Abstract
Patients with end-stage renal disease (ESRD) show a high incidence of bacterial translocation and impaired gastrointestinal motility. The intestinal tract is believed to be the most crucial source of translocated bacteria. To evaluate the risk of colonic diverticulitis in patients with ESRD, we conducted a nationwide population-based cohort study. Patients who met the following 3 criteria were defined as patients with ESRD: patients diagnosed with ESRD who received regular hemodialysis between 2000 and 2005, patients who received hemodialysis for more than 90% of the time during the observation period (2000–2011), and patients with no prior history of hemodialysis between 1997 and 1999. We matched every patient with ESRD with 1 matched control on the basis of propensity scores. The first diagnosis of diverticulitis (ICD-9-CM codes 562.11 and 562.13) within the follow-up period was defined as the primary endpoint. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were calculated using the patients in the control group as the reference. We included 32,547 and 32,547 patients in the ESRD and matched control cohorts, respectively. The 12-year cumulative incidence of acute colonic diverticulitis for patients with ESRD was significantly higher than that for the controls (P < 0.001). After adjustment for age, sex, comorbidities, and medication use, the HR of acute colonic diverticulitis in the ESRD cohort was 11.20 times greater than that in the control cohort (95% CI: 8.14–15.42). The results indicated that patients with ESRD are at an increased risk for acute colonic diverticulitis.
Keywords: bacterial translocation, diverticulitis, end-stage renal disease (ESRD), gastrointestinal motility, hemodialysis, intestinal tract
1. Introduction
Mucosal and submucosal herniations via the circular muscular layer cause diverticulosis.[1,2] Both asymptomatic and symptomatic presentations increase in diverticulosis.[2] However, few diverticulosis patients develop diverticulitis in Western and Asian populations.[3,4] The risk factors for diverticulitis include physical inactivity, constipation, obesity, smoking, and nonsteroidal anti-inflammatory drug (NSAID) use.[5]
Obstruction at the neck of the diverticulum, low-grade inflammation, and bacterial translocation (BT) result in diverticulitis. Bacterial overgrowth is due to stasis or obstruction in the narrow-necked diverticulum.[6] Recent findings suggest that diverticulitis may be related to long-term alterations in the patient's diet and microecology.[7] BT occurs in patients with end-stage renal disease (ESRD).[8] In 1 study, gastrointestinal motility was impaired in patients with ESRD.[9] Certain studies have revealed that the guts of patients with ESRD and uremic animals exhibit similar damage to the mechanical barriers, such as conspicuous mucosal edema and erosion.[10,11] The intestinal tract is considered the most crucial source of translocated bacteria.[12]
No large population-based studies have evaluated the occurrence of colonic diverticulitis on the basis of the presence of comorbidities and medication administered in patients with ESRD and their respective controls. We conducted a nationwide population-based cohort study to investigate the association between ESRD and diverticulitis by using data obtained from the Taiwan National Health Insurance Research Database (NHIRD).
2. Materials and methods
2.1. Ethical considerations
The NHIRD is a secondary database. The identities of patients in the database were scrambled before the data were released for research purposes. Patient records and information were anonymized and deidentified before analysis. This study was approved by the NHRI and the Institutional Review Board (IRB) of Taipei City Hospital (IRB No.: TCHIRB-1021102-E). The IRB waived the requirement of written consent.
2.2. Data source
This nationwide cohort study was based on patient data obtained from the NHIRD, which is managed by the Taiwan National Health Research Institute (NHRI). The NHIRD contains health care data on 99% of the Taiwanese population (approximately 23 million people). We used the International Classifications of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes to define the diseases. The NHIRD contains comprehensive information, including demographic data, dates of clinical visits, diagnostic codes, and details of prescriptions.
2.3. Study population
In Taiwan, patients with ESRD requiring dialysis can apply for a catastrophic illness card. Patients in the NHIRD meeting the following 3 criteria were defined as patients with newly diagnosed ESRD: patients diagnosed with ESRD receiving regular hemodialysis between 2000 and 2005 (half the whole 12-year observation period) and excluding emergent hemodialysis without ESRD, patients receiving hemodialysis (excluding peritoneal dialysis) for more than 90% of the time during the observation period (2000–2011), and patients with no prior history of hemodialysis between 1997 and 1999. Patients with no chronic kidney disease (CKD) in their inpatient records or in the ambulatory care claims between 1997 and 2011 were defined as the cohort members group. The following ICD-9-CM codes denoted CKD: 580 to 589, 250.4, 274.1, 283.11, 403.x1, 404.x2, 404.x3, 440.1, 442.1, 447.3, 572.4, 642.1x, 646.2x, and 794.4.
2.4. Study cohorts
Among the eligible patients, patients younger than 20 years and patients diagnosed with colonic diverticulitis before 2000 were excluded from the study. Patients diagnosed with colon cancer, celiac disease, or inflammatory bowel disease (IBD), and patients who underwent kidney transplantation during the observation period (1997–2011) were also excluded. In addition, patients who underwent colectomy before the event were excluded. The demographic data of patients in the ESRD and control cohorts were first compared. Subsequently, each patient with ESRD was matched with 1 control on the basis of propensity scores. Figure 1 illustrates a flow chart of the patient selection process.
Figure 1.
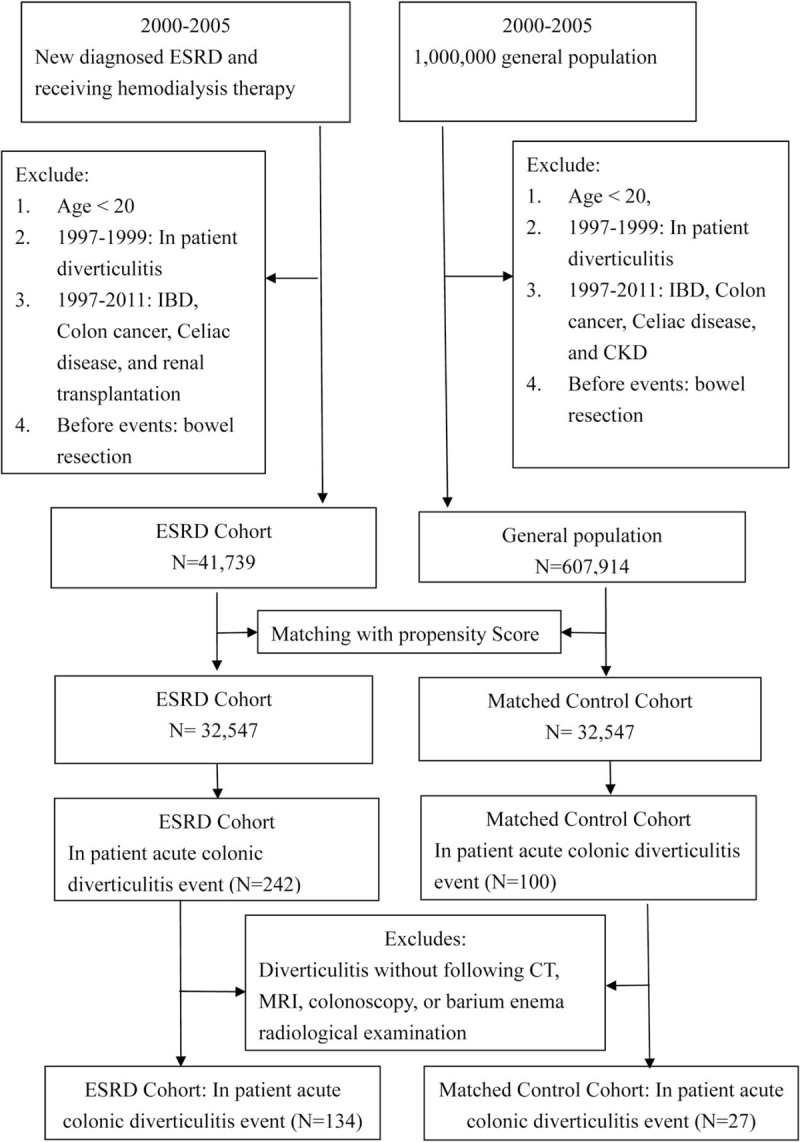
Flow chart depicting the selection of the participants.
2.5. Assessing cofounders and covariates
The propensity score was calculated using logistic regression as described by Rosenbaum and Rubin.[13] The mean and median propensity scores were compared between the 2 cohorts. All diseases included in the Charlson comorbidity index were analyzed.[14] Age, gender, comorbidities, and Charlson score were included in the propensity score. Conditions that required inpatient care between January 1, 1997, and December 31, 2011, were defined as comorbidities in our study. The comorbidities identified in our cohort and their corresponding ICD-9-CM diagnosis codes are listed as follows: diabetes mellitus (DM; ICD-9-CM code 250), coronary artery disease (CAD; ICD-9-CM codes 410–414), congestive heart failure (CHF; ICD-9-CM code 428), cerebral vascular disease (CVD; ICD-9-CM codes 430–438), dyslipidemia (ICD-9-CM code 272.xx), liver cirrhosis (ICD-9-CM codes 571.2, 571.5, and 571.6), and chronic obstructive pulmonary disease (COPD; ICD-9-CM codes 490–492, 494, and 496). Certain drugs that may alter the risk of diverticulitis, such as aspirin, NSAIDs, cyclooxygenase-2-specific inhibitors, steroids, and laxative drugs, were analyzed. Exposure to these drugs was defined as the frequency of exposure for more than 10% of the observation period (1997–2011).
2.6. End point
All inpatient records for each patient in the propensity score matched ESRD and control cohorts were followed up until the occurrence of acute colonic diverticulitis (the top 3 diagnoses), death, or the end of 2011. The colonic diverticulitis was diagnosed after undergoing computed tomography (CT), magnetic resonance imaging (MRI), colonoscopy, or barium radiological examination. The date of the first diagnosis of colonic diverticulitis (ICD-9-CM codes 562.11 and 562.13) within the follow-up period was defined as the primary endpoint.
2.7. Statistical analysis
The Chi-square test and Student t test were used to examine the differences in the demographic variables, comorbidities, and propensity scores between the ESRD and control cohorts. Stratified Cox proportional hazard regression analysis on age, gender, comorbidities, Charlson score, and medications was used to estimate the effect of ESRD on the occurrence of acute colonic diverticulitis. An alpha level of 0.05 was considered statistically significant for all analyses. Cumulative incidence analyses were performed using the Kaplan–Meier method, and the differences between the curves were calculated using the 2-tailed log rank test. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were calculated using patients in the control cohort as the reference. Models with adjusted variables are presented. All statistical analyses were performed using the SAS System for Windows, Version 9.2 (SAS Institute, Cary, NC).
3. Results
3.1. Demographic characteristics
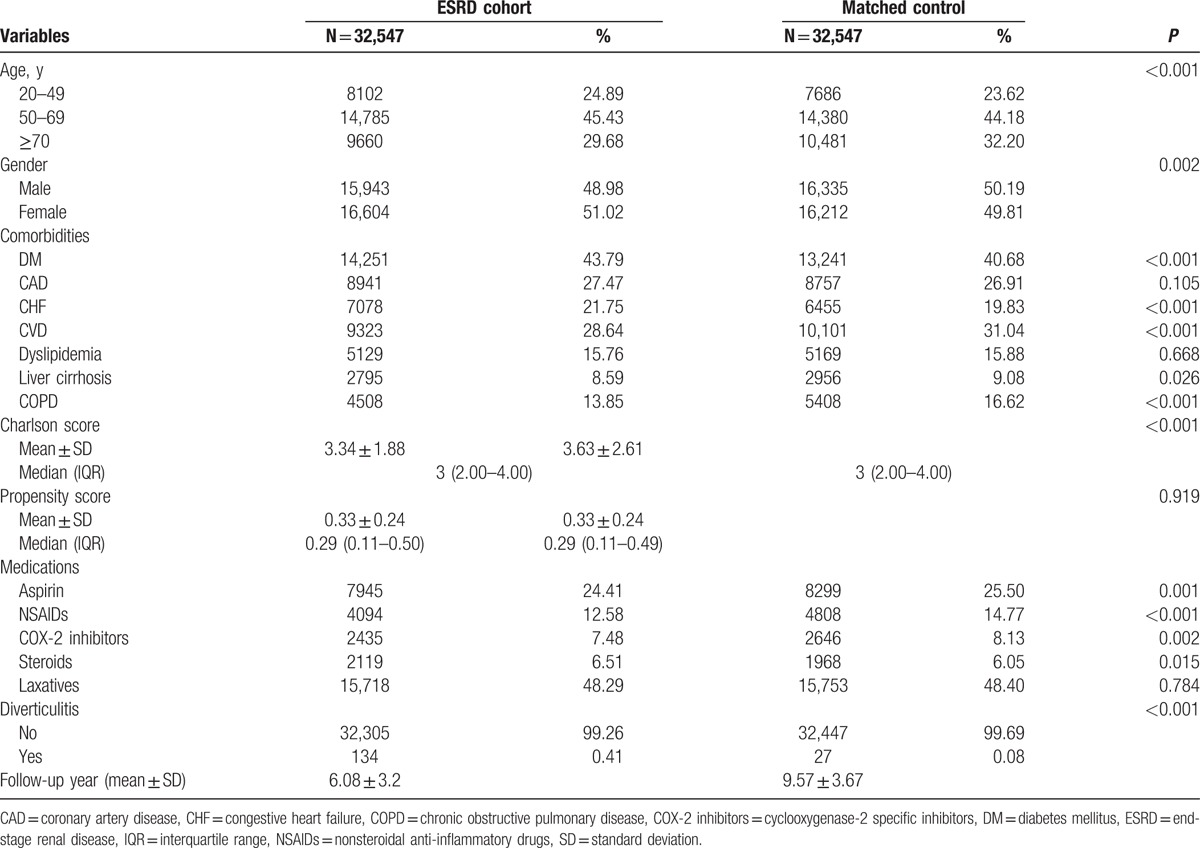
We first selected 41,739 patients diagnosed with ESRD and 607,914 cohort members from 2000 to 2005. Table 1 lists the demographic data, including age, sex, comorbidities, and medications. The original propensity scores of the controls (mean, 0.07) were significantly lower than those of patients with ESRD (mean, 0.39) (P < 0.001). We used propensity scores to match 1 patient in the ESRD cohort with 1 patient in the matched control cohort. The 2 groups had comparable distributions of propensity scores after the patients were matched. We included a total of 32,547 patients in the ESRD cohort and 32,547 patients in the matched control cohort (Table 2). The mean follow-up durations for the ESRD and matched control cohorts were 6.08 and 9.57 years, respectively. The incidence of acute colonic diverticulitis was significantly higher in the ESRD cohort [n = 134 (0.41%)] than in the control cohort [n = 27 (0.08%)] (Table 2). In addition, the overall all-cause 3-month mortality cumulative incidence in the ESRD cohort (n = 13) was significantly higher than that in the control cohort (n = 3). The number of patients who underwent surgery for acute colonic diverticulitis was higher in the ESRD cohort than in the control cohort (8 vs 0, respectively). Drainage therapy for acute colonic diverticulitis was performed for 7 patients in the ESRD cohort and 1 patient in the control cohort (data not shown).
Table 1.
Demographic characteristics of ESRD and control cohort before propensity score matching.
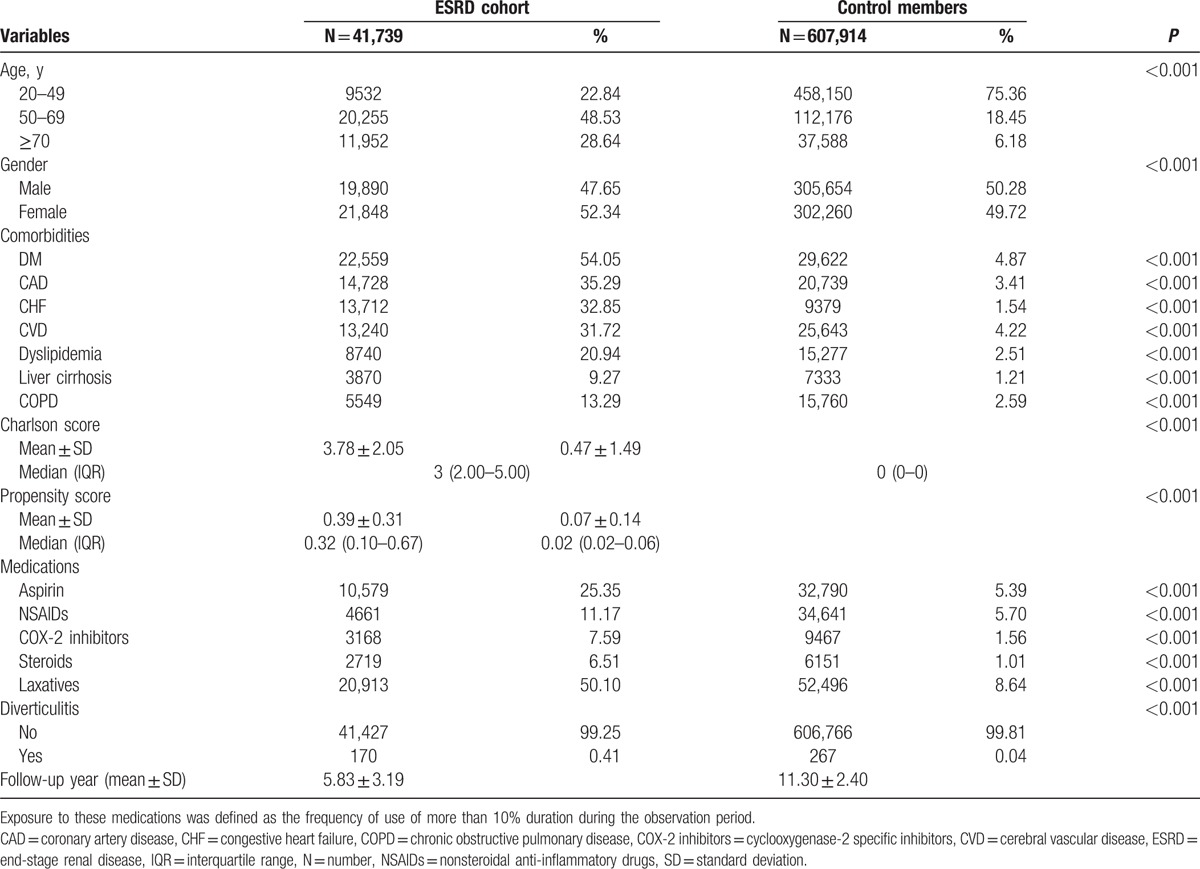
Table 2.
Demographic characteristics of ESRD and matched control cohort after propensity score matching.
3.2. Acute colonic diverticulitis (12-year cumulative incidence)
The 1-year and 5-year cumulative incidences of acute colonic diverticulitis were significantly higher in the ESRD cohort than in the control cohort (1 year: 0.065% vs 0.006%, P < 0.001; 5 years: 0.522% vs 0.055%, P < 0.001) (data not shown). In addition, the 12-year cumulative incidence of acute colonic diverticulitis was higher in the ESRD cohort than in the control cohort (P < 0.001) (Fig. 2).
Figure 2.
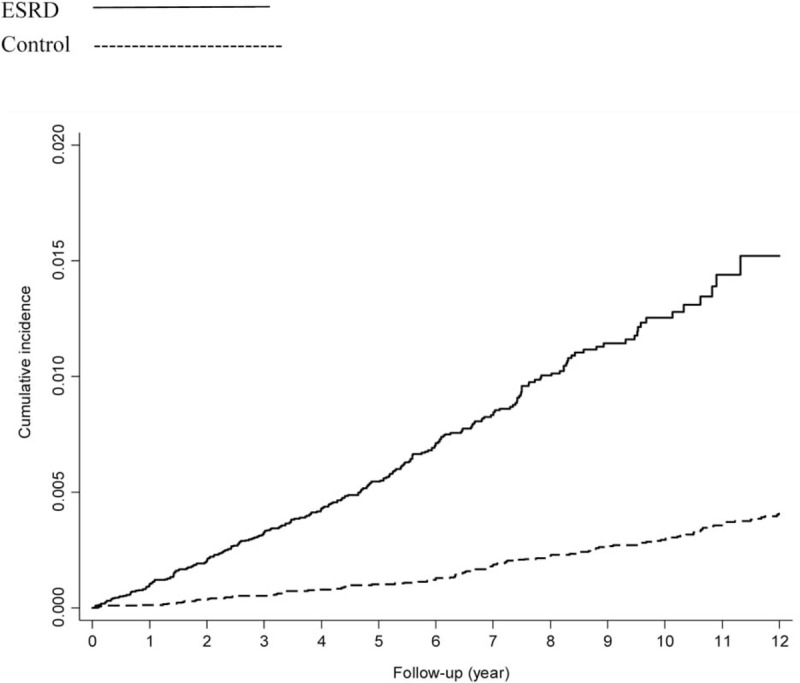
Twelve-year cumulative incidences of acute colonic diverticulitis. (Log rank: P < 0.001).
3.3. Risk factors of symptomatic colonic diverticulitis in the study population
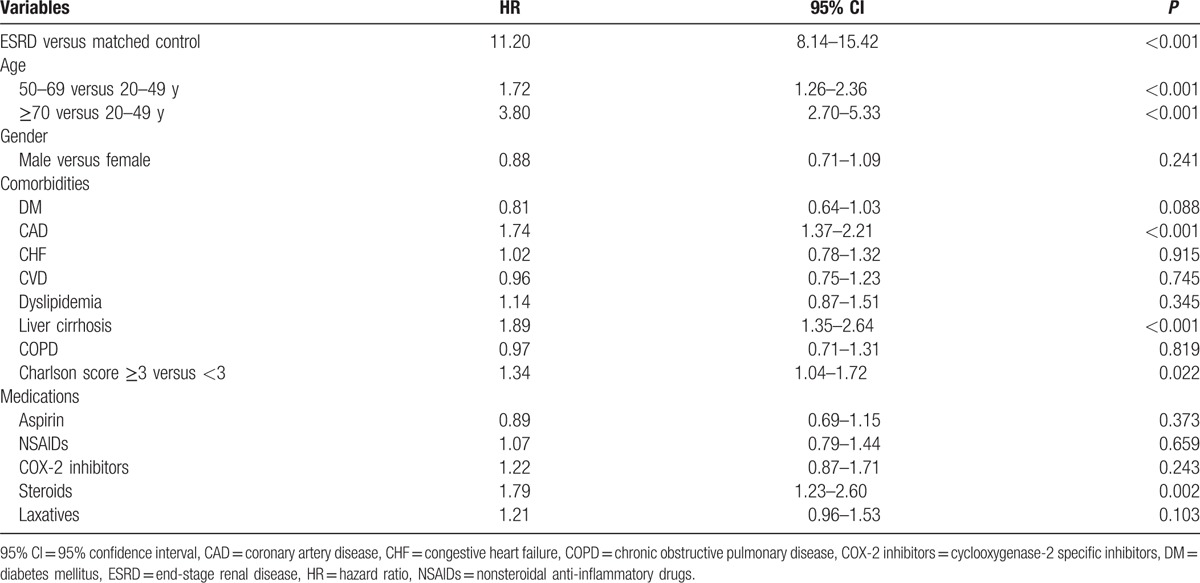
After adjustment for age, sex, the presence of comorbidities, and medication use, the HR of acute colonic diverticulitis in the ESRD cohort was 11.20 times greater (95% CI: 8.14–15.42) (Table 3) than that in the control cohort. Moreover, we also analyzed the HR of acute colonic diverticulitis without following CT, MRI, colonoscopy, or barium radiological examination in the ESRD cohort, which was 4.84 times greater [95% CI: 3.76–6.22 (data not shown)] than that in the control cohort. In addition, factors such as old age (50–69 vs 20–49 years: HR = 1.72, 95% CI: 1.26–2.36; ≥70 vs 20–49 years: HR = 3.80, 95% CI: 2.70–5.33), CAD (HR = 1.74, 95% CI: 1.37–2.21), liver cirrhosis (HR = 1.89, 95% CI: 1.35–2.64), Charlson score more than 3 (HR = 1.34, 95% CI = 1.04–1.72), and steroid use (HR = 1.79, 95% CI: 1.23–2.60) were associated with an increased risk of acute colonic diverticulitis (Table 3).
Table 3.
Multivariate Cox regression analysis for prediction acute colonic diverticulitis in the overall study group.
3.4. Multivariate stratified analysis
The results of a multivariate analysis stratified by age, gender, comorbidities, Charlson score, and medications are illustrated in Fig. 3. In each stratum, ESRD was significantly associated with high risks of acute colonic diverticulitis, regardless of age, sex, the presence or absence of comorbidities, the Charlson comorbidity index score, and medications.
Figure 3.
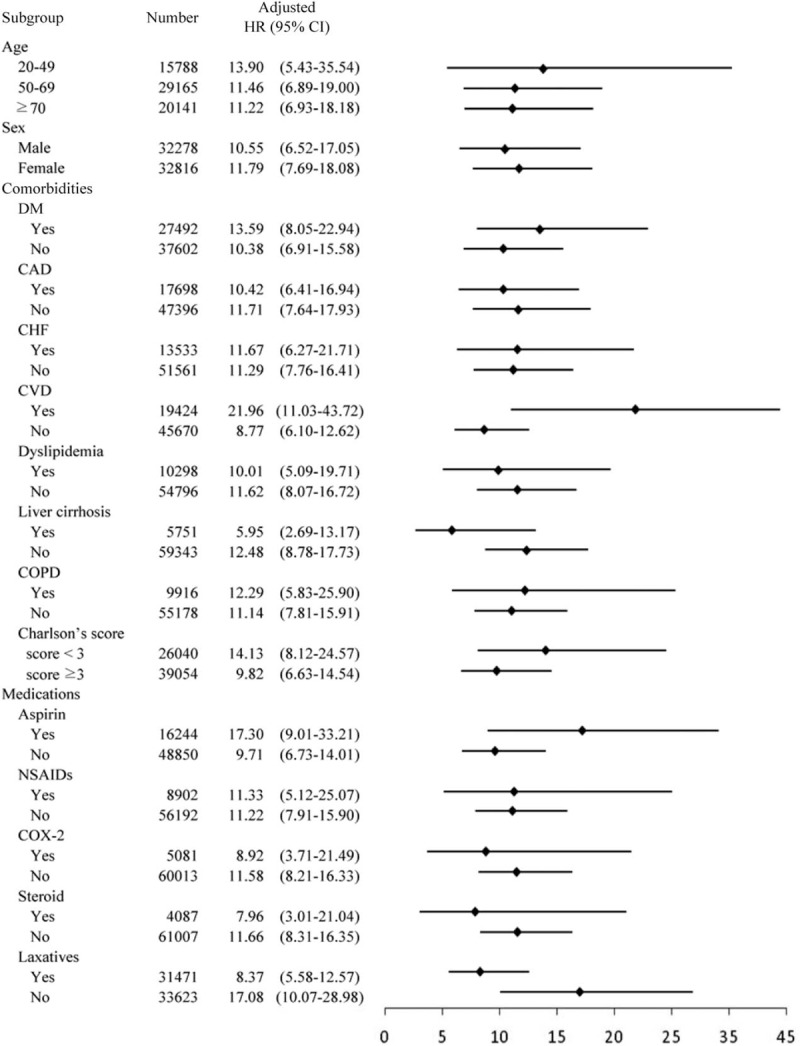
Multivariate stratified analysis for prediction acute colonic diverticulitis: HR for ESRD in subgroups of patients adjusting for other factors.
4. Discussion
The results of this population-based cohort study show that patients with ESRD are associated with an increased risk of acute colonic diverticulitis. After adjusting for multiple confounding factors, we observed that patients in the ESRD cohort were associated with an adjusted HR of 11.20 for acute colonic diverticulitis compared with those in the matched control cohort. In the stratified analysis, we found that ESRD was associated with a significantly increased risk of acute colonic diverticulitis regardless of comorbidities and medications. The association between ESRD and an increased risk of diverticulitis was observed in all subgroups of patients with ESRD.
This current study shows that ESRD, old age, CAD, liver cirrhosis, Charlson score more than 3, and steroid use are crucial risk factors of acute colonic diverticulitis. The impact of ESRD was higher than that of the other factors. Scheff et al[15] reported that patients with chronic renal failure (CRF) caused by polycystic kidney disease (PCKD) had a high incidence of diverticulosis and diverticulitis, but no such association was observed for diverticulitis in patients with non-PCKD CRF. Therefore, Scheff et al[15] recommended that diverticulitis be an initial consideration in the differential diagnosis of abdominal pain in patients with PCKD. The previous study enrolled a low number of patients receiving hemodialysis (n = 151). By contrast, the current study was a nationwide 12-year longitudinal study that enrolled a high number (n = 32,547) of patients receiving hemodialysis and focused on acute colonic diverticulitis in patients with ESRD.
Wang et al[16] proposed that immune defense system suppression occurs in ESRD and that the mechanisms of BT are gastrointestinal microflora alteration, gut mucosal barrier disruption, and host defense impairment. Our current study confirmed the high risk of acute colonic diverticulitis in patients with ESRD. These results are of clinical value and significance. Previous studies have reported that in clinical practice, probiotics use and digestive tract decontamination with antibiotics may alleviate BT in inflammatory diseases.[17,18] Therefore, conducting a prospective randomized controlled study to elucidate whether microinflammation mitigation through antibiotics or probiotics can reduce acute colonic diverticulitis in patients with ESRD is warranted.
Our observations are consistent with those of previous reports regarding the role of old age and steroid use in diverticulitis. Comparato et al[19] reported that people younger than 40 years seldom suffer from diverticulitis, and the incidence increases to 65% in people older than 65 years. Chapman et al[20] determined that steroid use increased perforation rates and mortality in complicated diverticulitis patients. A low-fiber diet and high-fat food, which are risk factors of colonic diverticulitis, were associated with an increased risk of CAD. Gut flora and BT play crucial roles in the pathogenesis of cirrhosis-related complications. Alteration of intestinal flora and mucosal barrier function cause BT in cirrhosis.[21] Therefore, a prospective randomized controlled study must be performed to evaluate whether CAD and liver cirrhosis increase the risk of acute colonic diverticulitis, with or without the presence of obesity and comorbidities.
Diverticulitis is an acute illness and generally does not progress but rather resolves; therefore, we analyzed only hospitalization cases for acute colonic diverticulitis by using inpatient databases. Not only symptomatic uncomplicated diverticular disease but also complicated diverticulitis, patients contracting with diverticulitis, and failing in outpatient therapy or unable to tolerate oral intake are our endpoint. The cases diagnosed in emergency department without admission is not our endpoint. Although imaging results are not available in the NHIRD, ICD-9-CM coding was strictly audited to verify diagnosis for the reimbursement purpose by the Bureau of NHI.
In the present study, unmeasurable bias may have existed. We analyzed the propensity scores for comparing the 2 cohorts. Propensity score matching was applied to select comparable controls to imitate a randomized clinical trial. Although we used all potential confounders in the model to create a propensity score, there were some significant differences in the distributions, such as those of age, sex, DM, CHF, CVD, COPD, and medications. The large sample size in the present study may be the reason for the statistical significance. For example, the difference in the number of male patients in the ESRD and control cohorts (48.98% vs 50.19%) and the difference in steroid use in the ESRD and control cohorts (6.51% vs 6.05%) were statistically significant, but may not be clinically significant.
This study had several limitations. First, some factors, such as the patients’ lifestyle, dietary habits (particularly, fiber intake),[22] body mass index,[23] and smoking[24] could not be adjusted. However, we performed a multivariate analysis to adjust for potential confounders and to examine the risk of acute colonic diverticulitis in different strata. Although unmeasured confounders may still have existed, we believe that the methodology used in the present study is robust. Second, in addition, the locations of diverticulitis are unavailable in the NHIRD. Left-sided diverticular disease is most commonly observed in western populations. Diverticulosis in Asia is predominantly a right-sided phenomenon and that right-sided diverticulitis differs from left-sided disease (less severe presentation, rarely complicated). Therefore, these results may not be generalizable to western populations where right-sided diverticulitis is uncommon. Third, we did not have data regarding the severity of diverticulitis graded according to Hinchey criteria. Finally, patients contracting with colonic diverticulitis may admit for elective surgery followed multiple attacks. Such cases should have been excluded. Only 8 cases underwent surgery in the ESRD cohort; therefore, sensitivity analysis with worst case scenario will not bias our result.
In conclusion, our results provide evidence to support the view that patients with ESRD are at an increased risk of acute colonic diverticulitis. Close attention should be paid to patients with ESRD who contract diverticulitis to prevent life-threatening events. We recommend that physicians be highly vigilant for acute colonic diverticulitis and initiate early intervention to reduce morbidity and mortality in patients with ESRD experiencing abdominal pain or discomfort.
Acknowledgments
We thank the Taiwan Ministry of Education for their support for this work through its “Aim for the Top University Plan.” We also thank the Bureau of NHI and the NHRI for making the NHIRD available to us for this study. In addition, we thank the anonymous reviewers and editors for their comments.
Footnotes
Abbreviations: BT = Bacterial translocation, CAD = coronary artery disease, CHF = congestive heart failure, CI = confidence interval, CKD = chronic kidney disease, COPD = chronic obstructive pulmonary disease, CRF = chronic renal failure, CT = computed tomography, CVD = cerebral vascular disease, DM = diabetes mellitus, ESRD = End-stage renal disease, HR = hazard ratio, IBD = inflammatory bowel disease, ICD-9-CM = International Classifications of Diseases, Revision 9, and Clinical Modification, IRB = Institutional Review Board, MRI = magnetic resonance imaging, NHIRD = National Health Insurance Database, NHRI = National Health Research Institute, NSAIDs = nonsteroidal anti-inflammatory drugs, PCKD = polycystic kidney disease.
Authorship: H-YH performed database management, data analysis, and statistic testing.
S-SC did the design and implementation of the study. NH did the critical revision of the manuscript for important intellectual content. The manuscript was written mainly by S-SC. H-YH and S-S C contributed equally to this study. All authors approved the final version of the manuscript.
Funding/support: This study was supported by the Department of Health, Taipei City Government (104-05).
The authors have no funding and conflicts of interest to disclose.
References
- 1.Slack WW. The anatomy, pathology, and some clinical features of divericulitis of the colon. Br J Surg 1962; 50:185–190. [DOI] [PubMed] [Google Scholar]
- 2.Delvaux M. Diverticular disease of the colon in Europe: epidemiology, impact on citizen health and prevention. Aliment Pharmacol Ther 2003; 18 suppl 3:71–74. [DOI] [PubMed] [Google Scholar]
- 3.Shahedi K, Fuller G, Bolus R, et al. Long-term risk of acute diverticulitis among patients with incidental diverticulosis found during colonoscopy. Clin Gastroenterol Hepatol 2013; 11:1609–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang SS, Hu HY. Long-term use of steroids protects from the development of symptomatic diverticulitis requiring hospitalization in the Asian population. PLoS One 2015; 10:e0124598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stollman NH, Raskin JB. Diverticular disease of the colon. J Clin Gastroenterol 1999; 29:241–252. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs DO. Clinical practice. Diverticulitis. N Engl J Med 2007; 357:2057–2066. [DOI] [PubMed] [Google Scholar]
- 7.Floch MH, White J. Diverticulitis: new concepts and new therapies. J Clin Gastroenterol 2005; 39:355–356. [DOI] [PubMed] [Google Scholar]
- 8.Schindler R. Causes and therapy of microinflammation in renal failure. Nephrol Dial Transplant 2004; 19 suppl 5:V34–V40. [DOI] [PubMed] [Google Scholar]
- 9.Hirako M, Kamiya T, Misu N, et al. Impaired gastric motility and its relationship to gastrointestinal symptoms in patients with chronic renal failure. J Gastroenterol 2005; 40:1116–1122. [DOI] [PubMed] [Google Scholar]
- 10.Sotoudehmanesh R, Ali Asgari A, Ansari R, et al. Endoscopic findings in end-stage renal disease. Endoscopy 2003; 35:502–505. [DOI] [PubMed] [Google Scholar]
- 11.de Almeida Duarte JB, de Aguilar-Nascimento JE, Nascimento M, et al. Bacterial translocation in experimental uremia. Urol Res 2004; 32:266–270. [DOI] [PubMed] [Google Scholar]
- 12.Naaber P, Smidt I, Tamme K, et al. Translocation of indigenous microflora in an experimental model of sepsis. J Med Microbiol 2000; 49:431–439. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 79:516–524. [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies. J Chronic Dis 1987; 40:373–383. [DOI] [PubMed] [Google Scholar]
- 15.Scheff RT, Zuckerman G, Harter H, et al. Diverticular disease in patients with chronic renal failure due to polycystic kidney disease. Ann Intern Med 1980; 92:202–204. [DOI] [PubMed] [Google Scholar]
- 16.Wang F, Jiang H, Shi K, et al. Gut bacterial translocation is associated with microinflammation in end-stage renal disease patients. Nephrology 2012; 17:733–738. [DOI] [PubMed] [Google Scholar]
- 17.Mennigen R, Bruewer M. Effect of probiotics on intestinal barrier function. Ann N Y Acad Sci 2009; 1165:183–189. [DOI] [PubMed] [Google Scholar]
- 18.Silvestri L, van Saene HK, Zandstra DF, et al. Impact of selective decontamination of the digestive tract on multiple organ dysfunction syndrome: systematic review of randomized controlled trials. Crit Care Med 2010; 38:1370–1376. [DOI] [PubMed] [Google Scholar]
- 19.Comparato G, Pilotto A, Franze A, et al. Diverticular disease in the elderly. Dig Dis 2007; 25:151–159. [DOI] [PubMed] [Google Scholar]
- 20.Chapman J, Davies M, Wolff B, et al. Complicated diverticulitis: is it time to rethink the rules? Ann Surg 2005; 242:576–581.discussion 581-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology 2005; 41:422–433. [DOI] [PubMed] [Google Scholar]
- 22.Strate LL, Modi R, Cohen E, et al. Diverticular disease as a chronic illness: evolving epidemiologic and clinical insights. Am J Gastroenterol 2012; 107:1483–1486. [DOI] [PubMed] [Google Scholar]
- 23.Bose KP, Khorshidi I, Southern WN, et al. The impact of ethnicity and obesity on the course of colonic diverticulitis. J Clin Gastroenterol 2013; 47:160–164. [DOI] [PubMed] [Google Scholar]
- 24.Humes DJ, Spiller RC. Review article: the pathogenesis and management of acute colonic diverticulitis. Aliment Pharmacol Ther 2014; 39:359–370. [DOI] [PubMed] [Google Scholar]


