Abstract
A meta-analysis was performed to ascertain to what extent hepatitis B surface antigen (HBsAg)-negative/anti-hepatitis B core (anti-HBc)-positive subjects with chronic liver disease are at a higher risk of developing hepatocellular carcinoma (HCC) than the anti-HBc-negative.
All studies included had to fulfill the following characteristics and inclusion criteria: they investigated the relationship between HBsAg-negative/anti-HBc-positive serology and the occurrence of HCC, whether a case–control or cohort study, they provided relative risk (RR) or odds ratios (ORs) and 95% confidence intervals (CIs), were available as a full text written in English, and were published and indexed up to April 2015.
Twenty-six original studies met the inclusion criteria, allowing a meta-analysis on 44,553 patients. The risk of HCC among the 9986 anti-HBc-positive subjects was 67% higher than in the 34,567 anti-HBc-negative (95% CI = 1.44–1.95, P < 0.0001). The results were similar when groups of patients with a different stage of liver disease (patients with chronic liver disease, patients with cirrhosis), with different ethnicity (Asian and non-Asian) and etiology (HCV and non-HCV) were considered. The risk of HCC was significantly higher in the 651 anti-HBs/anti-HBc-positive patients (RR = 1.36; 95% CI = 1.17–1.58, P = 0.03) and in the 595 anti-HBs-negative/anti-HBc-positive subjects (RR = 2.15; 95% CI = 1.58–2.92, P < 0.0001) than in the 1242 anti-HBs/anti-HBc negative. However, the RR from 8 studies indicated that the risk of HCC was 35% lower among the anti-HBs/anti-HBc-positive subjects compared to the anti-HBs-negative/anti-HBc-positive (RR = 0.65; 95% CI = 0.52–0.8, P < 0.0001).
This meta-analysis shows that in HBsAg-negative subjects with chronic liver disease, anti-HBc positivity is strongly associated with the presence of HCC, an association observed in all subgroups according to the stage of the disease, etiology, and ethnicity.
Keywords: anti-HBc positivity, chronic hepatitis, hepatocellular carcinoma, liver cirrhosis, occult HBV infection, silent HBV infection
1. Introduction
Hepatitis B virus (HBV) affects over 350 million people worldwide and is one of the leading causes of cirrhosis and hepatocellular carcinoma (HCC).[1–3] The severity of chronic hepatitis B (CHB) is variable, with a clinical presentation ranging from a healthy HBV carriage to the more severe expressions of the disease and with a clinical course ranging from a benign indolent progression over decades to a rapid evolution to liver cirrhosis and HCC.[4,5]
The development of sensitive assays to detect small amounts of HBV DNA has favored the identification of occult hepatitis B infection characterized by HBV DNA detectable in liver tissue and hepatitis B surface antigen (HBsAg) undetectable in serum.[6] In patients with chronic hepatitis C, occult HBV infection has been associated with more severe liver damage and a higher risk of HCC.[7–9] In immunosuppressed patients, occult hepatitis B may reactivate,[10–12] particularly in onco-hematological patients receiving chemotherapy.[13] Reactivation of occult HBV infection has been more frequently observed in patients showing anti-HBc as the only HBV marker (“isolated” anti-HBc).[14]
The presence of anti-HBc in HBsAg-negative subjects, a serological condition suggesting a resolved HBV infection, has been used by several authors as a surrogate marker of occult HBV infection in several case–control and cohort studies that investigated the association between serum HBsAg negativity/anti-HBc positivity and the onset of HCC,[7,8,11,14] but the results are inconsistent. A meta-analysis was conducted on 10 observational studies in 2010,[15] but the question of whether and to what extent HBsAg-negative patients with chronic liver disease and anti-HBc positivity are at higher risk of HCC is still open. Since watertight conclusions on this topic are of great clinical and therapeutic value, a meta-analysis has been conducted to evaluate the association between anti-HBc positivity and the occurrence of HCC in HBsAg-negative subjects with chronic liver disease.
2. Methods
The present meta-analysis was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)[16] and of the Meta-Analysis of Observational Studies in Epidemiology (MOOSE).[17]
2.1. Data sources and literature search
Two researchers (LO and CS) conducted a comprehensive computerized literature search to identify original reports using MEDLINE, EMBASE, LILACS, and the Cochrane Library from 1995 to April 2015, involving both medical subject heading (MeSH) terminology and relevant keywords for search strings to locate articles that analyzed the contribution of serum anti-HBc positivity to the occurrence of HCC. The following items were used to search the studies: “HCC,” “hepatocellular carcinoma,” “anti-HBc,” “occult HBV infection,” “latent HBV infection,” and “silent HBV infection.” In addition, the reference lists of all studies meeting the inclusion criteria, of the studies excluded and of the published review articles were manually searched to identify any other study that might merit inclusion.
2.2. Study selection
All studies included had to fulfill the following characteristics and inclusion criteria: they investigated the association between HBsAg-negative/anti-HBc-positive serology and the occurrence of HCC, whether a case–control or cohort study, they reported the relative risk (RR) or odds ratios (ORs) and their 95% confidence intervals (CIs) or sufficient data to calculate them, were available as a full text manuscript, were written in the English language, and were published online and indexed up to April 2015. The exclusion criteria of the meta-analysis were: meta-analyses, letters, reviews, meeting abstracts, or editorial comments; studies investigating HBsAg-positive patients and not reporting separate data for HBsAg-negative patients; and included patients with HIV infection. If more than 1 publication dealt with the same patient population and offered the same outcome messages, only the most recent or most complete article was included in the analysis.
Potentially eligible articles were selected in 2 rounds. Firstly, 2 reviewers (LO and CS) independently screened the title, abstract, and key words from all citations identified in the search to select the relevant articles that would meet the criteria outlined above. An inclusion/exclusion form for all papers was filled out. Reasons for the exclusion of any study were recorded independently and cross-checked for agreement. Secondly, studies that satisfied the inclusion criteria were retrieved for a full text evaluation performed independently by the same reviewers. In the case of disagreement, the 2 reviewers reevaluated the article together and a consensus on whether to include or exclude a study was always reached.
2.3. Data extraction
Two reviewers (LO and CS) working independently extracted the data using a standard protocol and data-collection form according to the inclusion criteria. The following relevant information was collected from every article selected according to the inclusion criteria: last name of the first author, year of publication, country where the population was investigated, study design, sample size (cases and controls or cohort size), duration of follow-up for cohort studies, variables adjusted for in the analysis, RR or OR and their 95% CIs or sufficient data to allow their calculation if not directly available. The discrepancies between these reviewers were resolved with discussion. The corresponding author was contacted via email if the data presentation was incomplete or if was necessary to resolve an apparent conflict or inconsistency in the article.
2.4. Quality assessment
Two reviewers (IFA and NC) independently determined the methodological quality of each cohort or case–control study according to the Newcastle-Ottawa Scale (NOS) for assessing nonrandomized studies.[18] Using a star system with a maximum of 9 stars for each study, this scale assesses every aspect of an observational epidemiological study from a methodological point of view. Eight items are categorized in 3 broad study components: selection of the study groups (4 items, 1 star each), comparability of the study groups (1 item, up to 2 stars) and method of ascertaining both the exposure and outcome of interest (3 items, 1 star each). Studies with a score from 7 to 9 stars were considered at a low risk of bias, studies with 4 to 6 stars at a moderate risk and those with 3 stars or less at a high risk of bias. Any discrepancies in assessing the risk of bias scores between the reviewers were addressed with a joint reevaluation of the original article. If a consensus was not reached, a third reviewer decided.
2.5. Statistical analysis
For each study not reporting the estimates of RR, RR estimates and their 95% CIs were calculated based on the reported numbers of participants. Statistical heterogeneity between studies included in the meta-analysis was assessed using the Cochran Q test, and the proportion of total variation in study estimates due to heterogeneity was quantified with the I2 statistic. I2 values between 25% and 49% indicated low heterogeneity, between 50% and 75% indicated moderate heterogeneity and an I2 value of 75% or above indicated high heterogeneity.[19] A threshold P-value less than 0.1 was considered statistically significant. The Mantel–Haenszel method for a fixed-effects model was applied in the absence of heterogeneity between the studies (Q-statistic: P > 0.1; I2 < 50%),[20] otherwise, the DerSimonian and Laird method for a random-effects model was used if substantial heterogeneity was detected (Q-statistic: P < 0.1; I2 > 50%).[21] Subgroup analyses were additionally conducted based on the study quality (low risk of bias vs moderate risk of bias), study design (cohort vs case–control studies), and length of the follow-up in the cohort studies (at least 5 years vs less than 5 years). Several methods were used to statistically assess the potential for small study effects such as publication bias. Potential publication bias was assessed by visual inspections of the Begg funnel plots and then, more formally, Egger linear regression test of asymmetry[22] and the Begg and Mazumdar adjusted rank correlation test[23] were also used. A 2-tailed P-value of less than 0.05 was considered representative of a statistically significant publication bias. All statistical analyses were performed using Stata/SE, version 10.1 software (Stata Corporation, College Station, TX).
2.6. Ethics statement
Approval for the specific study was not required. However, all procedures used in the study were in accordance with the current international guidelines, with the standards on human experimentation of the Ethics Committee of the Azienda Ospedaliera of the Second University of Naples, Italy, and with the Helsinki Declaration of 1975, revised in 1983.
3. Results
3.1. Literature search
Figure 1 shows a flow diagram of the process of identification and selection of the articles included in the meta-analysis. A total of 58,261 potentially relevant articles were identified from the search of electronic databases. Of these, 58,202 articles were excluded after the first screening on the basis of the title and abstracts, 59 were considered potentially valuable and full texts were retrieved for detailed evaluation. After further evaluation and manual search of the bibliography references of the relevant publications, a total of 26 articles met the inclusion criteria, of which 10 were case–control[24–33] and 16 were cohort studies,[34–49] and were included in this meta-analysis.
Figure 1.
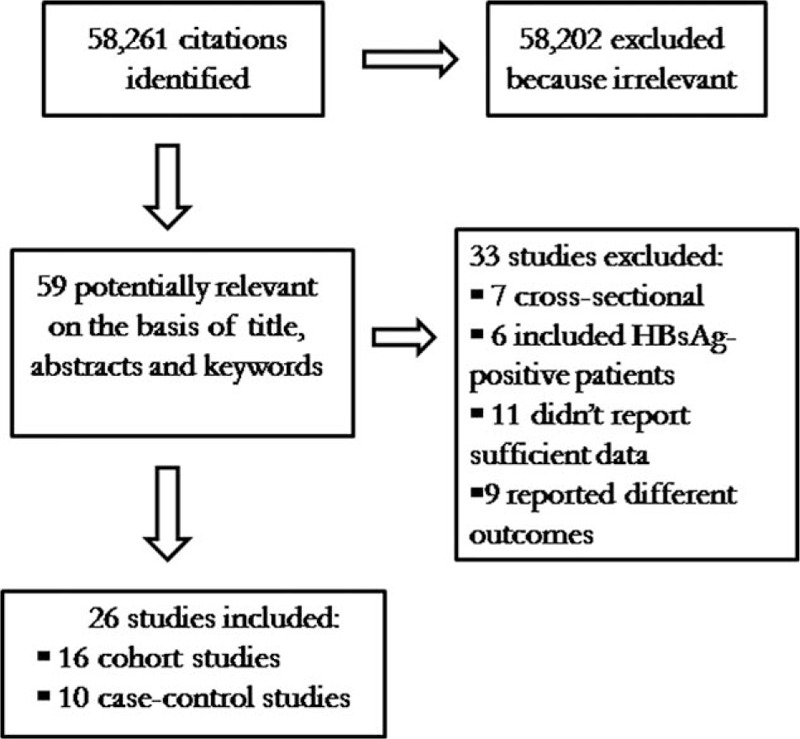
Flow chart of the published studies evaluated for inclusion in the meta-analysis.
3.2. Study characteristics
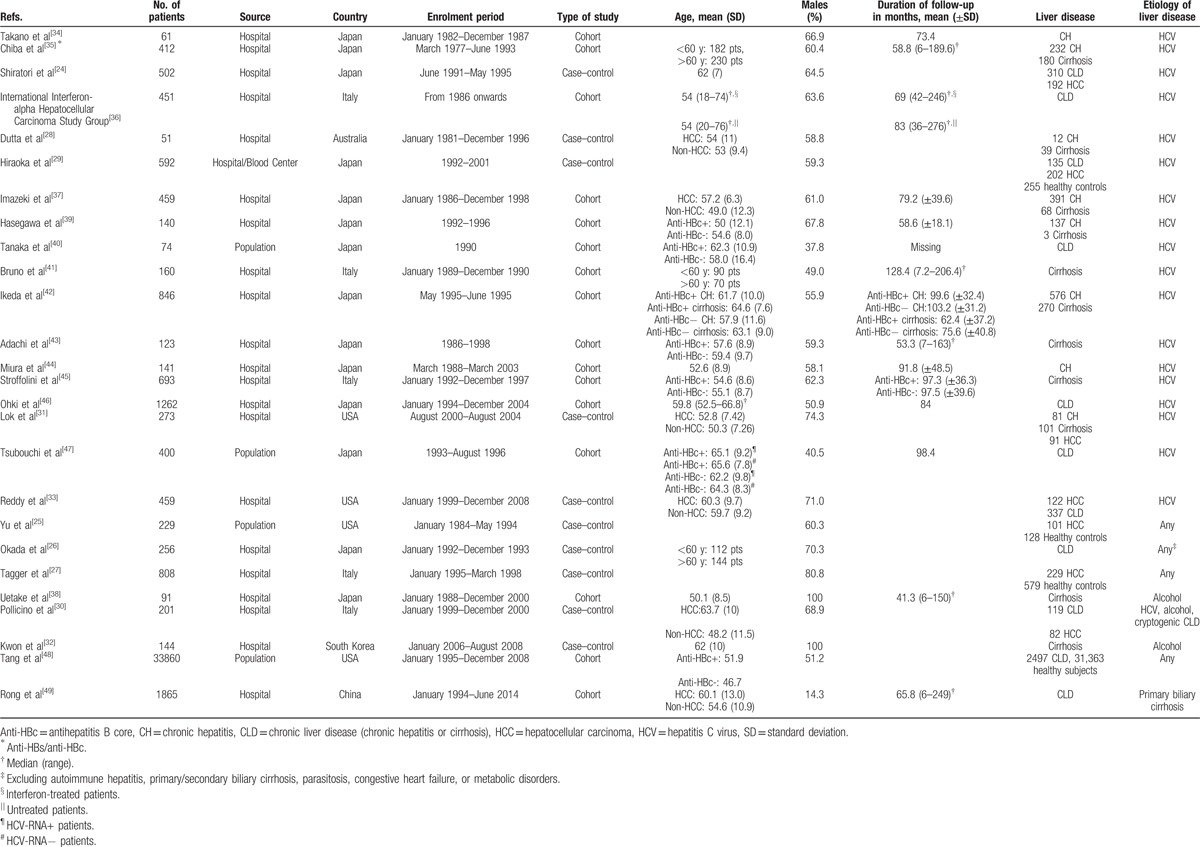
The main characteristics of the 26 studies included in the meta-analysis are summarized in Table 1. The studies had been published between 1995 and 2015 and the number of patients per study ranged from 51 to 33,860 patients. Overall, the studies included a total of 44,553 HBsAg-negative subjects, 9986 anti-HBc-positive and 34,567 anti-HBc-negative. Of the 26 studies included, 14 were conducted in Japan,[24,26,29,34,35,37–40,42–44,46,47] 5 in Italy,[27,30,36,41,45] 4 in the United States,[25,31,33] 1 each in Australia,[28] China,[49] and South Korea.[32] Fifteen studies enrolled patients with different stages of liver disease (chronic hepatitis or cirrhosis),[24,26,28,30,31,33,35–37,39,40,42,46,47,49] 2 studies involved patients with chronic hepatitis,[34,44] 5 studies patients with cirrhosis,[32,38,41,43,45] and 4 included also healthy controls.[25,27,29,48] The etiologic agent of liver disease was HCV in 18 studies,[24,28,29,31,33–37,39–47] alcohol in 2 studies,[32,38] primary biliary cirrhosis in 1,[49] whereas 5 studies[25–27,30,48] enrolled patients with a mixed etiology, HCV infection, alcohol abuse, and/or other nonspecified etiology.
Table 1.
Characteristics of the studies included in the meta-analysis.
3.3. Quality assessment
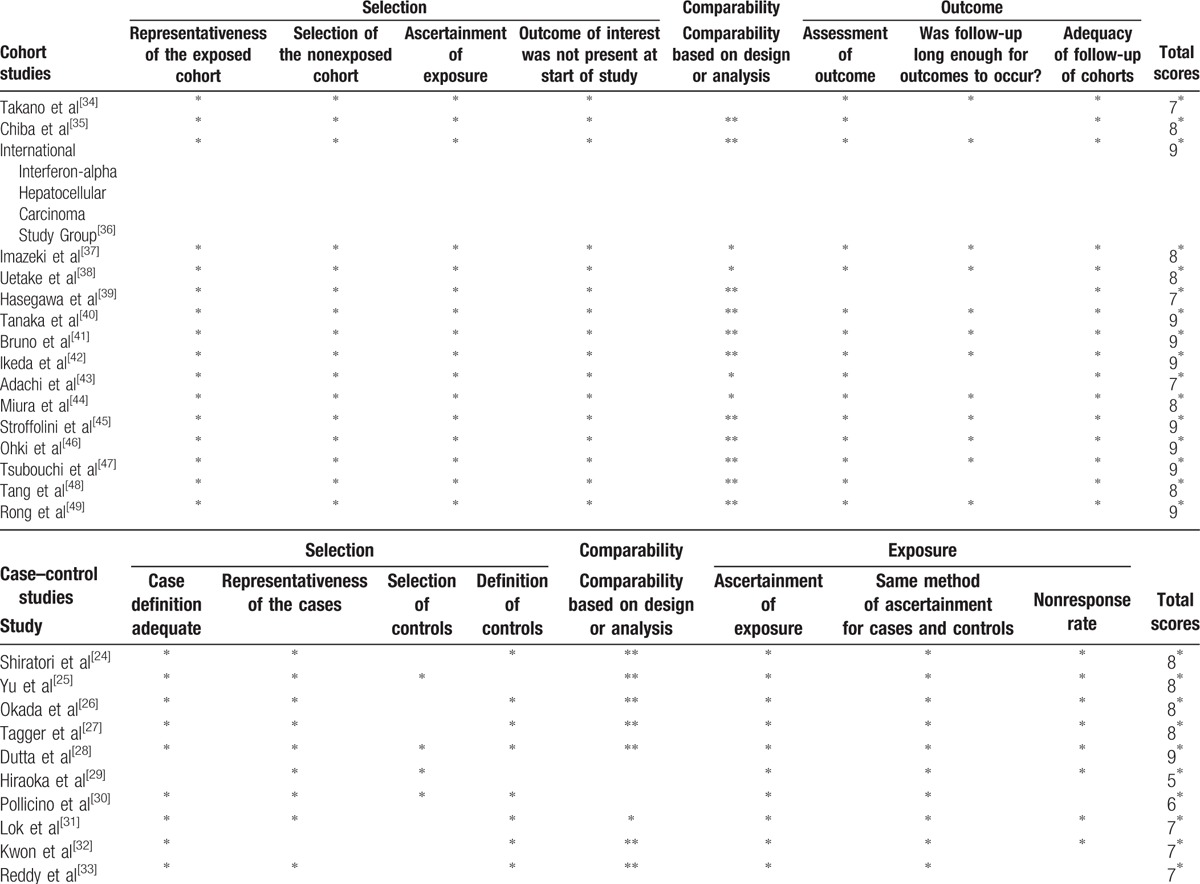
The results of the methodological quality assessments using the NOS scores of all case–control and cohort studies included in the meta-analysis are shown in Table 2. NOS scores ranged from 7 to 9 stars for the cohort studies and from 5 to 9 stars for the case–control studies, with 8[36,40–42,45–47,49] out of the 16 cohort studies and 1[28] out of the 10 case–control studies with 9 stars. As regards the case–control studies, the majority had an adequate definition of the cases and controls and therefore got 2 stars. All but 1 of the studies recruited a clearly representative or a consecutive sample of cases, and only 4 studies recruited adequate community controls without an obvious source of bias. The most common selection bias was that the control series used in the study was not taken from the same population as the cases, with only 40% of the studies having a low risk of bias. All HCC cases were stated to have been diagnosed over a certain period, in certain medical centers, and thus the representativeness of cases qualified for another star. HCC was identified by histology and/or radiographic methods (ultrasound and/or computed tomography and/or magnetic resonance) and/or high serum level of alpha-protein, so 2 more stars were assigned to all studies for the “ascertainment of exposure” and the “same method of ascertainment for the cases and controls.” For the comparability criteria, all except 2 case–control studies included[29,30] earned a star for matching for age, which was considered the most important factor for adjustment, and the majority of them earned a second star for additional adjustment. The same nonresponse rate between groups was not shown or not mentioned in 2 studies,[30,33] and thus they failed to get a star for “nonresponse rate.” In the cohort studies, in terms of selection bias, 100% of the studies met all the high-quality criteria. All cohort studies included, except 1,[34] earned a star for comparability based on design or analysis with regard to age, and the majority of them earned a second star for additional adjustment. Only 1 study[33] did not earn a star for the assessment of outcome. The average follow-up time was similar for all studies reporting it and was judged to be appropriate (at least 5 years) in 12 of the 16 included.
Table 2.
Newcastle-Ottawa Scale (NOS) assessment of the quality of the studies.
3.4. Meta-analyses of the data
The results of the meta-analysis for the association between anti-HBc positivity and HCC are shown in Table 3. Considering all 44,553 subjects included in the 26 studies, the risk of HCC among the 9986 anti-HBc-positive subjects was 67% higher than in the 34,567 anti-HBc-negative (95% CI = 1.44–1.95, P < 0.0001).
Table 3.
Meta-analysis data on the development of HCC in the anti-HBc-positive or anti-HBc-negative subjects.
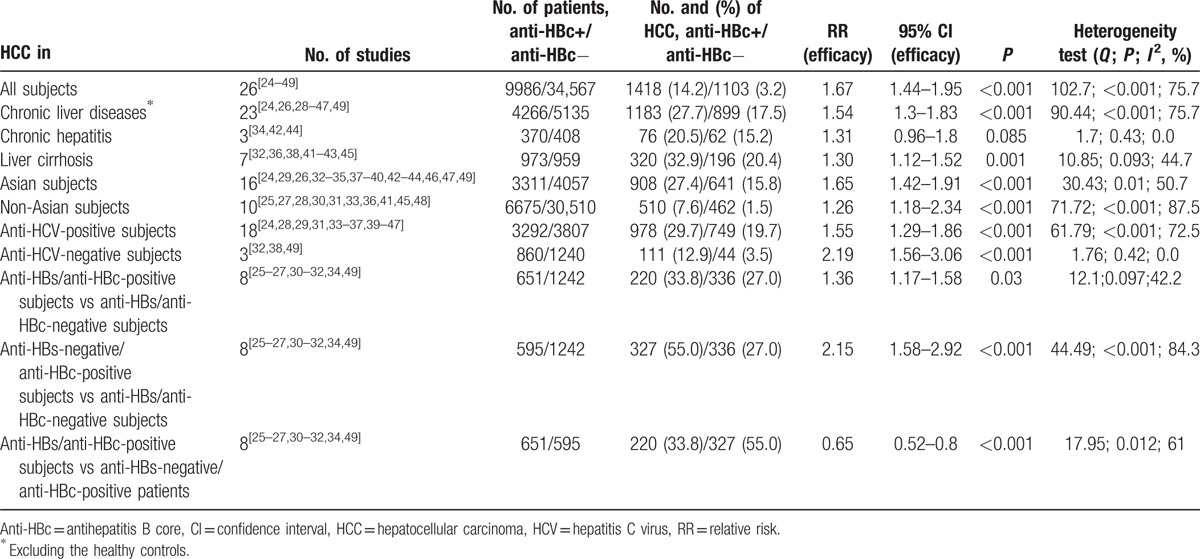
The results were similar when groups of patients with a different stage of liver disease were considered. In those with a chronic liver disease, HCC was more frequently observed in the 4266 anti-HBc-positive patients than in the 5135 anti-HBc-negative (RR = 1.54; 95% CI = 1.3–1.83, P < 0.0001). Anti-HBc positivity was associated with HCC also in patients with cirrhosis (RR = 1.30; 95% CI = 1.12–1.52, P = 0.001). An independent association of anti-HBc positivity with the risk of HCC was similar both in the 16 studies carried out in Asia and in the 10 studies from other countries. In fact, in the Asian studies the 3311 anti-HBc-positive subjects were at a higher risk of HCC than the 4057 anti-HBc-negative (RR = 1.65; 95% CI = 1.42–1.91, P < 0.0001). The results were similar in the 10 non-Asian studies, where the risk of HCC was higher in the 6675 anti-HBc-positive than in the 30,510 anti-HBc-negative patients (RR = 1.62; 95% CI = 1.18–2.34, P < 0.0001).
As regards the etiology, the association between anti-HBc positivity and the risk of HCC was investigated in HCV-related chronic liver diseases pooling 18 studies with 7099 anti-HCV-positive subjects. HCC was observed more frequently in the 3292 anti-HBc-positive subjects than in the 3807 anti-HBc-negative (RR = 1.55; 95% CI = 1.29–1.86, P < 0.0001). Other etiologies of liver diseases (alcohol or primary biliary cirrhosis in anti-HCV-negative subjects) were investigated in 3 studies and the risk of HCC was more frequently observed in the 860 anti-HBc-positive subjects than in the 1240 anti-HBc-negative (RR = 2.19; 95% CI = 1.56–3.06, P < 0.0001).
The risk of HCC was significantly higher in the 651 anti-HBs/anti-HBc-positive patients (RR = 1.36; 95% CI = 1.17–1.58, P = 0.03) and in the 595 anti-HBs-negative/anti-HBc-positive patients (RR = 2.15; 95% CI = 1.58–2.92, P < 0.0001) than in the 1242 anti-HBs/anti-HBc negative. However, the RR from 8 studies indicated that the risk of HCC was 35% lower among the anti-HBs/anti-HBc-positive subjects compared to that of the anti-HBs-negative/anti-HBc-positive (RR = 0.65; 95% CI = 0.52–0.8, P < 0.0001).
Heterogeneity was calculated among all studies using the Q-statistic and the I2 test. As shown in Table 3, heterogeneity in all meta-analyses was found except for the meta-analyses for liver cirrhosis, chronic hepatitis, and anti-HBs/anti-HBc-positive versus -negative status. The between-study heterogeneity was significant (I2 = 75.7%, Q < 0.001) when all 26 studies were pooled in the meta-analysis. In the subgroup analyses by ethnicity, severity, and etiology of liver disease, heterogeneity was also significant in Asian (I2 = 50.7%, Q = 0.01) and non-Asian groups (I2 = 87.5%, Q < 0.001), in chronic liver disease (I2 = 75.7%, Q < 0.001), and anti-HCV-positive subgroups (I2 = 72.5%, Q < 0.001).
Visual inspection of the funnel plots and Begg and Egger tests were performed to assess the potential publication bias of the studies included in this meta-analysis (Figs. 2–5). The shapes of the funnel plots did not reveal any clear evidence of obvious asymmetry in the analysis. The Begg and Egger test results showed no significant statistical evidence of publication bias in this analysis of the association between anti-HBc positivity and the risk of HCC in HBsAg-negative subjects, which indicated a low risk of publication bias, with the exception of the meta-analysis for liver cirrhosis and Asian ethnicity, in which evidence of publication bias was noted (P-value for Egger 0.044 and 0.009, respectively).
Figure 2.
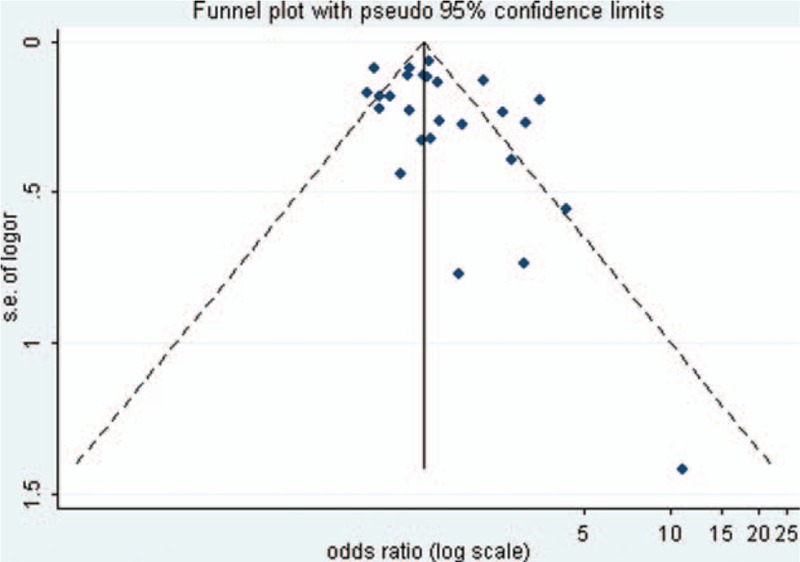
Funnel plot of the risk ratios versus the reciprocal of their standard errors of studies evaluating the risk of HCC in all patients (anti-HBc positive vs anti-HBc negative).
Figure 5.
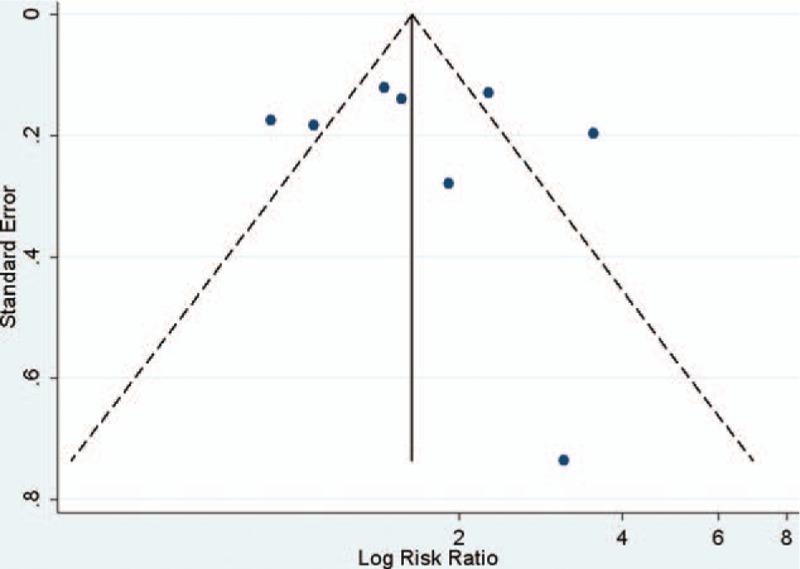
Funnel plot of the risk ratios versus the reciprocal of their standard errors of studies evaluating the risk of HCC in all patients (anti-HBc positive/anti-HBs positive vs anti-HBc/anti-HBs positive).
Figure 3.
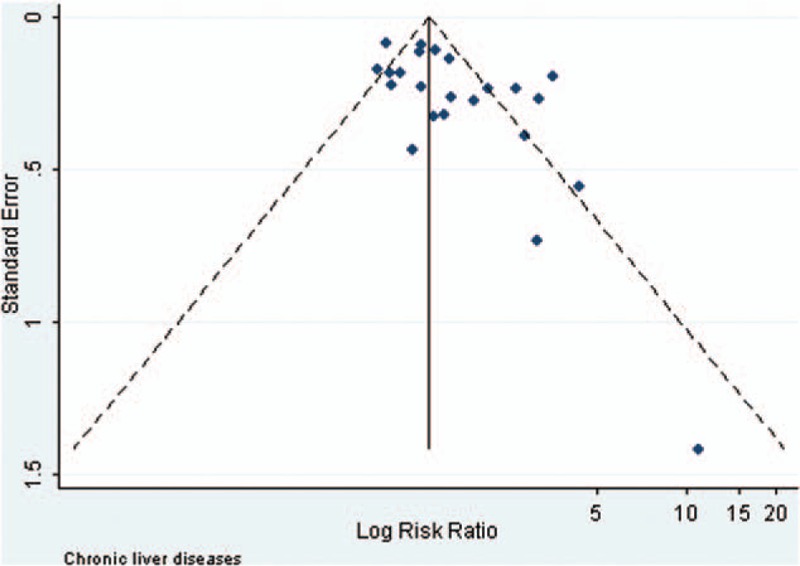
Funnel plot of the risk ratios versus the reciprocal of their standard errors of studies evaluating the risk of HCC in the patients with chronic liver diseases (anti-HBc positive vs anti-HBc negative).
Figure 4.
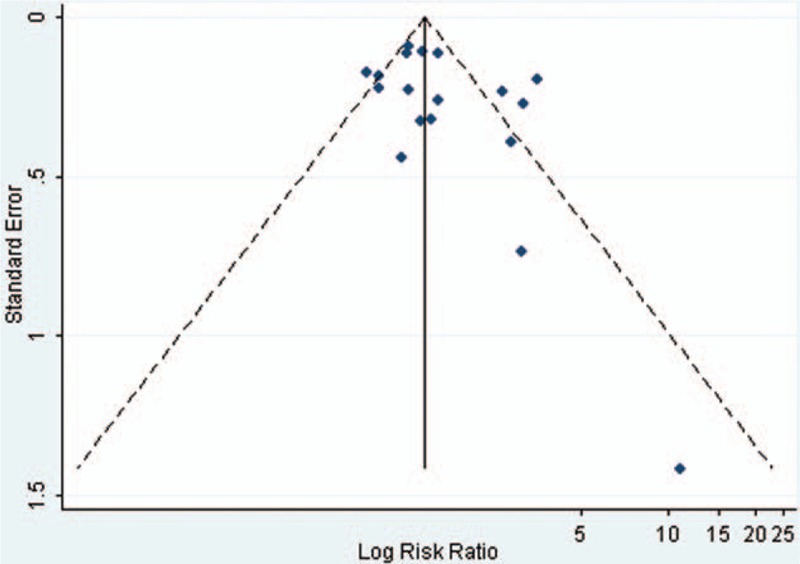
Funnel plot of the risk ratios versus the reciprocal of their standard errors of studies evaluating the risk of HCC in the anti-HCV positive patients (anti-HBc positive vs anti-HBc negative).
In subgroup analyses, compared to the overall analysis, the pooled estimate was similar when the studies were analyzed separately according to the level of risk of bias, to the study design, and the length of the follow-up in the cohort studies. There was no evidence of significant heterogeneity between subgroups (data not shown).
4. Discussion
This meta-analysis sought to evaluate the literature on the role of anti-HBc positivity in the risk of HCC in HBsAg-negative subjects with chronic liver disease. It presents substantial differences from a previous meta-analysis[15] that demonstrated the association between anti-HBc positivity and presence of HCC in patients with chronic HCV infection, in that the present meta-analysis includes chronic hepatitis of different etiologies, a larger number of studies (26 vs 10) and of participants (44,553 vs 4845), and presents analyses performed also for subgroups of patients regarding the stages of the liver diseases, the geographical area of origin, and the anti-HBs status. The results clearly show that in HBsAg-negative chronic hepatitis patients, serum anti-HBc, an indirect serological sign of occult HBV infection, is strongly associated with the presence of HCC and may even predict liver cancer. This association was observed in all subgroups irrespective of the stage of the disease (chronic liver disease, liver cirrhosis), area of origin (Asian and non-Asian), and etiology (anti-HCV-positive and anti-HCV-negative subjects).
The presence of anti-HBc in serum is now considered a reliable surrogate marker of the persistence of occult HBV infection,[4,8,50] but the mechanisms leading to more severe fibrosis and to the risk of HCC in HBsAg-negative/anti-HBc-positive patients have not been completely elucidated. The persistence of occult HBV infection should, however, be considered an unfavorable event since it produces 2 deleterious effects: an increased risk of progression to liver cirrhosis due to a longer immunological attack, a greater risk of HCC due to prolonged exposure to HBV, a virus of known oncogenicity.[6]
This meta-analysis also demonstrates that in HBsAg-negative chronic hepatitis, both anti-HBs/anti-HBc-positive and anti-HBs-negative/anti-HBc-positive patients have a significantly higher risk of HCC than the anti-HBs/anti-HBc-negative. However, patients with “isolated” anti-HBc were found to have a significantly higher risk of HCC than the anti-HBs/anti-HBc-positive, most probably because these subjects showed more frequently an occult HBV infection in the liver. In fact, in our previous observation HBV DNA can be detected in plasma, peripheral blood mononuclear cells, or liver tissue of 80% of patients with “isolated” anti-HBc and of 60% of the anti-HBs/anti-HBc-positive,[14] suggesting a prolonged exposure to occult HBV replication that may favor the onset of HCC. However, it may also be hypothesized that circulating anti-HBs may prevent the risk of HCC, most probably by controlling HBV replication, as already suggested.[7,50–53] Recently in a prospective study Lee et al[53] showed that in 196 HCV-related-cirrhotic patients the development of HCC was less frequently observed in the anti-HBs-positive patients, suggesting a protective role of the circulating anti-HBs. Moreover, in animal models it was shown that adoptive transfer of immunity against HBsAg facilitates the suppression of experimental human HCC-expressing HBsAg in athymic mices.[54,55]
At present, no datum is available, at least to our best knowledge, on the strategies to be adopted for anti-HBc-positive patients, that is, whether close monitoring should be applied for an early identification of HCC or whether anti-HBV nucleot(s)ides should be administered to prevent the risk of liver cirrhosis and possibly the onset of HCC, a problem of considerable clinical impact since anti-HBc positivity accounted for around 15% of the cases with HCC and the presence of “isolated” anti-HBc for around 37%. The results of this meta-analysis indicate that, at least for HBsAg-negative/anti-HBc-positive patients with chronic liver disease, a more accurate monitoring for HCC is hypothesized.
In this meta-analysis, a validated risk of bias assessment tool, the NOS, was used to evaluate the quality of the studies included. The overall individual study quality was good. In general, the quality of the measurements in the studies included was generally high and the source studies were published in peer-reviewed journals. Overall, the studies included provide evidence for the association of anti-HBc positivity with the risk of HCC in HBsAg-negative subjects. It is necessary, however, to take into account that these studies may be prone to bias, particularly as they have a nonrandomized design.
Heterogeneity is a potential problem when interpreting the results of all meta-analyses, and finding the sources of heterogeneity is one of the most important goals. In the present meta-analysis, the between-study heterogeneity was assessed using different methods, including the Cochran Q statistic and the I2 statistic. There was significant between-study heterogeneity in the pooled meta-analysis of all eligible studies, which suggested obvious consistency of effects across the studies included. Subgroup analyses by ethnicity, severity of liver disease, and etiology showed that heterogeneity was still significant in the subgroup analyses for Asian and non-Asian ethnicity, in patients with chronic liver disease, with cirrhosis, and with HCV chronic infection.
This meta-analysis has several strengths. First, a comprehensive literature search strategy was applied to minimize identification and selection bias and a large number of studies from 6 countries covering 4 continents (Asia, Australia, Europe, and North America) were identified as evaluating the role of anti-HBc positivity in the risk of HCC in HBsAg-negative subjects. Second, the extensive amount of data reviewed and the large sample size. Third, the majority of the 26 studies included were of average to high quality, as assessed by the NOS. However, there are some limitations which should be addressed when interpreting the findings of this meta-analysis. First, the NOS quality assessment scale has been criticized for potential interoperator variability. In response to this limitation, an independent evaluation by 2 investigators combined with arbitrage was performed to increase the reliability of scoring. Second, caution is warranted in interpreting some of the overall estimates provided in this study as there was significant heterogeneity. Third, the findings are based on the results of observational studies and, therefore, as in observational studies themselves, recall and selection biases cannot be ruled out, and it is not possible to exclude potential confounding by various variables associated with exposure. Although almost all the studies included adjusted for confounding factors relevant to the outcome of interest, the number and types of adjusted factors were different and, therefore, we cannot rule out the possibility that some other unmeasured factor might have been partly responsible for the association observed. Finally, we did not search for unpublished studies, and this meta-analysis included only studies which were published in English and, as in any meta-analysis of published data, a publication bias may have occurred because small studies with null results tend not to be published, but there was no statistical evidence of a nonpublication bias from the visualization of the funnel plot or from Begg and Egger tests. Despite the above limitations, the findings of this meta-analysis indicate an increased risk of HCC in HBsAg-negative chronic liver disease.
In conclusion, the present meta-analysis showed that the presence of anti-HBc, a reliable marker of occult HBV replication, is significantly associated with the risk of HCC in HBsAg-negative chronic liver disease. This is evident in Asian and non-Asian populations, in different stages of chronic hepatitis, in HCV etiology, and in patients with or without circulating anti-HBs. The risk of HCC seems to be lower in anti-HBs/anti-HBc-positive patients than in those with “isolated” anti-HBc, suggesting some inhibitory effect of anti-HBs on occult HBV replication.
Footnotes
Abbreviations: CI = confidence interval, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, NOS = Newcastle-Ottawa Assessment Scale, OR = odds ratio; RR = relative risk, SE = standard error.
Author contributions: NC was responsible for the conception and design of the study, performed the data extraction, assessed the quality, interpreted the data, and wrote the manuscript. LO participated in the conception of the study, performed the data extraction and interpreted the data. CS performed the literature search and interpreted the data. ES critically revised the manuscript for important intellectual contribution. IFA was responsible for the conception and design of the study, assessed the quality of the studies, analyzed the data, and wrote the manuscript. All authors read and approved the final manuscript.
The authors have no conflicts of interest to disclose.
References
- 1.Sagnelli E, Stroffolini T, Mele A, et al. Chronic hepatitis B in Italy: new features of an old disease—approaching the universal prevalence of hepatitis B e antigen-negative cases and the eradication of hepatitis D infection. Clin Infect Dis 2008; 46:110–113. [DOI] [PubMed] [Google Scholar]
- 2.Dienstag JL. Hepatitis B virus infection. N Engl J Med 2008; 359:1486–1500. [DOI] [PubMed] [Google Scholar]
- 3.Sagnelli E, Sagnelli C, Pisaturo M, et al. Epidemiology of acute and chronic hepatitis B and Delta over the last 5 decades in Italy. World J Gastroenterol Hepatol 2014; 20:7635–7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fattovich G, Olivari N, Pasino M, et al. Long-term outcome of chronic hepatitis B in Caucasian patients: mortality after 25 years. Gut 2008; 57:84–90. [DOI] [PubMed] [Google Scholar]
- 5.Sagnelli E, Stroffolini T, Mele A, et al. The impact of different cofactors on the severity of chronic hepatitis B at presentation. World J Gastroenterol Hepatol 2012; 18:1616–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raimondo G, Allain JP, Brunetto MR, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol 2008; 49:652–657. [DOI] [PubMed] [Google Scholar]
- 7.Cacciola I, Pollicino T, Squadrito G, et al. Occult hepatitis B virus infection in patients with chronic hepatitis C liver disease. N Engl J Med 1999; 341:22–26. [DOI] [PubMed] [Google Scholar]
- 8.Sagnelli E, Coppola N, Scolastico C, et al. HCV genotype and “silent” HBV coinfection: two main risk factors for a more severe liver disease. J Med Virol 2001; 64:350–355. [DOI] [PubMed] [Google Scholar]
- 9.Squadrito G, Pollicino T, Cacciola I, et al. Occult hepatitis B virus infection is associated with the development of hepatocellular carcinoma in chronic hepatitis C patients. Cancer 2006; 106:1326–1330. [DOI] [PubMed] [Google Scholar]
- 10.Filippini P, Coppola N, Pisapia R, et al. Impact of occult hepatitis B virus infection in HIV patients naive for antiretroviral therapy. AIDS 2006; 20:1253–1260. [DOI] [PubMed] [Google Scholar]
- 11.Sagnelli E, Pisaturo M, Martini S, et al. Clinical impact of occult hepatitis B virus infection in immunosuppressed patients. World J Hepatol 2014; 6:384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeo W, Chan TC, Leung NW, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol 2009; 27:605–611. [DOI] [PubMed] [Google Scholar]
- 13.Coppola N, Tonziello G, Pisaturo M, et al. Reactivation of overt and occult hepatitis B infection in various immunosuppressive settings. J Med Virol 2011; 83:1909–1916. [DOI] [PubMed] [Google Scholar]
- 14.Sagnelli E, Imparato M, Coppola N, et al. Diagnosis and clinical impact of occult hepatitis B infection in patients with biopsy proven chronic hepatitis C: a multicenter study. J Med Virol 2008; 80:1547–1553. [DOI] [PubMed] [Google Scholar]
- 15.Nan X, Shi S, Yu C, et al. Meta-analysis of the association between anti-HBc seropositivity and a poor prognosis of chronic HCV infection. Hepatol Res 2010; 40:1176–1187. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stroup DF, Berlin JA, Morton SC, et al. Metaanalysis of observational studies in epidemiology: a proposal for reporting. Metaanalysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 18.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the Quality of Non Randomised Studies in Meta-Analyses. 2011. Available from http://www.ohri.ca/programs/clinical epidemiology/oxford.asp. [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959; 22:719–748. [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 22.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiratori Y, Shiina S, Zhang PY, et al. Does dual infection by hepatitis B and C viruses play an important role in the pathogenesis of hepatocellular carcinoma in Japan? Cancer 1997; 80:2060–2067. [PubMed] [Google Scholar]
- 25.Yu MC, Yuan JM, Ross RK, et al. Presence of antibodies to the hepatitis B surface antigen is associated with an excess risk for hepatocellular carcinoma among non-Asians in Los Angeles County, California. Hepatology 1997; 25:226–228. [DOI] [PubMed] [Google Scholar]
- 26.Okada S, Sato T, Okusaka T, et al. Past exposure to hepatitis B virus as a risk factor for hepatocellular carcinoma in patients with chronic liver disease. Br J Cancer 1998; 77:2028–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tagger A, Donato F, Ribero ML, et al. Case-control study on hepatitis C virus (HCV) as a risk factor for hepatocellular carcinoma: the role of HCV genotypes and the synergism with hepatitis B virus and alcohol. Brescia HCC Study. Int J Cancer 1999; 81:695–699. [DOI] [PubMed] [Google Scholar]
- 28.Dutta U, Byth K, Kench J, et al. Risk factors for development of hepatocellular carcinoma among Australians with hepatitis C: a case-control study. Aust N Z J Med 1999; 29:300–307. [DOI] [PubMed] [Google Scholar]
- 29.Hiraoka T, Katayama K, Tanaka J, et al. Lack of epidemiological evidence for a role of resolved hepatitis B virus infection in hepatocarcinogenesis in patients infected with hepatitis C virus in Japan. Intervirology 2003; 46:171–176. [DOI] [PubMed] [Google Scholar]
- 30.Pollicino T, Squadrito G, Cerenzia G, et al. Hepatitis B virus maintains its pro-oncogenic properties in the case of occult HBV infection. Gastroenterology 2004; 126:102–110. [DOI] [PubMed] [Google Scholar]
- 31.Lok AS, Everhart JE, Di Bisceglie AM, et al. Occult and previous hepatitis B virus infection are not associated with hepatocellular carcinoma in United States patients with chronic hepatitis C. Hepatology 2011; 54:434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon OS, Jung YK, Bae KS, et al. Anti-hepatitis B core positivity as a risk factor for hepatocellular carcinoma in alcoholic cirrhosis: a case-control study. Alcohol 2012; 46:537–541. [DOI] [PubMed] [Google Scholar]
- 33.Reddy A, May E, Ehrinpreis M, et al. Latent hepatitis B is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C. World J Gastroenterol 2013; 19:9328–9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takano S, Yokosuka O, Imazeki F, et al. Incidence of hepatocellular carcinoma in chronic hepatitis B and C: a prospective study of 251 patients. Hepatology 1995; 21:650–655. [PubMed] [Google Scholar]
- 35.Chiba T, Matsuzaki Y, Abei M, et al. The role of previous hepatitis B virus infection and heavy smoking in hepatitis C virus-related hepatocellular carcinoma. Am J Gastroenterol 1996; 91:1195–1203. [PubMed] [Google Scholar]
- 36.Effect of interferon-α on progression of cirrhosis to hepatocellular carcinoma: a retrospective cohort study. International Interferon-alpha Hepatocellular Carcinoma Study Group. Lancet 1998; 351:1535–1539. [PubMed] [Google Scholar]
- 37.Imazeki F, Yokosuka O, Fukai K, et al. Significance of prior hepatitis B virus infection in the development of hepatocellular carcinoma in patients with chronic hepatitis C. Dig Dis Sci 2003; 48:1786–1792. [DOI] [PubMed] [Google Scholar]
- 38.Uetake S, Yamauchi M, Itoh S, et al. Analysis of risk factors for hepatocellular carcinoma in patients with HBs antigen- and anti-HCV antibody-negative alcoholic cirrhosis: clinical significance of prior hepatitis B virus infection. Alcohol Clin Exp Res 2003; 27:47S–51S. [DOI] [PubMed] [Google Scholar]
- 39.Hasegawa I, Orito E, Tanaka Y, et al. Impact of occult hepatitis B virus infection on efficacy and prognosis of interferon-α therapy for patients with chronic hepatitis C. Liver Int 2005; 25:247–253. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka K, Nagao Y, Ide T, et al. Antibody to hepatitis B core antigen is associated with the development of hepatocellular carcinoma in hepatitis C virus-infected persons: a 12-year prospective study. Int J Mol Med 2006; 17:827–832. [PubMed] [Google Scholar]
- 41.Bruno S, Crosignani A, Maisonneuve P, et al. Hepatitis C virus genotype 1b as a major risk factor associated with hepatocellular carcinoma in patients with cirrhosis: a seventeen-year prospective cohort study. Hepatology 2007; 46:1350–1356. [DOI] [PubMed] [Google Scholar]
- 42.Ikeda K, Marusawa H, Osaki Y, et al. Antibody to hepatitis B core antigen and risk for hepatitis C-related hepatocellular carcinoma: a prospective study. Ann Intern Med 2007; 146:649–656. [DOI] [PubMed] [Google Scholar]
- 43.Adachi S, Shibuya A, Miura Y, et al. Impact of occult hepatitis B virus infection and prior hepatitis B virus infection on development of hepatocellular carcinoma in patients with liver cirrhosis due to hepatitis C virus. Scand J Gastroenterol 2008; 43:849–856. [DOI] [PubMed] [Google Scholar]
- 44.Miura Y, Shibuya A, Adachi S, et al. Occult hepatitis B virus infection as a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C in whom viral eradication fails. Hepatol Res 2008; 38:546–556. [DOI] [PubMed] [Google Scholar]
- 45.Stroffolini T, Almasio PL, Persico M, et al. Lack of correlation between serum anti-HBcore detectability and hepatocellular carcinoma in patients with HCV-related cirrhosis. Am J Gastroenterol 2008; 103:1966–1972. [DOI] [PubMed] [Google Scholar]
- 46.Ohki T, Tateishi R, Goto E, et al. Influence of anti-HBc seropositivity on the risk of hepatocellular carcinoma in HCV-infected patients after adjusting for confounding factors. J Vir Hepat 2010; 17:91–97. [DOI] [PubMed] [Google Scholar]
- 47.Tsubouchi N, Uto H, Kumagai K, et al. Impact of antibody to hepatitis B core antigen on the clinical course of hepatitis C virus carriers in a hyperendemic area in Japan: a community-based cohort study. Hepatol Res 2013; 43:1130–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang J, Sharma R, Lamerato L, et al. Is previous exposure to hepatitis B a risk factor for pancreatic cancer or hepatocellular carcinoma? J Clin Gastroenterol 2014; 48:729–733. [DOI] [PubMed] [Google Scholar]
- 49.Rong G, Wang H, Bowlus CL, et al. Incidence and risk factors for hepatocellular carcinoma in primary biliary cirrhosis. Clin Rev Allergy Immunol 2015; 48:132–141. [DOI] [PubMed] [Google Scholar]
- 50.Coppola N, Gentile I, Pasquale G, et al. Anti-HBc positivity was associated with histological cirrhosis in patients with chronic hepatitis C. Ann Hepatol 2014; 13:20–26. [PubMed] [Google Scholar]
- 51.Matsue K, Kimura S, Takanashi Y, et al. Reactivation of hepatitis B virus after rituximab-containing treatment in patients with CD20-positive B-cell lymphoma. Cancer 2010; 116:4769–4776. [DOI] [PubMed] [Google Scholar]
- 52.Kim SJ, Hsu C, Song YQ, et al. Hepatitis B virus reactivation in B-cell lymphoma patients treated with rituximab: analysis from the Asia Lymphoma Study Group. Eur J Cancer 2013; 49:3486–3496. [DOI] [PubMed] [Google Scholar]
- 53.Lee SS, Jeong SH, Jang ES, et al. Prospective cohort study on the outcomes of hepatitis C virus-related cirrhosis in South Korea. J Gastroenterol Hepatol 2015; 30:1281–1287. [DOI] [PubMed] [Google Scholar]
- 54.Ilan Y, Gabay E, Amit G, et al. Suppression of human hepatoma in mice through adoptive transfer of immunity to the hepatitis B surface antigen. J Hepatol 1997; 27:170–175. [DOI] [PubMed] [Google Scholar]
- 55.Gotsman I, Alper R, Klein A, et al. Inducing oral immune regulation of hepatitis B virus envelope proteins suppresses the growth of hepatocellular carcinoma in mice. Cancer 2002; 94:406–414. [DOI] [PubMed] [Google Scholar]


