Abstract
There are limited data regarding the efficacy of β-blockers for secondary prevention in patients with coronary chronic total occlusion (CTO). Therefore, we investigated the association of β-blocker therapy with long-term clinical outcomes in CTO patients. From March 2003 to February 2012, a total of 2024 CTO patients treated with either medical therapy alone or revascularization were enrolled in the study. We assessed 1596 patients with stable ischemic heart disease and divided them into the β-blocker group (n = 932) and the no-β-blocker group (n = 664). The primary outcome was all-cause death. The median follow-up duration was 3.9 (interquartile range: 2.0–6.2) years. All-cause death occurred in 11.6% patients in the β-blocker group and 13.6% patients in the no-β-blocker group (hazard ratio [HR]: 0.81, 95% confidence interval [CI]: 0.61–1.08; P = 0.15). In the propensity score-matched population (570 pairs), all-cause death occurred in 12.3% patients in the β-blocker group and 12.8% patients in the no-β-blocker group (HR: 0.93, 95% CI: 0.67–1.29; P = 0.66). In subgroup analysis, β-blocker therapy was associated with better outcome, in terms of all-cause death, in patients with CTO of the left anterior descending coronary artery and Synergy Between PCI with Taxus and Cardiac Surgery (SYNTAX) score ≥23 (P for interaction = 0.01 and 0.02, respectively). In conclusion, β-blocker therapy was not associated with favorable long-term clinical outcomes in stable CTO patients, regardless of treatment strategy. However, β-blocker therapy might be beneficial in a highly selective group of CTO patients with a high ischemic burden.
Keywords: β-blockers, chronic total occlusion, coronary artery bypass graft, coronary intervention, medication
1. Introduction
The prevalence of coronary chronic total occlusion (CTO) varies in the literature. According to a recent study, up to 20% of patients that undergo coronary angiography are diagnosed with CTO.[1] Although successful CTO revascularization has improved cardiovascular outcome and quality of life in recent observational studies, the absence of randomized trials has led to controversy.[2–5] Many CTO patients still rely on medical treatments such as statins, renin–angiotensin system blockers, and β-blockers even after successful revascularization. β-blocker treatment in patients with coronary artery disease (CAD) is a cornerstone of secondary prevention therapy, especially in acute myocardial infarction (MI).[6,7] Based on these approaches, β-blockers have been widely prescribed in stable CAD patients for secondary prevention as well as in patients after MI. However, several large-scale studies such as the Reduction of Atherothrombosis for Continued Health (REACH) registry suggested that, in stable CAD patients, the use of β-blockers was not associated with a lower risk of cardiovascular events.[8] To date, the influence of β-blocker therapy on long-term clinical outcomes in stable CAD remains unclear, and, in particular, the clinical impact of β-blocker therapy has not been evaluated in stable CTO patients, who exhibit a high atherosclerotic burden. Therefore, we investigated the association of β-blocker therapy with long-term clinical outcomes in stable CTO patients treated with either optimal medical therapy (OMT) alone or OMT with revascularization.
2. Methods
2.1. Study population
From March 2003 to February 2012, a total of 2024 consecutive patients with CTO were enrolled in the Samsung Medical Center CTO registry, a prospective single-center cohort. The inclusion criteria for this registry were: patients over 18 years old, 1 or more CTO vessels identified with diagnostic coronary angiography, and symptomatic angina and/or a positive result on functional ischemia study. Exclusion criteria for the registry were: a previous history of coronary artery bypass graft (CABG), cardiogenic shock or cardiopulmonary resuscitation at initial presentation, and ST-segment elevation MI during the preceding 48 hours. We selected the study population from among these patients, after applying additional exclusion criteria: in-hospital death (n = 18) and patients who initially presented with acute coronary syndrome (n = 410). Patient follow-up occurred in the out-patient clinic up to every 6 months, and the interval might be shortened according to the patient's condition. Baseline characteristics, angiographic and procedural findings, and clinical outcome data were collected prospectively using web-based reporting system by the research coordinators of the dedicated registry. Additional information was obtained by reviewing the medical records or by telephone contact, if necessary. The institutional review board of Samsung Medical Center approved this study.
2.2. Treatment strategy
All patients were taking 1 or more antianginal medications, including a long-acting β-blocker, calcium channel blocker and nitrate, alone or in combination, along with either an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker, and statins as standard secondary prevention. Each dose of all medications was based on heart rate, blood pressure, and symptoms. Duration of dual antiplatelet therapy was determined by each physician. The medication regimens of all patients were prescribed in the absence of reasonable contraindications and were considered optimal. Revascularization of CTO was performed by CABG or percutaneous coronary intervention (PCI) with drug-eluting stent, and selection for revascularization strategy was based on patient and physician preference. All preparation and techniques for revascularization were performed as previously described.[9,10]
2.3. Definitions and outcomes
The definition of a CTO lesion was an obstruction of a native coronary artery with a thrombolysis in myocardial infarction (TIMI) flow grade of 0 for an estimated duration of more than 3 months. The duration of coronary occlusion was determined by the interval from the last episode of acute coronary syndrome or, in patients without a history of acute coronary syndrome, from the first episode of effort angina consistent with the location of the occlusion or by previous coronary angiogram.[11,12] Successful PCI was defined as final residual stenosis less than 20% and TIMI flow grade ≥2 after drug-eluting stent implantation by visual estimation of angiograms.[11] Calculation and determination of the cutoff value of Synergy Between PCI with Taxus and Cardiac Surgery (SYNTAX) score and Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) score were based on previous publications.[13,14] The primary outcome was all-cause death during the follow-up period. The secondary outcomes were cardiac death, nonfatal MI, any coronary revascularization, and a composite of major adverse cardiac events (MACE) including cardiac death, nonfatal MI, or any coronary revascularization during the follow-up period. All deaths were considered cardiac death unless a definite noncardiac cause could be established. MI was defined as elevated cardiac enzyme level such as troponin I or MB fraction of creatine kinase greater than the upper limit of the normal range with ischemic symptoms or electrocardiographic changes, which implicated ischemia, that were irrelevant to the index procedure.[15] Any coronary revascularization was a composite of target vessel or nontarget vessel revascularization treated with PCI or CABG.
2.4. Statistical analysis
Continuous variables were presented as mean ± standard deviation and differences were assessed by independent t test or Wilcoxon rank sum test. Categorical variables were described as a number (n) with a percentage (%) and differences were analyzed by Pearson χ2 or Fisher exact test. The Cox proportional hazard model was used to compare the risks of composite adverse cardiac events and individual clinical outcomes between the β-blocker group and the no-β-blocker group. Cumulative incidence rates of MACE were estimated by the Kaplan–Meier method and compared by log-rank test. Propensity scores were estimated using multiple logistic-regression analysis. Full nonparsimonious models were developed and all variables included as covariates are listed in Table 1. Cox regression analysis using pairs matched by a greedy algorithm and the nearest available pair-matching method among patients with an individual propensity score was also performed to evaluate the reduction in outcome risk. The covariate balance achieved by matching was assessed by calculating the absolute standardized differences in covariates between the 2 groups. An absolute standardized difference <10.0% for the measured covariate suggests appropriate balance between the groups. The “psmatching” custom dialog was used in conjunction with SPSS version 20 (IBM, Armonk, NY). The psmatching program performs all analyses in R (R Foundation for Statistical Computing, Vienna, Austria) through the SPSS R-Plugin (version 2.12.1). All tests were 2-tailed, and P < 0.05 was considered statistically significant. All analyses were performed using SPSS version 20 for windows.
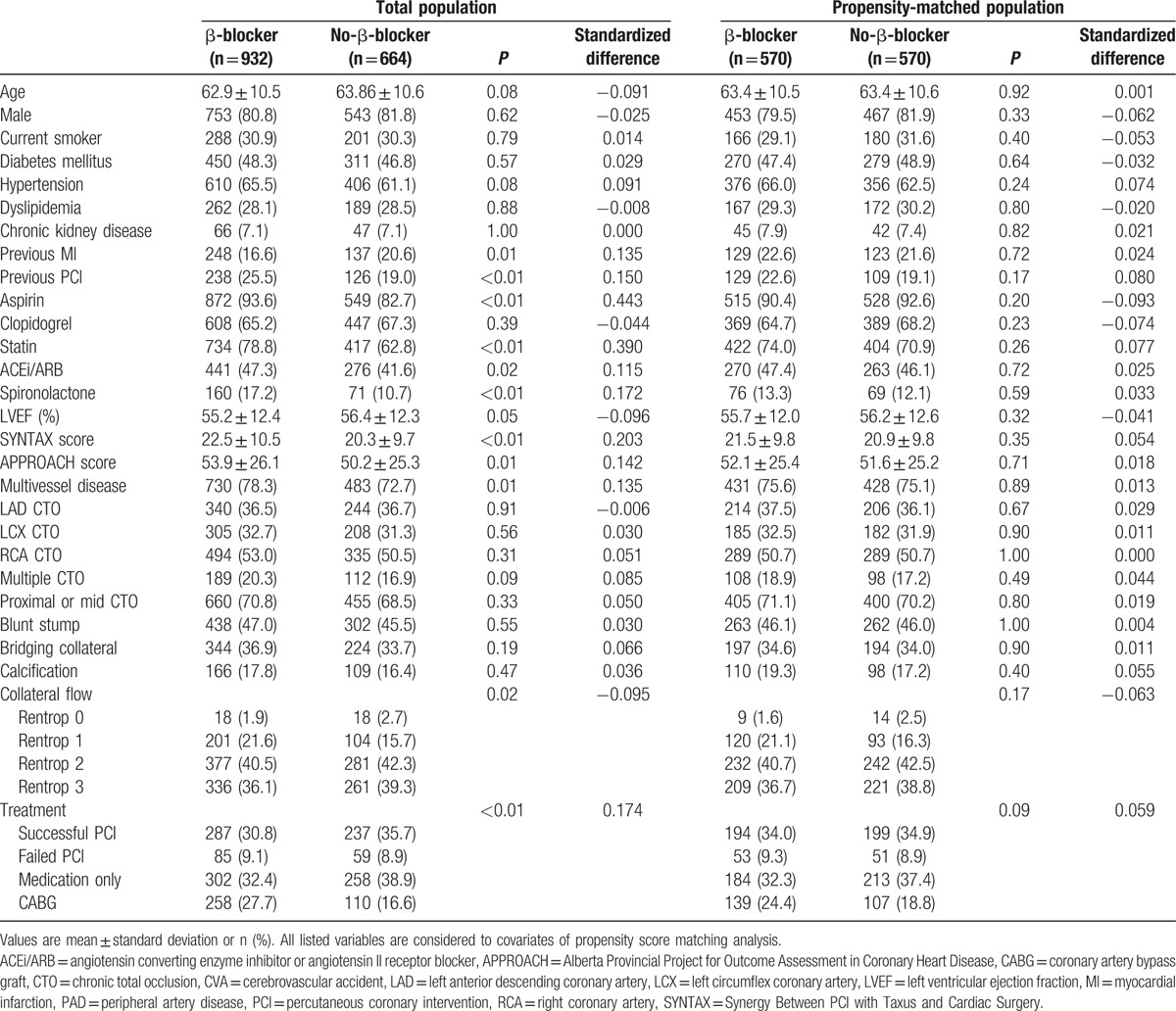
Table 1.
Baseline and angiographic characteristics.
3. Results
3.1. Baseline and angiographic characteristics
From the 2024 patients in the registry, a total of 1596 patients were enrolled (Fig. 1). CABG surgery and PCI were performed in 368 (23.1%) patients and 668 (41.9%) patients, respectively. Among the patients undergoing PCI, 144 (21.6%) patients failed revascularization and treated with OMT. Of the remaining patients, 560 (35.1%) were treated with OMT alone. The patients who completed the follow up at 6 months were 1509 (97%), and 1366 (91.1%) patients performed follow up at 12 months after enrollment. The study population was divided into the β-blocker group (n = 932) and the no-β-blocker group (n = 664) based on the selected discharge medication (Table 1). Median heart rate at the discharge was 69.0 (interquartile range [IQR]: 61.0–78.5) beat per minute in the β-blocker group and 72.0 (IQR: 64.0–81.0) beat per minute in the no-β-blocker group (P < 0.01). The prevalence of previous MI in the β-blocker group was higher than in the no-β-blocker group. The β-blocker group patients took a greater number of aspirin, statins, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and had a greater frequency of spironolactone prescription than the no-β-blocker group when they were enrolled in the registry. Left ventricular ejection fraction was lower in the β-blocker group compared to the no-β-blocker group. In angiographic findings, well-developed collateral flows were observed more in no-β-blocker group patients than in the β-blocker group. For propensity score-matched analysis, a total of 570 patient-pairs were matched. The c-statistic for the propensity score model was 0.71, and the matching estimated by overall balance test was adequate (Chi-square = 13.27, df = 29.00, and P = 0.99). There were no significant differences in the baseline and angiographic characteristics of the β-blocker and no-β-blocker groups in the propensity score-matched population.
Figure 1.
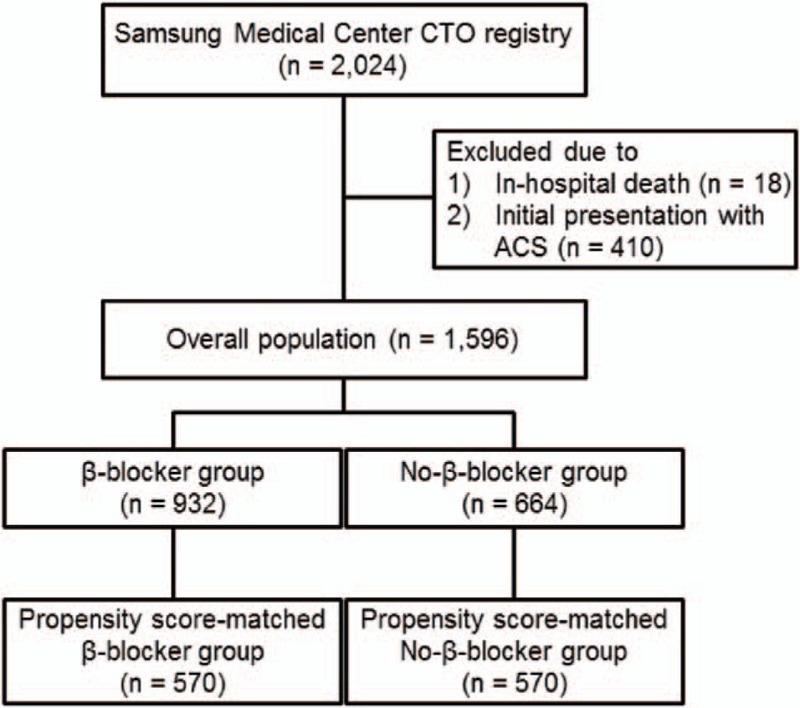
Study scheme. ACS = acute coronary syndrome, CTO = coronary chronic total occlusion.
3.2. Clinical outcomes
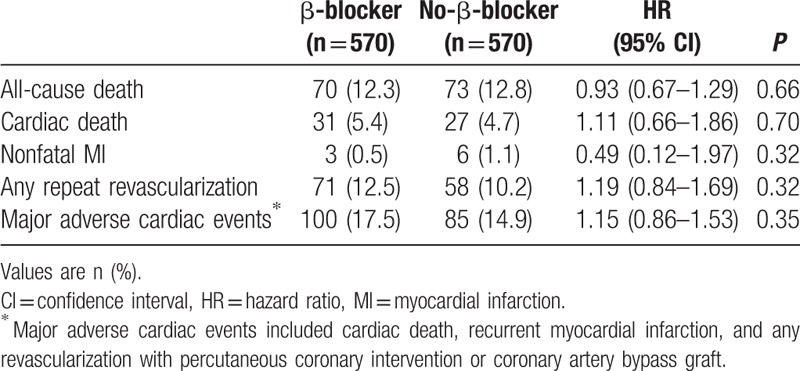
The median follow-up duration was 3.9 (IQR: 2.0–6.2) years. During the follow-up period, median heart rate measured at outpatient clinic was 73.0 (IQR: 66.0–82.0) beat per minute in the β-blocker group and 76.0 (IQR: 67.0–84.5) beat per minute in the no-β-blocker group. There were no significant differences in all-cause death (β-blocker group vs no-β-blocker group: 11.6% vs 13.6%, unadjusted hazard ratio [HR]: 0.81, 95% confidence interval [CI]: 0.61–1.08; P = 0.15) (Table 2 and Fig. 2A). After 1:1 propensity score-matched analysis, all-cause death during a median follow-up duration of 3.9 years in the matched patients was not significantly different between the 2 groups (12.3% vs 12.8%, HR: 0.93, 95% CI: 0.67–1.29; P = 0.66) (Table 3 and Fig. 2B). In addition, there were no differences between the 2 groups in the rates of cardiac death, nonfatal MI, any coronary revascularization, or MACE.
Table 2.
Clinical outcomes of β-blocker group compared with no-β-blocker group in total population during the follow-up period.
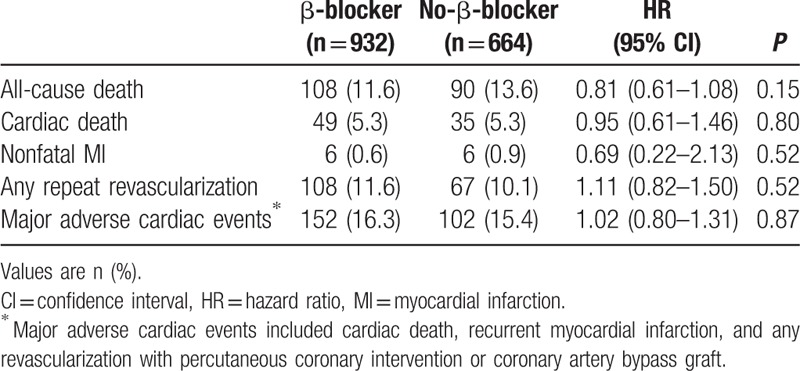
Figure 2.
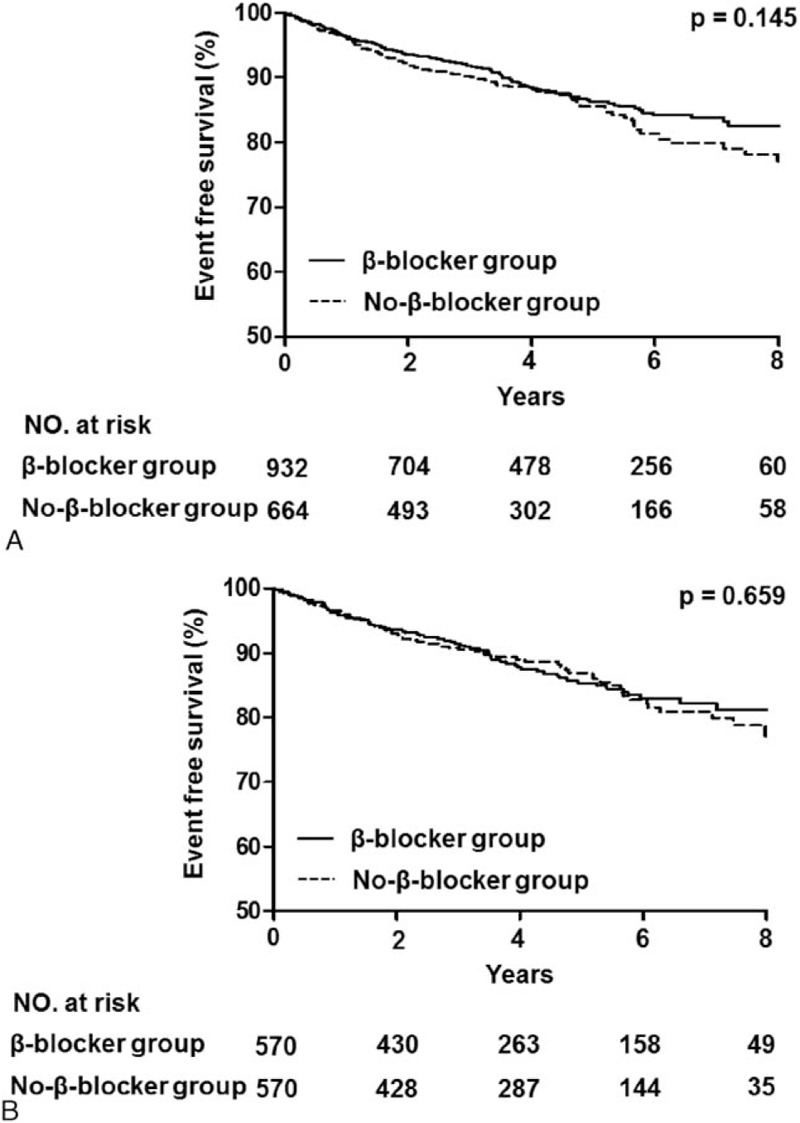
Kaplan–Meier curves of the β-blocker versus no-β-blocker groups. (A) Kaplan–Meier curves for all-cause death in the β-blocker group (solid line) versus the no-β-blocker group (dashed line). (B) Kaplan–Meier curves for all-cause death in the β-blocker group (solid line) versus the no-β-blocker group (dashed line) in the propensity score-matched population.
Table 3.
Clinical outcomes of β-blocker group compared with no-β-blocker group in a propensity score-matched population during follow-up period.
3.3. Subgroup analysis
We analyzed the benefit of β-blocker therapy in various complex subgroups (Fig. 3). Compared with the non-β-blocker group in terms of all-cause death, the outcomes in the β-blockers group were significantly better among patients with CTO of the left anterior descending (LAD) coronary artery and a SYNTAX score ≥23 (P for interaction = 0.01 and 0.02, respectively). In addition, β-blocker therapy showed a trend toward improvement in all-cause death in patients with an APPROACH score ≥60 (P for interaction = 0.05).
Figure 3.
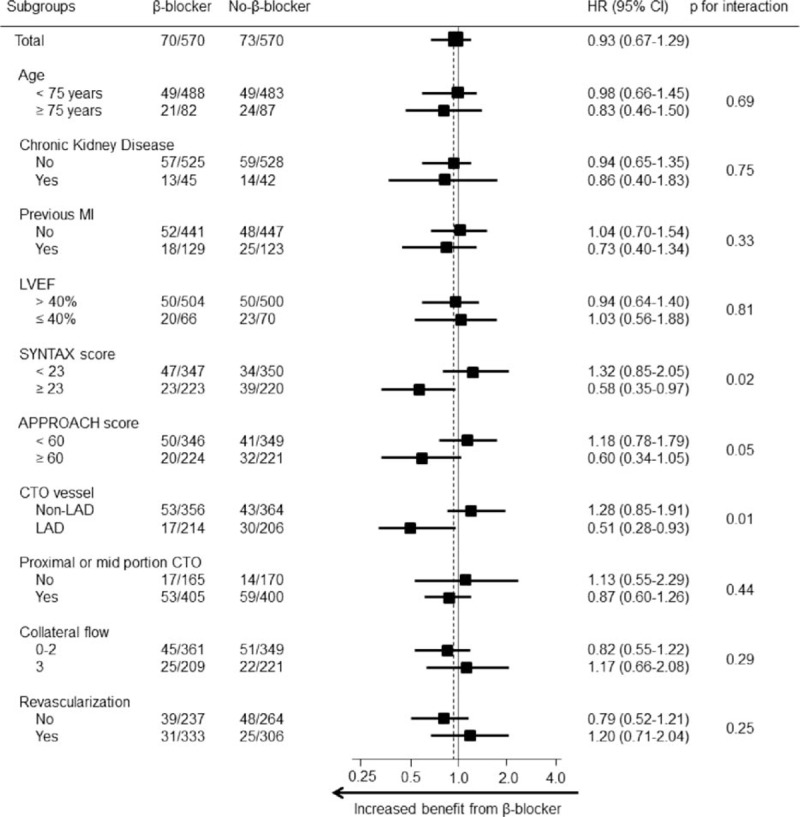
Comparative unadjusted hazard ratios of all-cause death between the β-blocker group and no-β-blocker group for each subgroup in the propensity score-matched population. ∗Higher risk for CHD means patients who had either prior myocardial infarction or left ventricular ejection fraction ≤40%. APPROACH = Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease, CHD = coronary heart disease, CI = confidence interval, CTO = chronic total occlusion, HR = hazard ratio, LAD = left anterior descending coronary artery, LVEF = left ventricular ejection fraction, MI = myocardial infarction, SYNTAX = Synergy Between PCI with Taxus and Cardiac Surgery.
4. Discussion
This is the first study to investigate the long-term clinical impacts of β-blockers on adverse cardiovascular events in stable CTO patients treated with either OMT after revascularization or OMT alone. The major findings of this study were as follows: β-blocker therapy did not reduce all-cause death compared with no-β-blocker therapy during the follow-up period, and the results were consistent after propensity score-matched analysis, β-blocker therapy was not associated with lower cardiac death, nonfatal MI, repeat coronary revascularization, or MACE in the total and the propensity score-matched population, compared with the non-β-blocker group in terms of all-cause death, outcomes after β-blocker therapy were significantly better among CTO patients with a high ischemic burden, such as those with CTO of the LAD, a high SYNTAX score or a high APPROACH score, as identified on subgroup analysis.
Many studies have discussed the benefits of β-blockers for reducing life-threatening arrhythmias, recurrent ischemia, and cardiac mortality in patients that suffer from MI.[16–19] Based on the evidence, recent American Heart Association and American College of Cardiology Foundation (AHA/ACCF) guidelines recommend β-blocker therapy for up to 3 years for secondary prevention in all patients after MI or ACS (Class I).[20,21] According to these guidelines, β-blocker therapy may be considered for all patients with coronary or other vascular disease (Class IIb).[20,21] The recent European Society of Cardiology guidelines recommended β-blockers as a first-line treatment in stable CAD patients to control heart rate and symptoms (Class I); however, there is no evidence to support β-blocker therapy for event prevention.[22] To date, there have been no well-designed randomized controlled trials that supported the effect of β-blockers on mortality or adverse cardiac events in stable CAD. Furthermore, previous studies from large-scale registries that investigated the efficacy of β-blocker therapy in stable CAD patients have shown mixed results.[8,23,24]
Recently, several large cohort analyses used a propensity score-matching system to adjust for the limitations of a nonrandomized study. The REACH registry showed that the use of β-blockers was not associated with a lower risk of composite cardiovascular events in either CAD patients with prior MI or without prior MI.[8] In the prior MI cohort from the REACH registry, a composite of cardiovascular death, nonfatal MI, and nonfatal stroke was numerically lower in the β-blocker group, but was not significantly different to that of the no-β-blocker group. The absolute difference in the event rate between the 2 groups in the prior MI cohort (1.67%) was higher than that in the CAD without MI cohort (0.61%). Similarly, in post hoc analysis from the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial, using β-blockers in patients with prior MI, but not heart failure was associated with better clinical outcomes; the results were driven mainly by a reduction in recurrent MI, and the use of β-blockers was not associated with lower cardiovascular events in patients without MI.[23] In the present study, we focused on a study population of CTO patients that presented with stable angina or silent ischemia, with the exception of acute coronary syndrome. Many clinicians recommend medical therapy alone for treating CTO based on the theoretical protective effect of distal collateral circulation. In particular, OMT such as β-blocker therapy in CTO patients is very important because a substantial portion of CTO patients are unsuitable for PCI due to blunt stump, heavy calcification, tortuosity, or long occlusion length, which are associated with a low probability of PCI success.[25,26] Therefore, we investigated the association of β-blocker therapy at discharge with long-term clinical outcomes in stable CTO patients treated with or without revascularization, and we demonstrated that β-blocker therapy at discharge was not associated with improved all-cause mortality in this setting.
In this study, subgroup analysis showed the positive effects of β-blocker therapy on all-cause death in patients with a high ischemic burden, such as those with CTO of the LAD and a high SYNTAX or APPROACH score. According to the SYNTAX score algorithm, the segment weighing score of the CTO lesion at the LAD ranges from 5 to 17.5, and the score increases compared to that of CTO at non-LAD locations with additional adverse lesion characteristics, such as multivessel involvement and bifurcation lesions.[13] Although SYNTAX score focuses on the anatomical complexity of the coronary vasculature, it is possible to presume that a high SYNTAX score of CTO patients also signifies a high ischemic burden. Moreover, according to the autopsy study providing the basis of APPROACH lesion scoring, 41% of the entire ventricular myocardium is supplied by the LAD.[27] The APPROACH score is calculated from the summation of all jeopardized territories, and a higher score is associated with a large amount of myocardium at risk. Based on the evidence from previous studies and the results of subgroup analysis in our study, we suggest that the beneficial effects of β-blocker therapy are confined to a highly selective group of CTO patients with high ischemic burden, as represented by CTO of the LAD and a high SYNTAX or APPROACH score.
4.1. Limitations
Our study had several limitations. First, the study lacked data on specific β-blockers and doses. Additionally, we do not know how long β-blockers were continued after discharge. However, when we compared heart rate measured at discharge with which measured at follow up, the patients in β-blockers group had consistently lower heart rate than those in no-β-blockers group. Therefore, we could infer the effect of β-blockers treatment was sustained during follow-up period. Second, the study design was nonrandomized, retrospective, and observational, which may have affected the results due to confounding factors. Patients with higher risk for cardiovascular events may have tendency to be prescribed β-blockers and also have better other pharmacological treatment. Such heterogeneity between 2 groups may effect on the study results, despite we performed propensity score-matching analysis to adjust for these potential confounding factors, because we were not able to correct for unmeasured variables. Third, this study is an underpowered study due to relatively small sample size. Fourth, we have no information on accurate documentation of variability or ischemic burden in the territory supplied by the CTO. Regardless of these limitations, we tried to assess the association between β-blocker therapy and long-term clinical outcomes in patients with stable CTO and the results may support an appropriate management plan for some selected patients.
5. Conclusion
β-blocker therapy did not reduce all-cause mortality in stable CTO patients treated with either revascularization with OMT or OMT alone. However, in patient with a high ischemic burden, β-blocker therapy appears to be associated with improved long-term survival. Further large-scale, prospective, randomized, controlled trials are needed to clarify the effects of long-term β-blocker therapy in this setting.
Acknowledgment
We would like to thank Seonwoo Kim, PhD, and Joonghyun Ahn, MS, from the Samsung Biomedical Research Institute for their excellent statistical support.
Footnotes
Abbreviations: AHA/ACCF = American Heart Association and American College of Cardiology Foundation, APPROACH = Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease, CABG = coronary artery bypass graft, CAD = coronary artery disease, CHARISMA = the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance trial, CI = confidence interval, CTO = coronary chronic total occlusion, HR = hazard ratio, LAD = left anterior descending coronary artery, MACE = major adverse cardiac events, MI = myocardial infarction, OMT = optimal medical therapy, PCI = percutaneous coronary intervention, REACH = the Reduction of Atherothrombosis for Continued Health registry, SYNTAX = Synergy Between PCI with Taxus and Cardiac Surgery, TIMI = thrombolysis in myocardial infarction.
JKH and JHY contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- 1.Fefer P, Knudtson ML, Cheema AN, et al. Current perspectives on coronary chronic total occlusions: the Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol 2012; 59:991–997. [DOI] [PubMed] [Google Scholar]
- 2.George S, Cockburn J, Clayton TC, et al. Long-term follow-up of elective chronic total coronary occlusion angioplasty: analysis from the U.K. Central Cardiac Audit Database. J Am Coll Cardiol 2014; 64:235–243. [DOI] [PubMed] [Google Scholar]
- 3.Safley DM, Grantham JA, Hatch J, et al. Quality of life benefits of percutaneous coronary intervention for chronic occlusions. Catheter Cardiovasc Interv 2014; 84:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wijeysundera HC, Norris C, Fefer P, et al. Relationship between initial treatment strategy and quality of life in patients with coronary chronic total occlusions. EuroIntervention 2014; 9:1165–1172. [DOI] [PubMed] [Google Scholar]
- 5.Di Serafino L, Borgia F, Maeremans J, et al. The age, creatinine, and ejection fraction score to risk stratify patients who underwent percutaneous coronary intervention of coronary chronic total occlusion. Am J Cardiol 2014; 114:1158–1164. [DOI] [PubMed] [Google Scholar]
- 6.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001; 357:1385–1390. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Radford MJ, Wang Y, et al. Are beta-blockers effective in elderly patients who undergo coronary revascularization after acute myocardial infarction? Arch Intern Med 2000; 160:947–952. [DOI] [PubMed] [Google Scholar]
- 8.Bangalore S, Steg G, Deedwania P, et al. beta-Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA 2012; 308:1340–1349. [DOI] [PubMed] [Google Scholar]
- 9.Kim BS, Yang JH, Jang WJ, et al. Clinical outcomes of multiple chronic total occlusions in coronary arteries according to three therapeutic strategies: bypass surgery, percutaneous intervention and medication. Int J Cardiol 2015; 197:2–7. [DOI] [PubMed] [Google Scholar]
- 10.Jang WJ, Yang JH, Choi SH, et al. Long-term survival benefit of revascularization compared with medical therapy in patients with coronary chronic total occlusion and well-developed collateral circulation. JACC Cardiovasc Interv 2015; 8:271–279. [DOI] [PubMed] [Google Scholar]
- 11.Godino C, Bassanelli G, Economou FI, et al. Predictors of cardiac death in patients with coronary chronic total occlusion not revascularized by PCI. Int J Cardiol 2013; 168:1402–1409. [DOI] [PubMed] [Google Scholar]
- 12.Valenti R, Vergara R, Migliorini A, et al. Predictors of reocclusion after successful drug-eluting stent-supported percutaneous coronary intervention of chronic total occlusion. J Am Coll Cardiol 2013; 61:545–550. [DOI] [PubMed] [Google Scholar]
- 13.Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005; 1:219–227. [PubMed] [Google Scholar]
- 14.Graham MM, Faris PD, Ghali WA, et al. Validation of three myocardial jeopardy scores in a population-based cardiac catheterization cohort. Am Heart J 2001; 142:254–261. [DOI] [PubMed] [Google Scholar]
- 15.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J 2012; 33:2551–2567. [DOI] [PubMed] [Google Scholar]
- 16.Kontos MC, Diercks DB, Ho PM, et al. Treatment and outcomes in patients with myocardial infarction treated with acute beta-blocker therapy: results from the American College of Cardiology's NCDR(®). Am Heart J 2011; 161:864–870. [DOI] [PubMed] [Google Scholar]
- 17.Randomised trial of intravenous atenolol among 16 027 cases of suspected acute myocardial infarction: ISIS-1. First International Study of Infarct Survival Collaborative Group. Lancet 1986; 2:57–66. [PubMed] [Google Scholar]
- 18.Freemantle N, Cleland J, Young P, et al. beta Blockade after myocardial infarction: systematic review and meta regression analysis. BMJ 1999; 318:1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen ZM, Pan HC, Chen YP, et al. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1622–1632. [DOI] [PubMed] [Google Scholar]
- 20.Smith SC, Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation 2011; 124:2458–2473. [DOI] [PubMed] [Google Scholar]
- 21.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2012; 60:e44–e164. [DOI] [PubMed] [Google Scholar]
- 22.Montalescot G, Sechtem U, et al. Task Force Members. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013; 34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 23.Bangalore S, Bhatt DL, Steg PG, et al. beta-Blockers and cardiovascular events in patients with and without myocardial infarction: post hoc analysis from the CHARISMA trial. Circ Cardiovasc Qual Outcomes 2014; 7:872–881. [DOI] [PubMed] [Google Scholar]
- 24.Bauters C, Lemesle G, Meurice T, et al. Prognostic impact of β-blocker use in patients with stable coronary artery disease. Heart 2014; 100:1757–1761. [DOI] [PubMed] [Google Scholar]
- 25.Puma JA, Sketch MH, Jr, Tcheng JE, et al. Percutaneous revascularization of chronic coronary occlusions: an overview. J Am Coll Cardiol 1995; 26:1–11. [DOI] [PubMed] [Google Scholar]
- 26.Carlino M, Magri CJ, Uretsky BF, et al. Treatment of the chronic total occlusion: a call to action for the interventional community. Catheter Cardiovasc Interv 2014. [DOI] [PubMed] [Google Scholar]
- 27.Kalbfleisch H, Hort W. Quantitative study on the size of coronary artery supplying areas postmortem. Am Heart J 1977; 94:183–188. [DOI] [PubMed] [Google Scholar]


