Abstract
Recently, several studies reported increased signal intensity (SI) in the dentate nucleus (DN) after repeated application of gadolinium-based contrast agents (GBCAs), suggesting a deposition of gadolinium in this location. Patients with relapsing–remitting multiple sclerosis (RRMS) frequently show increased permeability of the blood–brain barrier as part of the inflammatory process in the brain parenchyma, which theoretically might increase the risk of gadolinium deposition. In this retrospective study, we investigated a possible increasing SI in the DN after repeated administrations of the macrocyclic contrast agent gadoterate meglumine.
Forty-one RRMS patients (33 women, mean age 38 years) with at least 6 prior gadolinium-enhanced examinations (single dose gadoterate meglumine) were identified. A total of 279 unenhanced T1-weighted examinations were analyzed.
SI ratio differences did not differ between the first and last MRI examination, neither for the DN-to-pons ratio (P = 0.594) nor for the DN-to-cerebellum ratio (P = 0.847). There was no correlation between the mean DN-to-pons, or between the mean DN-to-cerebellum SI ratio and the number of MRI examinations (P = 0.848 and 0.891), disease duration (P = 0.676 and 0.985), and expanded disability status scale (EDSS) (P = 0.639 and 0.945).
We found no signal increases in the DN after a minimum of 6 injections of the macrocyclic GBCA gadoterate meglumine in RRMS patients. This warrants further investigations in regard to the true pathophysiologic basis of intracerebral gadolinium deposition.
Keywords: dentate nucleus, gadolinium, MRI, multiple sclerosis
1. Introduction
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system and the most common cause of nontraumatic neurological disability in young adults. Magnetic resonance imaging (MRI) allows an insight into the pathophysiology of MS and represents currently the most important biomarker for early diagnosis and for disease monitoring. It has therefore been incorporated into the diagnostic criteria by McDonald[1] in 2001 and its importance has been substantiated in the 2005 and 2010[2] revisions. It is well established that MRI disease activity (contrast-enhancing lesions, new or enlarging T2 lesions) is more sensitive than clinical disease activity (relapses).[3] Furthermore, MRI parameters have become important secondary outcome measures in therapeutic clinical trials.[4] Besides, MRI plays an important role in MS-related drug surveillance, for example, in patients treated with natalizumab as a valuable screening method for the detection of progressive multifocal leukoencephalopathy in a presymptomatic early stage.[5]
Recently, several studies reported an increased signal intensity (SI) of the dentate nucleus (DN) on unenhanced T1-weighted MRI images after multiple applications of gadolinium-based contrast agents (GBCAs) independent of renal function suggesting a deposition of gadoliniumin this location. Previous studies reported increased SI after multiple doses of linear GBCAs[6–9] suggesting gadolinium deposition, which subsequently has been confirmed in autopsy studies.[10] This raised safety concerns on the use of GBCAs.[11] As a consequence, the European Medicines Agency has recently announced that its Pharmacovigilance Risk Assessment Committee (PRAC) will carry out an in-depth review of the risk of brain deposits and of the overall safety of these products.[12] Recent studies might provide evidence that SI in the DN is associated with the previous administration of linear GBCAs, but possibly not to the same extent with macrocyclic GBCAs.[13–16]
In MS, increased permeability of the blood–brain barrier (BBB) is a common finding and a part of the inflammatory process in the brain parenchyma, which might influence the risk of gadolinium deposition. An increase of the permeability of the BBB represents a crucial step in the development of newly developing inflammatory focal brain parenchyma lesions, that are associated with the formation of vasogenic edema and are correlated histopathologically with an inflammatory lymphocytic infiltrate.[17] The opening of the BBB in MS allows GBCAs to cross the BBB and results in the detection of contrast-enhanced lesions.[18] Contrast-enhancement in acute MS lesions varies in shape and size with a mean duration of 3 weeks.[19] Therapeutic intervention by steroid treatment can reduce the extent and shorten the duration of contrast enhancement.[20] Due to these pathophysiological characteristics, MS patients might theoretically pose a higher risk for gadolinium deposition in the brain parenchyma. The aim of this study was to investigate a possible increasing SI in the DN after repeated administrations of GBCAs in MS patients.
2. Methods
2.1. Patients
From our prospectively collected database, we retrospectively screened patients eligible for further analysis. Inclusion criteria were as follows: diagnosis of definite MS according to the current diagnostic criteria presenting with a relapsing–remitting type,[2] at least 18 years of age, a minimum of 6 gadolinium-enhanced examinations (analogous to the initial study by Kanda et al,[6] who had chosen this cut-off criterion) in our institution on the same MRI system throughout (either 1.5T or 3T) that were all exclusively performed with the macrocyclic contrast agent gadoterate meglumine, and no contrast agent had been previously applied before the first MRI examination. Exclusion criteria were as follows: presence of neurological conditions other than MS (in particular brain hemorrhage, stroke, and tumor); cardiovascular or respiratory disease; current or past substance abuse; current or past radiation or chemotherapy; abnormal renal (estimated glomerular filtration rate <60 mL/min) or liver function (abnormal serum concentrations of aspartate aminotransferase, alanine aminotransferase, total bilirubin, y-glutamyl transpeptidase); lesions located in the dentate nucleus, cerebellum or pons causing difficulties for accurate placement of regions of interest (ROIs); missing or unsatisfactory unenhanced T1-weighted MRI images (e.g., due to motion artifacts); and missing documentation of the applied contrast agent.
The local ethics committee approved this study. Patient consent was not required due to the retrospective nature of the study and the lack of patient interaction.
2.2. Magnetic resonance imaging protocol
Initial and follow-up MRI studies were performed on a 3.0-T MR system (MAGNETOM Skyra, Siemens Healthcare GmbH, Erlangen, Germany) or a 1.5-T system (MAGNETOM Sonata, Siemens Healthcare GmbH, Erlangen, Germany).
The standard MRI protocol on the 3.0T system included a high resolution 3D magnetization-prepared rapid acquisition gradient-echo (MPRAGE) sequence (TE = 2.49 ms, TR = 1900 ms, TI = 900 ms, field of view 240 × 240 mm2, spatial resolution = 0.9 × 0.9 × 0.9 mm3), a 3D fluid-attenuated inversion recovery (FLAIR) data set, proton density (PD) images, T1-weighted images (TE = 2.48 ms, TR = 225 ms, field of view 220 × 220 mm2, voxel size 0.7 × 0.7 × 3.0 mm, slice thickness 3 mm) 10 minutes after manual injection of single dose contrast agent (gadoterate meglumine).
On the 1.5T system the protocol included T2-weighted images (slice thickness 3 mm), T1-weighted images (TE = 11 ms, TR = 540 ms, field of view 240 × 240 mm2, voxel size = 0.9 × 0.9 × 3.0 mm3), FLAIR images, PD-weighted images, followed by T1-weighted images 10 minutes after manual injection of single dose contrast agent, identical to T1-weighted images mentioned previously.
2.3. Data processing and analysis
Image post-processing was performed offline on our picture archiving and communication system. Image analysis was conducted on all MRI time points by an experienced reader who was blinded to the serial number of the MRI scan and clinical data. Data analysis was performed in a comparable manner to that reported in previous studies:[6,16] Oval ROIs were placed on the unenhanced T1-weighted images around the left and right DN, the cerebellum next to the DN, and on the central pons. To improve accuracy, we calculated the mean of the left and right DN and cerebellum as proposed previously.[16] If the DN was not clearly visible on unenhanced T1-weighted images, T2-weighted images were used to guide ROI placement on T1-weighted images. First, the average SI of the right and left DN and cerebellum was obtained. Then, the DN-to-pons SI ratio was calculated by dividing the mean SI of the DN by that of the central pons. The DN-to-cerebellum SI ratio was calculated analogue by dividing the mean SI of the DN by the mean SI of the cerebellum.
2.4. Statistical analysis
Statistical analysis was performed with IBM SPSS Statistics version 22.0 (IBM Corp., Armonk, NY). A paired t test was used to compare the DN-to-pons and DN-to-cerebellum SI ratio between the first and last MRI scans. Correlations between DN-to-pons, DN-to-cerebellum SI, and disease duration and number of MRI examinations were assessed using Pearson correlation coefficient. Correlations between DN-to-pons, DN-to-cerebellum SI and expanded disability status scale (EDSS) were assessed using Spearman rank correlation coefficient.
3. Results
A total of 41 MS patients (33 women, 8 men; mean age 38 years [range 18–59 years]) fulfilled the inclusion criteria and were included in the study. The number of contrast-enhanced MRI examinations ranged from 6 to 12 (mean 6.8 examinations, a total of 279 MRI examinations). Mean disease duration was 6.5 years (range 2–16 years) and the median EDSS was 1.5 (range 0–7.5). Thirty-six (88%) patients were on best individually selected treatment either with interferon-beta, glatiramer acetate, fingolimod, natalizumab, or dimethyl fumarate. Twenty patients showed acute contrast-enhancing lesions on post-contrast T1-weighted images (overall 80 contrast-enhancing lesions).
We found no significant differences between the mean DN-to-pons SI ratio of 0.0015 ± 0.0059 (P = 0.594) and the mean DN-to-cerebellum SI ratio of 0.007 ± 0.0067 (P = 0.847) between the first and last MRI. There was no correlation neither between the mean DN-to-pons nor between the mean DN-to-cerebellum SI ratio and the number of MRI examinations (P = 0.848 and 0.891), disease duration (P = 0.676 and 0.985), and the EDSS (P = 0.639 and 0.945, respectively).
T1-weighted MRI examinations in 2 patients with relapsing–remitting multiple sclerosis (RRMS), at the level of the dentate nucleus, are depicted in Figs. 1 and 2 with 7 and 6 consecutive slices, respectively. Figure 3 shows a plot of the DN-to-pons SI ratio of 6 MRI examinations in chronological order of all included patients.
Figure 1.
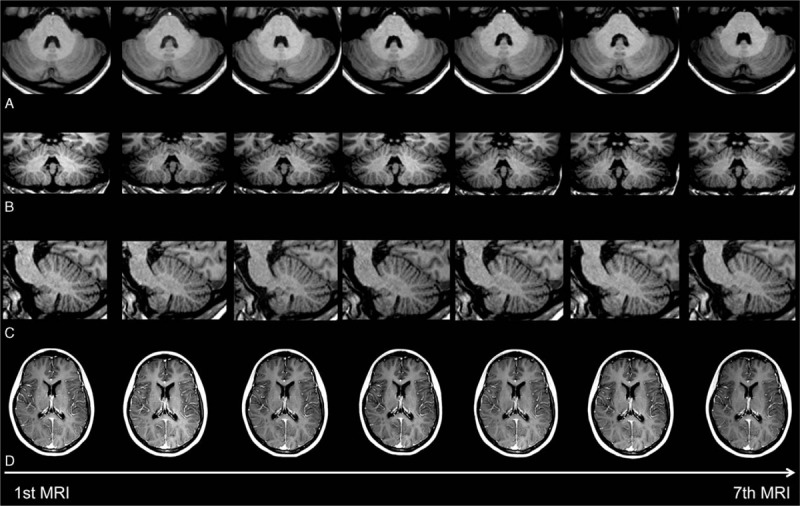
Consecutive (A) axial, (B) coronal, and (C) sagittal unenhanced T1-weighted images of an MS patient at the level of the dentate nucleus. No increase of the signal intensity in the dentate nucleus is observed throughout the examinations. (D) Consecutive MRI examinations of this patient without contrastenhancing lesions.
Figure 2.
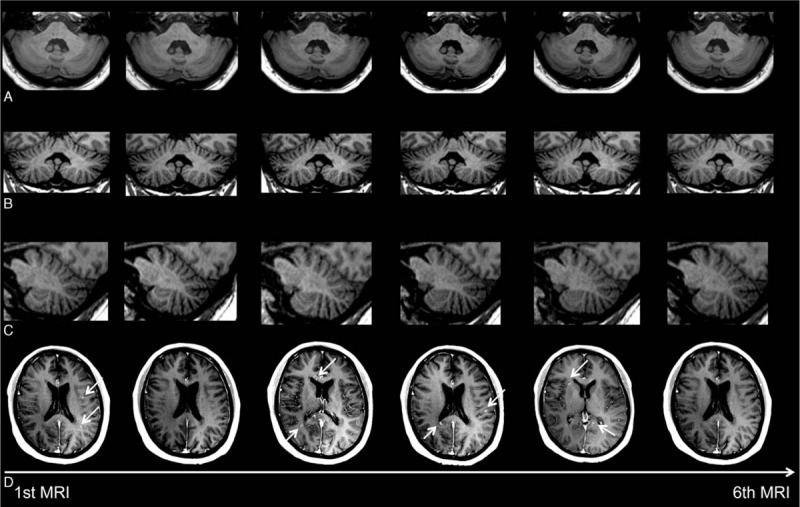
Consecutive (A) axial, (B) coronal, and (C) sagittal unenhanced T1-weighted images of an MS patient at the level of the dentate nucleus. No increase of the signal intensity in the dentate nucleus is observed throughout the examinations. (D) Consecutive MRI examinations of this patient showing multiple contrast-enhancing lesions (white arrows) throughout the 6 examinations.
Figure 3.
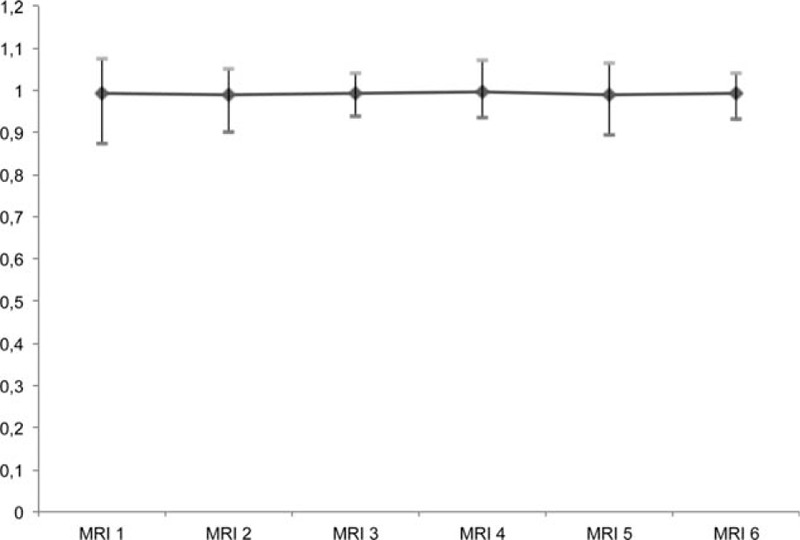
Plot of the dentate nucleus-to-pons signal intensity ratio of 6 MRI examinations in chronological order of all included patients demonstrating stable signal intensities throughout the observation period.
4. Discussion
The present study adds to the growing database of SI changes in the DN after serial applications of GBCAs. In our study, we found no differences of SIs in the DN after multiple administrations of the macrocyclic contrast agent gadoterate meglumine. Our results confirm previous studies that failed to demonstrate an SI increase for the applied macrocyclic GBCAs.[13–16]
In patients with MS, in whom repeated contrast-enhanced MRI is routinely performed to diagnose and monitor the disease activity, the experience of abnormal SI in the DN is limited until now. Abnormally hyperintense appearance of the DN on unenhanced T1-weighted images in MS was already described in 2009.[21] These findings were associated with a secondary-progressive disease course, disability, lower normalized brain volume, and higher lesion load, but no association with administration of GBCAs was reported, though patients received either the linear GBCA gadodiamide or the macrocyclic gadoterate meglumine.[21] Errante et al[7] reported an increase of the SI in 38 MS patients after serial application of the linear contrast agent gadodiamide, but no patients receiving macrocyclic GBCAs were included. Recently, a study by Stojanov and colleagues[22] reported for the first time signal abnormalities within the DN after multiple injections of the macrocyclic GBCA gadobuterol in 58 MS patients. However, their study had significant limitations, for example, they could not exclude the use of other contrast agents suggesting a confounding contamination prior to the first MRI examination that was included in the study. In addition, no abnormally hyperintense signal in the DN was visible on the presented unenhanced T1-weighted images.[23]
There is another interesting aspect in regard to the findings of increased SI of the DN after serial application of GBCAs. There is increasing evidence that the previously reported signal abnormalities due to T1-shortening after application of contrast agents represent a result of gadolinium deposition in preferred brain areas, as confirmed by animal[24] and autopsy studies,[10,25] although the exact underlying mechanisms are yet unknown. This would be in line with a recent study evaluating effects in T1 and T2∗ relaxometry of the DN in 74 RRMS patients with respect to the number of previous applied GBCAs.[26] The authors found a correlation of increased R1 (=1/T1) values with repeated administration of GBCAs, mainly related to linear contrast agents, while T2∗ relaxometry was not affected, supporting the hypothesis that the observed T1-shortening is related to gadolinium administration and not to iron deposition. In MS, an increase of the permeability of the BBB represents a crucial step in the development of new acute contrast-enhancing lesions. A current study by McDonald and colleagues evaluated autopsy specimens from patients in whom at least 4 MRI examinations with the linear GBCA gadodiamide were performed.[10] Besides the detection of gadolinium clusters in the endothelial walls, they reported that up to 42% of gadolinium had crossed the intact BBB and deposited into the neural tissue interstitium.[10] We found 80 acute contrast-enhancing lesions but no abnormal SIs in the DN after multiple administrations of the macrocyclic contrast agent gadoterate meglumine. One might have assumed that in a disorder that is characterized by repeated focal brain tissue inflammation associated with BBB opening, residuals of gadolinium due to leakage might also be detectable. However, it still remains unclear in what form gadolinium deposition (free ionic form, chelated state) occurs, but the most prevalent hypothesis[11] is that the observed SI changes are a result of dechelation and release of the Gd3+ ion from its ligand molecule.[26]
After a minimum of 6 applications of the macrocyclic contrast agent gadoterate meglumine, we could not detect gadolinium accumulation in the brain of MS patients. This is in keeping with the results of other recent studies.[13–16] These findings are also motivation to extend our findings to patients with more frequent GBCA applications and longer observations periods.
Acknowledgment
We acknowledge financial support by Deutsche Forschungsgemeinschaft and Ruprecht-Karls-Universität Heidelberg within the funding programme Open Access Publishing.
Footnotes
Abbreviations: BBB = blood–brain barrier, DN = dentate nucleus, EDSS = expanded disability status scale, FLAIR = fluid-attenuated inversion recovery, GBCA = gadolinium-based contrast agent, MPRAGE = magnetization-prepared rapid acquisition gradient-echo, MRI = magnetic resonance imaging, MS = multiple sclerosis, ROI = region of interest, RRMS = relapsing–remitting multiple sclerosis, SI = signal intensity.
PE has received travel expenses from Bayer Health Care. AA is on the editorial board for Advances in Neuroscience and Cerebrovascular Diseases. KS serves on the editorial board for Cerebrovascular Diseases and has received research support from the German Research Foundation (DFG). AE, MO reports no disclosures.
SOS reports that the Institute of Clinical Radiology and Nuclear Medicine has research agreements with Siemens Healthcare GmbH. AG has received honoraria for lecturing, travel expenses for attending meetings, and financial support for research from Bayer Schering, Biogen Idec, Merck Serono, Novartis, and TEVA Neurosciences.
The authors declare no conflicts of interest relevant to the manuscript.
References
- 1.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001; 50:121–127. [DOI] [PubMed] [Google Scholar]
- 2.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol 2015; 25 suppl 2:157–165. [DOI] [PubMed] [Google Scholar]
- 4.Fox EJ, Rhoades RW. New treatments and treatment goals for patients with relapsing-remitting multiple sclerosis. Curr Opin Neurol 2012; 25 (suppl):S11–19. [DOI] [PubMed] [Google Scholar]
- 5.Wattjes MP, Vennegoor A, Steenwijk MD, et al. MRI pattern in asymptomatic natalizumab-associated PML. J Neurol Neurosurg Psychiatry 2015; 86:793–798. [DOI] [PubMed] [Google Scholar]
- 6.Kanda T, Ishii K, Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014; 270:834–841. [DOI] [PubMed] [Google Scholar]
- 7.Errante Y, Cirimele V, Mallio CA, et al. Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Invest Radiol 2014; 49:685–690. [DOI] [PubMed] [Google Scholar]
- 8.Weberling LD, Kieslich PJ, Kickingereder P, et al. Increased signal intensity in the dentate nucleus on unenhanced T1-weighted images after gadobenate dimeglumine administration. Invest Radiol 2015; 50:743–748. [DOI] [PubMed] [Google Scholar]
- 9.Adin ME, Kleinberg L, Vaidya D, et al. Hyperintense dentate nuclei on T1-weighted MRI: relation to repeat gadolinium administration. AJNR Am J Neuroradiol 2015; 36:1859–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015; 275:772–782. [DOI] [PubMed] [Google Scholar]
- 11.Kanal E, Tweedle MF. Residual or retained gadolinium: practical implications for radiologists and our patients. Radiology 2015; 275:630–634. [DOI] [PubMed] [Google Scholar]
- 12.EMA. EMA reviewing gadolinium contrast agents used in MRI scans. 2016; http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/gadolinium_contrast_agents_31/Procedure_started/WC500203487.pdf Accessed April 11, 2016. [Google Scholar]
- 13.Kanda T, Osawa M, Oba H, et al. High signal intensity in dentate nucleus on unenhanced T1-weighted MR images: association with linear versus macrocyclic gadolinium chelate administration. Radiology 2015; 275:803–809. [DOI] [PubMed] [Google Scholar]
- 14.Radbruch A, Weberling LD, Kieslich PJ, et al. High-signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evaluation of the macrocyclic gadolinium-based contrast agent gadobutrol. Invest Radiol 2015; 50:805–810. [DOI] [PubMed] [Google Scholar]
- 15.Cao Y, Huang DQ, Shih G, et al. Signal change in the dentate nucleus on T1-weighted MR images after multiple administrations of gadopentetate dimeglumine versus gadobutrol. AJR Am J Roentgenol 2016; 206:414–419. [DOI] [PubMed] [Google Scholar]
- 16.Radbruch A, Weberling LD, Kieslich PJ, et al. Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 2015; 275:783–791. [DOI] [PubMed] [Google Scholar]
- 17.Katz D, Taubenberger JK, Cannella B, et al. Correlation between magnetic resonance imaging findings and lesion development in chronic, active multiple sclerosis. Ann Neurol 1993; 34:661–669. [DOI] [PubMed] [Google Scholar]
- 18.Guttmann CR, Ahn SS, Hsu L, et al. The evolution of multiple sclerosis lesions on serial MR. AJNR Am J Neuroradiol 1995; 16:1481–1491. [PMC free article] [PubMed] [Google Scholar]
- 19.Cotton F, Weiner HL, Jolesz FA, et al. MRI contrast uptake in new lesions in relapsing-remitting MS followed at weekly intervals. Neurology 2003; 60:640–646. [DOI] [PubMed] [Google Scholar]
- 20.Burnham JA, Wright RR, Dreisbach J, et al. The effect of high-dose steroids on MRI gadolinium enhancement in acute demyelinating lesions. Neurology 1991; 41:1349–1354. [DOI] [PubMed] [Google Scholar]
- 21.Roccatagliata L, Vuolo L, Bonzano L, et al. Multiple sclerosis: hyperintense dentate nucleus on unenhanced T1-weighted MR images is associated with the secondary progressive subtype. Radiology 2009; 251:503–510. [DOI] [PubMed] [Google Scholar]
- 22.Stojanov DA, Aracki-Trenkic A, Vojinovic S, et al. Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur Radiol 2016; 26:807–815. [DOI] [PubMed] [Google Scholar]
- 23.Agris J, Pietsch H, Balzer T. What evidence is there that gadobutrol causes increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W MRI in patients with RRMS? Eur Radiol 2016; 26:816–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robert P, Lehericy S, Grand S, et al. T1-weighted hypersignal in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats: difference between linear and macrocyclic agents. Invest Radiol 2015; 50:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015; 276:228–232. [DOI] [PubMed] [Google Scholar]
- 26.Tedeschi E, Palma G, Canna A, et al. In vivo dentate nucleus MRI relaxometry correlates with previous administration of Gadolinium-based contrast agents. Eur Radiol 2016; [Epub ahead of print] doi:10.1007/s00330-016-4245-2. [DOI] [PubMed] [Google Scholar]


