Abstract
In Crohn disease, bowel-preserving surgery is necessary to prevent short bowel syndrome due to repeated operations. This study aimed to determine the remnant small bowel length cut-off and to evaluate the clinical factors related to nutritional status after small bowel resection in Crohn disease.
We included 394 patients (69.3% male) who underwent small bowel resection for Crohn disease between 1991 and 2012. Patients who were classified as underweight (body mass index < 17.5) or at high risk of nutrition-related problems (modified nutritional risk index < 83.5) were regarded as having a poor nutritional status. Preliminary remnant small bowel length cut-offs were determined using receiver operating characteristic curves. Variables associated with poor nutritional status were assessed retrospectively using Student t tests, chi-squared tests, Fisher exact tests, and logistic regression analyses.
The mean follow-up period was 52.9 months and the mean patient ages at the time of the last bowel surgery and last follow-up were 31.2 and 35.7 years, respectively. The mean remnant small bowel length was 331.8 cm. Forty-three patients (10.9%) underwent ileostomy, 309 (78.4%) underwent combined small bowel and colon resection, 111 (28.2%) had currently active disease, and 105 (26.6%) underwent at least 2 operations for recurrent disease. The mean body mass index and modified nutritional risk index were 20.6 and 100.8, respectively. The independent factors affecting underweight status were remnant small bowel length ≤240 cm (odds ratio: 4.84, P < 0.001), ileostomy (odds ratio: 4.70, P < 0.001), and currently active disease (odds ratio: 4.16, P < 0.001). The independent factors affecting high nutritional risk were remnant small bowel length ≤230 cm (odds ratio: 2.84, P = 0.012), presence of ileostomy (odds ratio: 3.36, P = 0.025), and currently active disease (odds ratio: 4.90, P < 0.001).
Currently active disease, ileostomy, and remnant small bowel length ≤230 cm are risk factors affecting the poor nutritional status of patients with Crohn disease after small bowel resection.
Keywords: body mass index, Crohn disease, ileostomy, nutritional risk index, small bowel length
1. Introduction
Crohn disease (CD) is an inflammatory bowel disease that can involve the whole gastrointestinal tract, although the most common location of involvement is the terminal ileum.[1] The major therapeutic approach to CD is conservative medical treatment; however, patients with CD undergoing surgery are up to 80% during their lifetime.[2] Moreover, the possibility of repeated surgery can reach up to 40% after 10 years,[3] and bowel preserving surgery is necessary to prevent short bowel syndrome (SBS).
CD can induce a variety of nutritional problems that affect patient health and quality of life. Nutritional problems in patients with CD are common and vary depending on the disease activity, disease location, the existence of stoma, range of bowel resection, and associated complication after surgery.[4] Additionally, other causes of poor nutritional status in patients with CD include low nutrient absorption, increased nutrient requirements, poorly dietary intake.[5] These nutritional problems are not limited to the term of active phase in CD; a significant number of patients have nutritional deficiencies during the period of remission, even if macronutrient requirements are being met.[6–8] Overall, 20% to 85% of patients suffering from inflammatory bowel disease have nutritional deficiencies.[4,9]
In addition to CD, several other conditions requiring intestinal resection can also lead to SBS in adults, such as postoperative causes, irradiation/cancer, mesenteric vascular disease, and other benign causes.[10,11] Because of the relapsing and intractable nature of CD, surgeons are often reluctant to perform a massive bowel resection and tend to try and preserve as much bowel tissue as possible during the resection procedure. However, there has been no investigation of the cut-off remnant small bowel length (r-SBL) that has a critical effect on the nutritional status of patients with CD. Therefore, the aims of our present study were to determine the cut-off value of r-SBL and evaluate the other factors that affect nutritional status after small bowel resection (SBR) in patients with CD, based on cross-sectional study with retrospective design.
2. Materials and methods
2.1. Patients
Data were collected from 510 patients who underwent SBR for CD at our institution between 1991 and 2012. Clinical data were collected during a follow-up period of at least 12 months after the last surgery. A total of 116 patients were excluded, leading to the inclusion of 394 patients in the study. The exclusion criteria were as follows: follow-up period <12 months (n = 52), cancer development (n = 11), unknown r-SBL (n = 47), age at last follow-up ≥70 (n = 5), or repeated admission due to persistent complications of CD (n = 1). The diagnosis of CD was confirmed by postoperative pathologic analyses. The study was approved by the Institutional Review Board of Asan Medical Center. This study was conducted in accordance with the guidelines in the Declaration of Helsinki.
2.2. Nutritional status parameters
The body mass index (BMI) and modified nutritional risk index (mNRI) were used to evaluate the nutritional status. The BMI parameter was established by the World Health Organization in 1997 and was published in 2000.[12] A BMI < 18.5 was classified as underweight and was used as one of the criteria for poor nutritional status. A World Health Organization expert consultation reviewed scientific evidence suggesting that associations between the BMI, percentage of body fat, and health risks differ between Asian and Western populations. The definition of underweight is a BMI < 17.5 for Asian populations; accordingly, we performed a risk analysis for a BMI < 17.5.[13] NRI scores were categorized as “well nourished” (>100), “mildly malnourished” (97.5–100), “moderately malnourished” (83.5 to <97.5), and “severely malnourished” (<83.5).[14,15] To generate the mNRI, the usual body weight in the NRI formula was replaced with the ideal body weight calculated using the Lorentz equations. The mNRI was calculated as follows: mNRI = [1.519 × serum albumin (g/L)] + 41.7 (present weight/ideal body weight). An mNRI < 83.5 (“severely malnourished”) was used as the other criterion for poor nutritional status.
2.3. Data analysis
The independent variables were sex, age at last follow-up, duration after last surgery, r-SBL, ileostomy status, resection of the ileocecal valve (ICV), colon resection-related parameters, existence of active CD, number of surgeries (single vs multiple), and surgical method (laparoscopic vs open). The dependent variables were underweight (BMI < 17.5) and high nutritional risk (mNRI < 83.5). The SBL was measured during surgery using a sterile paper ruler. The total small bowel length (t-SBL) before initial SBR was identified in 298 patients. The existence of active CD was defined as a Crohn disease activity index (CDAI) ≥150. The CDAI is considered the gold standard for the assessment of disease activity for clinical trials.[16]
2.4. Statistical analysis
The preliminary r-SBL cut-off values for subjects classified as underweight (BMI < 17.5) and high nutritional risk (mNRI < 83.5) were determined using receiver operating characteristic (ROC) curves. Risk analyses were performed for these 2 dependent variables. The clinical parameters of the patients were evaluated by a cross-table analysis using a Pearson chi-squared test or Fisher exact test. The relationships between poor nutritional status and currently active CD, ileostomy, cut-off value of the r-SBL, and other independent variables were assessed using logistic regression analysis. Statistical significance was defined as P < 0.05. All calculations were performed using SPSS software (ver. 19; SPSS Inc., Chicago, IL).
3. Results
The patient characteristics are summarized in Table 1. A total of 394 (69.3% male) patients who underwent SBR for CD were included in the study. The mean age at the time of the last operation was 31.2 ± 9.2 years, and the mean age at the last follow-up was 35.7 ± 9.0 years. The mean follow-up period was 52.9 ± 31.2 months. The mean values of the initial t-SBL, the fully resected SBL, and the final r-SBL were 435.0, 85.7, and 331.8 cm, respectively. The initial t-SBL was determined in 298 patients. A total of 300 patients underwent ICV resection. At the time of the last follow-up, 43 patients (10.9%) had undergone ileostomy, 111 patients (28.2%) had currently active CD, and 105 patients (26.6%) had undergone at least 2 operations due to recurrent CD. Overall, 309 patients (78.4%) had combined SBR and colon resection; of these, 68.9% underwent right colon resection and 28.5% right and left colon resection. In addition, 61 patients (15.5%) underwent total colectomy or total proctocolectomy (absence of remnant colon). The mean BMI and mNRI values were 20.6 ± 3.4 and 100.8 ± 14.4, respectively. More patients received open surgery (343, 87.1%) than laparoscopic surgery (51, 12.9%).
Table 1.
Patient characteristics.
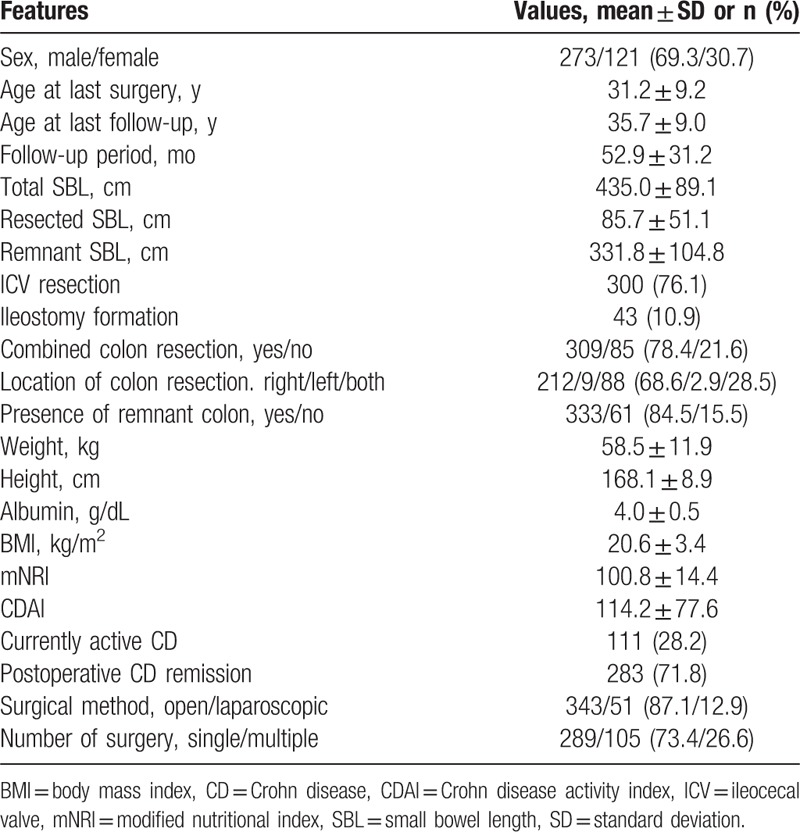
First, the maximum Youden indices of the ROC curves were used to determine preliminary cut-off values of the r-SBL for the underweight and high nutritional risk statuses. The cut-off value of the r-SBL for the underweight status (BMI < 17.5) was 242.5 cm (maximum Youden index = 0.384; Fig. 1), and that for the high nutritional risk status (mNRI < 83.5) was 232.5 cm (maximum Youden index = 0.273; Fig. 2). These cut-off values were used for the grouping of independent variables in the univariate and multivariate analyses (Tables 2 and 3).
Figure 1.
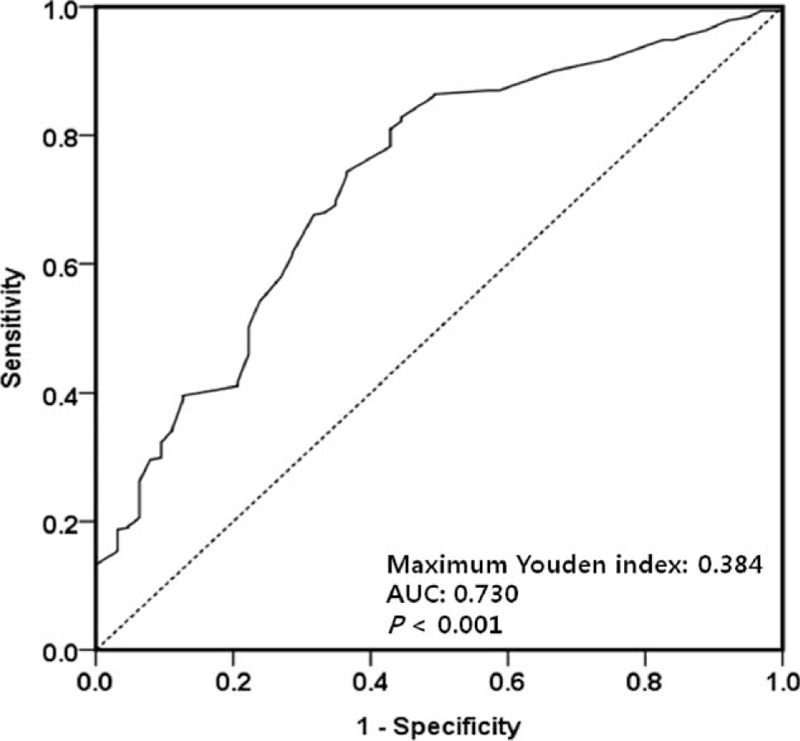
Receiver operating characteristic curve used to identify the preliminary cut-off value of the remnant small bowel length relative to poor nutritional status (body mass index < 17.5). The length with the maximum Youden index (0.384) was 242.5 cm. AUC = area under the curve.
Figure 2.
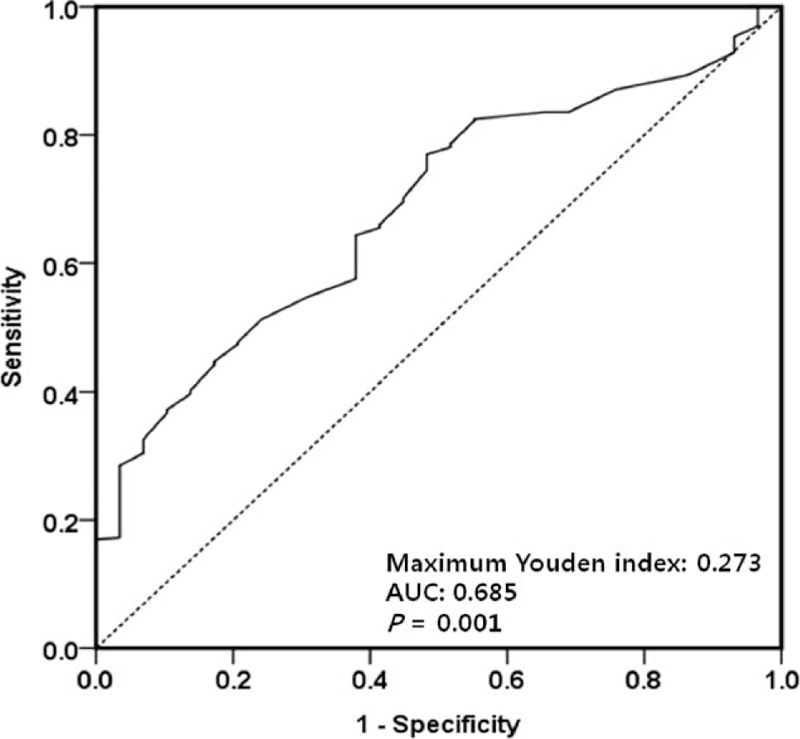
Receiver operating characteristic curve used to identify the preliminary cut-off value of the remnant small bowel length relative to poor nutritional status (modified nutritional risk index < 83.5). The length with the maximum Youden index (0.273) was 232.5 cm. AUC = area under the curve.
Table 2.
Clinical factors affecting underweight (BMI < 17.5).
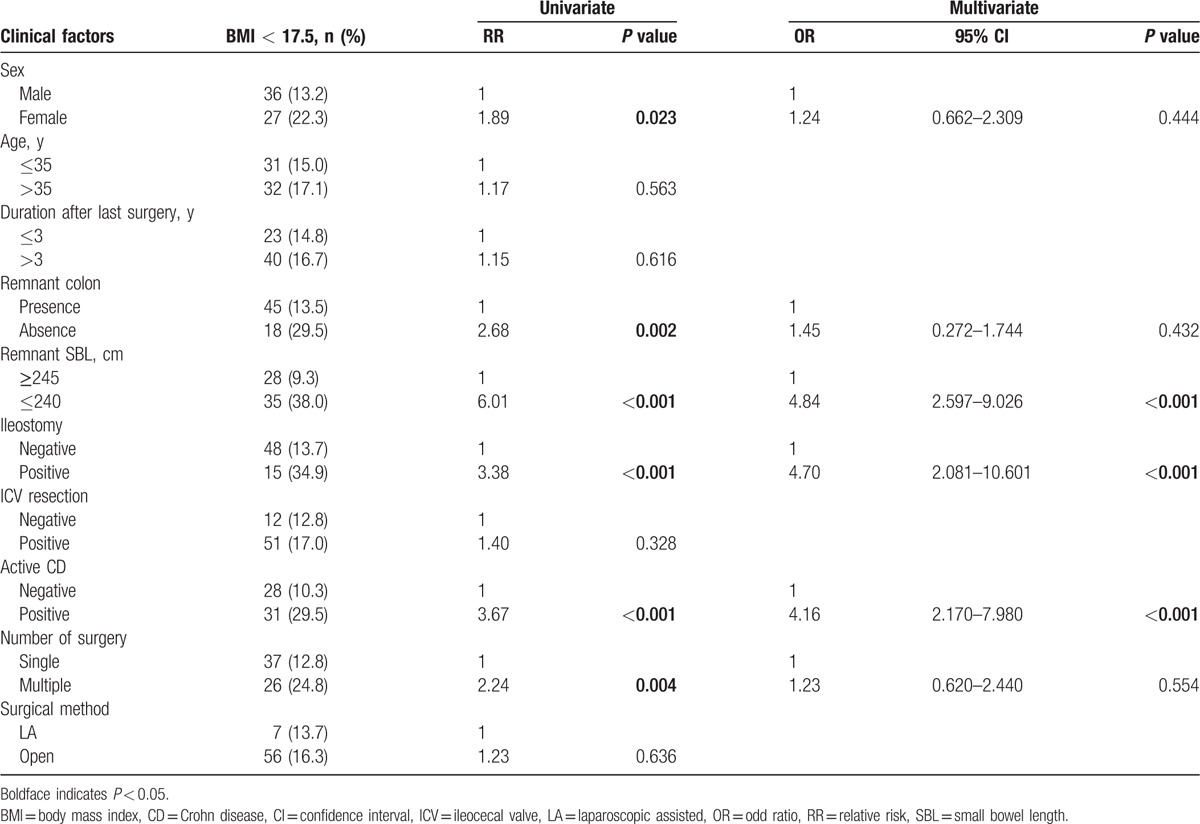
Table 3.
Clinical factors affecting high nutritional risk (mNRI < 83.5).
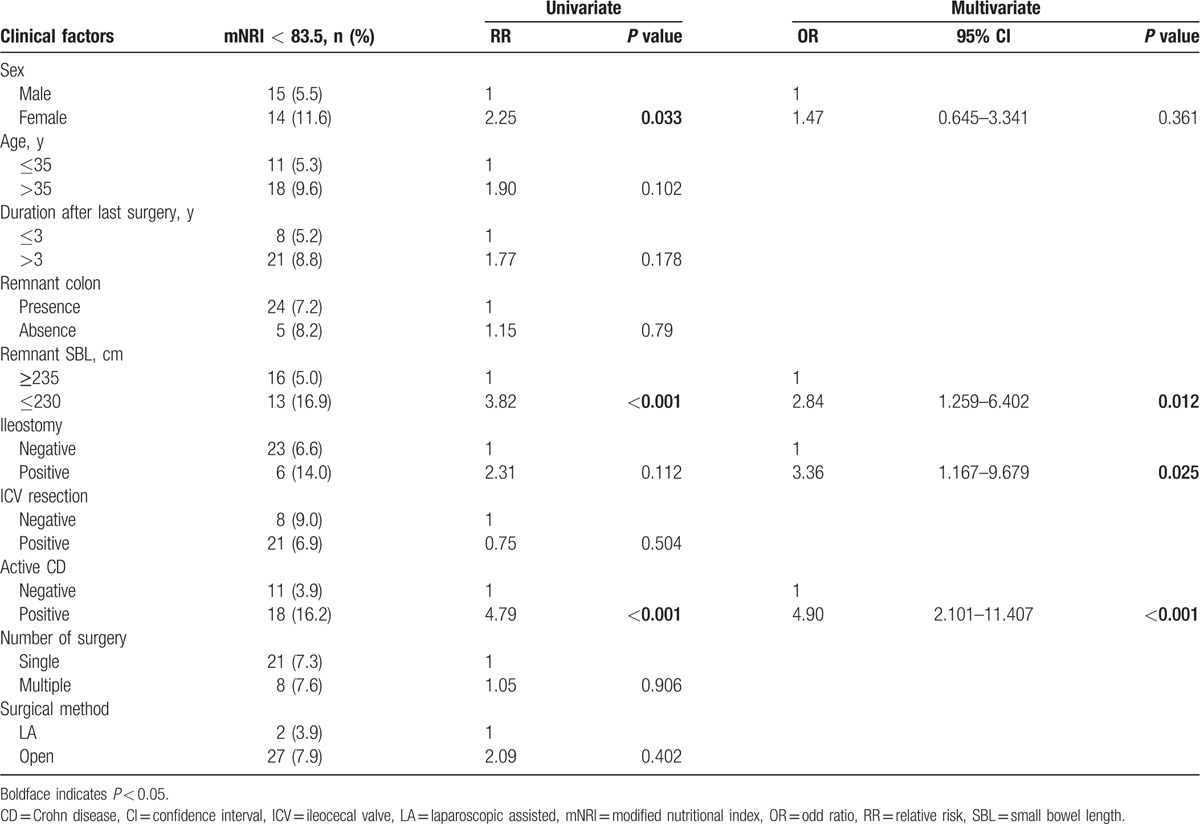
By univariate analysis, the clinical factors affecting the underweight status (BMI < 17.5) were female sex (P = 0.023), absence of remnant colon (P = 0.002), r-SBL ≤ 240 cm (P < 0.001), presence of ileostomy (P < 0.001), currently active CD (P < 0.001), and multiple surgeries (P = 0.004). Multivariate analysis identified r-SBL ≤ 240 cm (odds ratio [OR]: 4.84, P < 0.001), presence of ileostomy (OR: 4.70, P < 0.001), and currently active CD (OR: 4.16, P < 0.001) as statistically significant independent risk factors for the underweight status (Table 2). By univariate analysis, the clinical factors affecting high nutritional risk (mNRI < 83.5) were female sex (P = 0.033), r-SBL ≤ 230 cm (P < 0.001), and currently active CD (P < 0.001). Multivariate analysis revealed that currently active CD (OR: 4.90, P < 0.001), presence of ileostomy (OR: 3.36, P = 0.025), and r-SBL ≤ 230 cm (OR: 2.84, P = 0.012) were statistically significant independent risk factors for high nutritional risk (Table 3).
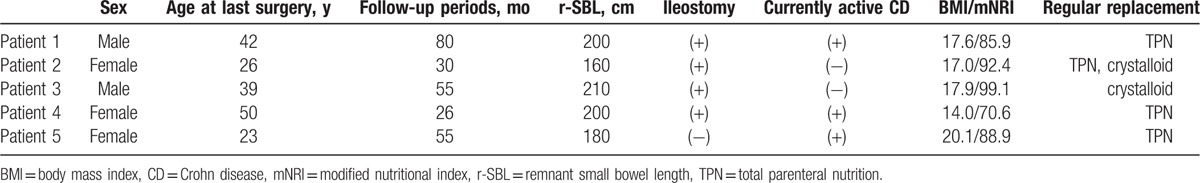
Five (1.3%) of the patients included in this study received regular parenteral nutrition; the clinical data for these patients are summarized in Table 4. The group included 2 male and 3 female patients who were 23 to 50 years old at the time of the last SBR and 27 to 51 years old at the time of the last follow-up. Four of the patients underwent ileostomy and 3 had currently active CD. In addition, all of 5 patients had an r-SBL < 230 cm and 2 or more risk factors for a poor nutritional status. In spite of regular parenteral nutrition, the 4th patient, who had all 3 risk factors, displayed a very poor nutritional status (BMI = 14.0 and mNRI = 70.6).
Table 4.
Patients who received regular parenteral nutrition or crystalloid intravenous infusion.
4. Discussion
After SBR, the intestines undergo an adaptation process that lasts 1 to 2 years and involves maximal stimulation of nutrient absorption by gradually increasing intestinal nutrient exposure.[17] For this reason, a minimum follow-up period of 12 months after the last operation was used in our present study. According to the correlation analysis, there was no correlation between duration after last surgery (follow-up period) and nutritional status (BMI; r = −0.039, mNRI; r = 0.026, r = Pearson correlation coefficient). Additionally, when divided into 2 groups (≤3 vs >3 years), this factor did not affect the nutritional status (Tables 2 and 3).
Changes in body composition that occur with aging and intrinsic factors related to aging render older patients more susceptible to nutritional complications. Moreover, comorbidities are frequently present in the elderly, these can have a significant impact on the nutritional status.[18,19] For this reason, patients ages 70 years and over were excluded from our current analyses.
A sterile paper ruler was used to measure the SBL before and after SBR. Without tension, the t-SBL and r-SBL were measured before and after SBR, respectively. Although the r-SBL was measured accurately in all patients, the resected SBL and t-SBL were difficult to measure in conglomerated or tangled small bowels, due to inflammation. Therefore, in spite of meticulous measurements, the resected SBL and t-SBL data may be slightly inaccurate. Furthermore, the total length of the original small bowel was only known in 298 patients (75.6%), due to incomplete records or previous surgery in other hospitals.
The variety of triggers for the development of malnutrition has led scientists to develop nutritional screening tools and methods for early recognition of the problem.[20–22] However, it is difficult to apply these screening tools to retrospective studies, and there is no objective index for nutritional status in patients with CD; therefore, the BMI and mNRI were used to evaluate the nutritional status of the patients included in this study.
As described earlier, the mNRI is a modified form of the NRI, the latter of which was first described by Buzby et al, which is used to score nutritional risk in surgical patients, and is a simple, accurate, and validated tool for predicting the risks of morbidity and mortality. The NRI is calculated as follows: NRI = [(1.519 × serum albumin (g/L)) + 41.7 (present weight/usual weight)]. The formula takes into account the usual body weight and is related to a history of recent weight loss.[14] However, we had difficulties identifying the usual body weights of patients with CD due to prolonged affected periods and early onset characteristics of this disease. Hence, we replaced the usual body weight in the NRI formula with the ideal body weight to generate the mNRI. Similarly, the geriatric nutritional risk index (GNRI), a new index for evaluating at-risk elderly medical patients described by Bouillanne et al, uses the ideal body weight and is calculated as follows: GNRI = [1.489 × serum albumin (g/L)] + 41.7 (present weight/ideal body weight)]. As in the case of patients with CD, it is difficult to obtain the usual body weights of elderly patients; hence, Bouillanne et al hypothesized that this value in the NRI formula could be replaced by the ideal body weight. Bouillanne et al also changed the constant multiplier of the albumin concentration based on the results of regression analysis.[23]
SBS is associated with permanent parenteral nutrition dependency due to fluid and electrolyte disorder, progressive malnutrition, and weight loss after extensive SBR. According to previous studies, SBS may occur after resection of more than 50% of the initial length of small bowel and essentially caused by resection of more than 70%, or if <100 cm of the small bowel remains after resection. Simultaneous resection of colon and small bowel or resection of the ileocecal region can lead to worse conditions.[11,17,24,25] However, although numerous studies have been performed, there is no consistent definition of SBS. Because small bowel function is not dependent on length alone, some definitions of SBS are based on the functional capacity of the remnant bowel.[26,27] In patients with CD, persistent or recurrent small bowel diseases make it difficult to assess the cut-off value of SBL affecting nutritional status; therefore, we attempted to define a cut-off value of the r-SBL. Using ROC analyses, we were able to determine preliminary cut-off values for 2 dependent variables (242.5 cm for BMI < 17.5 and 232.5 cm for mNRI < 83.5); however, the direct application of these cut-off values to patients with CD may not be ideal due to their slightly low maximum Youden indices (0.384 and 0.273, respectively; Figs. 1 and 2). Variable factors affecting the nutritional status of patients with CD made it difficult to define clear cut-off values using ROC analyses; however, the preliminary cut-off values were valuable points of separation for independent variables in univariate and multivariate analyses. These analyses identified r-SBL cut-off values of 240 and 230 cm for a BMI < 17.5 and an mNRI < 83.5, respectively (Tables 2 and 3). It should be noted that these values are higher than the cut-off values reported in previous studies of SBS.[24,25,28] The cause of this discrepancy may be the influence of other factors affecting nutritional status that were not included in our present study, such as combined perianal disease, psychological components, effects of medication, or long-term dietary intake habits. Of the 394 patients included in our current analyses, only 24 and 8 individuals had an r-SBL <180 and <150 cm, respectively; therefore, it was difficult to identify lower cut-off values that were statistically significant. A more reliable lower cut-off value may have been identified if more patients had undergone extensive SBR; however, this assumption is difficult to prove due to the prevalence of bowel-preserving surgery for patients with CD.
There is the similar concept to SBS. In 2015, the European Society for Clinical Nutrition and Metabolism presented recommendations for definition and classification of “intestinal failure (IF)” in adults. According to this presentation, the definition of IF is the reduction of gut function below the minimum necessary for the absorption of macronutrients and/or water and electrolytes. As a result, intravenous supplementation is required to maintain patient health. Additionally, they presented that IF is classified in terms of the following 3 aspects: functional, pathophysiological, and clinical aspects.[29] As shown in Table 4, 5 (1.3%) of the patients included in our study received regular parenteral nutrition. These 5 patients are included in the definition of the IF, their classifications can be included as follows: Type III (chronic condition, in metabolically stable condition, requiring intravenous supplementation over months or years) in functional classification, short bowel and/or extensive small bowel mucosal disease in pathophysiological classification. The patients with ileostomy can be classified as intestinal fistula, and the patients with severe adhesion due to repeated surgery or postinflammatory scar change may be classified as intestinal dysmotility or mechanical obstruction.
Intestinal adaptation after massive bowel resection depends on a number of factors, including the length of the remaining small bowel, as well as the presence of the ileum, ICV, and/or colon. In particular, loss of the ileum is much more deleterious than loss of the jejunum. Indeed, nutritional problems are much less common in patients exhibiting loss of the jejunum but an intact ileum and colon, whereas patients with large ileal resections usually require a number of medical interventions and continuous medical care and supervision.[24,25,28] In our present study, 47 patients (11.9%) underwent resection of the jejunum and 371 patients (94.2%) received an ileal resection; there was no significant difference between the nutritional statuses of these 2 groups in univariate analysis (P = 0.802 for BMI < 17.5, P = 0.145 for mNRI < 83.5). However, the descriptions of the jejunoileal junction resections and the distinction between the jejunum and the ileum were ambiguous in the operative records; therefore, we excluded this parameter (jejunum vs ileum) from our current analyses. On the other hand, resection of ICV, a site that is commonly involved in CD, was performed in 300 patients (76.1%), but this factor did not affect the nutritional status in our analysis (Tables 2 and 3).
The colon has important roles for water and electrolyte absorption. Also, it plays as regulator of stomach and small bowel function. In healthy individuals, peptide YY, which is secreted by colonocytes upon colonic contact of the small bowel contents, inhibits gastric emptying and transit of small bowel content.[30,31] Regardless of colonic resection, patients with an ileostomy have a similar situation to patients who undergo total colectomy. The functions of peptide YY are reduced after ileostomy formation or colectomy, with a consequent time reduction for the digestion and absorption of food. Furthermore, glucagon-like-peptide 2, which is secreted largely by colonocytes, have the trophic effects on the small bowel.[32,33] After colectomy or ileostomy, patients obtain lesser trophic effects of glucagon-like-peptide 2. In certain conditions (total colectomy or end-ileostomy), patients lose the trophic effects, almost completely. Also, they cannot use energy from the fermentation of unabsorbed carbohydrates to short-chain fatty acids by colonic bacteria.[34,35] However, in our present study, factors related to colon resection (combined colon resection, location of colon resection, and presence of remnant colon) had no significant effect on nutritional status. In a previous study, patients with an ileostomy tended to have a low BMI, lean body mass, bone mineral density, and urine volume, and some had sodium, calcium, and magnesium deficiencies.[36] Here, the risk of poor nutritional status was found to be 3 to 5 times higher for patients with CD with an ileostomy than those without an ileostomy (Tables 2 and 3).
The risk of poor nutritional status was also 4 to 5 times higher for patients with currently active CD than those without active CD, and the presence of active disease was the most dangerous factor affecting nutritional status. In our institute, the CDAI is an essential survey item during each outpatient visit. Depending on outpatient visit records, we confirmed the CDAI of all patients in our study. We divided the patients in 2 groups (active CD: CDAI ≥ 150; CD remission: CDAI < 150) and performed a risk analysis based on the CDAI (Tables 1–3). Because of the multifactorial characteristics of CD, the classification of active disease cannot be seen as absolute. However, we think there is sufficient evidence to dichotomize patients using the CDAI as the gold standard for assessment of disease activity.
Some limitations of this study must be addressed. The major limitation lies in the retrospective design of our analysis. A number of other factors will affect the nutritional status of patients with CD. In addition, considering the multifactorial characteristics of CD, there may be other factors affecting the patient nutritional status that we did not take into account. It is difficult to evaluate most of these factors in retrospective studies. Additional prospective studies are needed on the dietary factors (macronutrients and micronutrients), psychological components, medications, and quality of life for patients with CD. In particular, the nutritional status of patients with CD is closely associated with the quality of life and future studies therefore required to identify proper nutritional indicators in these patients. All surgeons in our institution measure the SBL during abdominal surgery in patients with CD. However, the measurement by a surgeon of the SBL by a sterile ruler is not standardized, and there may be some variations. However, all patients in our present study were operated on by the same group of surgeons, who used similar procedures in the measurement of SBL, likely making any variations mostly negligible.
In conclusion, the existence of currently active CD, the presence of ileostomy, and an r-SBL ≤ 230 cm are risk factors that affect poor nutritional status after SBR in patients with CD. These findings will aid intraoperative decisions regarding the range of bowel resection and preparations for the risk of nutritional imbalance in patients with CD.
Footnotes
Abbreviations: BMI = body mass index, CD = Crohn disease, CDAI = Crohn disease activity index, ICV = ileocecal valve, IF = intestinal failure, mNRI = modified nutritional risk index, NRI = nutritional risk index, OR = odds ratio, ROC = receiver operating characteristic, r-SBL = remnant small bowel length, SBL = small bowel length, SBR = small bowel resection, SBS = short bowel syndrome, t-SBL = total small bowel length.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Harper PH, Fazio VW, Lavery IC, et al. The long-term outcome in Crohn's disease. Dis Colon Rectum 1987; 30:174–179. [DOI] [PubMed] [Google Scholar]
- 2.Bernell O, Lapidus A, Hellers G. Risk factors for surgery and recurrence in 907 patients with primary ileocaecal Crohn's disease. Br J Surg 2000; 87:1697–1701. [DOI] [PubMed] [Google Scholar]
- 3.Post S, Herfarth C, Bohm E, et al. The impact of disease pattern, surgical management, and individual surgeons on the risk for relaparotomy for recurrent Crohn's disease. Ann Surg 1996; 223:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucendo AJ, De Rezende LC. Importance of nutrition in inflammatory bowel disease. World J Gastroenterol 2009; 15:2081–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Sullivan M, O’Morain C. Nutrition in inflammatory bowel disease. Best Pract Res Clin Gastroenterol 2006; 20:561–573. [DOI] [PubMed] [Google Scholar]
- 6.Filippi J, Al-Jaouni R, Wiroth JB, et al. Nutritional deficiencies in patients with Crohn's disease in remission. Inflamm Bowel Dis 2006; 12:185–191. [DOI] [PubMed] [Google Scholar]
- 7.Vaisman N, Dotan I, Halack A, et al. Malabsorption is a major contributor to underweight in Crohn's disease patients in remission. Nutrition 2006; 22:855–859. [DOI] [PubMed] [Google Scholar]
- 8.Hengstermann S, Valentini L, Schaper L, et al. Altered status of antioxidant vitamins and fatty acids in patients with inactive inflammatory bowel disease. Clin Nutr 2008; 27:571–578. [DOI] [PubMed] [Google Scholar]
- 9.Carter MJ, Lobo AJ, Travis SP, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2004; 53 suppl 5:V1–V16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson JS, DiBaise JK, Iyer KR, et al. Postoperative short bowel syndrome. J Am Coll Surg 2005; 201:85–89. [DOI] [PubMed] [Google Scholar]
- 11.Gouttebel MC, Saint-Aubert B, Astre C, et al. Total parenteral nutrition needs in different types of short bowel syndrome. Dig Dis Sci 1986; 31:718–723. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization Obesity: preventing, managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000; 894 : i-xii, 1-253. [PubMed] [Google Scholar]
- 13.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363:157–163. [DOI] [PubMed] [Google Scholar]
- 14.Buzby GP, Knox LS, Crosby LO, et al. Study protocol: a randomized clinical trial of total parenteral nutrition in malnourished surgical patients. Am J Clin Nutr 1988; 47 (suppl):366–381. [DOI] [PubMed] [Google Scholar]
- 15.Schneider SM, Hebuterne X. Use of nutritional scores to predict clinical outcomes in chronic diseases. Nutr Rev 2000; 58:31–38. [DOI] [PubMed] [Google Scholar]
- 16.Winship DH, Summers RW, Singleton JW, et al. National Cooperative Crohn's Disease Study: study design and conduct of the study. Gastroenterology 1979; 77:829–842. [PubMed] [Google Scholar]
- 17.Keller J, Panter H, Layer P. Management of the short bowel syndrome after extensive small bowel resection. Best Pract Res Clin Gastroenterol 2004; 18:977–992. [DOI] [PubMed] [Google Scholar]
- 18.Labossiere R, Bernard MA. Nutritional considerations in institutionalized elders. Curr Opin Clin Nutr Metab Care 2008; 11:1–6. [DOI] [PubMed] [Google Scholar]
- 19.Norman K, Pichard C, Lochs H, et al. Prognostic impact of disease-related malnutrition. Clin Nutr 2008; 27:5–15. [DOI] [PubMed] [Google Scholar]
- 20.Cereda E, Pedrolli C. The Geriatric Nutritional Risk Index. Curr Opin Clin Nutr Metab Care 2009; 12:1–7. [DOI] [PubMed] [Google Scholar]
- 21.Kondrup J, Allison SP, Elia M, et al. ESPEN guidelines for nutrition screening 2002. Clin Nutr 2003; 22:415–421. [DOI] [PubMed] [Google Scholar]
- 22.Russell CA, Elia M. Malnutrition in the UK: where does it begin? Proc Nutr Soc 2010; 69:465–469. [DOI] [PubMed] [Google Scholar]
- 23.Bouillanne O, Morineau G, Dupont C, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr 2005; 82:777–783. [DOI] [PubMed] [Google Scholar]
- 24.Jeejeebhoy KN. Management of short bowel syndrome: avoidance of total parenteral nutrition. Gastroenterology 2006; 130 suppl 1:S60–S66. [DOI] [PubMed] [Google Scholar]
- 25.Spencer AU, Neaga A, West B, et al. Pediatric short bowel syndrome: redefining predictors of success. Ann Surg 2005; 242:403–409.discussion 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crenn P, Vahedi K, Lavergne-Slove A, et al. Plasma citrulline: a marker of enterocyte mass in villous atrophy-associated small bowel disease. Gastroenterology 2003; 124:1210–1219. [DOI] [PubMed] [Google Scholar]
- 27.Jeppesen PB, Mortensen PB. Intestinal failure defined by measurements of intestinal energy and wet weight absorption. Gut 2000; 46:701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchman AL. Etiology and initial management of short bowel syndrome. Gastroenterology 2006; 130 suppl 1:S5–S15. [DOI] [PubMed] [Google Scholar]
- 29.Pironi L, Arends J, Baxter J, et al. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin Nutr 2015; 34:171–180. [DOI] [PubMed] [Google Scholar]
- 30.Allen JM, Fitzpatrick ML, Yeats JC, et al. Effects of peptide YY and neuropeptide Y on gastric emptying in man. Digestion 1984; 30:255–262. [DOI] [PubMed] [Google Scholar]
- 31.Nightingale JM, Kamm MA, van der Sijp JR, et al. Gastrointestinal hormones in short bowel syndrome. Peptide YY may be the “colonic brake” to gastric emptying. Gut 1996; 39:267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drucker DJ, Erlich P, Asa SL, et al. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci USA 1996; 93:7911–7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gregor M, Menge H, Stossel R, et al. Effect of monoclonal antibodies to enteroglucagon on ileal adaptation after proximal small bowel resection. Gut 1987; 28 (suppl):9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levitt MD. Malabsorption of starch: a normal phenomenon. Gastroenterology 1983; 85:769–770. [PubMed] [Google Scholar]
- 35.Mortensen PB, Clausen MR. Short-chain fatty acids in the human colon: relation to gastrointestinal health and disease. Scand J Gastroenterol Suppl 1996; 216:132–148. [DOI] [PubMed] [Google Scholar]
- 36.Ng DH, Pither CA, Wootton SA, et al. The “not so short-bowel syndrome”: potential health problems in patients with an ileostomy. Colorectal Dis 2013; 15:1154–1161. [DOI] [PubMed] [Google Scholar]


