Supplemental Digital Content is available in the text
Keywords: mortality, Parkinson's disease, risk factors, socioeconomic status
Abstract
Little is known about the role of socioeconomic status in relation to Parkinson's disease (PD) risk, and no study has investigated whether the impact of socioeconomic status on all-cause mortality differs between individuals with and without PD.
In this population-based prospective study, over 4.6 million Swedish inhabitants who participated in the Swedish census in 1980 were followed from 1981 to 2010. The incidence rate of PD and incidence rate ratio were estimated for the association between socioeconomic status and PD risk. Age-standardized mortality rate and hazard ratio (HR) were estimated for the association between socioeconomic status and all-cause mortality for individuals with and without PD.
During follow-up, 66,332 incident PD cases at a mean age of 76.0 years were recorded. Compared to individuals with the highest socioeconomic status (high nonmanual workers), all other socioeconomic groups (manual or nonmanual and self-employed workers) had a lower PD risk. All-cause mortality rates were higher in individuals with lower socioeconomic status compared with high nonmanual workers, but relative risks for all-cause mortality were lower in PD patients than in non-PD individuals (e.g., for low manual workers, HR: 1.12, 95% confidence interval [CI]: 1.09–1.15 for PD patients; HR: 1.36, 95% CI: 1.35–1.36 for non-PD individuals).
Individuals with lower socioeconomic status had a lower PD incidence compared to the highest socioeconomic group. Lower socioeconomic status was associated with higher all-cause mortality among individuals with and without PD, but such impact was weaker among PD patients.
1. Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disorder after dementia and affects over 1% of individuals >60 years of age.[1] The underlying mechanisms for PD remain unclear, and current treatments are symptomatic and cannot halt the disease progression. As the disease progresses, motor as well as nonmotor symptoms result in poor quality of life and disability,[2,3] placing a large burden on the health care system and leading to on an average 2-fold increased mortality rate among PD patients compared to the general population.[1,4]
Research about socioeconomic status (SES) in relation to disease risk is important both in terms of formulating new hypotheses about the role of environment and social factors in disease etiology, and developing equitable health care social policies. Numerous studies have well demonstrated that low SES is associated with risk of chronic diseases, including cardiovascular diseases and dementia.[5,6] However, very few studies investigated the role of SES in relation to PD risk and with conflicting results.[7,8] In addition, although it is known that lower SES is associated with increased mortality in the general population,[9] no study has examined whether the impact of SES on all-cause mortality is modified by PD.
Apart from being a progressive disease substantially impairing patients’ activities of daily living and quality of life even in early to mid-stage,[10,11] PD is also of great public health importance especially given the aging worldwide population.[12,13] In Sweden, total direct healthcare cost for PD patients was estimated at 1.7 billion Swedish kronor during 2009, out of which 48% were costs for outpatient healthcare and drugs.[14] We therefore conducted a population-based cohort study of over 4.6 million Swedish inhabitants between 1981 and 2010, to examine the association between SES and PD risk as well as the impact of SES on all-cause mortality in individuals with and without PD.
2. Methods
2.1. Study population
In 1960, 1970, 1980, and 1990, population and housing censuses were performed by Statistics Sweden. The census questionnaires were sent to all Swedish inhabitants who were above the age of 16 at the time of the census, with a mandatory answering request. The present study was based on the 1980 census (n = 8,318,187, response rate 99%).[15] The questionnaires included detailed information on housing, marital status, highest level of education, income, occupation, and social class. Using the unique Swedish personal identification number assigned to all residents, we linked all persons in the censuses to several Swedish nationwide registers. The study was approved by The Regional Ethics Review Board in Stockholm.
Previous studies have reported that individuals >30 years of age have a stable occupation and social class throughout life course.[16–18] We therefore excluded participants <30 years of age, leaving 5,016,713 participants in the study. In total, 818 participants were also excluded due to incorrect or missing record of personal identification number, date of death or migration, and SES information. Among the remaining participants, we further excluded those with a record of PD diagnosis (n = 8,406), death (n = 27,066), and migration (n = 349,595) before start of follow-up on January 1, 1981. Thus, 4,630,828 individuals were included in the study population, including 2,243,574 (48.5%) men and 2,387,254 (51.5%) women (Fig. 1).
Figure 1.

Flowchart describing the study population.
2.2. Assessment of socioeconomic status and covariates
The Swedish socioeconomic index (SEI) distinguishes employers and employees, and classifies occupations into different social classes.[19] We used SEI information primarily from the 1980 census. For individuals who could not be classified according to SEI in the 1980 census (n = 1,173,184, of which pensioners accounted for 85.3%), whenever possible, we obtained this information from the 1970 or 1960 censuses.[20] We then categorized the SEI into 8 socioeconomic groups: higher nonmanual workers (including intermediate nonmanual, SEI-46, 56), lower nonmanual workers (SEI-33, 36), higher manual workers (SEI-21, 22), lower manual workers (SEI-11, 12), self-employed workers (company or farm owners, SEI-60, 79), farmers (SEI-89), pensioners (SEI-95), and unclassifiable employed workers (house-workers and employed individuals that could not be assigned to an occupational class, SEI-91, 96, 97, 98, 99). The first 4 groups can be considered ordered from the highest to the lowest SES, whereas the remaining may not be ordered.
Information on age, sex, marital status, and area of living was also collected from the 1980 census. Marital status was categorized into 2 groups: unmarried/divorced/widow/living alone or married/cohabiting. Area of living was categorized into urban (3 largest cities in Sweden) or rural.
2.3. Ascertainment of Parkinson's disease and all-cause death
The Swedish National Patient Register (NPR) started to collect hospital discharge records in 1964 and became nationwide in 1987; since 2001, this register also collects information on hospital-based outpatient specialist visits.[21] Diagnoses in the NPR were coded according to the Swedish revisions of the International Classification of Diseases (ICD).[22] We identified PD patients as individuals with any PD diagnosis in the NPR, considering both primary and secondary diagnoses. The ICD codes used for PD were: 350 (ICD-7, 1964–68), 342 (ICD-8, 1969–86), 332.0 (ICD-9, 1987–96), and G20 (ICD-10, 1997–2010). As PD has gradual onset, the index date for a PD diagnosis was defined as date of first hospital admission or outpatient contact. Our previous validation of hospital discharge diagnosis of PD against clinical diagnosis showed a positive predictive value of 70.8% and a sensitivity of 72.7%.[23]
The Swedish Cause of Death Register (CDR), with nationwide coverage since 1961, includes information on date of death as well as underlying and contributory causes of death.[24] In the present study, all-cause death was defined as any death record in the CDR.
2.4. Statistical analyses
2.4.1. Association between SES and PD risk
To examine the association between SES and PD risk, we followed all individuals who were free of PD at baseline until index date of PD diagnosis, date of emigration, date of death, or end of follow-up on December 31, 2010. First, we calculated incidence rates (IRs) of PD per 100,000 person-years for different SES groups. To estimate incidence rate ratios with 95% confidence intervals (CIs) for PD risk by different SES, we used Cox proportional hazards regression with attained age as the underlying timescale. We present a multivariable model further adjusting for sex, marital status, and area of living. The highest socioeconomic group, higher nonmanual workers, was used as the reference category in all analyses. We performed both analyses in the entire population and analyses stratified by sex. To examine whether the association between SES and PD risk varied across different ages, we further stratified the analyses by age group (30–64, 65–74, and 75+ years). Finally, to examine whether health-seeking behavior influences the observed association with PD, we conducted sensitivity analyses by adjusting for total number of hospital visits for causes other than PD during the follow-up period.
2.4.2. Association between SES and all-cause mortality among individuals with and without PD
To assess the association between SES and all-cause mortality, all participants were followed from baseline until date of death, date of emigration, or end of follow-up on December 31, 2010. First, we calculated age-standardized all-cause mortality rates per 100,000 person-years. We then estimated hazard ratios (HRs) with 95% CIs for all-cause mortality in a multivariable Cox proportional hazards regression model with attained age as the underlying timescale. To explore whether PD modified the association between SES and all-cause mortality, we treated PD diagnosis as a time-varying covariate and performed interaction tests by stratifying participants by PD diagnosis. For example, the follow-up time for an individual who did not have a PD diagnosis at baseline but developed PD during the follow-up was categorized into a PD-free period and a PD period.
All statistical analyses were performed with Stata, Version 13 (StataCorp LP, College Station, TX).
3. Results
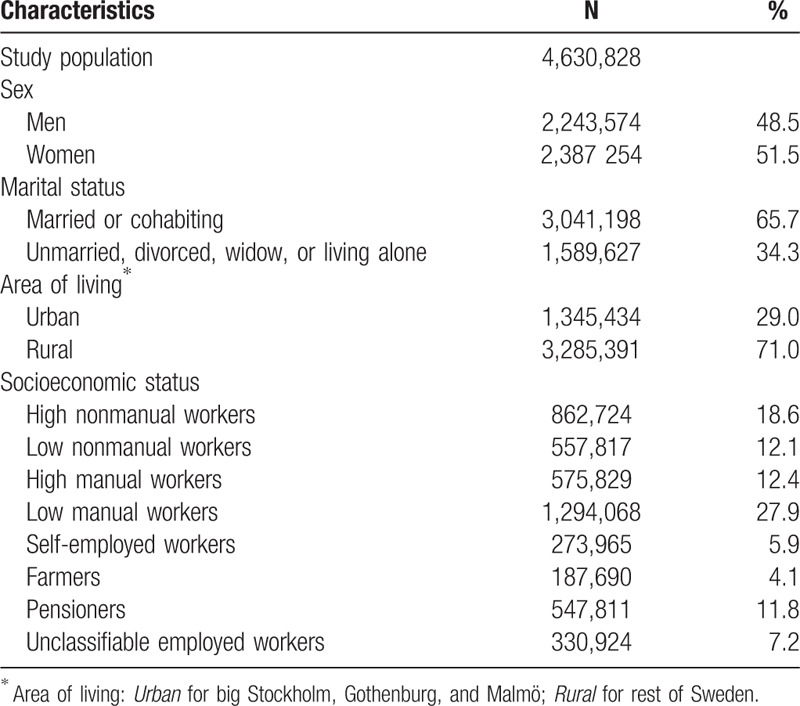
Table 1 shows baseline characteristics of the study population. Men were slightly older at start of follow-up than women (mean age 55.7 [standard deviation {SD} 16.0] years for men and 53.5 [SD 15.2] years for women). About one-third of the participants lived alone, in an urban area, or were classified as low manual workers.
Table 1.
Baseline characteristics of the study population.
3.1. Association between SES and PD risk
During a mean of 21.2 (SD 10.0) years of follow-up, we identified 66,332 incident PD cases (IR: 67.3/100,000 person-years), including 36,807 men (IR: 79.1/100,000 person-years) and 29,525 women (IR: 56.8/100,000 person-years). Mean age at the index date of PD diagnosis was 76.0 (SD 8.2) years, 75.5 (SD 8.3) for men, and 76.7 (SD 8.1) for women.
Individuals with lower SES had generally lower incidence of PD (Table 2). Compared to high nonmanual workers, high manual and low manual workers had a 12% and 7% lower incidence of PD, respectively. There was no clear dose–response relationship between SES and incidence of PD. The age group was a statistically significant effect modifier for the association between SES and PD risk (P < 0.05 for the interaction test; see Figure, Supplementary Digital Content 1 which demonstrates the age-dependent effect of SES for PD risk). In older ages, the inverse associations appeared to be weaker for low nonmanual and high manual workers (see Table, Supplementary Digital Content 2 which illustrates the associations between SES and PD risk by different age groups). Results from sensitivity analyses by adjusting for hospital visits as a proxy of health-seeking behavior were similar to the main analyses (data not shown).
Table 2.
Multivariable-adjusted incidence rate ratios and 95% confidence intervals of socioeconomic status in relation to Parkinson's disease risk.

3.2. Association between SES and all-cause mortality
In total 2,547,058 participants died during a mean of 21.3 (SD 10.0) years of follow-up, including 1,270,967 men and 1,276,091 women. Mean age at death was 77.0 (SD 11.1) years for men and 81.5 (SD 10.7) years for women. Mean survival time from the index date of PD diagnosis was 4.5 (SD 4.0) years for PD patients, with female PD patients surviving on average 1 year longer compared to male PD patients (5.0 [SD 4.2] years for women and 4.1 [SD 3.8] years for men).
Among individuals without PD, individuals with lower SES had higher all-cause mortality rate compared to high nonmanual workers (Table 3). The inverse associations between SES and all-cause mortality were also observed among PD patients, although attenuated (P < 0.01 for the interaction test). However, the standardized mortality rates among PD patients were on average 3-fold higher compared to individuals without PD (see Figure, Supplementary Digital Content 3 which demonstrates the estimated morality rate by SES). Results were similar in the analyses stratified by sex (data not shown).
Table 3.
Multivariable-adjusted hazard ratios and 95% confidence intervals of socioeconomic status in relation to all-cause mortality.

4. Discussion
With >4.6 million participants, to our knowledge, this is the first and largest nationwide cohort study investigating the impact of SES on PD risk and all-cause mortality for PD patients. Compared to the highest socioeconomic group, we observed a lower incidence of PD in individuals with lower SES. All-cause mortality was higher in individuals with lower SES and PD patients had in general 3-fold higher all-cause mortality rate compared to non-PD individuals. Our findings also showed that the association between SES and all-cause mortality was weaker in PD patients than non-PD individuals.
4.1. Association between SES and PD risk
A possible explanation for the lower risk of PD in lower socioeconomic groups is factors related to SES, such as smoking and physical activity. There is a well-documented inverse association between smoking and PD risk, and during the past decades, the prevalence of daily smoking has been greater among lower socioeconomic groups in Sweden.[25] In addition, physical activity, also associated with lower PD risk,[26] may be related to SES such that individuals with lower SES (e.g., manual workers) may have higher physical activity level than nonmanual workers.
Few studies have examined the association between SES and PD risk. Most of these studies used a surrogate (e.g., a specific occupation) for SES, or included the surrogate as a covariate in the analyses, reporting conflicting results.[27–31] A previous Swedish nationwide study used education as a marker for SES and reported that individuals with higher educational level had increased risk of PD among men,[29] corroborating our results. Another study showed that some occupational groups such as construction and extractive workers (e.g., miners, oil well drillers) and production workers (e.g., machine operators, fabricators), which were similar to low-manual workers in our study, were associated with a decreased PD risk.[30] A third study in the U.S. reported that PD diagnoses were more likely to be recorded on death records among individuals with higher income than individuals with lower income.[31]
4.2. Association between SES and all-cause mortality
The present study, to our knowledge, is the first examining whether the association between SES and all-cause mortality differs in PD patients compared to non-PD individuals. We observed that lower socioeconomic groups had a higher mortality rate, and a similar pattern in PD patients as in non-PD individuals but the effect of SES on all-cause mortality was weaker in PD patients.
The observed attenuation of the relative effect of SES on all-cause mortality in PD patients can be explained by different underlying mortality rates between non-PD individuals and PD patients. As the underlying mortality rates are much lower in non-PD individuals than PD patients on an absolute scale, in the comparison on a relative scale with the underlying mortality rates as denominator, small differences in mortality rates in the numerator can have greater impact, resulting in larger HR differences in non-PD individuals than PD patients. In addition, PD patients were regularly followed-up within the health care system in Sweden, making them better controlled regarding other diseases, such as diabetes, hypertension, and so on, which are likely to be associated with SES.
There are several possible explanations for the higher mortality rates in lower socioeconomic groups in PD patients as well as in the general population. Lifestyle factors such as unhealthy dietary habits, lack of physical activity, obesity, and smoking are associated with chronic diseases such as cardiovascular diseases including hypertension and diabetes, as well as cancer, and tend to be more common in lower socioeconomic groups, leading to higher all-cause mortality.[25,32] Although welfare programs have been implemented, socioeconomic inequalities still exist in several western European countries, indicating that more efficient welfare policies may be needed.[33] Among PD patients, infections such as pneumonia, accidental falls, and fractures can lead to higher mortality and may be associated with SES if there is less support from caregivers (e.g., family members) and lack of health care resources in low socioeconomic groups. These factors may also induce depression and stress that disrupt social connectedness,[12] which, in turn, may trigger other diseases such as cardiovascular and psychiatric diseases leading to high mortality.[34]
4.3. Strengths and limitations
Strengths of our study include the population-based cohort design with a nationwide study sample and up to 30 years of follow-up. The population-based design can minimize selection bias, of particular importance when studying a variable such as SES. Other strengths include our ability to study both PD incidence and all-course mortality among PD patients as well as non-PD individuals by different socioeconomic groups. In addition, all exposure information was obtained independently from disease ascertainment, limiting potential for recall bias and reverse causation. Further, information on SES was classified according to a standard index, ensuring high validity.
This study has some limitations. First, one may question the lack of updated information on SES during our long follow-up period. However, although the Swedish labor market had undergone great changes during the follow-up period, most workers retained the same occupation with similar SES over time.[35] Admittedly, although quite rare, misclassification of SES may still exist in the census data. Given the prospective design and the standard classification of SES, we believe that potential misclassification of the exposure would be nondifferential, theoretically leading to underestimation of the observed associations. Second, we obtained PD diagnoses from the National Patient Register in which inpatient PD diagnoses have been validated against clinical diagnoses showing good accuracy; however, misclassification between PD and other parkinsonian disorders occurs.[23] We have no reason to believe that the magnitude of this misclassification differs by SES, therefore resulting in a bias toward the null. Another potential limitation in our study is surveillance bias;[36] participants in higher socioeconomic groups may be more health-conscious, more likely to seek care, resulting in earlier detection of PD. We examined this potential bias in the sensitivity analyses by adjusting for number of hospital visits for reasons other than PD as a proxy for health-seeking behavior; however, results were similar. Last, the present study lacked information on possible individual risk factors such as smoking, limiting the possibility to examine underlying mechanisms.
5. Conclusions
In Sweden, compared to the highest socioeconomic group, individuals with lower SES were associated with a lower incidence of PD identified from national registries. Our findings also showed that lower SES was associated with higher all-cause mortality among individuals with and without PD, but this association was weaker among PD patients.
Supplementary Material
Footnotes
Abbreviations: CDR = Swedish Cause of Death Register, ICD = International Classification of Diseases, NPR = Swedish National Patient Register, PD = Parkinson's disease, SES = socioeconomic status, SEI = Swedish socioeconomic index.
Funding: This work was supported by the Swedish Research Council (grant number 521-2013-2488) and the Swedish Society of Medicine. FF was supported by the Swedish Society of Medical Research and the Karolinska Institutet. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Wirdefeldt K, Adami HO, Cole P, et al. Epidemiology and etiology of Parkinson's disease: a review of the evidence. Eur J Epidemiol 2011; 26 suppl 1:S1–S58. [DOI] [PubMed] [Google Scholar]
- 2.Soh SE, Morris ME, McGinley JL. Determinants of health-related quality of life in Parkinson's disease: a systematic review. Parkinsonism Relat D 2011; 17:1–9. [DOI] [PubMed] [Google Scholar]
- 3.Hechtner MC, Vogt T, Zollner Y, et al. Quality of life in Parkinson's disease patients with motor fluctuations and dyskinesias in five European countries. Parkinsonism Relat D 2014; 20:969–974. [DOI] [PubMed] [Google Scholar]
- 4.Findley L, Aujla M, Bain PG, et al. Direct economic impact of Parkinson's disease: a research survey in the United Kingdom. Mov Disord 2003; 18:1139–1145. [DOI] [PubMed] [Google Scholar]
- 5.Cox AM, McKevitt C, Rudd AG, et al. Socioeconomic status and stroke. Lancet Neurol 2006; 5:181–188. [DOI] [PubMed] [Google Scholar]
- 6.Yaffe K, Falvey C, Harris TB, et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ 2013; 347:f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horsfall L, Petersen I, Walters K, et al. Time trends in incidence of Parkinson's disease diagnosis in UK primary care. J Neurol 2013; 260:1351–1357. [DOI] [PubMed] [Google Scholar]
- 8.Wirdefeldt K, Gatz M, Pawitan Y, et al. Risk and protective factors for Parkinson's disease: a study in Swedish twins. Ann Neurol 2005; 57:27–33. [DOI] [PubMed] [Google Scholar]
- 9.Mackenbach JP, Stirbu I, Roskam AJ, et al. Socioeconomic inequalities in health in 22 European countries. N Engl J Med 2008; 358:2468–2481. [DOI] [PubMed] [Google Scholar]
- 10.Kluger BM, Brown RP, Aerts S, et al. Determinants of objectively measured physical functional performance in early to mid-stage Parkinson disease. PM R 2014; 6:992–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chekani F, Bali V, Aparasu RR. Quality of life of patients with Parkinson's disease and neurodegenerative dementia: a nationally representative study. Res Social Adm Pharm 2016; 12:604–613. [DOI] [PubMed] [Google Scholar]
- 12.Soleimani MA, Negarandeh R, Bastani F, et al. Disrupted social connectedness in people with Parkinson's disease. Br J Community Nurs 2014; 19:136–141. [DOI] [PubMed] [Google Scholar]
- 13.Jennum P, Zoetmulder M, Korbo L, et al. The health-related, social, and economic consequences of parkinsonism: a controlled national study. J nNur 2011; 258:1497–1506. [DOI] [PubMed] [Google Scholar]
- 14.Lokk J, Borg S, Svensson J, et al. Drug and treatment costs in Parkinson's disease patients in Sweden. Acta Neurol Scand 2012; 125:142–147. [DOI] [PubMed] [Google Scholar]
- 15.Statistic Sweden. Population and Housing Census 1980. Stockholm: Statistic Sweden; 2009. http://www.scb.se/folkochbostadsrakningen1980_sos_/. (in Swedish: Folk- och bostadsräkningen 1980). [Google Scholar]
- 16.Bohlmark A, Lindquist MJ. Life-cycle variations in the association between current and lifetime income: replication and extension for Sweden. J Labor Econ 2006; 24:879–896. [Google Scholar]
- 17.Harkonen J, Bihagen E. Occupational attainment and career progression in Sweden. Eur Soc 2011; 13:451–479. [Google Scholar]
- 18.Breen R, Jonsson JO. Explaining change in social fluidity: educational equalization and educational expansion in twentieth-century Sweden. Am J Sociol 2007; 112:1775–1810. [Google Scholar]
- 19.Statistic Sweden. Report on co-ordination issues: Socioeconomic index (SEI) 1982:4. Stockholm: Statistic Sweden; 1982. http://www.scb.se/sei/. (in Swedish: Meddelanden i Samordningsfrågor: Socioekonomisk indelning (SEI) 1982:4). [Google Scholar]
- 20.Erikson R, Jonsson JO. Commission Report to the Swedish Government: “Origin and education. Social selection to higher education”. Stockholm:Fritzes. SOU 1993:85. (in Swedish: Ursprung och utbildning. Social snedrekrytering till hogre studier). [Google Scholar]
- 21.The National Board of Health and Welfare. The Swedish National Patient Register. Stockholm: Socialstyrelsen; 2015. http://www.socialstyrelsen.se/register/halsodataregister/patientregistret/. [Google Scholar]
- 22.The National Board of Health and Welfare. International Statistical Classification of Diseases, Injuries, and Causes of Death (Swedish version). Stockholm: Socialstyrelsen; 2015. http://www.socialstyrelsen.se/klassificeringochkoder/. [Google Scholar]
- 23.Feldman AL, Johansson AL, Gatz M, et al. Accuracy and sensitivity of Parkinsonian disorder diagnoses in two Swedish national health registers. Neuroepidemiology 2012; 38:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The National Board of Health and Welfare. The Swedish Cause of Death Register. Stockholm: Socialstyrelsen; 2015. http://www.socialstyrelsen.se/register/dodsorsaksregistret/. [Google Scholar]
- 25.Public Health Agency of Sweden. Tobacco Habits - National Survey of Public Health 2014. Stockholm: Folkhälsomyndigheten; 2014. https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/statistikdatabaser-och-visualisering/nationella-folkhalsoenkaten/levnadsvanor/tobaksvanor/ Accessed September 16 2015. [Google Scholar]
- 26.Yang F, Trolle Lagerros Y, Bellocco R, et al. Physical activity and risk of Parkinson's disease in the Swedish National March Cohort. Brain 2015; 138 (pt 2):269–275. [DOI] [PubMed] [Google Scholar]
- 27.Lix LM, Hobson DE, Azimaee M, et al. Socioeconomic variations in the prevalence and incidence of Parkinson's disease: a population-based analysis. J Epidemiol Community Health 2010; 64:335–340. [DOI] [PubMed] [Google Scholar]
- 28.Caslake R, Taylor K, Scott N, et al. Age-, gender-, and socioeconomic status-specific incidence of Parkinson's disease and parkinsonism in northeast Scotland: the PINE study. Parkinsonism Relat Disord 2013; 19:515–521. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Sundquist J, Sundquist K. Socioeconomic and occupational groups and Parkinson's disease: a nationwide study based on hospitalizations in Sweden. Int Arch Occup Environ Health 2009; 82:235–241. [DOI] [PubMed] [Google Scholar]
- 30.Frigerio R, Elbaz A, Sanft KR, et al. Education and occupations preceding Parkinson disease: a population-based case-control study. Neurology 2005; 65:1575–1583. [DOI] [PubMed] [Google Scholar]
- 31.Pressley JC, Tang MX, Marder K, et al. Disparities in the recording of Parkinson's disease on death certificates. Movement Disord 2005; 20:315–321. [DOI] [PubMed] [Google Scholar]
- 32.Sommer I, Griebler U, Mahlknecht P, et al. Socioeconomic inequalities in non-communicable diseases and their risk factors: an overview of systematic reviews. BMC Public Health 2015; 15:914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackenbach JP. The persistence of health inequalities in modern welfare states: the explanation of a paradox. Soc Sci Med 2012; 75:761–769. [DOI] [PubMed] [Google Scholar]
- 34.Kawachi I. Stress and the Heart: Psychosocial Pathways to Coronary Heart Disease. BMJ 2002; 324:176. [Google Scholar]
- 35.Warnryd B, Ostlin P, Thorslund M. Living conditions. Appendix 11. Quality in retrospective questions on previous occupational exposures: an evaluation of occupational histories in the investigation on living conditions. Stockholm: Statistics Sweden; 1989. [Google Scholar]
- 36.Sackett DL. Bias in analytic research. J Chronic Dis 1979; 32:51–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


