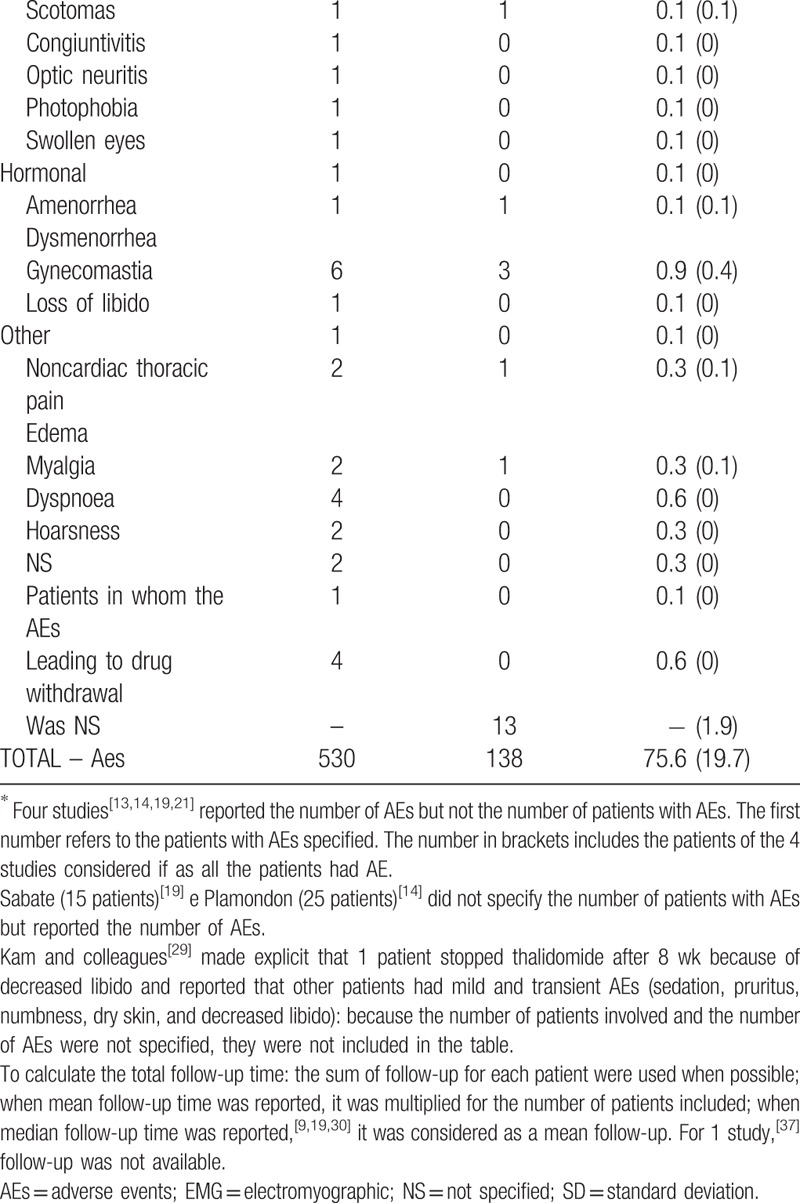
Neurological disturbances account for 64.3% of the AE reported. Peripheral neuropathy was the most common side effect observed (109 cases, incidence 15.6/1000 patient-months) and led to drug withdrawal in 56.8% patients who suffered from it.
Sedation or somnolence, of various degrees, were reported in ∼25% of the patients and were usually complained of in the first period of therapy. To prevent these symptoms during the day time, some authors administered the drug in the evening before sleeping or managed them by reducing the drug dosage.[16,18,19,21,34]
Mood disturbances and anxiety were rare but in the reported cases severe enough to interfere with daily activities.[13,18,23,25]
Acute severe neurological events like seizure and stroke-like episodes were reported overall in 3 cases (incidence rate 0.3: 1000 patient-months) and determined immediate drug suspension in 2 (0.2: 1000 patient-months).
Cutaneous manifestations were the second most common category of AE reported and account for 15.8% of the AE overall. These include dry dermatitis and rashes and were generally mild; xerostomia was also reported quite commonly.
Constipation was reported by ∼10% of patients (incidence rate 3.0:1000 patient-months) but caused thalidomide suspension only in 2 cases. Ocular abnormalities accounted for 2% of all AE.
Secondary amenorrhea was reported in a 6 female patients and had a cumulative incidence rate in the female sex of 1.8/1000 patient-months; in half of the reported cases, the events led to immediate drug withdrawal.
Few patients reported potentially severe AE such as myelosuppression (2 cases), venous thrombosis (2 cases), infarct (1 case), or cardiac rhythm disturbances (7 cases) but none of the patients died as a result of thalidomide treatment.
4. Discussion
With the present review, we aimed at systematically evaluating existing evidence on the efficacy and safety of thalidomide in patients with either CD or UC. The effects of thalidomide were reported in 31 studies (2 RCTs and 29 case series), for a total of 489 patients. Overall, thalidomide appeared to be a promising therapy for IBD: thalidomide induced clinical remission in 51.4% of 427 cases (25 studies), whereas in 69.3% a clinical response was observed in the first months of treatment (27 studies). In almost 50% of the cases in which endoscopy was performed, complete mucosal healing was observed and a further 15% of patients showed a macroscopic mucosal improvement (8 studies). IBD remission was maintained in 72.2% after 12 months (13 studies) and in 54.5% of patients after 2 years of treatment (11 studies). AE leading to drug suspension had a cumulative incidence of 19.7/1000 patients-months, with neurological disturbances being the most frequent cause of drug withdrawal.
Limitations of this review are mostly related to the quality of the existing evidence. The review identified 31 primary studies for inclusion, but only 2 randomized controlled trials. Additionally, there was a certain degree of case selection by type of disease (over 80% of patients were affected by CD), and by disease severity (patients refractory to standard medical therapies and in some case to biological drugs). No comparative study was available which evaluated thalidomide versus other treatment strategies. Clearly, more RCTs are needed to further evaluate the effects of thalidomide in patients with IBD, in particular, in those with UC, as well as in patients in mild or moderate activity and/or at early stages of the disease. Comparative studies would also help to clarify the role of thalidomide in relation to other treatment strategies.
Despite these limitations, the review has the merit of synthesizing all available evidence on thalidomide for treating IBD. A very comprehensive search strategy was used to identify relevant studies, and efforts were made at all steps of the process to reduce possible bias in data synthesis.
Overall this review shows that evidence is accumulating on the use of thalidomide in patients with IBD. The efficacy of thalidomide appears to be not negligible, and worth investing in future research.
This review highlighted that thalidomide was effective even when used after the failure of biological therapies, with response and remission rates similar to biologically naive patients. These results can be explained by the different mechanism of action of thalidomide compared to other anti-TNF alpha biological agents, and supports the use of thalidomide in patients refractory/intolerant to other anti-TNF alpha agents.
This review confirms to a great extent what is already known about the safety of thalidomide, but also provides a more detailed insight into observed AE in patients with IBD. Adverse events were the most common cause of thalidomide withdrawal in the long-term, whereas the drug's loss of efficacy accounted for only 12% of the drug suspensions. Neurological AE are the most frequent complain during thalidomide treatment. Thalidomide was first commercialized as a sedative drug and it is therefore not surprising that sedation is a common AE. Sedation/somnolence are generally observed in the first weeks of treatment and are subsequently tolerated probably with a mechanism of tachyphylaxis.[45] The degree of sedation is usually proportional to the daily dose and can be further decreased by assuming the drug late in the evening, before going to sleep at night.[16,21,45]
On the opposite, as reported in studies in multiple myeloma patients or in other inflammatory diseases,[46–49] peripheral neuropathy is generally detected after several months of treatment, as it seems to be associated more with thalidomide cumulative dose, rather than with the daily dosage. The frequency of thalidomide-induced peripheral neuropathy varies in the current literature depending on the age of patients, the primary disease, the drug doses, the concomitant treatments, and the length of follow-up. In 135 adult patients with various dermatological conditions treated with thalidomide at daily dosages comparable to those used in the studies included in the present review (mean thalidomide starting dose 97.5 ± 25.6 mg/d), clinical signs of peripheral neuropathy accompanied by electromyographic signs were observed in 25.2% of cases during a median 11 month follow-up.[47] Data for children are limited: Priolo and colleagues evaluated 13 patients treated with thalidomide for rheumatological conditions or for Crohn's disease and found a clinical neuropathy in 35.8% of cases and the presence of electromyographic subclinical alterations in 53.8% of cases.[49] Thalidomide-induced peripheral neuropathy is a predominantly sensory polyneuropathy affecting mainly long and large fibers;[39,49,50] it has been described as reversible although few cases presented persistent clinical and electrophysiologic alterations after thalidomide suspension during a short follow-up time.[49] Nerve conduction studies are useful to monitor the development and the evolution of neurotoxicity once it has become clinically apparent, although it is not fully clear if electromyographic abnormalities in the absence of symptoms are predictive of a developing clinical neuropathy.[45] While awaiting further safety data in patients treated with IBD with low doses of thalidomide, it remains important to warn patients of the need to report symptoms suggestive of neuropathy (tremors, numbness and tingling), and to perform careful routine neurological evaluations including sensitivity to vibration. In cases of mild symptoms or in the case of persistent nerve conduction abnormalities without clinical signs/symptoms, reducing the daily dose may be used as a strategy to arrest or slow down the progression of clinical neuropathy.[10]
This review highlighted that amenorrhea due to hypergonadotropic hypogonadism was a relatively frequent AE in female patients with IBD treated with thalidomide. A previous review highlighted that the risk of amenorrhea in patients with different inflammatory diseases under thalidomide treatment may be higher than in the general population of woman treated with thalidomide.[44] Though in most cases amenorrhea has been described as reversible, patients need to be carefully informed and strictly monitored.
Despite the fact that thalidomide is known to increase the risk of deep vein thrombosis in patients with multiple myeloma,[50] and despite IBD with active diseases having per se a higher risk of venous and arterial thromboembolism compared to the general population,[51] only 2 cases of deep venous thrombosis were identified by this review. This may be explained by the fact that the risk of thrombosis associated with thalidomide is low, if the drug is used alone (and not in association with the other drugs used in multiple myeloma) and at a low dosage. Additionally, it is possible that the increased pro-thrombotic risk associated with thalidomide is balanced by the capacity of the drug to control the inflammatory response. More studies on large samples of patients are needed to further evaluate the real risk of thrombosis in patients with IBD at different stages of disease activity.
Interestingly, no infection was reported under thalidomide treatment, even when thalidomide was used at high dosages (>150 mg/d). This highlights that the immunomodulatory effects of thalidomide are not, or are only slightly, immunosuppressive, as compared with anti-TNF alpha biologics.
Most of the other AE reported in this review were mild and did not require the drug to be withdrawn. However, larger studies are needed to detect rare although potentially severe AE.
Toxicity is certainly the main concern of thalidomide treatment. Reducing thalidomide dose to a minimum effective dose, after achieving stable remission, may potentially be a successful strategy to reduce long-term AE and to delay the onset of neuropathy. Despite the fact that at present there is no evidence on efficacy of lenalidomide,[52] in the future, the development of other thalidomide analogs with a lower incidence of AE may further improve the risk and benefit profile of this therapy.
In conclusion, according to the results of this review, thalidomide appears to be a valuable treatment option for patients with CD refractory to other first- and second-line treatments.
Further randomized controlled trials are needed to adequately explore the efficacy and safety of thalidomide in patients with UC, as well as to evaluate thalidomide in comparison with other therapies for patients with less severe diseases or at earlier stages of their natural course.
Supplementary Material
Footnotes
Abbreviations: AE = adverse effect, CD = Crohn disease, IBD = inflammatory bowel disease, IC = indeterminate colitis, RCT = randomized controlled trial, RR = risk ratio, TNF = tumor necrosis factor, UC = ulcerative colitis.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Sheskin J. Thalidomide in the treatment of lepra reaction. Clin Pharmacol Ther 1965; 6:303–306. [DOI] [PubMed] [Google Scholar]
- 2.Teo SK, Resztak KE, Scheffler MA, et al. Thalidomide in the treatment of leprosy. Microbes Infect 2002; 4:1193–1202. [DOI] [PubMed] [Google Scholar]
- 3.Yang CS, Kim C, Antaya RJ. Review of thalidomide use in the pediatric population. J Am Acad Dermatol J Am Acad Dermatol 2015; 72:703–711. [DOI] [PubMed] [Google Scholar]
- 4.Akobeng AK, Stokkers PC. Thalidomide and thalidomide analogues for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev 2009i; 2: CD007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srinivasan R, Akobeng AK. Thalidomide and thalidomide analogues for induction of remission in Crohn's disease. Cochrane Database Syst Rev 2009; 2: CD007350. [DOI] [PubMed] [Google Scholar]
- 6.Yang C, Singh P, Singh H, et al. Systematic review: thalidomide and thalidomide analogues for treatment of inflammatory bowel disease. Aliment Pharmacol Ther 2015; 41:1079–1093. [DOI] [PubMed] [Google Scholar]
- 7.xxx http://prisma-statement.org/documents/PRISMA%202009%20checklist.pdf. [Google Scholar]
- 8.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons; 2008. [Google Scholar]
- 9.Simon M, Pariente B, Lambert J, et al. Long-term Outcomes of Thalidomide Therapy for Adults With Refractory Crohn's Disease. Clin Gastroenterol Hepatol 2016; 14:966–972. [DOI] [PubMed] [Google Scholar]
- 10.Lazzerini M, Martelossi S, Magazzù G, et al. Effect of thalidomide on clinical remission in children and adolescents with refractory Crohn disease: a randomized clinical trial. JAMA 2013; 310:2164–2173. [DOI] [PubMed] [Google Scholar]
- 11.Gerich ME, Yoon JL, Targan SR, et al. Long-term outcomes of thalidomide in refractory Crohn's disease. Aliment Pharmacol Ther 2015; 41:429–437. [DOI] [PubMed] [Google Scholar]
- 12.Luo HQ, Tan B, Lü H, et al. An analysis of adverse drug reactions of thalidomide in treatment of immune-related bowel diseases. Zhonghua Nei Ke Za Zhi 2013; 52:726–729. [PubMed] [Google Scholar]
- 13.Lazzerini M, Martelossi S, Marchetti F, et al. Efficacy and safety of thalidomide in children and young adults with intractable inflammatory bowel disease: long-term results. Aliment Pharmacol Ther 2007; 25:419–427. [DOI] [PubMed] [Google Scholar]
- 14.Plamondon S, Ng SC, Kamm MA. Thalidomide in luminal and fistulizing Crohn's disease resistant to standard therapies. Aliment Pharmacol Ther 2007; 25:557–567. [DOI] [PubMed] [Google Scholar]
- 15.Lazzerini M, Martelossi S, Magazzù G, et al. Effect of thalidomide on clinical remission in children and adolescents with ulcerative colitis refractory to other immunosuppressives: pilot randomised clinical trial. Inflamm Bowel Dis 2015; 21:1739–1749. [DOI] [PubMed] [Google Scholar]
- 16.Ehrenpreis ED, Kane SV, Cohen LB, et al. Thalidomide therapy for patients with refractory Crohn's disease: an open-label trial. Gastroenterology 1999; 117:1271–1277. [DOI] [PubMed] [Google Scholar]
- 17.Leung YK, Huang Y. Clinical efficacy and mechanism of action of thalidomide in pediatric patients with refractory Crohn's disease. Gastroenterology 2014; 146:S–452. [Google Scholar]
- 18.Scribano ML, Cantoro L, Mangiarotti R, et al. Thalidomide in immunosuppressors and anti-TNF unresponsive Crohn's disease patients: efficacy and side effects. J Crohn's Colitis 2011; 3:183. [Google Scholar]
- 19.Sabate JM, Villarejo J, Lemann M, et al. An open-label study of thalidomide for maintenance therapy in responders to infliximab in chronically active and fistulizing refractory Crohn's disease. Aliment Pharmacol Ther 2002; 16:1117–1124. [DOI] [PubMed] [Google Scholar]
- 20.Tang J, Gao X, Zhi M, et al. Intravenous cyclophosphamide combined with thalidomide has a promising effect in refractory Crohn's disease. J Crohn's Colitis 2014; 8:S247–S248. [Google Scholar]
- 21.Vasiliauskas EA, Kam LY, Abreu-Martin MT, et al. An open-label pilot study of low-dose thalidomide in chronically active, steroid-dependent Crohn's disease. Gastroenterology 1999; 117:1278–1287. [DOI] [PubMed] [Google Scholar]
- 22.Felipez LM, Gokhale R, Tierney MP, et al. Thalidomide use and outcomes in pediatric patients with Crohn disease refractory to infliximab and adalimumab. J Pediatr Gastroenterol Nutr 2012; 54:28–33. [DOI] [PubMed] [Google Scholar]
- 23.Bariol C, Meagher AP, Vickers CR, et al. Early studies on the safety and efficacy of thalidomide for symptomatic inflammatory bowel disease. J Gastroenterol Hepatol 2002; 17:135–139. [DOI] [PubMed] [Google Scholar]
- 24.Bauditz J, Wedel S, Lochs H. Thalidomide reduces tumour necrosis factor alpha and interleukin 12 production in patients with chronic active Crohn's disease. Gut 2002; 50:196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macumber C, Wettstein AP, Vickers CR, et al. Thalidomide-effective therapy in chronic resistant inflammatory bowel disease. Gastroenterology 1999; 116:A767. [Google Scholar]
- 26.Gupta P, Gokhale R, Andrew H, et al. Thalidomide for refractory Crohn's disease failing infliximab. J Pediatr Gastroenterol Nutr 2003; 37:338–342. [Google Scholar]
- 27.Ng SC, Plamondon S, Gupta A, et al. Prospective evaluation of anti-tumor necrosis factor therapy guided by magnetic resonance imaging for Crohn's perineal fistulas. Am J Gastroenterol 2009; 104:2973–2986. [DOI] [PubMed] [Google Scholar]
- 28.Scribano M, Monterubbianesi R, Cantoro L, et al. Association of thalidomide with anti-TNF-α therapy in Crohn's disease patients who lost response to anti-TNF-α: A case series. Dig Liver Dis 2014; 46 suppl 2:S27. [Google Scholar]
- 29.Kam LY, Vasiliauskas EA, Abreu MT, et al. Open labeled pilot study of thalidomide(thal) as a novel therapy for medically resistant ulcerative colitis (UC). Gastroenterology 2000; 118:A582. [Google Scholar]
- 30.Srinivasan R, Casson D. Thalidomide treatment for maintainance of infliximab induced response in refractory paediatric Crohn's disease. J Pediatr Gastroenterol Nutr 2007; 44:110. [Google Scholar]
- 31.Ahmed M, El-Hadi S, Jenkins HR. Thalidomide in Crohn disease and the risk of peripheral neuropathy. J Pediatr Gastroenterol Nutr 2003; 37:522. [DOI] [PubMed] [Google Scholar]
- 32.Trebble T, Johns T, Duncan HD, et al. An open trial of thalidomide in refractory Crohn's colitis. Gut 2001; 48:A89–A90. [Google Scholar]
- 33.Zheng CF, Xu JH, Huang Y, et al. Treatment of pediatric refractory Crohn's disease with thalidomide. World J Gastroenterol 2011; 17:1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hegarty A, Hodgson T, Porter S. Thalidomide for the treatment of recalcitrant oral Crohn's disease and orofacial granulomatosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003; 95:576–585. [DOI] [PubMed] [Google Scholar]
- 35.Lazzerini M, Martelossi S, Cont G, et al. Orofacial granulomatosis in children: think about Crohn's disease. Dig Liver Dis 2015; 47:338–341. [DOI] [PubMed] [Google Scholar]
- 36.Kane S, Stone LJ, Ehrenpreis E. Thalidomide as “salvage” therapy for patients with delayed hypersensitivity response to infliximab: a case series. J Clin Gastroenterol 2002; 35:149–150. [DOI] [PubMed] [Google Scholar]
- 37.Cohen LB. Re: Disappearance of Crohn's ulcers in the terminal ileum after thalidomide therapy. Can J Gastroenterol 2004; 18:101–104.419. [DOI] [PubMed] [Google Scholar]
- 38.Bauditz J, Schachschal G, Wedel S, et al. Thalidomide for treatment of severe intestinal bleeding. Gut 2004; 53:609–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fleming FJ, Vytopil M, Chaitow J, et al. Thalidomide neuropathy in childhood. Neuromuscul Disord 2005; 15:172–176. [DOI] [PubMed] [Google Scholar]
- 40.Simon M, Gornet JM, Plane C, et al. Long term efficacy and toxicity of thalidomide in Crohn's disease. Gastroenterology 2004; 126:A629. [Google Scholar]
- 41.Qian J, Li Y. Efficacy and safety of thalidomide in Chinese patients with refractory Crohn's disease. Gastroenterology 2013; 144:S435–S1435. [Google Scholar]
- 42.Facchini S, Candusso M, Martelossi S, et al. Efficacy of long-term treatment with thalidomide in children and young adults with Crohn disease: preliminary results. J Pediatr Gastroenterol Nutr 2001; 32:178–181. [DOI] [PubMed] [Google Scholar]
- 43.Scribano ML, Cantoro L, Marrollo M, et al. Mucosal healing with thalidomide in refractory Crohn's disease patients intolerant of anti-TNF-α drugs: report of 3 cases and literature review. J Clin Gastroenterol 2014; 48:530–533. [DOI] [PubMed] [Google Scholar]
- 44.Lazzerini M, Bramuzzo M, Martelossi S, et al. Amenorrhea in women treated with thalidomide: report of two cases and literature review. Inflamm Bowel Dis 2013; 19:E10–E11. [DOI] [PubMed] [Google Scholar]
- 45.Ghobrial IM, Rajkumar SV. Management of thalidomide toxicity. J Support Oncol 2003; 1:194–205. [PMC free article] [PubMed] [Google Scholar]
- 46.Chaudhry V, Cornblath DR, Corse A, et al. Thalidomide-induced neuropathy. Neurology 2002; 59:1872–1875. [DOI] [PubMed] [Google Scholar]
- 47.Cavaletti G, Beronio A, Reni L, et al. Thalidomide sensory neurotoxicity: a clinical and neurophysiologic study. Neurology 2004; 62:2291–2293. [DOI] [PubMed] [Google Scholar]
- 48.Bastuji-Garin S, Ochonisky S, Bouche P, et al. Thalidomide Neuropathy Study Group. Incidence and risk factors for thalidomide neuropathy: a prospective study of 135 dermatologic patients. J Invest Dermatol 2002; 119:1020–1026. [DOI] [PubMed] [Google Scholar]
- 49.Priolo T, Lamba LD, Giribaldi G, et al. Childhood thalidomide neuropathy: a clinical and neurophysiologic study. Pediatr Neurol 2008; 38:196–199. [DOI] [PubMed] [Google Scholar]
- 50.Carrier M, Le Gal G, Tay J, et al. Rates of venous thromboembolism in multiple myeloma patients undergoing immunomodulatory therapy with thalidomide or lenalidomide: a systematic review and meta-analysis. J Thromb Haemost 2011; 9:653–663. [DOI] [PubMed] [Google Scholar]
- 51.Lazzerini M, Bramuzzo M, Maschio M, et al. Thromboembolism in pediatric inflammatory bowel disease: systematic review. Inflamm Bowel Dis 2011; 17:2174–2183. [DOI] [PubMed] [Google Scholar]
- 52.Mansfield JC, Parkes M, Hawthorne AB, et al. A randomized, double-blind, placebo-controlled trial of lenalidomide in the treatment of moderately severe active Crohn's disease. Aliment Pharmacol Ther 2007; 26:421–430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


