Supplemental Digital Content is available in the text
Keywords: CVID, gamma delta T cells, immune activation, IVIg, PD-1, Vδ1, Vδ2
Abstract
Common variable immunodeficiency (CVID) is defined by low levels of IgG and IgA, but perturbations in T cells are also commonly found. However, there is limited information on γδ T cells in CVID patients. Newly diagnosed CVID patients (n = 15) were enrolled before and after intravenous IgG (IVIg) replacement therapy. Cryopreserved peripheral blood mononuclear cells were then used to study γδ T cells and CVID patients were compared to healthy controls (n = 22). The frequency and absolute count of Vδ1 γδ T cells was found to be increased in CVID (median 0.60% vs 2.64%, P <0.01 and 7.5 vs 39, P <0.01 respectively), while they were decreased for Vδ2 γδ T cells (median, 2.36% vs 0.74%, P <0.01 and 37.8 vs 13.9, P <0.01 respectively) resulting in an inversion of the Vδ1 to Vδ2 ratio (0.24 vs 1.4, P <0.001). Markers of immune activation were elevated on all subsets of γδ T cells, and HLA-DR expression was associated with an expansion of Vδ1 γδ T cells (r = 0.73, P = 0.003). Elevated PD-1 expression was found only on Vδ2 γδ T cells (median 1.15% vs 3.08%, P <0.001) and was associated with the decrease of Vδ2 γδ T cells (r = −0.67, P = 0.007). IVIg had no effect on the frequency of Vδ1 and Vδ2 γδ T cells or HLA-DR expression, but alleviated CD38 expression on Vδ1 γδ T cells (median MFI 965 vs 736, P <0.05). These findings suggest that immunological perturbations of γδ T cells are a general feature associated with CVID and are only partially reversed by IVIg therapy.
1. Introduction
Common variable immunodeficiency (CVID) is characterized by low levels of IgG and IgA and/or IgM (2 standard deviations below the normal level) and by an impaired antibody response to vaccination.[1] While some genetic mutations causing CVID have been identified, it is thought to be a polygenic disease with multiple susceptibility loci involved.[2] The poor antibody response in CVID patients results in recurrent infections of the respiratory and gastrointestinal tracts. CVID patients also have an increased incidence of some forms of cancer and autoimmunity,[3,4] probably due to the chronic state of immune activation and loss of key regulatory cells.[5–8] Treatment for CVID consists of IgG replacement that can be given subcutaneous or intravenously (IVIg). Replacement therapy significantly reduces the number of infections[9] and corrects some immune abnormalities,[10] while other perturbations are only partially restored or unchanged.[6]
In humans, γδ T cells represent between 0.5% and 16% of T cells in the blood with an average of approximately 4%.[11] The major subsets are designated Vδ1 and Vδ2, the latter being the most abundant. Similar to αβ T cells, γδ T cells develop in the thymus and undergo gene rearrangement, but they are not subjected to positive selection.[12] They can produce similar cytokines to conventional αβ T cells, including IL-17,[13] and can also have cytotoxic functions. Following infection, γδ T cells are activated earlier than αβ T cells and are involved in the initiation of the inflammatory response.[11] The antigens recognized by γδ T cells are very diverse and include phosphoantigens, peptides, and glycolipids.[11,14] The γδ TCR can also recognize stress-induced ligands. For instance, a Vγ4Vδ5 T cell clone has been shown to recognize EPCR expression on tumor cell and CMV-infected cells.[15]
HIV infection leads to a profound perturbation of the γδ T cells population,[16] characterized by an early expansion of the Vδ1 T cells, while Vδ2 T cells are greatly reduced in number.[17] Vδ2 T cells from HIV patients were also found to have a lower capacity to respond to phosphoantigens.[18] The expansion of Vδ1 T cells has been associated with increased microbial translocation,[19] now recognized as a major contributor of chronic immune activation in HIV infection.[20] CVID patients share several immunological features with HIV patients, namely CD4+ and CD8+ T cell activation, loss of iNKT cells and of regulatory T cells.[5,6] In contrast to CVID, HIV is characterized by hypergammaglobulinemia, but HIV disease progression is characterized by loss of memory B cell subsets and impaired antibody response to vaccination.[21,22] Increased microbial translocation has also been suggested to occur in CVID.[23] Two case report studies have shown an increase in γδ T cells in 1 CVID patient each[24,25]; however, no γδ T cells study has been performed on a larger cohort of CVID patients. We hypothesized that chronic immune activation in CVID patients would lead to perturbations in γδ T cells populations. Thus, we evaluated the frequency and phenotype of γδ T cells in a cohort of CVID patients.
2. Materials and methods
2.1. Study cohort and samples
Fifteen CVID patients (10 females and 5 males aged 6–51, average 34) from the Primary Immunodeficiency Outpatient Clinic of Clinical Immunology and Allergy Division of HC-FMUSP, fulfilling the PAGID/ESID criteria (1999) for CVID diagnosis and 22 healthy controls (14 females, 8 males aged 25–45, average 36) were enrolled in the study. Group size was based on sample availability. All patients were HIV negative. The study was approved by the Hospital das Clínicas, University of São Paulo Medical School Ethics Committee (CAPPesq), and written informed consent was provided by all participants or their legal guardians. Peripheral blood mononuclear cells (PBMC) were isolated by density-gradient sedimentation using Ficoll-Paque (Lymphoprep; Nycomed Pharma, Oslo, Norway). Isolated PBMC were washed twice in Hank balanced salt solution (Gibco, Grand Island, NY), and cryopreserved in RPMI 1640 (Gibco), supplemented with 20% heat inactivated fetal bovine serum (FBS; Hyclone Laboratories, Logan, UT), 50 U/mL of penicillin (Gibco), 50 μg/mL of streptomycin (Gibco), 10 mM glutamine (Gibco) and 7.5% dimethylsulphoxide (DMSO; Sigma, St Louis, MO). Cryopreserved PBMC from all subjects were stored in liquid nitrogen until used in the assays. For CVID patients, samples were collected before and after initiation of IVIg treatment.
2.2. Flow cytometry and mAbs
Cryopreserved specimens were thawed, washed, and counts and viability were assessed using the Countless Automated Cell Counter system (Invitrogen, Carlsbad, CA). PBMC were washed and stained in Brilliant Violet Stain Buffer (BD Biosciences, San Jose, CA) at room temperature for 15 min in 96-well V-bottom plates in the dark. Samples were then washed and fixed using Cytofix/Cytoperm (BD Biosciences) before flow cytometry data acquisition. mAbs used in flow cytometry; CD3 AF700 (clone UCHT1), CD4 PE-CF594 (clone RPA-T4), CD8 BV711 (clone RPA-T8), CD38 APC-H7 (clone HB7), CD161 BV421 (clone DX12), HLA-DR APC (clone L243), TCR γδ BV650 (clone B1), and PD-1 PE-Cy7 (clone EH12.1) were all from BD Biosciences, TCR Vδ1 FITC (clone TS8.2) was from Abcam (Cambridge, MA), and TCR Vδ2 PE (clone B6) was from Biolegend (San Diego, CA). Live/dead aqua fixable cell stain was from Life Technologies (Eugene, OR). All antibodies were used together in 1 panel. A minimum number of 200 events were recorded for all subsets of γδ T cells. Data were acquired on a BD LSRFortessa instrument (BD Biosciences) and analyzed using FlowJo Version 9.8.5 software (TreeStar, Ashland, OR).
2.3. Statistical analysis
All statistical analyses were performed using Graph Pad Prism version 6.0f for Mac OSX (GraphPad Software, La Jolla, CA). The comparison between healthy controls and CVID patients was analyzed using Mann–Whitney U test and changes after IVIg initiation in CVID patients were analyzed with Wilcoxon matched-pairs signed rank test. Nonparametric analyses were performed because the data were not normally distributed. Associations between groups were determined by Spearman rank correlation. P values <0.05 were considered statistically significant.
3. Results
3.1. Expansion of Vδ1+ and loss of Vδ2+ in CVID
Table 1 shows the demographic details of the subjects included in this study. We used cryopreserved PBMCs samples from a treatment naive cohort of CVID patients followed 9 to 15 months after initiation of monthly intravenous immunoglobulin replacement therapy (400–600 mg/k) to study γδ T cells by flow cytometry. The viability of the thawed PBMC was over 95% and there was no difference in the viability between the groups. First, we evaluated the frequency of the Vδ1+, Vδ2+, and Vδ1− Vδ2− γδ+ subsets in healthy controls and in treatment naive CVID patients. In healthy controls, Vδ1+ cells typically represented less than 1% of the T cells and Vδ2+ represented the major subset of γδ T cells (Supplementary file 1). Supplementary file 2 summarized all comparisons between CVID patients and healthy controls. In CVID patients Vδ1+ were significantly increased (median 0.60% vs 2.64%, P <0.01) while Vδ2+ were reduced (median, 2.36% vs 0.74%, P <0.01) compared with healthy controls (Fig. 1A). The frequency of the Vδ1− Vδ2− γδ+ subsets was also increased in CVID patients (Fig. 1A, median 0.39% vs 0.59%, P <0.05). Overall, this led to a significant increase in the ratio of Vδ1+ to Vδ2+ (Fig. 1B, 0.24 vs 1.4, P <0.001). Importantly, the frequency of all subsets of γδ T cells was not changed after IgG replacement therapy (median 2.64% vs 5.25%, P = 0.17; 0.74% vs 1.33% P = 0.58; 0.59% vs 0.60%, P = 0.21 for Vδ1+, Vδ2+, and Vδ1− Vδ2− γδ+ T cell respectively).
Table 1.
Subjects demographics.
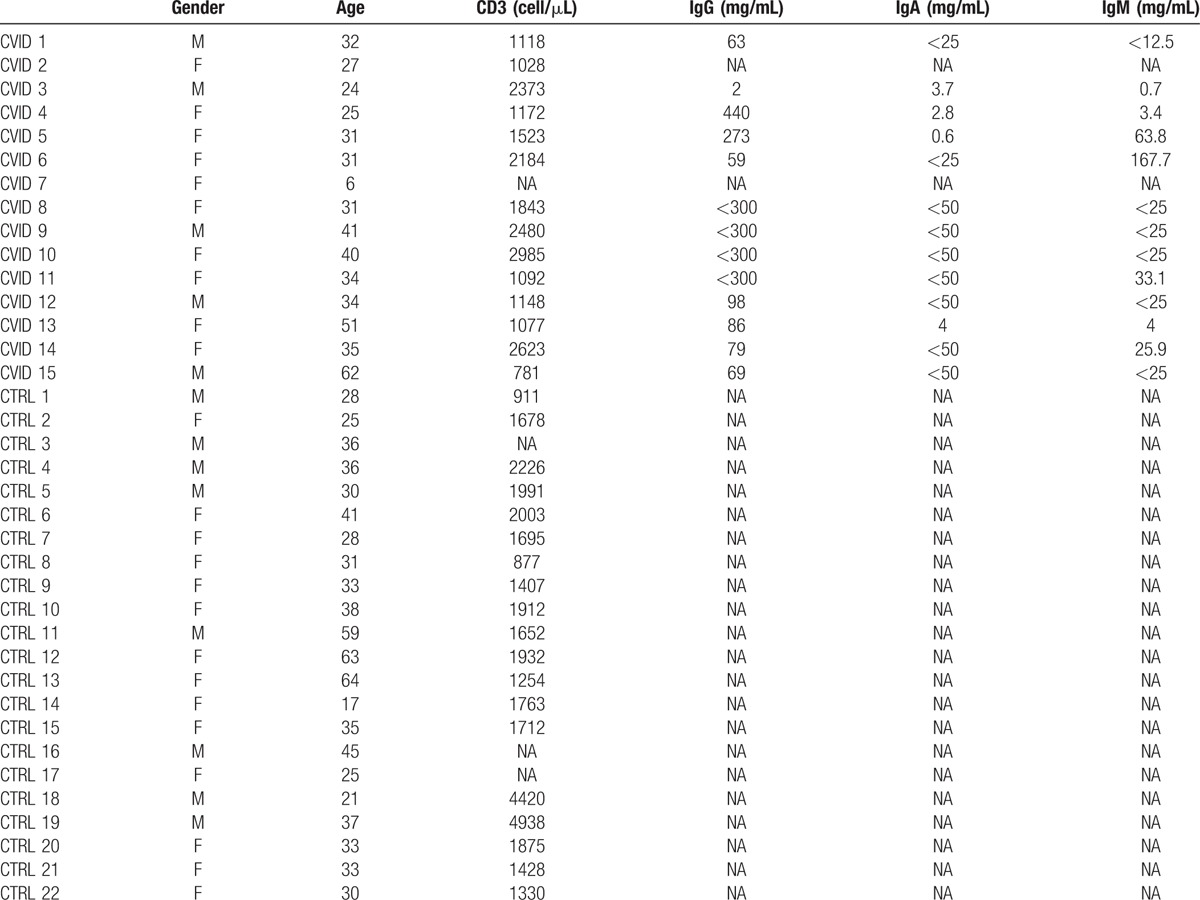
Figure 1.
Inverted Vδ1+/Vδ2+ ratio in CVID. Frequency of Vδ1+, Vδ2+, Vδ1− Vδ2− γδ+ T cells (A), Vδ1+/Vδ2+ ratio (B), and absolute count of Vδ1+, Vδ2+, and Vδ1− Vδ2− γδ+ T cells (C) in healthy controls, treatment naive, and IVIg-treated CVID patients. ∗ indicates P <0.05, the lines and whiskers represent the median and interquartile range respectively. CVID = common variable immunod, IVIg = intravenous immunoglobulin.
Next, we compared the absolute count of each subset of γδ T cells in CVID patients to those observed in healthy controls. Again, Vδ1+ were increased (median 7.5 vs 39, P <0.01) and Vδ2+ were decreased (median 37.8 vs 13.9, P <0.01; Fig. 1B). However, the absolute count of Vδ1− Vδ2− γδ+ was normal in CVID patients (median 7.3 vs 9.5, P = 0.69; Fig. 1B). The absolute count of all subsets of γδ T cells was not affected by IVIg (median, 39 vs 78.3 P = 0.81; 13.9 vs 23.9, P = 0.58; 9.5 vs 7.9 P = 0.69 for Vδ1+, Vδ2+, and Vδ1− Vδ2− γδ+ T cell respectively). Thus, our results suggest a specific expansion of Vδ1+ and loss of Vδ2+ subsets in CVID.
3.2. Differential redistribution of CD4+ and CD8+ on γδ T cells in CVID
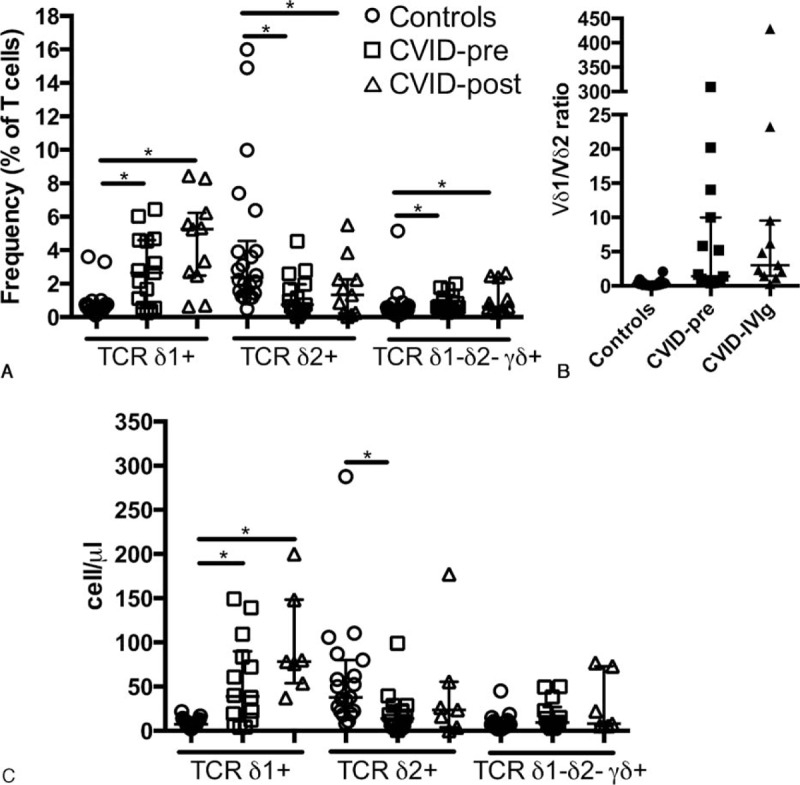
We evaluated the distribution of CD4 and CD8 expression on γδ T cells for each of the γδ T cells subsets individually. As expected, the major population for all subsets of γδ T cells was double negative (DN). CD4 expression was reduced for Vδ1+ and Vδ1− Vδ2− γδ+ T cells in CVID (median 11.6% vs 1.74%, P <0.01; 27.5% vs 8.04%, P <0.001 respectively; Fig. 2A). Vδ1+ presented an increased DN population while the CD8 population was increased for Vδ1− Vδ2− γδ+ (median 45.5% vs 64.4%, P <0.01; 29.6% vs 32.4%, P <0.05; Fig. 2B and C). On the other hand, CD4 and CD8 expression was normal on Vδ2+ cells (median 2.62% vs 3.12%, P = 0.96; 29.6% vs 32.4%, P = 0.4 respectively). Expression of CD4 and CD8 on all subsets of γδ T cells was not changed after IVIg. Thus, our results show that γδ T cells subsets in CVID have differential distribution of CD4 and CD8 expression.
Figure 2.
Altered CD4 and CD8 expression on γδ T cells in CVID. Proportion of γδ+ T cells expressing CD4 (A), CD8 (B), or DN (C) in healthy controls, treatment naive, and IVIg-treated CVID patients. ∗ indicates P <0.05, the lines and whiskers represent the median and interquartile range respectively.
3.3. High levels of γδ T cells activation in CVID
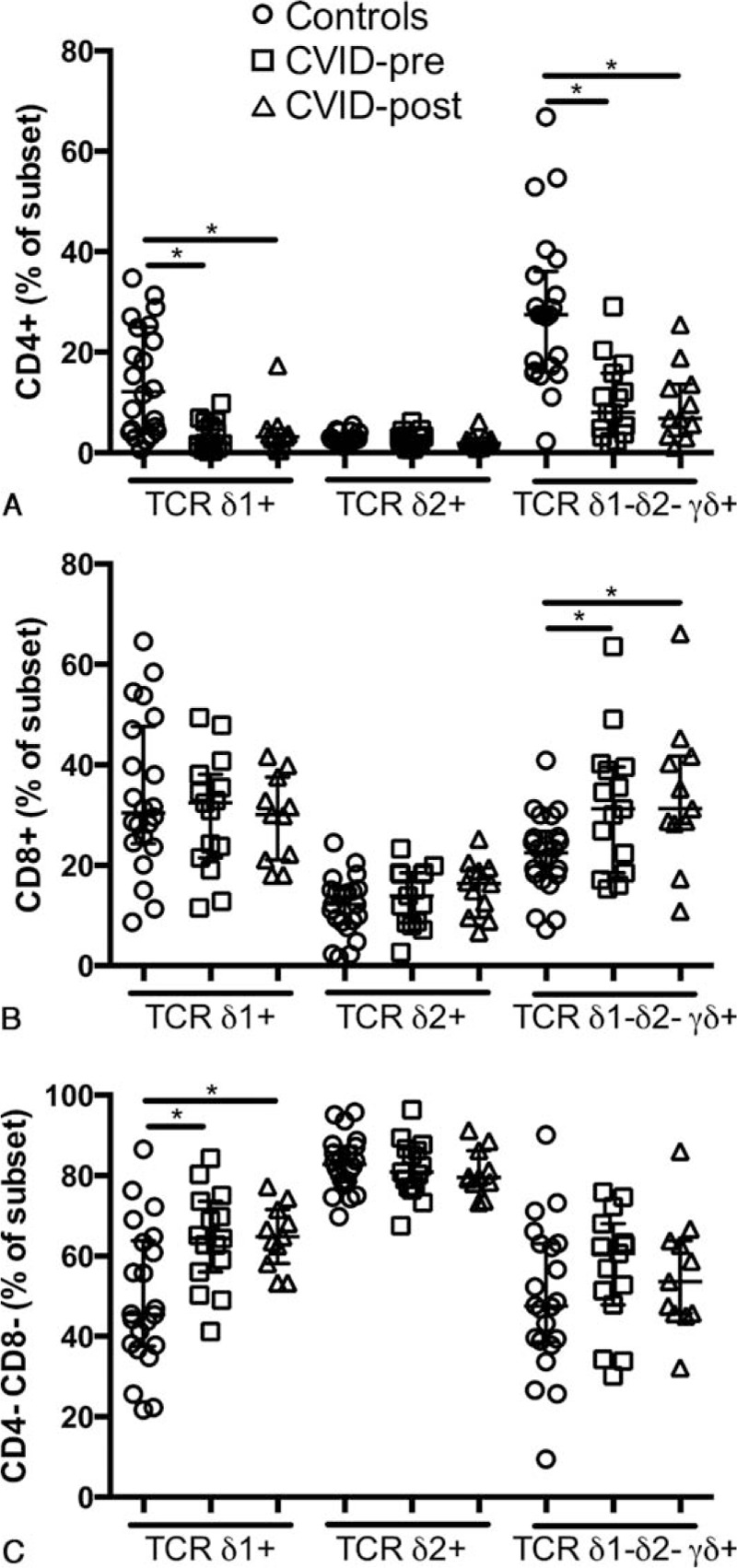
CVID is characterized by elevated CD4+ and CD8+ T cell activation,[6] therefore, we hypothesized that γδ T cells also had high levels of activation. We observed elevated levels of CD38, HLA-DR and coexpression of CD38 and HLA-DR on Vδ1+ (median MFI 576 vs 965, P < 0.001; 44.9% vs 73.1%, P <0.01; 30.5% vs 50.3%, P <0.001 respectively), Vδ2+ (median MFI 99.1 vs 299, P <0.01; 18.1% vs 44.9%, P <0.01; 8.41% vs 17.2%, P <0.001 respectively), and Vδ1− Vδ2− γδ+ T cells (median MFI 652 vs 968, P <0.01; 43.7% vs 65.6%, P <0.01; 26.1% vs 49%, P <0.001 respectively, Fig. 3A–C) confirming our hypothesis. Following replacement therapy CD38 levels and CD38 and HLA-DR coexpression were reduced only on Vδ1+ (median MFI 965 vs 736, P <0.001; 50.3% vs 38.3%, P <0.05 respectively; Fig. 3A and C) but remained higher compared with healthy controls (median MFI 576 vs 736, P <0.05; 30.5% vs 38.3%, P <0.05 respectively).
Figure 3.
Elevated γδ T cells activation in CVID. CD38 MFI (A), levels of HLA-DR (B), and coexpression of CD38 and HLA-DR (C) by γδ+ T cells in healthy controls, treatment naive, and IVIg-treated CVID patients. ∗ indicates P <0.05, the lines and whiskers represent the median and interquartile range respectively.
3.4. Elevated PD-1 expression by Vδ2+ T cells in CVID
Programmed death 1 (PD-1) is an inhibitory receptor and its expression is associated with immune exhaustion in chronic infections.[26] We have previously reported that PD-1 expression is elevated on CD4+ T cells in CVID.[6] Thus, we investigated PD-1 expression by γδ+ T cells in CVID. We observed that PD-1 expression was normal on Vδ1+ and Vδ1− Vδ2− γδ+ T cells (median, 33.0% vs 43.0%, P = 0.22; 33.9% vs 32.7%, P = 0.88 respectively) but elevated on Vδ2+ (median 1.15% vs 3.08%, P <0.001; Fig. 4A). PD-1 remained elevated on Vδ2+ after immune reconstitution (median 1.15% vs 3.09%, P <0.001) but was reduced on Vδ1+ (median 43.0% vs 36.5%, P <0.05).
Figure 4.
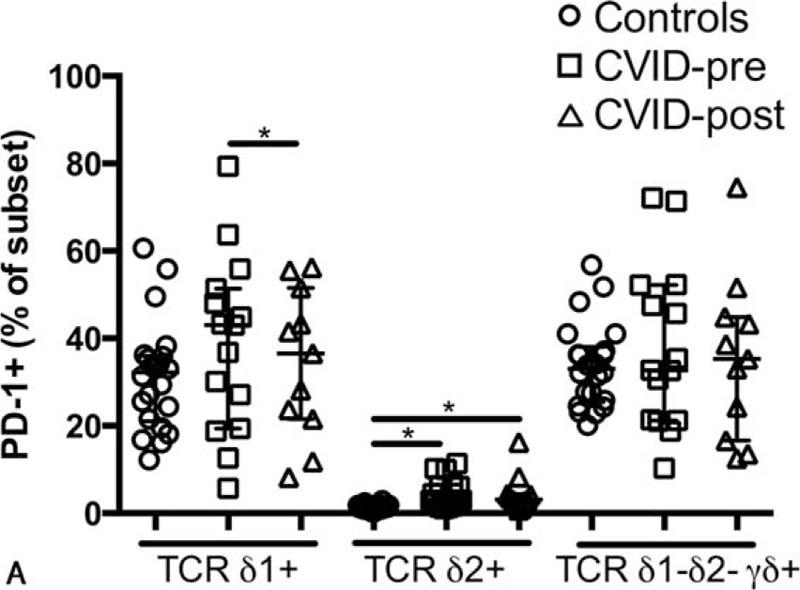
Expression of PD-1 by γδ T cells in CVID. Levels of PD-1 expression by γδ+ T cells (A) in healthy controls, treatment naive, and IVIg-treated CVID patients. ∗ indicates P <0.05, the lines and whiskers represent the median and interquartile range respectively. PD-1 = Programmed Death 1.
3.5. Activation and exhaustion are associated with γδ T cells frequency in CVID
Finally, we investigated if the elevated levels of activation and exhaustion could be associated with the change in γδ T cells frequency that we observed. HLA-DR expression was positively associated with the frequency of Vδ1+ and Vδ1− Vδ2− γδ+ T cells (r = 0.73, P = 0.003; r = 0.68, P = 0.006 respectively; Fig. 5A and B) but not with Vδ2+ T cell frequency (r = 0.022, P = 0.94; Fig. 5C) in treatment naive CVID patients. Vδ2+ T cell frequency was negatively associated with PD-1 expression (r = −0.67, P = 0.007; Fig. 5D).
Figure 5.
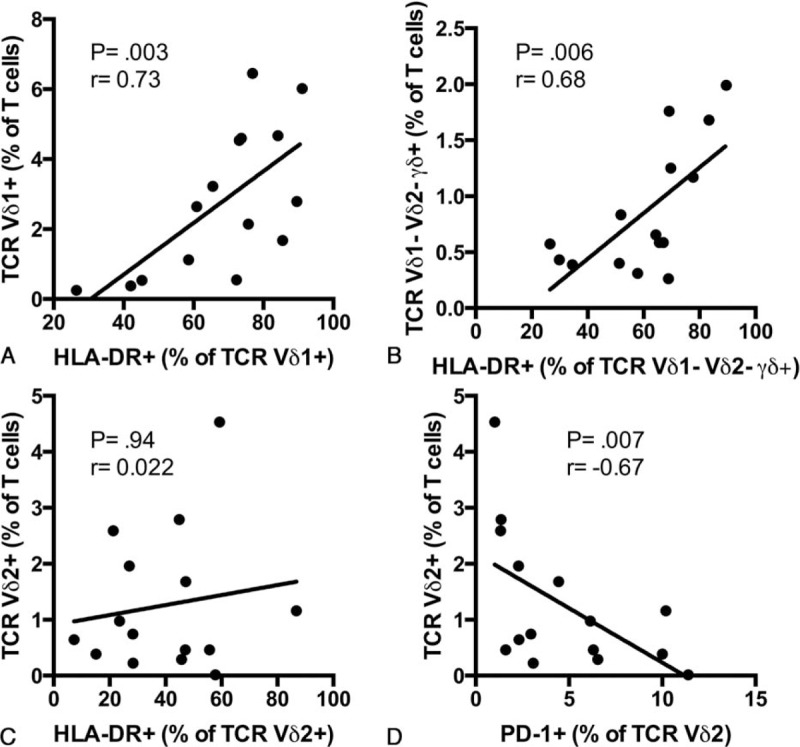
Associations between activation, exhaustion, and γδ T cells subsets frequency in CVID. Correlation between HLA-DR expression and Vδ1+ (A), Vδ1− Vδ2− γδ+ (B), Vδ2+ (C) T cells frequency in treatment naive CVID patients. Correlation between PD-1 expression and Vδ2+ T cells frequency in treatment naive CVID patients (D).
4. Discussion
Previous case reports described an expansion of γδ T cells in 2 CVID patients and this expansion was associated with Mycobacterium infection for one of them.[24,25] Our findings now suggest that perturbations in γδ T cells are a general feature of CVID patients. We observed an increase of Vδ1+ and a decrease of Vδ2+ γδ T cells in CVID, leading to an inversion of the Vδ1+ to Vδ2+ γδ T cell ratio. Interestingly, the majority of sulfatide reactive type II NKT cells have been described to express Vδ1,[27] however it remains to be determined if this population of type II NKT cells is expanded in CVID patients together with Vδ1+ γδ T cells or reduced like type I NKT cells.[28,29]
Vδ2+ γδ T cells are known to interact with many immune cells to shape immune responses, including B cells to stimulate humoral immunity[30] and activated Vδ2+ γδ T cells have been shown to act has antigen-presenting cells.[31,32] Furthermore, Vδ2+ T cells can recognize mevalonate metabolites in tumor cells and are believed to have an important antitumor activity.[33] Therefore, loss of this cell population could partially explain some of the immunological perturbation seen in CVID and the increased incidence of some forms of cancer. More studies are required to determine if the reduced frequency of Vδ2+ γδ T cells in the blood of CVID patients is due to redistribution to tissues or to a complete loss of those cells. More studies are also needed to evaluate the functionality of the remaining Vδ2+ γδ T cells. A mutation in Vav1 has been associated with impaired Th2 response in a subset of CVID patients,[34,35] more experiments are needed to assess the impact of this mutation on γδ T cell functionality.
The high levels of activation of all subsets of γδ T cells that we report here were not affected by IVIg treatment, suggesting that IVIg is not controlling the factors responsible for γδ T cells activation. However, it is possible that a longer period of time on replacement therapy is needed to observe a reduction in γδ T cells activation. There is now accumulating evidence that IgG replacement does not restore a normal immune system in CVID. Complementary therapies aiming to restore normal cellular immunity should be considered and could prevent some of the complications associated with CVID. Long-term low-dose IL-2 has been shown to enhance T cell function in CVID patients[36] but other compartments of the cellular immune system were not studied.
Interestingly, the expansion of Vδ1+ and the reduction in Vδ2+ γδ T cells in CVID is similar to that which has been described for HIV infection,[16] suggesting that common drivers in the pathology associated with primary and secondary immunodeficiency might exist. In this regard, microbial translocation has been implicated in the inversion of the Vδ1+ to Vδ2+ ratio in SIV infection[19] and in CD4 T cell exhaustion in CVID.[23] Expansion of Vδ1+ and Vδ1− Vδ2− γδ+ T cells in CVID was associated with activation, suggesting an implication for chronic inflammation in expending those subsets of γδ T cells. Therefore, we propose that inversion of the Vδ1 to Vδ2 ratio in CVID is a reflection of the infection burden.
Altogether, our results suggest that IVIg replacement therapy is not sufficient to normalize change in γδ T cells frequency and activation in CVID. Furthermore, our results add to the list of similarities between primary and secondary immunodeficiencies, such as HIV infection.
Acknowledgments
The authors thank all patients and healthy controls for their time and efforts toward this study. The authors thank Carla Alves for her support in the laboratory work.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CVID = common variable immunod, DN = double negative, IVIg = intravenous immunoglobulin, PD-1 = Programmed Death 1.
Author contributions: conceived and designed the experiments: DP-P, DFN, EGK. Performed the experiments: DP-P, NSB, BANS. Analyzed the data: DP-P, NSB, BANS. Contributed reagents/materials/analysis tools: AKBBM, CMK, MTB, KIC, JK, DFN, EGK. Wrote the paper: DP-P, DFN, EGK.
This work was partially supported by the National Institutes of Health grant of the District of Columbia Center for AIDS Research (P30AI087714 and P30AI117970), Fundação de Amparo a Pesquisa do Estado de São Paulo (04/15856-9/EGK and 2010/05845-0/EGK/DFN), CNPq/CAPES 056/2012 (DFN).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Park MA, Li JT, Hagan JB, et al. Common variable immunodeficiency: a new look at an old disease. Lancet 2008; 372:489–502. [DOI] [PubMed] [Google Scholar]
- 2.van Schouwenburg PA, Davenport EE, Kienzler AK, et al. Application of whole genome and RNA sequencing to investigate the genomic landscape of common variable immunodeficiency disorders. Clin Immunol 2015; 160:301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol 1999; 92:34–48. [DOI] [PubMed] [Google Scholar]
- 4.Resnick ES, Moshier EL, Godbold JH, et al. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood 2012; 119:1650–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paquin-Proulx D, Sandberg JK. Persistent immune activation in CVID and the role of IVIg in its suppression. Front Immunol 2014; 5:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paquin-Proulx D, Santos BA, Carvalho KI, et al. IVIg immune reconstitution treatment alleviates the state of persistent immune activation and suppressed CD4 T cell counts in CVID. PLoS One 2013; 8:e75199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vlkova M, Ticha O, Nechvatalova J, et al. Regulatory B cells in CVID patients fail to suppress multifunctional IFN-gamma+ TNF-alpha+ CD4+ T cells differentiation. Clin Immunol 2015; 160:292–300. [DOI] [PubMed] [Google Scholar]
- 8.Barsotti NS, Almeida RR, Costa PR, et al. IL-10-producing regulatory B cells are decreased in patients with common variable immunodeficiency. PLoS One 2016; 11:e0151761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duraisingham SS, Buckland M, Dempster J, et al. Primary vs. secondary antibody deficiency: clinical features and infection outcomes of immunoglobulin replacement. PLoS One 2014; 9:e100324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paquin-Proulx D, Santos BA, Carvalho KI, et al. Dysregulated CD1 profile in myeloid dendritic cells in CVID is normalized by IVIg treatment. Blood 2013; 121:4963–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chien YH, Meyer C, Bonneville M. gammadelta T cells: first line of defense and beyond. Annu Rev Immunol 2014; 32:121–155. [DOI] [PubMed] [Google Scholar]
- 12.Schweighoffer E, Fowlkes BJ. Positive selection is not required for thymic maturation of transgenic gamma delta T cells. J Exp Med 1996; 183:2033–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haas JD, Ravens S, Duber S, et al. Development of interleukin-17-producing gammadelta T cells is restricted to a functional embryonic wave. Immunity 2012; 37:48–59. [DOI] [PubMed] [Google Scholar]
- 14.Adams EJ, Gu S, Luoma AM. Human gamma delta T cells: evolution and ligand recognition. Cell Immunol 2015; 296:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willcox CR, Pitard V, Netzer S, et al. Cytomegalovirus and tumor stress surveillance by binding of a human gammadelta T cell antigen receptor to endothelial protein C receptor. Nat Immunol 2012; 13:872–879. [DOI] [PubMed] [Google Scholar]
- 16.Pauza CD, Poonia B, Li H, et al. Gammadelta T cells in HIV disease: past, present, and future. Front Immunol 2014; 5:687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Paoli P, Gennari D, Martelli P, et al. A subset of gamma delta lymphocytes is increased during HIV-1 infection. Clin Exp Immunol 1991; 83:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallace M, Scharko AM, Pauza CD, et al. Functional gamma delta T-lymphocyte defect associated with human immunodeficiency virus infections. Mol Med 1997; 3:60–71. [PMC free article] [PubMed] [Google Scholar]
- 19.Harris LD, Klatt NR, Vinton C, et al. Mechanisms underlying gammadelta T-cell subset perturbations in SIV-infected Asian rhesus macaques. Blood 2010; 116:4148–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–1371. [DOI] [PubMed] [Google Scholar]
- 21.Titanji K, De Milito A, Cagigi A, et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood 2006; 108:1580–1587. [DOI] [PubMed] [Google Scholar]
- 22.Hart M, Steel A, Clark SA, et al. Loss of discrete memory B cell subsets is associated with impaired immunization responses in HIV-1 infection and may be a risk factor for invasive pneumococcal disease. J Immunol 2007; 178:8212–8220. [DOI] [PubMed] [Google Scholar]
- 23.Perreau M, Vigano S, Bellanger F, et al. Exhaustion of bacteria-specific CD4 T cells and microbial translocation in common variable immunodeficiency disorders. J Exp Med 2014; 211:2033–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viallard JF, Bloch-Michel C, Caubet O, et al. Gammadelta T lymphocytosis associated with granulomatous disease in a patient with common variable immunodeficiency. Clin Infect Dis 2002; 35:e134–137. [DOI] [PubMed] [Google Scholar]
- 25.Katial RK, Lieberman MM, Muehlbauer SL, et al. Gamma delta T lymphocytosis associated with common variable immunodeficiency. J Clin Immunol 1997; 17:34–42. [DOI] [PubMed] [Google Scholar]
- 26.Khaitan A, Unutmaz D. Revisiting immune exhaustion during HIV infection. Curr HIV/AIDS Rep 2011; 8:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai L, Picard D, Anderson B, et al. The majority of CD1d-sulfatide-specific T cells in human blood use a semiinvariant Vdelta1 TCR. Eur J Immunol 2012; 42:2505–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carvalho KI, Melo KM, Bruno FR, et al. Skewed distribution of circulating activated natural killer T (NKT) cells in patients with common variable immunodeficiency disorders (CVID). PLoS One 2010; 5:e12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fulcher DA, Avery DT, Fewings NL, et al. Invariant natural killer (iNK) T cell deficiency in patients with common variable immunodeficiency. Clin Exp Immunol 2009; 157:365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tyler CJ, Doherty DG, Moser B, et al. Human Vgamma9/Vdelta2 T cells: innate adaptors of the immune system. Cell Immunol 2015; 296:10–21. [DOI] [PubMed] [Google Scholar]
- 31.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science 2005; 309:264–268. [DOI] [PubMed] [Google Scholar]
- 32.Muto M, Baghdadi M, Maekawa R, et al. Myeloid molecular characteristics of human gammadelta T cells support their acquisition of tumor antigen-presenting capacity. Cancer Immunol Immunother 2015; 64:941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gober HJ, Kistowska M, Angman L, et al. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med 2003; 197:163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capitani N, Ariani F, Amedei A, et al. Vav1 haploinsufficiency in a common variable immunodeficiency patient with defective T-cell function. Int J Immunopathol Pharmacol 2012; 25:811–817. [DOI] [PubMed] [Google Scholar]
- 35.Capitani N, Amedei A, Paccani SR, et al. Impaired TH2 response in patients with Vav1-deficient common variable immunodeficiency with T-cell defects. J Allergy Clin Immunol 2010; 126:671–675. [DOI] [PubMed] [Google Scholar]
- 36.Cunningham-Rundles C, Bodian C, Ochs HD, et al. Long-term low-dose IL-2 enhances immune function in common variable immunodeficiency. Clin Immunol 2001; 100:181–190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


