Supplemental Digital Content is available in the text
Keywords: first-ever stroke, growth differentiation factor 15, hypertension
Abstract
Growth differentiation factor 15 (GDF-15) is a relatively new biomarker that predicts adverse stroke outcomes. However, the association of GDF-15 with first-ever stroke in hypertensive patients has not yet been evaluated. The objective of this study was to evaluate the clinical implications of plasma GDF-15 on the development of first-ever stroke in patients with hypertension.
In total, 254 patients with hypertension without a history of stroke were included from March 2010 to August 2010 and followed up until June 2013. The baseline circulating GDF-15 was determined by enzyme-linked immunosorbent assays.
During a follow-up of 3.0 ± 0.6 years, 22 (8.7%) first-ever strokes were identified, including 12 ischemic strokes and 10 intracerebral hemorrhages (ICH). According to tertiles of GDF-15, survival free of first-ever stroke was lower in the highest tertile of GDF-15 (log-rank P = 0.001). By backward stepwise Cox-regression analysis, adjusted for age, gender, diabetes mellitus, hyperlipidemia, hypertension stage, body mass index, cigarette smoking, anti-hypertensive drugs, and uric acid, every 100 pg/mL-increase in plasma of GDF-15 predicted an 11% greater risk of first-ever stroke (hazard ratios [HR]: 1.11, 95% confidence interval [CI]: 1.03–1.20, P = 0.010) and an 18% increase in ischemic stroke risk (HR: 1.18, 95% CI: 1.07–1.30, P = 0.001). Receiver operating characteristic analysis indicated that GDF-15 had reasonable accuracy to predict first-ever stroke (area under curve = 0.73, 95% CI: 0.62–0.83, P < 0.001).
This study identifies that GDF-15 is an independent predictor of first-ever stroke, especially for ischemic stroke in the patients with hypertension.
1. Introduction
Stroke is the second most common cause of death among both urban and rural residents of China. Mortality due to stroke was 116 63 per 100,000 per year in cities and 111 74 per 100,000 in rural populations.[1] The annual incidence of first-ever stroke ranged from 178 to 260 per 100,000 in China.[2] Stroke is a preventable disease. Specific focuses on primary prevention and control strategies need to be highlighted in China as ∼77% of strokes are first events.[3] Stroke is influenced by genetic and environmental risk factors, including high blood pressure, hyperlipidemia, diabetes mellitus, smoking, and unhealthy life style.[4] Hypertension is the most important risk factor of stroke. An additional 10 mm Hg systolic blood pressure increase was significantly associated with 46% increase in ischemic stroke risk and 65% increase in hemorrhagic stroke risk.[5] However, the heterogeneity of stroke incidence has been noticed worldwide in patients with hypertension. Stroke is still largely unpredictable. Therefore, identifying potential risk factors of stroke is of great importance to reduce the burden of stroke.
Growth differentiation factor 15 (GDF-15) is a distant member of transforming growth factor β superfamily. Previous studies revealed that GDF-15 is related to tumor progression and invasiveness, cardiovascular injury, and cardiovascular diseases.[6–11] GDF-15 is also a predictor of cardiovascular death in a population of coronary artery disease patients after percutaneous coronary intervention.[12] During pathological stresses, GDF-15 expression can be increased and the protein secreted into the plasma to exert protective effects.[7,8] In an experimental animal model of brain injury, GDF-15 expression is increased.[13] In patients with ischemic stroke, circulating levels of GDF-15 are elevated and associated with neurological outcome.[14] GDF-15 is also reported to be an acute stroke biomarker, independently predicting unfavorable functional stroke outcome.[15] Likewise, our previous results demonstrated that genetic variants of GDF-15 are associated with cardiac remodeling in patients with hypertension.[16] These findings provide evidence that plasma levels of GDF-15 are closely involved in the development of stroke in hypertensive patients.
We hypothesized that plasma GDF-15 may confer the susceptibility to first-ever stroke in hypertensive patients and may serve as a biomarker to predict risk for first-ever stroke in hypertensive patients. To test these hypotheses, we investigated the potential relationship between plasma GDF-15 levels and first-ever stroke in community-based hypertensive subjects.
2. Methods
2.1. Study subjects
All subjects were recruited from a community-based population of in Xinyang County, a central region of China. In total, 1020 subjects were invited to participate in the study from 3 rural communities from March 2010 to August 2010. A total of 298 hypertensive patients were enrolled and 27 hypertensive patients with a history of stroke were excluded. Eventually, 271 hypertensive patients without a history of stroke were recruited in the present study (see Figure S1, Supplemental Content, which illustrates the patient flowchart). All patients underwent a complete cardiac evaluation, including a detailed history and clinical examination, 12-lead electrocardiogram, and echocardiogram. Blood pressure (BP) was measured by experienced doctors. Readings were obtained from 2 separate measurements recorded at least 30 seconds apart, after 5 minutes of rest. The diagnostic criteria for hypertension were systolic blood pressure ≥140 mm Hg or a diastolic blood pressure ≥ 90 mm Hg, or the use of antihypertensive medication.[17]
All patients gave informed consent to participate in this study, which was approved by the ethics committee of Fuwai Hospital. This study was performed in keeping with the requirements of the Declaration of Helsinki.
2.2. GDF-15 measurement
Baseline venous blood was obtained from all subjects at enrollment after fasting overnight (> 12 hours). All samples were collected into sodium ethylene diamine tetra-acetic acid (EDTA) tubes. After centrifugation at 3000 × g and 4 °C for 15 minutes, the supernatants were frozen in aliquots and stored in cryotubes at –80 °C until assayed. The procedure was completed within 20 minutes. Plasma levels of GDF-15 antigen were measured using a Sandwich Enzyme-linked Immunosorbent Assay (ELISA) Development kit (R&D Systems, Minneapolis, MN), according to the manufacturer's protocol. The detection limit was 23.4 pg/mL, the inter-assay coefficient of variation was ≤ 8%, and the intra-assay coefficient of variation was ≤ 5%. Recombinant human GDF-15 was used to prepare a standard curve. All the procedures were performed at room temperature (25 °C). Plasma samples and standards were applied to a 96-well plate precoated with capture antibody and incubated for 2 hours. The plate was washed and incubated with biotinylated polyclonal goat-antihuman corin antibody. After 2 hours, the plate was washed again 5 times and incubated with streptavidin-horseradish peroxidase for 30 minutes. After washing, horseradish peroxidase substrate was added, and the reaction was stopped after 30 minutes. Optical density was measured at 450 nm using a microplate reader. Biochemical variables, including triglycerides, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and uric acid were measured with an automatic analyzer (Hitachi 7060, Hitachi, Tokyo, Japan).
2.3. Follow-up and clinical endpoint
The clinical endpoint was first-ever stroke including ischemic stroke, intracerebral hemorrhage (ICH), and subarachnoid hemorrhage.[18] Follow-up data were obtained by phone calls or direct communication with patients by trained doctors. Follow-up was performed annually until June 2013. Seventeen patients (6.3%) were lost to follow-up during the course of the study. There is no significance difference between patients successfully followed up and lost to follow up in baseline clinical characteristics (see Table S1, Supplemental Content, which illustrates baseline clinical characteristics of the study patients and patients lost to follow up). The final study subjects consisted of 254 patients.
2.4. Statistical analysis
The baseline characteristics were analyzed by analysis of variance (ANOVA) for parametric variables, by the Kruskal–Wallis test for nonparametric variables, and by the chi-square test for categorical variables. Survival estimates were calculated by the Kaplan–Meier method according to tertiles of GDF-15 level, and the log-rank test was used for comparison. The association between GDF-15 and first-ever stroke was estimated with multivariate Cox proportional-hazards regression models. Multivariate Cox analysis adjusted for age, gender, diabetes mellitus, hyperlipidemia, hypertension stage (the Seventh Report of the Joint National Committee, JNC 7),[19] body mass index, cigarette smoking, antihypertensive drugs, and uric acid. Multivariable models were fitted using backward elimination of nonsignificant factors at a 10% level. A receiver operating characteristic (ROC) curve was generated to evaluate the accuracy of GDF-15 in the prediction of first-ever stroke.
All statistical testing was 2-tailed. Results were considered statistically significant at a level of P < 0.05. All analyses were performed with PASW Statistics18.0 software (SPSS Inc., Chicago, IL).
3. Results
3.1. Baseline characteristics
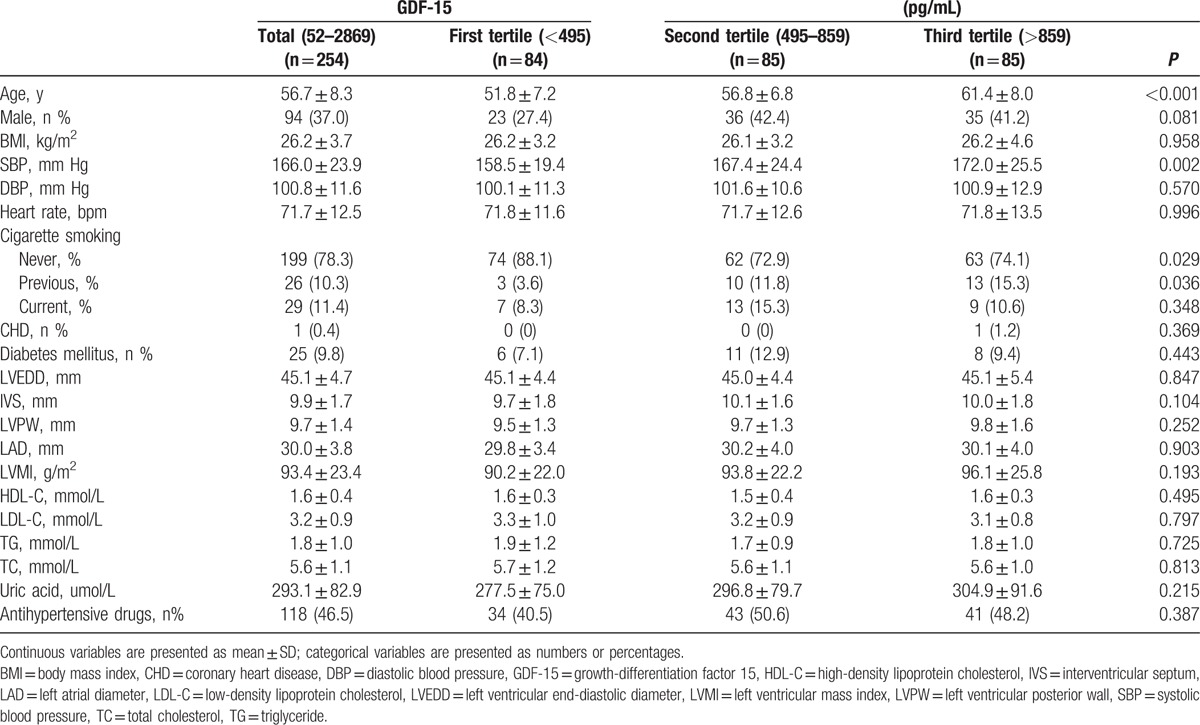
The baseline characteristics of the 254 patients with hypertension according to tertiles of GDF-15 are listed in Table 1. Patients were from 40 to 75 years old (mean age: 56.7 ± 8.3 years), 37.0% being male. Plasma GDF-15 ranged from 52 to 2869 pg/mL. Median GDF-15 for the entire study population was 650 pg/mL (interquartile range [IQR]: 386 to 1000 pg/mL). Subjects in the higher GDF-15 tertile group showed higher age, systolic BP, and percentage of smoking.
Table 1.
Baseline clinical characteristics of the study patients according to tertiles of GDF-15.
3.2. Survival analysis
During a follow-up of 3.0 ± 0.6 years, 22 (8.7%) first-ever strokes were identified, comprising 12 ischemic strokes and 10 ICH. According to tertiles of GDF-15, survival free of first-ever stroke was lower in the highest tertile of GDF-15 (log-rank P = 0.001, Fig. 1A) than that in other groups. Furthermore, survival free of ischemic stroke events and ICH events was lower in the highest tertile of GDF-15 (log-rank P = 0.026, Fig. 1B; log-rank P = 0.035, Fig. 1C, respectively) than that in other groups.
Figure 1.
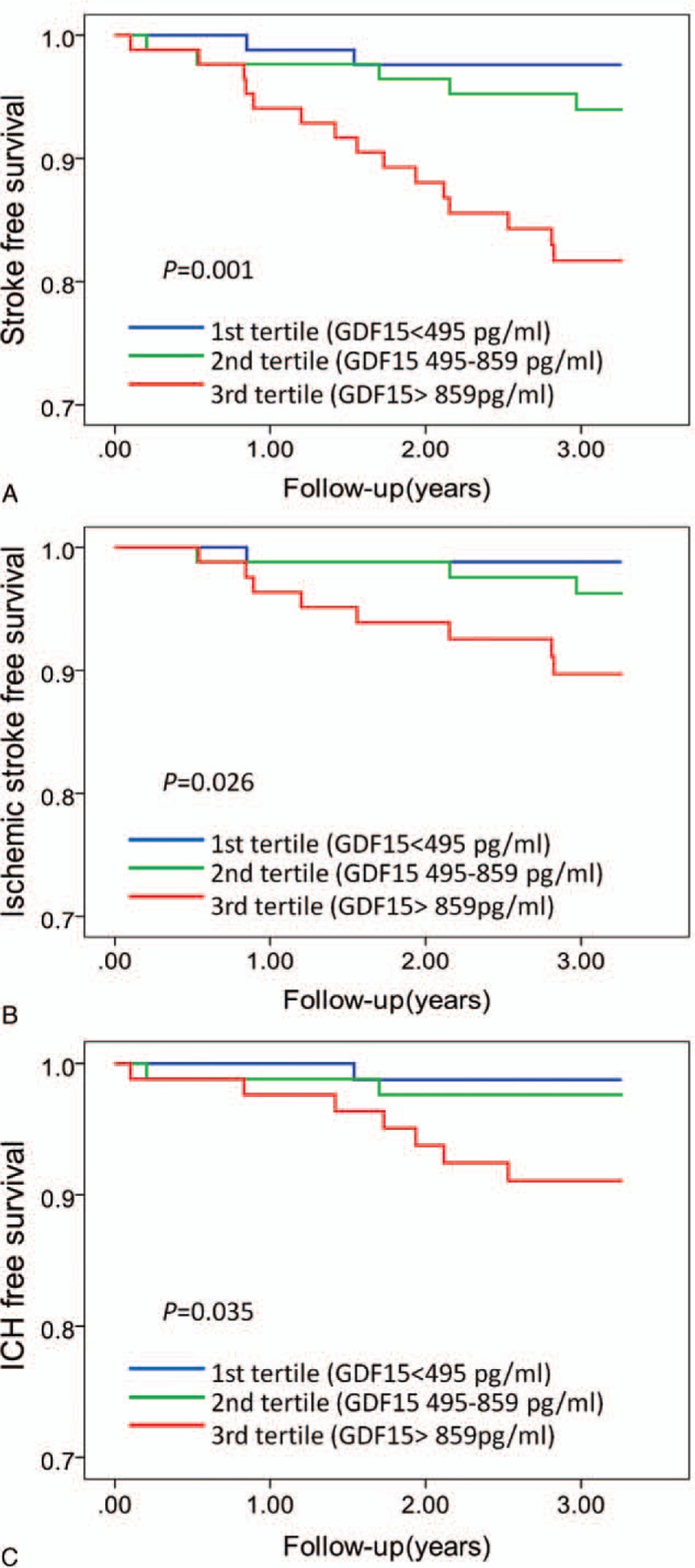
Kaplan–Meier analysis of first-ever stroke (A), ischemic stroke (B), and ICH (C) according to tertiles of GDF-15. Subjects in the highest tertile of GDF-15 had lower first-ever stroke-free survival (log-rank P = 0.001), ischemic stroke-free survival (log-rank P = 0.026), and ICH-free survival (log-rank P = 0.035) in the patients with hypertension. GDF-15 = growth differentiation factor 15; ICH = intracerebral hemorrhage.
3.3. Relation of GDF-15 to stroke
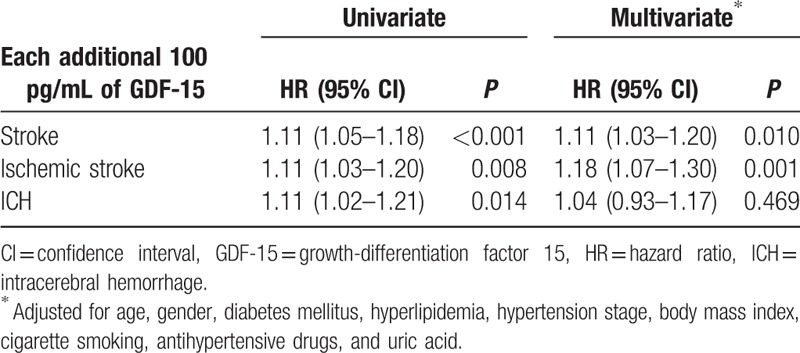
Univariate analysis showed that every 100 pg/mL increase in plasma of GDF-15 predicted an 11% increase in first-ever stroke risk (hazard ratios [HR]: 1.11, 95% confidence interval [CI]: 1.05–1.18, P < 0.001), an 11% greater risk of developing ischemic stroke (HR: 1.11, 95% CI: 1.03–1.20, P = 0.008), and 11% increase in ICH risk (HR: 1.11, 95% CI: 1.02–1.21, P = 0.014).
To further evaluate the effect of GDF-15 on stroke incidence, we used a multivariate Cox proportional hazards regression model to reveal whether the plasma GDF-15 level could predict the onset of stroke (Table 2). The continuous variable of GDF-15 (each additional 100 pg/mL) was also independently and positively related to first-ever stroke (HR: 1.11, 95% CI: 1.03–1.20, P = 0.010) and ischemic stroke (HR: 1.18, 95% CI: 1.07–1.30, P = 0.001) following adjustment for age, gender, diabetes mellitus, hyperlipidemia, hypertension stage, body mass index, cigarette smoking, antihypertensive drugs, and uric acid. After adjustment for conventional risk factors, GDF-15 was not associated with an increase in the risk of ICH (HR: 1.04, 95% CI: 0.93–1.17, P = 0.469).
Table 2.
Univariate and multivariate Cox analysis each additional 100 pg/mL of GDF-15.
3.4. Prognostic effects of GDF-15 on stroke
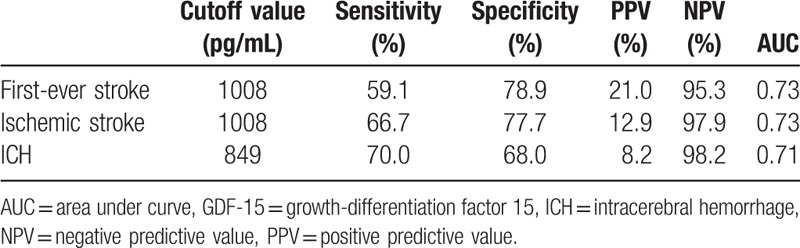
The ROC analysis indicated that GDF-15 had reasonable accuracy for prediction of stroke. The area under the ROC curve was 0.73 (95% CI: 0.62–0.83, P < 0.001) for first-ever stroke (see Figure S2, Supplemental Content, which indicates the accuracy of GDF-15 for prediction of stroke). The cutoff of GDF-15 level for predicting first-ever stroke was 1008 pg/mL and had a 95.3% negative predictive value (Table 3). The area under the ROC curve was 0.73 for ischemic stroke (95% CI: 0.58–0.87, P = 0.009) and 0.71 for ICH (95% CI: 0.56–0.85, P = 0.028) (see Figure S3 and S4, Supplemental Content, which indicate the accuracy of GDF-15 for prediction of ischemic stroke and ICH, respectively).
Table 3.
Operating characteristics of GDF-15 thresholds to predict stroke.
3.5. Application of cutoff points for risk prediction
The ROC curve-derived GDF-15 level of > 1008 pg/mL was associated with an increased risk of first-ever stroke (HR: 5.0, 95% CI: 2.1–11.8, P < 0.001) and ischemic stroke (HR: 7.1, 95% CI: 2.1–23.4, P = 0.001) (Table 4, Fig. 2A and B). Patients presenting with a GDF-15 level > 1008 pg/mL remained had a 3.9-fold increase in the risk of first-ever stroke (adjusted HR: 3.7, 95% CI: 1.4–9.9, P = 0.008) and 10.8-fold increase in the risk of ischemic stroke (adjusted HR: 10.8, 95% CI: 3.0–39.5, P < 0.001) after adjustment for age, gender, body mass index, diabetes mellitus, hyperlipidemia, cigarette smoking, hypertension stage, antihypertensive drugs, and uric acid (Table 4).
Table 4.
Univariate and multivariate Cox proportional hazards models for stroke according to cutoff value of GDF-15.
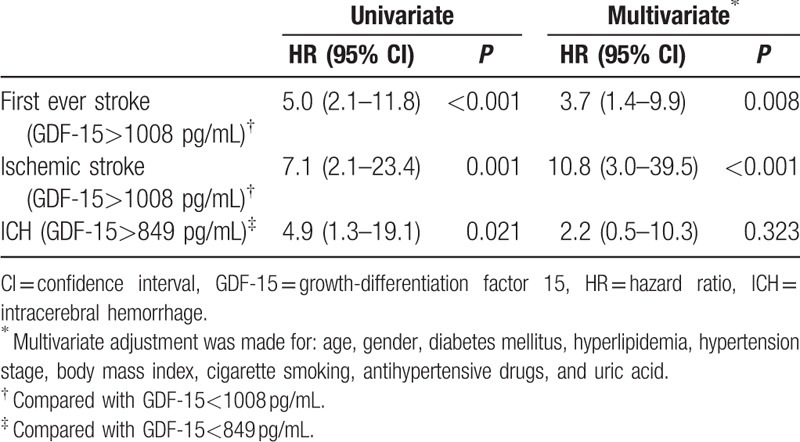
Figure 2.
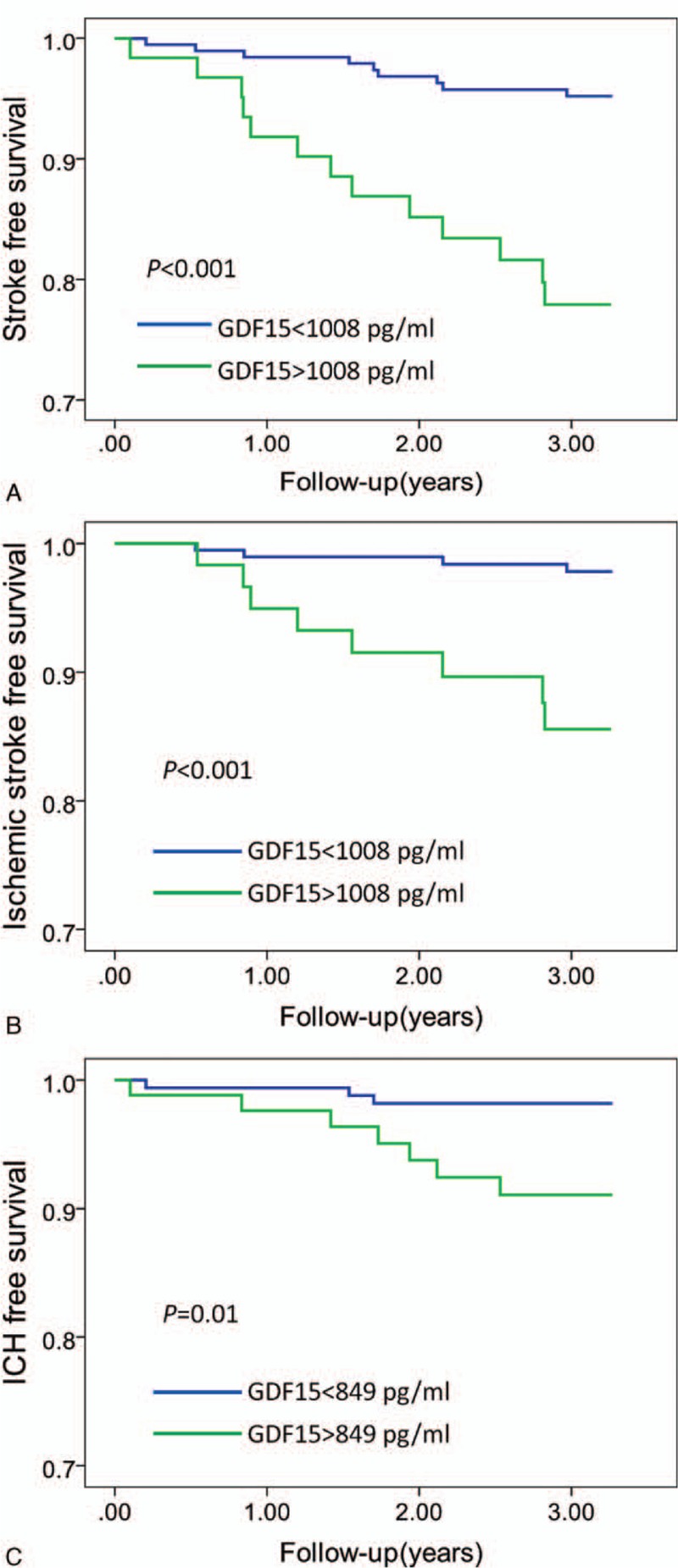
Kaplan–Meier analysis of first-ever stroke (A), ischemic stroke (B), and ICH (C) according to the cutoff value of GDF-15. Subjects with GDF-15 level > 1008 pg/mL had lower first-ever stroke-free survival (log-rank P < 0.001) and ischemic stroke-free survival (log-rank P < 0.001) in the patients with hypertension. Subjects with GDF-15 level > 849 pg/mL had lower ICH-free survival (log-rank P = 0.01) in patients with hypertension. GDF-15 = growth differentiation factor 15, ICH = intracerebral hemorrhage.
The cutoff of GDF-15 levels for predicting ICH was 849 pg/mL. Compared with that of patients with a GDF-15 level < 849 pg/mL, the HR was 4.9 (95% CI: 1.3–19.1, P = 0.021) for ICH in patients with a GDF-15 level > 849 pg/mL (Table 4, Fig. 2C). After adjustment for conventional risk factors, compared with patients having a GDF-15 level < 849 pg/mL, the association of GDF-15 level > 849 pg/mL with an increased risk of ICH was absent (adjusted HR: 2.2, 95% CI: 0.5–10.3, P = 0.323) (Table 4).
4. Discussion
In the present study, we found that elevated GDF-15 is an independent predictor for first-ever stroke, especially for ischemic stroke, in patients with hypertension. This was reasonably accurate by ROC analysis. The best ROC curve-derived GDF-15 cutoff level for predicting risk of first-ever stroke was > 1008 pg/mL, especially for ischemic stroke in patients with hypertension.
GDF-15 is closely related to plaque presence, which affects the incidence of ischemic stroke.[20] Previously, GDF-15 has been identified to be associated with neurological outcomes in patients with ischemic stroke. Circulating GDF-15 levels are increased at 6 hours after symptom onset in patients with acute ischemic stroke.[14] GDF-15 is also a stroke biomarker that independently predicts unfavorable functional stroke outcome.[15,21] In this study, we show that elevated GDF-15 level is associated with the development of first-ever stroke in the patients with hypertension. This association is independent of age, gender, and other clinical factors influencing stroke.
GDF-15 is a stress-induced cytokine. In pressure overload induced cardiac hypertrophy, the increase in GDF-15 might be protective.[8] During pathological stresses, the expression of GDF-15 can be increased and secreted into the plasma to exert protective function.[7,8] Taken together, these data indicate that GDF-15 might be a functionally protective factor in response to stresses. The increased plasma levels of GDF-15 may be induced by other pathological processes of stroke, such as hemodynamic and inflammatory changes,[7,22] which precede the onset of stroke. In other aspects, the increase of plasma GDF-15 level may reflect stroke severity, which affects the outcome of stroke in hypertensive patients. In this study, we showed that elevated GDF-15 was an independent predictor for ischemic stroke, but not for ICH, in patients with hypertension. Previous studies suggested that GDF-15 was one of the most important factors responsible for promoting angiogenesis under hypoxic conditions.[23] GDF-15 promotes angiogenesis under ischemic conditions, which may increase the plasma GDF-15 level in ischemia stroke. The increased plasma GDF-15 level may reflect the severity of ischemic stroke.
Given the relationship found with ischemic stroke, the lack of association of plasma GDF-15 level with ICH is intriguing and may be explained by the complexity, nature, and existing differences in the pathogenesis of large and small vessel diseases of the brain. The pathologies of different subtype of stroke are quite different.[24,25] Ischemic stroke mainly results from atherosclerosis of large arteries (those >0.1 mm in diameter); whereas ICH are caused primarily by disease of small arteries and arterioles that penetrate the brain cortex and reach the underlying structures of the white and deep gray matter. It is plausible that GDF-15 causes plaque vulnerability in large arteries and enhances the risk of intravascular thrombosis by promoting the angiogenesis process.
4.1. Limitations
There are several limitations of our study that should be noted in the interpretation of these results. First, the patients enrolled in this study come from a single center and were limited to native Chinese. These facts limit the generalizability of our findings. Second, the size of our population was small, and a prospective validation in a larger cohort is therefore recommended.
5. Conclusions
The importance of our study lies in the demonstration that a detectable circulatory factor, GDF-15, is a potential biomarker for first-ever stroke, especially for ischemic stroke in patients with hypertension.
Acknowledgments
We appreciate the cooperation of the patients and many investigators in the State Key Laboratory of Cardiovascular Disease of Fuwai Hospital.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, GDF-15 = growth-differentiation factor 15, HR = hazard ratios, ICH = intracerebral hemorrhage, ROC = receiver operating characteristic.
XJ W and LZ contributed equally to this work.
Funding: This study was supported by the Ministry of Science and Technology of China (grant no. 2012CB517804 and no. 2014CB541600), the National Natural Science Foundation of China (grant no. 91339116, and no. 81300184), the New-star program of Peking Union Medical College to Dr. Xiaojian Wang and the Key Project in the National Science & Technology Pillar Program during the 12th 5-Year Plan Period (2011BAI11B04).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Ministry of Healthy, Chinese Health Statistical Digest, 2006. Ministry of Health, People's Republic of China 2006; 45:1989–2005. [Google Scholar]
- 2.Liu M, Wu B, Wang WZ, et al. Stroke in China: epidemiology, prevention, and management strategies. Lancet Neurol 2007; 6:456–464. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation 2010; 121:e46–e215. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke 2006; 37:1583–1633. [DOI] [PubMed] [Google Scholar]
- 5.Tsukinoki R, Murakami Y, Huxley R, et al. Does body mass index impact on the relationship between systolic blood pressure and cardiovascular disease? Meta-analysis of 419 488 individuals from the Asia pacific cohort studies collaboration. Stroke 2012; 43:1478–1483. [DOI] [PubMed] [Google Scholar]
- 6.Bauskin AR, Brown DA, Kuffner T, et al. Role of macrophage inhibitory cytokine-1 in tumorigenesis and diagnosis of cancer. Cancer Res 2006; 66:4983–4986. [DOI] [PubMed] [Google Scholar]
- 7.Kempf T, Eden M, Strelau J, et al. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res 2006; 98:351–360. [DOI] [PubMed] [Google Scholar]
- 8.Xu J, Kimball TR, Lorenz JN, et al. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ Res 2006; 98:342–350. [DOI] [PubMed] [Google Scholar]
- 9.Stahrenberg R, Edelmann F, Mende M, et al. The novel biomarker growth differentiation factor 15 in heart failure with normal ejection fraction. Eur J Heart Fail 2010; 12:1309–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonaca MP, Morrow DA, Braunwald E, et al. Growth differentiation factor-15 and risk of recurrent events in patients stabilized after acute coronary syndrome: observations from PROVE IT-TIM. Arterioscler Thromb Vasc Biol 2011; 31:203–210. [DOI] [PubMed] [Google Scholar]
- 11.Lankeit M, Kempf T, Dellas C, et al. Growth differentiation factor-15 for prognostic assessment of patients with acute pulmonary embolism. Am J Respir Crit Care Med 2008; 177:1018–1025. [DOI] [PubMed] [Google Scholar]
- 12.Farhan S, Freynhofer MK, Brozovic I, et al. Determinants of growth differentiation factor 15 in patients with stable and acute coronary artery disease. A prospective observational study. Cardiovasc Diabetol 2016; 15:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schindowski K, von Bohlen und Halbach O, Strelau J, et al. Regulation of GDF-15, a distant TGF-beta superfamily member, in a mouse model of cerebral ischemia. Cell Tissue Res 2011; 343:399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Worthmann H, Kempf T, Widera C, et al. Growth differentiation factor 15 plasma levels and outcome after ischemic stroke. Cerebrovasc Dis 2011; 32:72–78. [DOI] [PubMed] [Google Scholar]
- 15.Groschel K, Schnaudigel S, Edelmann F, et al. Growth-differentiation factor-15 and functional outcome after acute ischemic stroke. J Neurol 2012; 259:1574–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Yang X, Sun K, et al. The haplotype of the growth-differentiation factor 15 gene is associated with left ventricular hypertrophy in human essential hypertension. Clin Sci (London) 2010; 118:137–145. [DOI] [PubMed] [Google Scholar]
- 17.Whitworth JA. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 2003; 21:1983–1992. [DOI] [PubMed] [Google Scholar]
- 18.Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:2064–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lenfant C, Chobanian AV, Jones DW, et al. Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): resetting the hypertension sails. Hypertension 2003; 41:1178–1179. [DOI] [PubMed] [Google Scholar]
- 20.Lind L, Siegbahn A, Lindahl B, et al. Discovery of new risk markers for ischemic stroke using a novel targeted proteomics chip. Stroke 2015; 46:3340–3347. [DOI] [PubMed] [Google Scholar]
- 21.Wallentin L, Zethelius B, Berglund L, et al. GDF-15 for prognostication of cardiovascular and cancer morbidity and mortality in men. PLoS One 2013; 8:e78797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bootcov MR, Bauskin AR, Valenzuela SM, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci U S A 1997; 94:11514–11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song H, Yin D, Liu Z. GDF-15 promotes angiogenesis through modulating p53/HIF-1alpha signaling pathway in hypoxic human umbilical vein endothelial cells. Mol Biol Rep 2012; 39:4017–4022. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg SM. Small vessels, big problems. N Engl J Med 2006; 354:1451–1453. [DOI] [PubMed] [Google Scholar]
- 25.Dufouil C, Chalmers J, Coskun O, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation 2005; 112:1644–1650. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


