Abstract
Background:
Vitrification is the standard method for cryopreserving human oocytes and embryos, but its effects on ovarian tissue are uncertain. The purpose of this meta-analysis was to compare the proportion of intact primordial follicles in ovarian tissue cryopreserved with vitrification versus slow freezing.
Methods:
Medline, Cochrane, EMBASE, and Google Scholar databases were searched until November 11, 2014 using combinations of the search terms: ovarian tissue, cryopreservation, vitrification, follicle, follicles. Inclusion criteria were randomized controlled trails, two-arm prospective studies, and retrospective studies in which ovarian tissues were preserved by vitrification or conventional slow freezing. The primary outcome was the proportion of intact primordial follicles.
Results:
Six studies were included in the meta-analysis. The number of patients ranged from 3 to 20, and age ranged from 20 to 43 years. Total number of morphologically intact follicles ranged from 14 to 2058, among which 6 to 724 were primordial. The pooled odds ratio (OR) showed no significant difference in the proportion of intact primordial follicles after slow freezing or vitrification (OR = 1.228, 95% confidence interval [CI]: 0.769–1.961, P = 0.390). Sensitivity analysis using the leave-one-out approach indicated no considerable changes in the direction and magnitude of the pooled estimates when individual studies were excluded one at a time, indicating good reliability of the current analysis.
Conclusions:
Vitrification and slow freezing produce equivalent results with respect to intact primordial follicles for the cryopreservation of human ovarian tissue. However, the included studies varied in the cryopreservation protocols used.
Keywords: cryopreservation, follicle, freezing, ovary, vitrification
1. Introduction
Cryopreservation of ovarian tissue is becoming an increasingly popular method of preserving fertility in women with cancer who require radiation and/or chemotherapy.[1,2] Autotransplantation of cryopreserved ovarian tissue has been shown to restore endocrine function,[3] and achieve live births.[1,4,5] Transplantation of ovarian tissue has also been used to restore endocrine function in cases of premature ovarian failure and ovarian dysgenesis.[6,7]
The standard method for cryopreservation of ovarian tissue is slow programmed freezing using a human serum albumin-containing medium, with propanediol, dimethyl sulphoxide, or ethylene glycol as a cryoprotectant, combined with sucrose.[4] Slow freezing, however, is problematic because the different cell types in ovarian tissue require different parameters to prevent damage from ice crystallization, and studies have reported negative effects on different ovarian tissues as a result of slow freezing.[8–12]
Vitrification is a process defined as the instant solidification of a solution as a result of an increase in viscosity during cooling without ice crystal formation.[13] Vitrification is now the most accepted method for the cryopreservation of human oocytes and embryos.[14] Vitrification seems to have advantages over slow freezing as animal studies have shown that it did not induce apoptosis in mouse and human ovarian tissue after warming.[15] However, studies on the cryopreservation of ovarian tissue with vitrification have provided conflicting results in comparison to those seen with conventional slow freezing.[16]
The purpose of this study was to perform a meta-analysis comparing the proportion of intact primordial follicles remaining in ovarian tissue cryopreserved with vitrification versus slow freezing.
2. Materials and methods
2.1. Literature search strategy
This systematic review and meta-analysis was conducted in accordance with PRISMA guidelines.[17] Medline, Cochrane, EMBASE, and Google Scholar databases were searched until November 11, 2014 using combinations of the search terms: ovarian tissue, cryopreservation, vitrification, follicle, follicles. Reference lists of relevant studies were hand-searched.
2.2. Selection criteria and data extraction
Inclusion criteria were randomized controlled trails (RCTs), 2-arm prospective studies, and retrospective studies in which ovarian tissues were retrieved from patients who underwent surgery for benign ovarian cysts and other gynecological conditions, cesarean section, or cancer and the ovarian tissue was preserved by vitrification or conventional slow freezing. Cohort study, letters, comments, editorials, case reports, proceedings, and personal communications were excluded. Studies were also excluded if there was no comparison between vitrification and conventional slow freezing, and if there were no quantitative primary outcome data reported. Studies were identified by the search strategy by 2 independent reviewers, and a third reviewer was consulted when disagreement arose.
Data extracted from studies that met the inclusion criteria were the name of the first author, year of publication, study design, number of participants in each group, participants’ age, method of ovarian tissue preservation, and proportion of intact primordial follicles. Data extraction was performed by 2 independent reviewers, and a third reviewer was consulted for any uncertainties.
2.3. Quality assessment
The Delphi list was used to assess the quality of the studies included in the meta-analysis.[18]
2.4. Outcome measures and data analysis
The primary outcome measure was the proportion of intact primordial follicles associated with vitrification versus slow freezing. Pooled odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated and compared between the vitrification and slow freezing groups, and a 2-sided value of P < 0.05 was considered statistically significant. An OR > 1 indicated that vitrification was associated with higher proportion of intact primordial follicles, whereas an OR < 1 indicated slow freezing was associated with higher proportion of intact primordial follicles. A χ2 based test of homogeneity was performed using Cochran Q statistic and I2. A P value of the Q statistic <0.10 indicates statistically significant heterogeneity. I2 indicates the percentage of the total variability in effect estimates among trials due to heterogeneity rather than chance, and an I2 > 50% indicates significant heterogeneity. A random-effects model of analysis according to DerSimonian–Laird method was used if heterogeneity was detected (I2 > 50% or Q statistic P < 0.1). Otherwise, a fixed-effects model of analysis was used.
Sensitivity analysis was performed based on the leave-one-out approach. Funnel plot and Egger test were used to evaluate the existence of publication bias. Absence of publication bias is indicated by the data points forming a symmetric funnel-shaped distribution, and a value of P > 0.05 in Egger test. All statistical analyses were performed using the statistical software Comprehensive Meta-Analysis, version 2.0 (Biostat, Englewood, NJ).
3. Results
3.1. Literature search and study characteristics
A flow diagram of study selection is shown in Fig. 1. A total of 229 studies were identified in the database searches, and 15 full text articles were reviewed after exclusion of 214 non-relevant studies. Of these 15 articles, 4 articles were excluded with reasons shown in Fig. 1. Six articles were eligible for the meta-analysis. The remaining 5 articles did not provide sufficient data for a quantitative study and were analyzed qualitatively.
Figure 1.
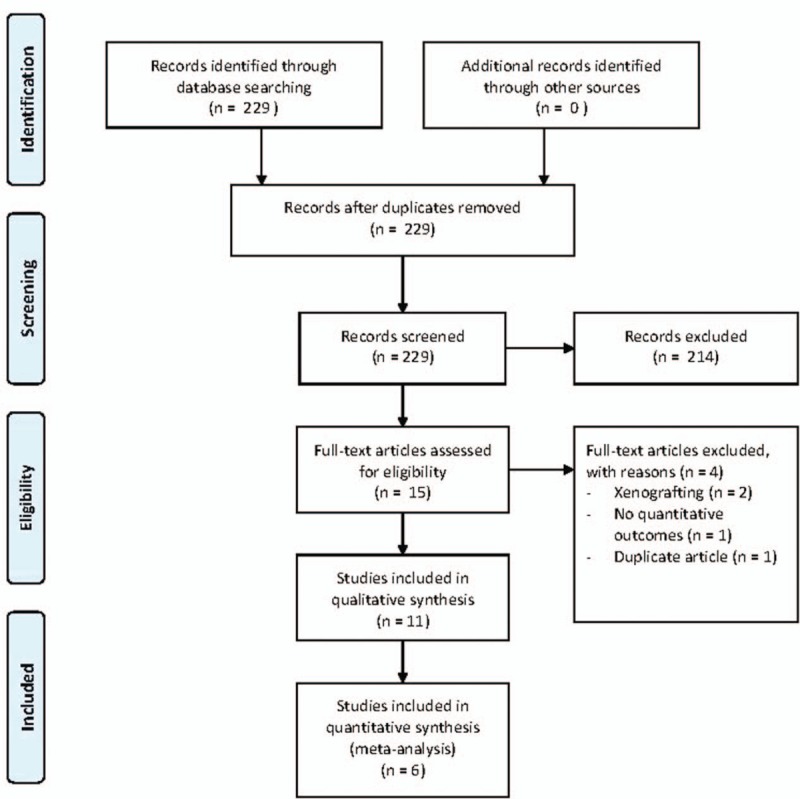
Flow diagram of study selection.
The characteristics and outcomes of the 6 studies[2,19–23] included in the meta-analysis are summarized in Table 1. The characteristics of the 5 studies[24–28] included in the qualitative review and their reasons for exclusion from the meta-analysis are summarized in Table 2. In the studies included in the meta-analysis, ovarian tissues were retrieved by laparoscopy or laparotomy performed for benign gynecologic conditions or at the time of caesarean section. The number of study patients ranged from 3 to 20, and patient age ranged from 20 to 43 years. Total number of morphologically intact follicles ranged from 14 to 2058, among which 6 to 724 were primordial.
Table 1.
Basic characteristics and outcomes of studies included in the meta-analysis.
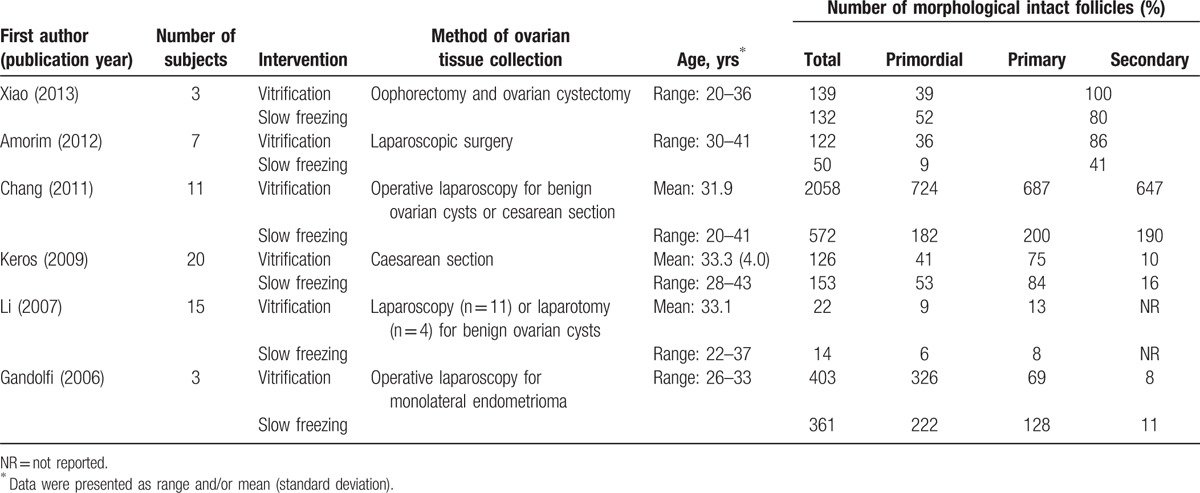
Table 2.
Basic characteristics and outcomes of studies included in the systematic review but not the meta-analysis.
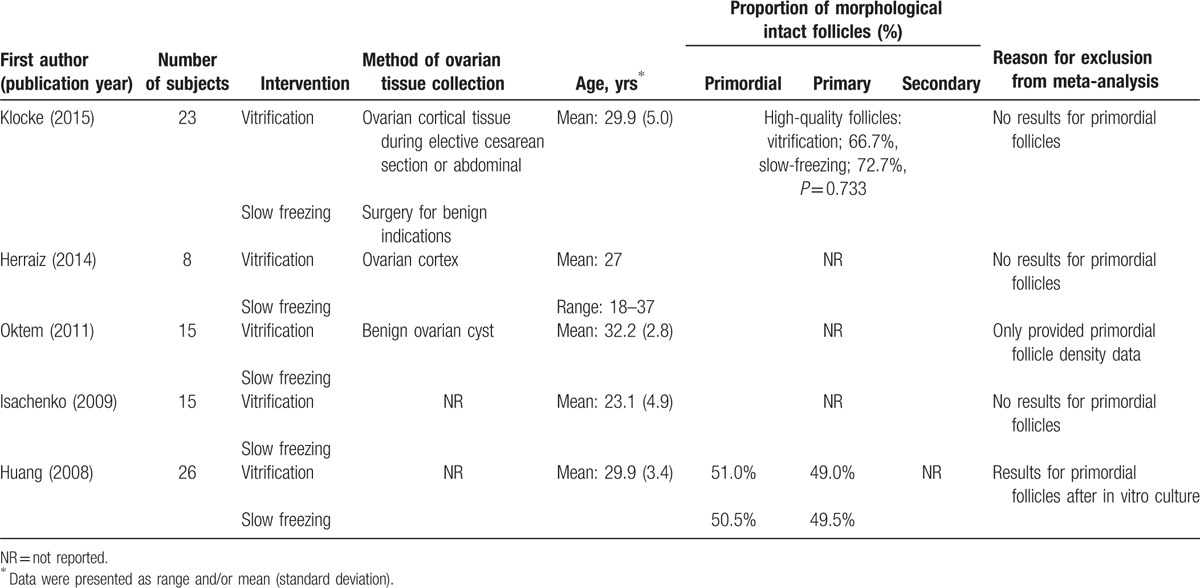
In the studies excluded from meta-analysis, Huang et al[24] compared slow-freezing and vitrification with histological observation and terminal deoxynucleotidyl transferase dUTP nick end labeling assay of the tissue after cryopreservation. They found no significant difference in the destructive effect for primordial follicles, follicular proportions, and concentration of estradiol and progesterone, but the results can’t be compared within our analysis since primordial follicle data were obtained after in vitro culture. Oktem et al[25] compared freezing and vitrification with outcomes of primordial follicle density, histological assessment, and estradiol production with in vitro culture. The vitrified strips contained significantly fewer primordial follicles compared with slow-frozen strips. The structures of the primordial follicles were preserved better in the slow-frozen ovaries compared with the vitrified ovaries, but the estradiol production was similar. The study was excluded because it only provided results with respect to primordial follicle density. Isachenko et al[26] studied the two methods with outcomes of hormone activity, follicle development, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene expression. No difference was found in hormone activity and follicle quality, but GAPDH gene expression in ovarian tissue after vitrification was dramatically decreased in contrast to conventional freezing. However, the study lack results with respect to primordial follicles. Study conducted by Klocke et al[27] only provided results of high-quality follicles and found no significant difference between methods of cryopreservation. High quality follicles were defined as follicles with an apparent intact nucleolus, clear cytoplasm, and round shape. Herraiz et al[28] compared slow-freezing and 4 vitrification methods with in vivo xenografting into nude mice. One vitrification method preserved more quiescent follicles than slow freezing, but did not provide any results for population of primordial follicles.
3.2. Proportion of intact primordial follicles
One study[23] indicated that slow freezing was associated with a significantly higher proportion of intact primordial follicles (OR = 0.600, 95% CI: 0.361–0.998, P = 0.049), while another study[19] showed that vitrification was associated with a significantly higher proportion of intact primordial follicles (OR = 2.651, 95% CI: 1.912–3.674, P < 0.001). The other 4 studies[2,20,21,22] did not find any difference in the number of intact primordial follicles between slow freezing and vitrification. There was significant evidence of heterogeneity across the 6 studies (Q = 31.42, P < 0.001, I2 = 84.09%); therefore, a random-effects model was used for the pooled estimates. The pooled OR showed no significant difference in the proportion of intact primordial follicles after slow freezing or vitrification (OR = 1.228, 95% CI: 0.769–1.961, P = 0.390; Fig. 2).
Figure 2.
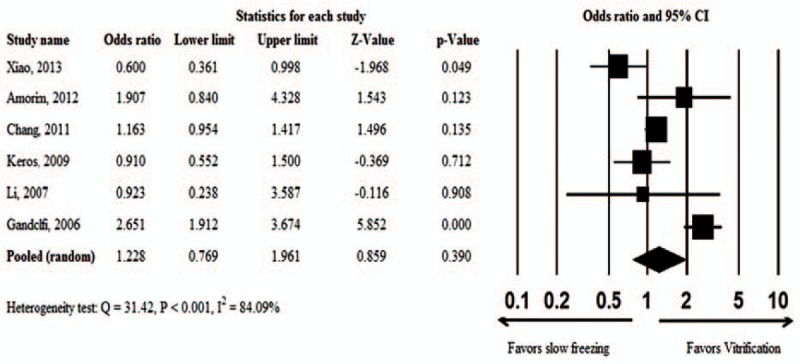
Meta-analysis of the proportion of intact primordial follicles associated with vitrification versus slow-freezing.
3.3. Sensitivity analysis, publication bias, and quality assessment
Results of the sensitivity analysis using the leave-one-out approach are shown in Fig. 3. No considerable changes in the direction and magnitude of the pooled estimates were found when individual studies were excluded one at a time, indicating good reliability of the current analysis. There was no significant evidence of publication bias based on the funnel plot (Fig. 4) or as assessed by Egger test (t = 0.188, P = 0.860).
Figure 3.
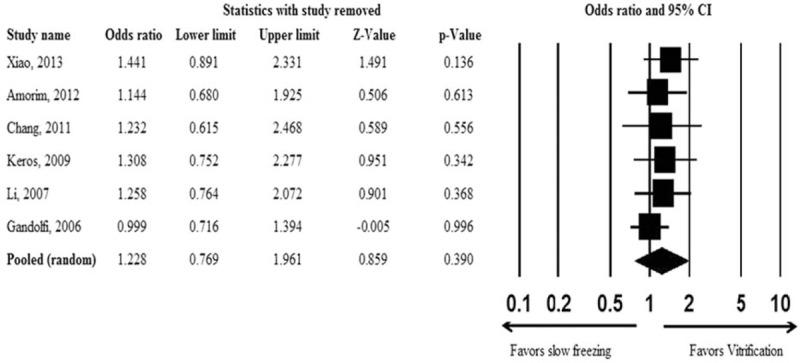
Sensitivity analysis examining the influence of individual studies on pooled estimates by the leave-one-out approach.
Figure 4.
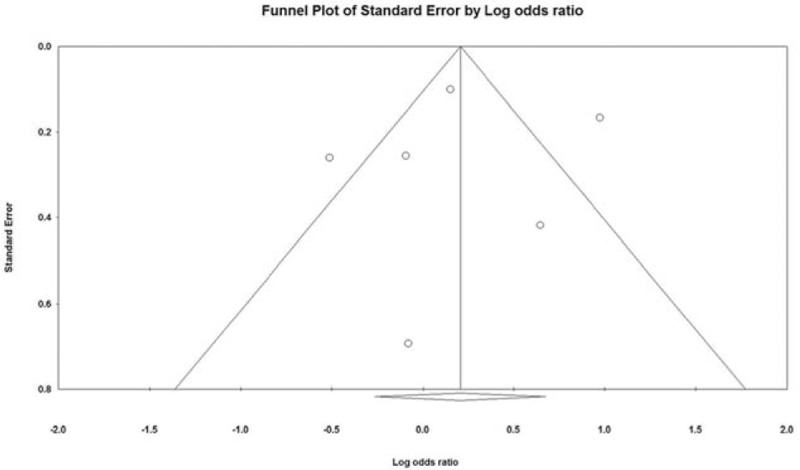
Funnel plot for examination of publication bias.
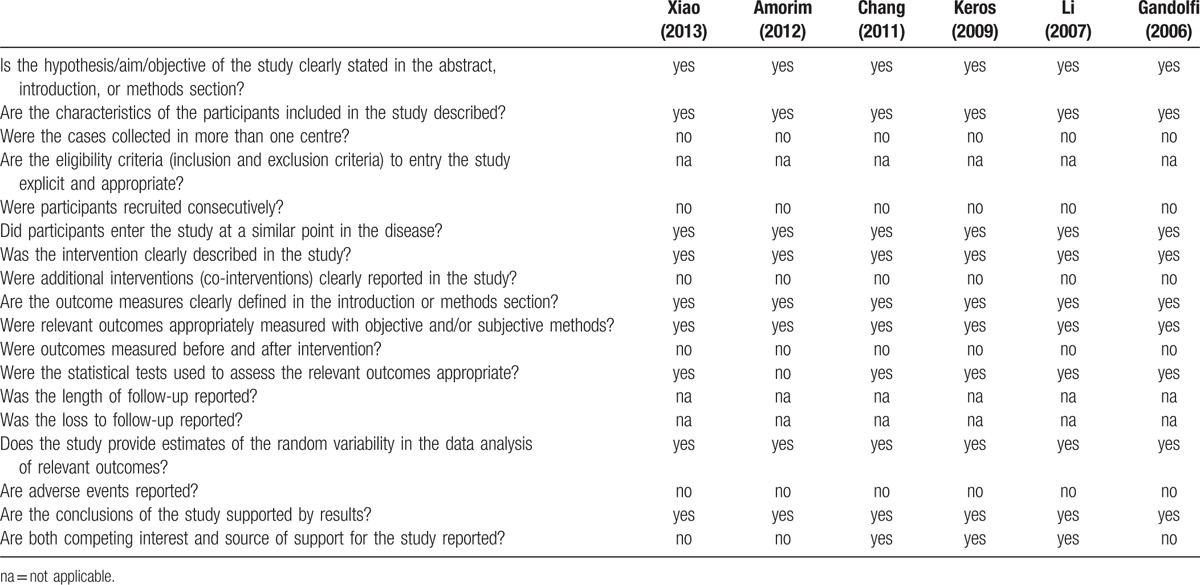
Results of the quality assessment of the 6 studies using the Delphi list are shown in Table 3. All the articles had clear description of intervention, and measured the outcomes with objective and/or subjective methods. All the studies collected the cases in one centre. However, no studies described the eligibility criteria to entry the study. Overall, the results indicated that the studies were of good quality.
Table 3.
Delphi list for quality assessment of studies included in the meta-analysis.
4. Discussion
The purpose of this study was to compare the proportion of intact primordial follicles in ovarian tissue cryopreserved with conventional slow freezing and vitrification. Six studies were included in the meta-analysis and the pooled OR showed there was no difference in the proportion of intact primordial follicles between ovarian tissue cryopreserved with slow freezing and vitrification. Of note, while 5 studies included in the qualitative synthesis did not provide data on primordial follicles and their outcome measures varied, their results were inconsistent in that some studies showed no difference between slow freezing and vitrification and some indicated a benefit of one procedure over the other.
Vitrification has superseded slow freezing as the primary method for cryopreserving oocytes, embryos, and sperm. Cryopreservation of ovarian tissue is more complicated, however, as the ovary is a composite tissue with various cell types requiring different parameters for preventing ice crystal formation during freezing.[16,29] Many factors can affect the outcome of cryopreservation including the cryoprotectant, size of tissue fragments, and speed of cooling.[16,29] Early studies indicated that slow freezing was superior to vitrification for preserving ovarian tissue.[19,30] Vitrification techniques, however, have advanced markedly in the past decade and more recent studies have indicated that vitrification produces equivalent or better results for the preservation of ovarian tissue that slow cooling.[21,24,29,31] Keros et al,[21] a study also included in this meta-analysis, reported that vitrification using a combination of 1,2-propanediol, ethylene glycol, dimethyl sulfoxide, and polyvinylpyrrolidone was comparable to slow freezing with respect to preserving follicles in human ovarian tissue.
While studies have shown that cryopreservation of ovarian tissue is possible and that tissue that has been cryopreserved can restore endocrine function and achieve live births, the developmental capacity of morphologically normal primordial follicles after cryopreservation is still not clear.[32] Chen et al[33] recently reported that ovarian cryopreservation by slow freezing compromises ovarian reserve by cryoinjury and ischemia, evident at an early stage after transplantation. Oktem et al[25] found that vitrified human ovaries have fewer primordial follicles and produce less anti-Müllerian hormone than slow-frozen ovaries. Castro et al[34] showed that fresh and vitrified bovine preantral follicles have different nutritional requirements during in vitro culture. After performing a systematic literature review in 2008, Bedaiwy et al[4] reported that fresh ovarian grafts were associated with an increased likelihood of return of ovarian function and a decreased likelihood for recurrent ovarian failure compared with cryopreserved grafts in patients with premature ovarian failure (hazard ratio [HR] = 2.44, 95% CI: 0.92–6.49 and HR = 0.47, 95% CI: 0.18–1.12, respectively).
Primordial follicles are the primary type in human ovarian fragments used for cryopreservation, and account for more than 90% of the entire population of follicles.[8,35] Thus, they are commonly used as an endpoint for determining the efficiency of cryopreservation.[26] However, though follicles may be morphologically intact after cryopreservation their ability to develop and subsequently be fertilized and achieve pregnancy and live birth may be affected by cryopreservation, whether performed with slow cooling or vitrification. Apoptosis has been suggested as a marker of the developmental capacity of primordial follicles after cryopreservation.[36,37]
There are limitations of this analysis that need to be considered. The 6 studies included in the analysis used different vitrification protocols (including the method and vitrification solution) for cryopreservation, and the data were not sufficient to examine the effect of the different protocols. The number of samples was small, and outcome was based on morphological examination of the ovarian tissue. Lastly, a larger number of primordial follicles do not necessarily mean better results in terms of pregnancy. The quality of these primordial follicles should be evaluated by their capacity for growth, maturation, and fertilization, but this was not the aim of the study.
In conclusion, the results of this meta-analysis indicate that vitrification and slow freezing produce equivalent results with respect to intact primordial follicles for the cryopreservation of human ovarian tissue. However, the included studies varied in the cryopreservation protocols used. Thus, further study is required to determine the best method for cryopreserving human ovarian tissue.
Footnotes
Abbreviations: CI = confidence interval, GAPDH = glyceraldehydes 3-phosphate dehydrogenase, HR = hazard ratio, NR = not reported, OR = odds ratio, RCT = randomized controlled trail, TUNEL = terminal deoxynucleotidyl transferase dUTP nick end labeling.
Ethic statement: Meta-analyses do not involve human subjects and do not require IRB review (J Grad Med Educ. 2011 March; 3 (1): 5–6).
Funding: None.
The authors have no conflicts of interest to disclose.
References
- 1.Donnez J, Silber S, Andersen CY, et al. Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Ann Med 2011; 43:437–450. [DOI] [PubMed] [Google Scholar]
- 2.Amorim CA, Dolmans MM, David A, et al. Vitrification and xenografting of human ovarian tissue. Fertil Steril 2012; 98:1291-8.e1–1291-8.e2. [DOI] [PubMed] [Google Scholar]
- 3.Donnez J, Squifflet J, Van Eyck AS, et al. Restoration of ovarian function in orthotopically transplanted cryopreserved ovarian tissue: a pilot experience. Reprod Biomed Online 2008; 16:694–704. [DOI] [PubMed] [Google Scholar]
- 4.Bedaiwy MA, El-Nashar SA, El Saman AM, et al. Reproductive outcome after transplantation of ovarian tissue: a systematic review. Hum Reprod 2008; 23:2709–2717. [DOI] [PubMed] [Google Scholar]
- 5.Revel A, Laufer N, Ben Meir A, et al. Micro-organ ovarian transplantation enables pregnancy: a case report. Hum Reprod 2011; 26:1097–1103. [DOI] [PubMed] [Google Scholar]
- 6.Mhatre P, Mhatre J, Magotra R. Ovarian transplant: a new frontier. Transplant Proc 2005; 37:1396–1398. [DOI] [PubMed] [Google Scholar]
- 7.Silber SJ, Gosden RG. Ovarian transplantation in a series of monozygotic twins discordant for ovarian failure. N Engl J Med 2007; 356:1382–1384. [DOI] [PubMed] [Google Scholar]
- 8.Gook DA, Edgar DH, Stern C. Effect of cooling rate and dehydration regimen on the histological appearance of human ovarian cortex following cryopreservation in 1,2-propanediol. Hum Reprod 1999; 14:2061–2068. [DOI] [PubMed] [Google Scholar]
- 9.Siebzehnrübl E, Kohl J, Dittrich R, et al. Freezing of human ovarian tissue- not the oocytes but the granulosa is the problem. Mol Cell Endocrinol 2000; 169:109–111. [DOI] [PubMed] [Google Scholar]
- 10.Eyden B, Radford J, Shalet SM, et al. Ultrastructural preservation of ovarian cortical tissue cryopreserved in dimethylsulfoxide for subsequent transplantation into young female cancer patients. Ultrastruct Pathol 2004; 28:239–245. [DOI] [PubMed] [Google Scholar]
- 11.Nottola SA, Camboni A, van Langendonckt A, et al. Cryopreservation and xenotransplantation of human ovarian tissue: an ultrastructural study. Fertil Steril 2008; 90:23–32. [DOI] [PubMed] [Google Scholar]
- 12.Schubert B, Canis M, Darcha C, et al. Follicular growth and estradiol follow-up after subcutaneous xenografting of fresh and cryopreserved human ovarian tissue. Fertil Steril 2008; 89:1787–1794. [DOI] [PubMed] [Google Scholar]
- 13.Merino O, Risopatrón J, Sánchez R, et al. Fish (Oncorshynchus mykiss) spermatozoa cryoprotectant-free vitrification: stability of mitochondrion as criterion of effectiveness. Anim Reprod Sci 2011; 124:125–131. [DOI] [PubMed] [Google Scholar]
- 14.Vajta G, Rienzi L, Ubaldi FM. Open versus closed systems for vitrification of human oocytes and embryos. Reprod Biomed Online 2015; 30:325–333. [DOI] [PubMed] [Google Scholar]
- 15.Sheikhi M, Hultenby K, Niklasson B, et al. Clinical grade vitrification of human ovarian tissue: an ultrastructural analysis of follicles and stroma in vitrified tissue. Hum Reprod 2011; 26:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amorim CA, Curaba M, Van Langendonckt A, et al. Vitrification as an alternative means of cryopreserving ovarian tissue. Reprod Biomed Online 2011; 23:160–186. [DOI] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151:W65–W94. [DOI] [PubMed] [Google Scholar]
- 18.Moga C, Guo B, Schopflocher D, et al. Development of a Quality Appraisal Tool for Case Series Studies Using a Modified Delphi Technique. Edmonton AB: Institute of Health Economics; 2012. [Google Scholar]
- 19.Gandolfi F, Paffoni A, Papasso Brambilla E, et al. Efficiency of equilibrium cooling and vitrification procedures for the cryopreservation of ovarian tissue: comparative analysis between human and animal models. Fertil Steril 2006; 85 suppl 1:1150–1156. [DOI] [PubMed] [Google Scholar]
- 20.Li YB, Zhou CQ, Yang GF, et al. Modified vitrification method for cryopreservation of human ovarian tissues. Chin Med J (Engl) 2007; 120:110–114. [PubMed] [Google Scholar]
- 21.Keros V, Xella S, Hultenby K, et al. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod 2009; 24:1670–1683. [DOI] [PubMed] [Google Scholar]
- 22.Chang HJ, Moon JH, Lee JR, et al. Optimal condition of vitrification method for cryopreservation of human ovarian cortical tissues. J Obstet Gynaecol Res 2011; 37:1092–1101. [DOI] [PubMed] [Google Scholar]
- 23.Xiao Z, Wang Y, Li LL, et al. In vitro culture thawed human ovarian tissue: NIV versus slow freezing method. Cryo Lett 2013; 34:520–526. [PubMed] [Google Scholar]
- 24.Huang L, Mo Y, Wang W, et al. Cryopreservation of human ovarian tissue by solid-surface vitrification. Eur J Obstet Gynecol Reprod Biol 2008; 139:193–198. [DOI] [PubMed] [Google Scholar]
- 25.Oktem O, Alper E, Balaban B, et al. Vitrified human ovaries have fewer primordial follicles and produce less antimüllerian hormone than slow-frozen ovaries. Fertil Steril 2011; 95:2661.e1–2664.e1. [DOI] [PubMed] [Google Scholar]
- 26.Isachenko V, Lapidus I, Isachenko E, et al. Human ovarian tissue vitrification versus conventional freezing: morphological, endocrinological, and molecular biological evaluation. Reproduction 2009; 138:319–327. [DOI] [PubMed] [Google Scholar]
- 27.Klocke S, Bündgen N, Köster F, et al. Slow-freezing versus vitrification for human ovarian tissue cryopreservation. Arch Gynecol Obstet 2015; 291:419–426. [DOI] [PubMed] [Google Scholar]
- 28.Herraiz S, Novella-Maestre E, Rodríguez B, et al. Improving ovarian tissue cryopreservation for oncologic patients: slow freezing versus vitrification, effect of different procedures and devices. Fertil Steril 2014; 101:775–784. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Wallberg KA, Oktay K. Recent advances in oocyte and ovarian tissue cryopreservation and transplantation. Best Pract Res Clin Obstet Gynaecol 2012; 26:391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gosden RG, Baird DT, Wade JC, et al. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at -196 degrees C. Hum Reprod 1994; 9:597–603. [DOI] [PubMed] [Google Scholar]
- 31.Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med 2009; 360:902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi JY, Lee JY, Lee EY, et al. Cryopreservation of the mouse ovary inhibits the onset of primordial follicle development. Cryobiology 2007; 54:55–62. [DOI] [PubMed] [Google Scholar]
- 33.Chen CH, Tan SJ, Tzeng CR. In vivo fate mapping of cryopreserved murine ovarian grafts. J Ovarian Res 2014; 7:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castro SV, Carvalho AA, Silva CM, et al. Fresh and vitrified bovine preantral follicles have different nutritional requirements during in vitro culture. Cell Tissue Bank 2014; 15:591–601. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt KL, Byskov AG, Nyboe Andersen A, et al. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod 2003; 18:1158–1164. [DOI] [PubMed] [Google Scholar]
- 36.Rimon E, Cohen T, Dantes A, et al. Apoptosis in cryopreserved human ovarian tissue obtained from cancer patients: a tool for evaluating cryopreservation utility. Int J Oncol 2005; 27:345–353. [DOI] [PubMed] [Google Scholar]
- 37.Xiao Z, Wang Y, Li L, et al. Cryopreservation of the human ovarian tissue induces the expression of Fas system in morphologically normal primordial follicles. Cryo Lett 2010; 31:112–119. [PubMed] [Google Scholar]


