Supplemental Digital Content is available in the text
Keywords: cysticercal encephalitis, disseminated, neurocysticercosis, Taenia solium
Abstract
In this study, we describe clinical and imaging spectrum, and the natural course of patients with disseminated cysticercosis. How albendazole affects the course of disease has also been evaluated. We assessed the Toll-like receptor-4 gene polymorphisms, to know the reason for the apparently higher prevalence of disseminated cysticercosis in India.
Sixty consecutive patients with disseminated cysticercosis were enrolled. Sixty age-and-sex-matched healthy controls were also enrolled for the purpose of genetic study. Twenty patients, who gave consent, were treated with albendazole along with corticosteroids. Forty patients did not give consent for antiparasitic therapy. Assessment for Toll-like receptor-4 gene polymorphisms (Asp299Gly and Thr399Ile genes) was done. Patients were followed for 6 months. We also performed a literature search of cases published in English language using PubMed electronic database and analyzed 56 cases thus available.
There was an increased risk (6.63 fold and 4.61 fold) of disseminated cysticercosis in the presence of Asp299Gly and Thr399Ile polymorphisms in Toll-like receptor-4, respectively. The allelic frequency of Gly (11% vs. 3%, P = 0.024, odds ratio [OR] = 3.52) and Ile alleles (11% vs. 2%, P = 0.009, OR = 4.738) in disseminated cysticercosis was high. Albendazole resulted in complete disappearance of all cerebral lesions in 35% (7/20) patients and reduction in lesion load in remaining 65% (13/20) patients. No significant change in number of cysticercal lesion was noted in patients who did not receive albendazole. No major adverse reaction following antiparasitic treatment was noted. Three deaths were recorded in patients who did not receive antiparasitic treatment.
Of the 56 cases reported in PubMed, 33 patients received antiparasitic treatment with follow-up data available for 31 patients. Most (24) of these patients received albendazole. A significant clinical and/or imaging improvements, on follow up, were observed in 27 patients. Of the 4 deaths recorded, 3 had a heavy parasitic load and died after praziquantel therapy.
Toll-like receptor-4 gene polymorphisms are associated with an increased susceptibility to disseminated cysticercosis, in the Indian population. Albendazole treatment seems to reduce the lesion load and improve symptoms.
1. Introduction
Human taeniasis/cysticercosis is caused by the tapeworm Taenia solium. Neurocysticercosis is an infection of the central nervous system caused by the larval stages of T solium. Disseminated cysticercosis is a relatively rarer form of cysticercosis in which the cysticercal larvae spread throughout the body.
Cysticercosis in human occurs following ingestion of eggs of T solium via contaminated food and water. The definitive host harboring the adult worm are humans. Normally, in the life cycle of T solium, eggs released from the human intestine are ingested by pigs (intermediated host) through a contaminated feed. Eggs evolve in the larval form in pig's muscles, which may then reach the human intestine following ingestion of inadequately-cooked pork. Disseminated cysticercosis, in contrast, occurs when eggs enter the human body (instead of that of pig) and via hematogenous dissemination reach several body parts like, brain, spinal cord, eyes, muscles, skin, lungs, and liver. The clinical picture usually depends upon the topography and size of cysts, lesion load, and intensity of host immune reaction against the parasite.[1]
Albendazole and praziquantel are two drugs currently used in the treatment of neurocysticercosis. In a randomized controlled study, it was demonstrated that in patients with seizures due to viable parenchymal cysts, antiparasitic therapy decreases the burden of parasites and helps in reducing the number of generalized seizures. In this study, however, patients with more than 20 viable cysts were excluded.[2] A recent report demonstrated that a combination of albendazole plus praziquantel is even better as it increased the antiparasitic effect in patients with multiple brain cysticercosis cysts (1–20 viable cysts) without increased side-effects.[3] No clear recommendation, however, about antiparasitic treatment in disseminated cysticercosis is available. In fact, treatment of disseminated cysticercosis with antiparasitic drugs was considered dangerous in the past.[4] Nonetheless, there are many reports (though isolated) where disseminated cysticercosis has been treated with albendazole, with variable success, but without any major adverse reaction.[5]
A review of available reports suggests that the majority of patients reported with disseminated cysticercosis belong to India. A genetic reason is likely for this apparently higher prevalence of disseminated cysticercosis in India. Toll-like receptors are inflammatory mediators and are crucial for immune modulation. Toll-like receptor-4 gene, in human, is located on chromosome 9q32-q33. Studies have demonstrated an association between toll-like receptor-4 gene polymorphism and an increased risk of symptomatic neurocysticercosis in the Indian population. It has been observed that subjects with a toll-like receptor-4 gene (Asp299Gly or Thr399Ile) polymorphism were more susceptible to neurocysticercosis than control subjects. It has also been shown that in patients with symptomatic neurocysticercosis a higher levels of toll like receptor-4 expression resulted in an enhanced proinflammatory cytokine production.[6,7] We hypothesize that genetic abnormalities may be responsible for a high incidence of disseminated cysticercosis in the Indian population.
Most of the knowledge about such patients is in the form of isolated case reports. In this study, we describe the spectrum of this entity with respect to its clinical and radiological characteristics, along with its natural course. How does albendazole affect the natural course has also been evaluated. We tried to assess the Toll-like receptor-4 gene polymorphisms to know the possible genetic reason for the higher prevalence of disseminated cysticercosis in India.
2. Material and methods
This prospective follow-up study was done in the Department of Neurology, in collaboration with Department of Microbiology, at King George Medical University, Lucknow, India. This is a highly endemic region for neurocysticercosis. The ethical approval was obtained from the Institutional Ethics Committee. Written informed consent was taken from all the cases and controls enrolled in the study, or by their legal guardians. An informed consent was sought for using albendazole for the treatment. Patients were enrolled between August 2013 and September 2015.
2.1. Inclusion criteria
All consecutive patients presenting with seizures, with or without subcutaneous lesions, were screened by neuroimaging of the brain parenchyma. Patients with multiple brain lesions, suggestive of neurocysticercosis, were further evaluated to look for dissemination of cysticercal infection in various body parts. Fig. 1 depicts the algorithm of the study.
Figure 1.
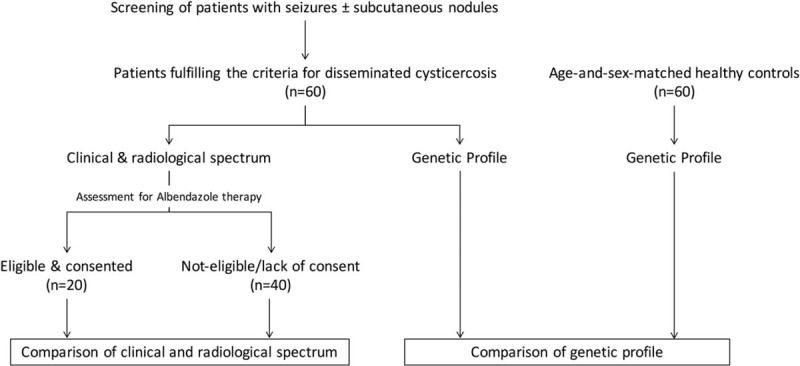
Algorithm of the study.
2.2. Case definition for disseminated cysticercosis
Patients of cysticercosis were diagnosed on the basis of absolute criteria consistent with cysticercosis.[8,9] All patients fulfilled at least one or more of the following 4 criteria. These criteria were histologic demonstration of the parasite in brain or spinal cord, cystic lesions showing the scolex, visualization of subretinal parasites, and multiple cystic lesions of brain (Fig. 2). Diagnosis of disseminated cysticercosis was made if there were multiple cystic/enhancing lesions in the brain, along with evidence of involvement of at least one extra site, like subcutaneous tissues, skeletal muscles, eyes, and presence in any visceral organ (like liver, lung, spleen, and heart).[8,9]
Figure 2.
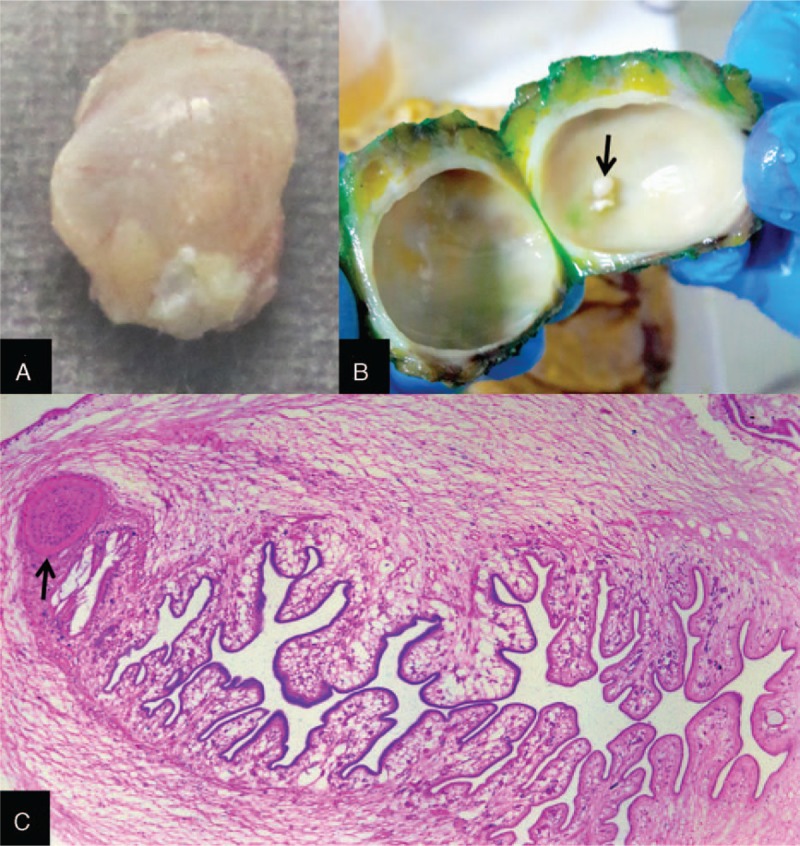
Gross image of the resected subcutaneous specimen shows a glistening cysticercus (A), the internal contents of which show the fibrotic cyst wall enclosing a pearly white scolex (B, arrow). Photomicrograph of the complete scolex (Hematoxylin & Eosin, ×50) depicts the sucker (C, arrow) and folded parenchyma.
Patients with prominent headache, vomiting, papilloedema, altered sensorium, and neuroimaging showing multiple enhancing lesions surrounded by variable amount of perilesional edema, were considered to have cysticercal encephalitis.
2.3. Exclusion criteria
The patients having evidence of systemic illness such as primary malignancy, pulmonary and systemic tuberculosis, and focus of pyogenic infection were excluded from the study.
2.4. Control population
Sixty age-and-sex-matched healthy controls were also enrolled for the purpose of genetic study.
2.5. Patient evaluation
A detailed clinical evaluation was performed in all included subjects. Routine hematological examination including complete hemogram and biochemical investigations, including renal and liver function tests, were done in all cases. All patients were subjected to enzyme-linked immunosorbent assay for human immunodeficiency virus. Serological tests for cysticercosis were not performed.
All included patients were subjected to magnetic resonance imaging of brain and spine (with gadolinium contrast). Fundus examination with ocular B-scan ultrasonography was done. X-rays of the chest, thighs, and the skull were done in all cases. Electrocardiogram was also done in all the cases.
Magnetic resonance imaging/ultrasonography of subcutaneous tissue, limbs, chest, and abdomen were done in selected cases, as per clinical decision. Whole body magnetic resonance imaging (MRI) was also performed in few cases. Biopsy of subcutaneous lesions, if present, was also done.
2.6. Polymorphism assay
2.6.1. Genomic DNA extraction
Peripheral blood in EDTA vial was collected from patients with disseminated cysticercosis and controls. By using salting out method, genomic DNA was extracted from the blood of patients of disseminated cysticercosis and purified by using the phenol-chloroform method.[10]
2.6.2. TLR4 (Asp299Gly and Thr399Ile) genotyping
Polymerase chain reaction followed by sequencing by chain termination (polymerase chain reaction [PCR]-sequencing) based method was done to determine toll-like receptor-4 gene polymorphisms. For both the Toll-like receptor-4 Asp299Gly and Toll-like receptor-4 Thr399Ile polymorphism a single set of primer sequences were designed: forward 5′-AGTCCATCGTTTGGTTCTGG-3′ and reverse 5′-TCAAATTGGAATGCTGGAAA-3′ (Integrated DNA technology, USA). Amplifications of all PCR were performed in a 25-μL volume containing 10× assay buffer, 200 μM each of dATP, dCTP, dGTP, dTTP, 0.1 μM of each primer, 1.0 U of Taq DNA polymerase (Finzymes, Thermo Scientific). Conditions for PCR were as follows: an initial denaturation at 94 °C for 10 minutes, followed by denaturation at 94 °C for 30 seconds for 35 cycles, annealing at 54.4 °C for 30 seconds, extension at 72 °C for 40 seconds, and a final extension at 72 °C for 7 minutes followed by cooling to 4 °C. For negative control Template free water was used. The products were subjected to exo–sap purification after amplification, and the purified 1 to 2 μL PCR products were subjected to sequencing PCR by using BigDye Terminator cycle sequencing Kit v3.1 and single primer, Thermo Fisher Scientific Inc., U.S. Again purification was done in these amplified sequencing PCR products by ethanol, EDTA, and sodium acetate precipitation. The second primer was used to repeat all the experiments.[11,12] After sequencing of the samples genotype analysis was carried out manually for each sample and each locus was analyzed individually using ABI Sequencing Analysis v5.4 software Applied Biosystems, U.S.
2.7. Treatment
All patients having seizures were treated with oxcarbazepine (10–15 mg/kg body weight). If seizure control was not achieved, clobazam was added. Patients with cysticercal encephalitis were treated with corticosteroids (intravenous dexamethasone, 4 mg every 6 hours).
Patients, who gave consent and did not have any contraindication, for example, involvement inside-of orbital muscle cone (without excision), cardiac conduction pathway involvement, sensitivity to albendazole, or comorbid ailment preventing corticosteroid therapy, were subjected to antiparasitic treatment. Patients with lesion/s abutting the ventricular outflow tract (deemed to cause obstruction and life-threatening increase in intracranial pressure on degeneration) and those with cysticercal encephalitis were also considered ineligible for albendazole therapy. It may be noted that cysticercal encephalitis represents a patient in an altered state of consciousness with a heavy first-contact lesion load and generalized cerebral edema; any type of antiparasitic therapy may prove lethal in such a situation. Albendazole (15 mg/kg/day in two divided doses) was given for 28 days. Three days before starting albendazole, oral corticosteroids (methylprednisolone 0.75–1 mg/kg) were started and continued throughout albendazole therapy; these were subsequently tapered off (0.25 mg/kg/wk) in the next 3 to 4 weeks. A close watch for any untoward event, like raised intracranial pressure, altered sensorium, visual disturbances, cardiac conduction defect, or occurrence of myelitis, was kept. In case of any complication, albendazole was stopped, corticosteroids were continued and re-imaging was performed.
2.8. Follow-up
Patients were followed for a minimum of 6 months. Besides an evaluation at 3 months of those having received albendazole, additional evaluations were done in the intervening periods in patients with seizure recurrence, new focal neurological deficit, or if there was an increase in headache.
A follow-up magnetic resonance imaging was performed after 6 months. On neuroimaging, the response to albendazole was assessed and categorized on the basis of visual impression. More than 50% reduction in the number of cysts was considered significant.
2.9. Search for published cases
We performed a literature search of cases or case series published in English language using PubMed electronic database. The key words used were “disseminated cysticercosis,” “disseminated neurocysticercosis,” and “miliary neurocysticercosis.” Second search through Google Scholar was performed utilizing the referencing and citation data. Data retrieved were tabulated and statistically analyzed.
2.10. Statistical analysis
Statistical analysis was done using SPSS version 16.0 (Chicago, LA). Qualitative variables were expressed as percentages. Qualitative variables were compared using chi-square/ fisher exact test, as applicable. Quantitative variables were expressed as mean ± SD, means were compared using independent “t” test. For genotype comparison age and sex adjusted odds ratio (OR) was calculated using binary logistic regression. Allele and haplotype comparisons were done using SNPstat (web tool for genetic analysis).[13]
3. Results
A total of 170 patients presenting with seizures and/or subcutaneous nodules were screened. The patients with multiple parenchymal cerebral cysticercal granulomas were further screened for extra sites of dissemination. Thus, 60 patients of disseminated cysticercosis were finally enrolled. None of the patient was human immunodeficiency virus positive. The demographic, clinical, and radiological characteristics of patients are detailed in Table 1 (Supplementary material 1). The depiction of the same is provided in Figs. 3–7.
Table 1.
Demographic, clinical, and radiological characteristics of 60 patients with disseminated cysticercosis.
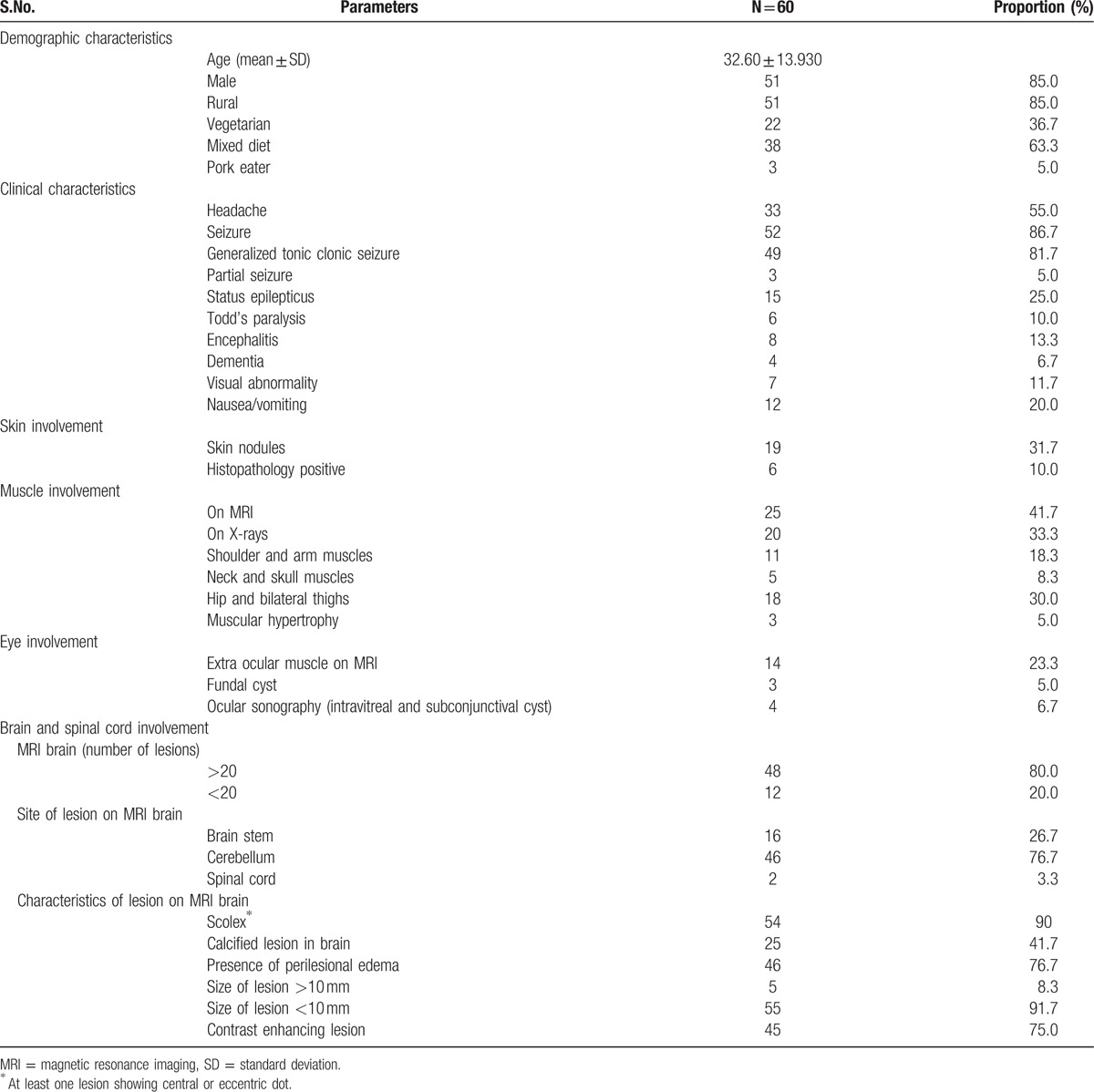
Figure 3.
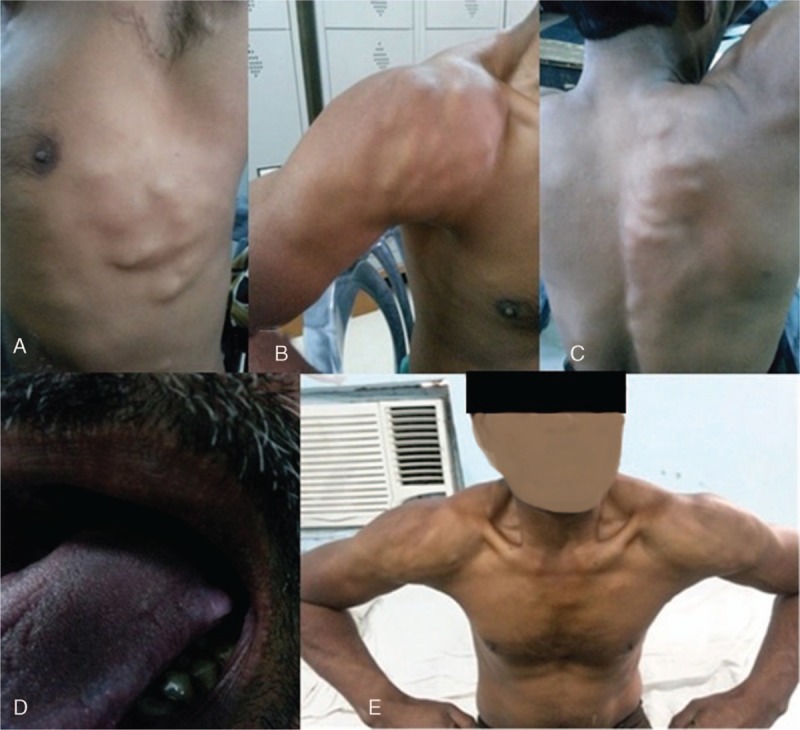
Subcutaneous nodules present on left side of the chest (A), right shoulder (B), and back (C); cyst on left lateral margins of tongue (D), and pseudo muscular hypertrophy (E). Biopsy and histopathology of the lesion was consistent with cysticercosis.
Figure 7.
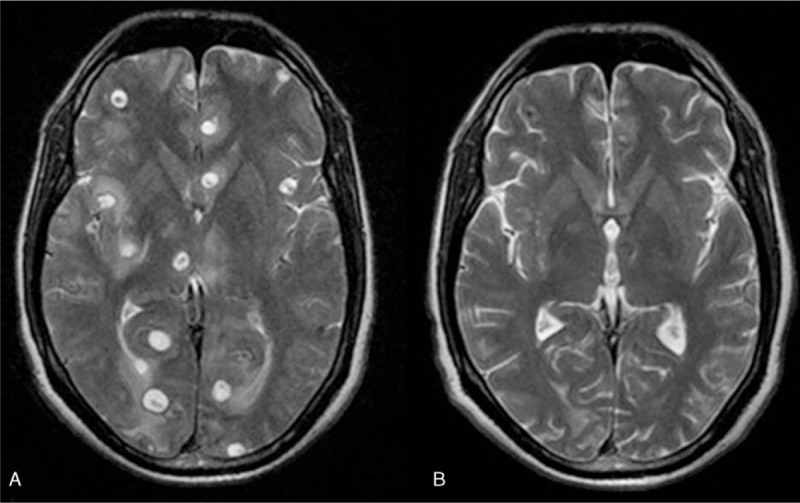
Axial T2-weighted image showing vesicular cysts (A) at baseline and (B) complete cysts resolution after albendazole therapy.
Figure 4.
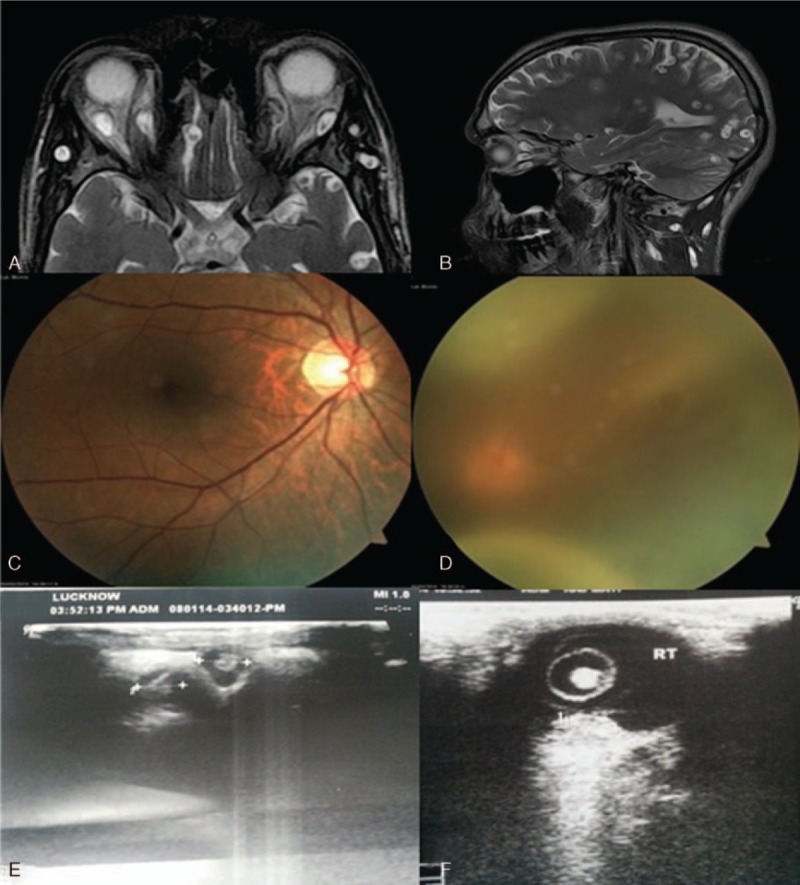
Evaluation of dissemination of cysticerci in the eye—MRI T2—weighted axial image (A) and sagittal (B) showing cysts in extra-ocular muscles. On Fundus examination there was a subretinal cyst with retinal detachment (C) and (D). Ocular B-scans showing ocular cyst (E) and (F). MRI = magnetic resonance imaging.
Figure 5.
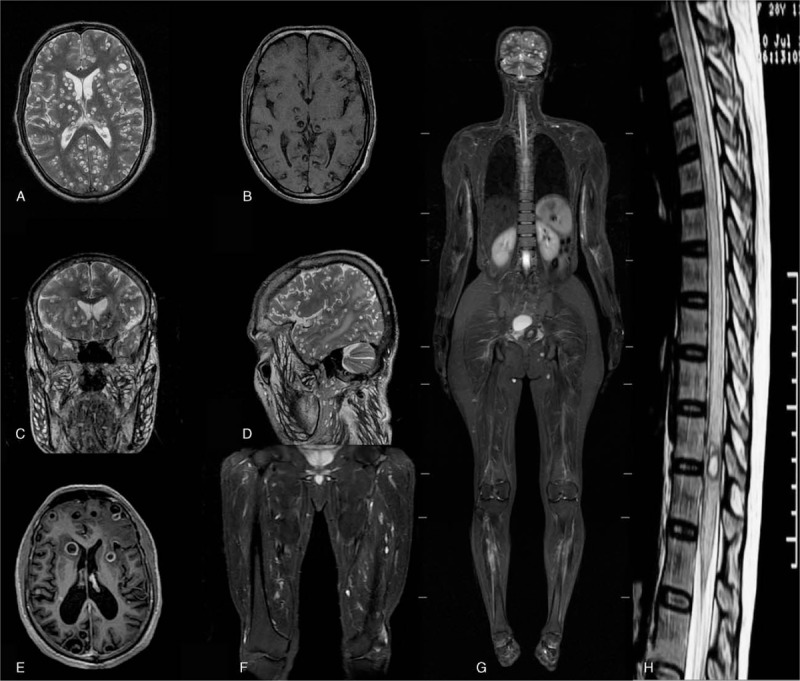
On MRI (A) axial T2-wieghted image showing multiple cysticercal cysts having scolex in bilateral brain parenchyma, (B) T1-weighted image with hyper intense scolex. (C) Coronal and (D) Saggital images showing multiple cysts in scalp, facial, and neck musculature along with parenchymal cysts. (E) MRI with SPGR (Spoiled gradient recalled echo) contrast showing ring enhancement, (F) and (G) STIR (Short tau inversion recovery) images as whole body protocol, and (H) showing intra medullary cyst in thoracic cord. MRI = magnetic resonance imaging.
Figure 6.
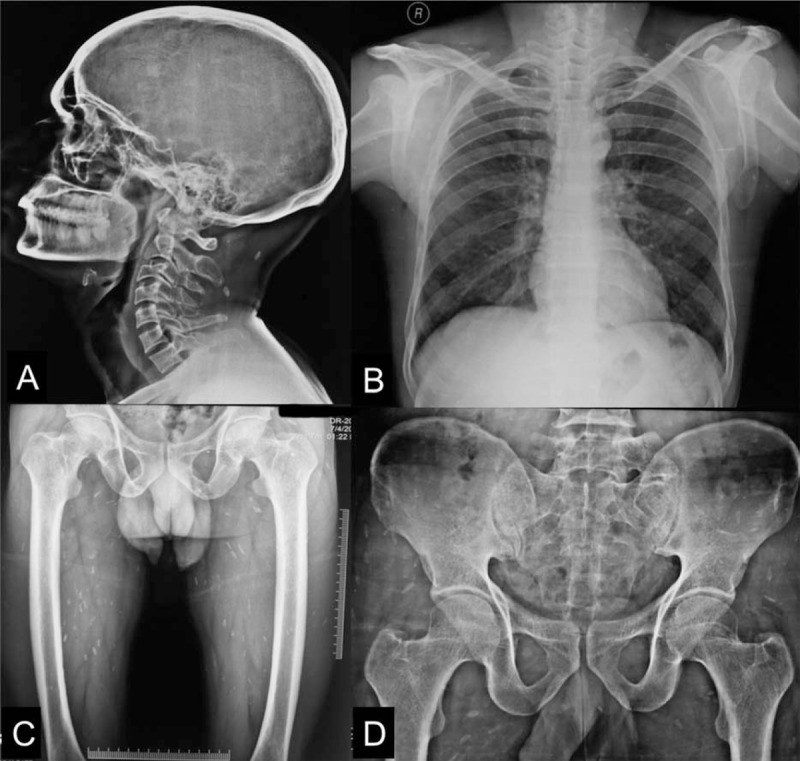
X-rays protocol—plain x-ray of skull, lateral view with neck (A), chest with bilateral shoulders (B), and hip with bilateral thighs (C) and (D) showing calcified granuloma as round, oblong, or cigar shaped radio-dense shadows involving the zone of muscles of the parts involved.
3.1. Epidemiology
The mean age of patients was 32.6 ± 13.9 years. In this study, 22 (36.7%) patients were strict vegetarian, while only 3 (5.0%) patients were pork eaters.
3.2. Clinical and imaging characteristics
Seizures (86.7%), headache (55%), raised intracranial tension (20.0%), encephalopathy (13.3%), visual abnormality (11.7%), dementia (6.7%), and muscular hypertrophy (5.0%) were common neurological presentations.
All cases of disseminated cysticercosis had multiple brain lesions; 48 (80%) patients had >20 lesions. Cerebellum involvement was seen in 46 (76.7%) patients. Brainstem was affected in 16 (26.7%) patients. Spinal cord involvement was seen in only 2 (3.3%) patients.
On MRI of the brain, scolex was present in 54 (90%) while perilesional edema was present in 46 (76.7%) patients; calcified lesions were present in 25 (41.1%) patients. In majority of patients (55, 91.7%), the size of an individual lesion was <10 mm.
3.3. Skin involvement
Skin nodules were present in 31.7% patients. In 3 patients, subcutaneous nodules were present throughout the body.
3.4. Muscle involvement
Muscular hypertrophy was demonstrated in 3 patients. On Xx-rays study, calcified lesion in muscles of the body was present in 20 (33.3%); majority of lesions were in hip and thigh muscles (18, 30.0%).
3.5. Eye involvement
Extra-ocular muscles were involved in 14 (23.3%) of patients. Subretinal cysts were present in 3 (5%) patients. In 2 out of 3 patients with subretinal cysts, surgical excision was done prior to albendazole therapy; consent for surgical therapy and subsequent albendazole administration could not be obtained in the third patient.
3.6. TLR-4 polymorphisms
Heterozygous form was more common in cases with disseminated cysticercosis as compared with healthy controls (P = 0.02, OR = 6.63, 95% confidence interval [CI] = 1.40–31.51) in the first polymorphism analyzed, that is, Asp/Gly genotype. In the second polymorphism, Thr/Ile genotype heterozygous form was, similarly, more common in cases with disseminated cysticercosis as compared with healthy controls (P = 0.003, OR = 4.61, 95% CI = 1.20–17.68) (Table 2).
Table 2.
Distribution of TLR-4 Asp299Gly and TLR-4 Thr399Ile polymorphisms in 60 patients with disseminated cysticercosis and 60 controls.
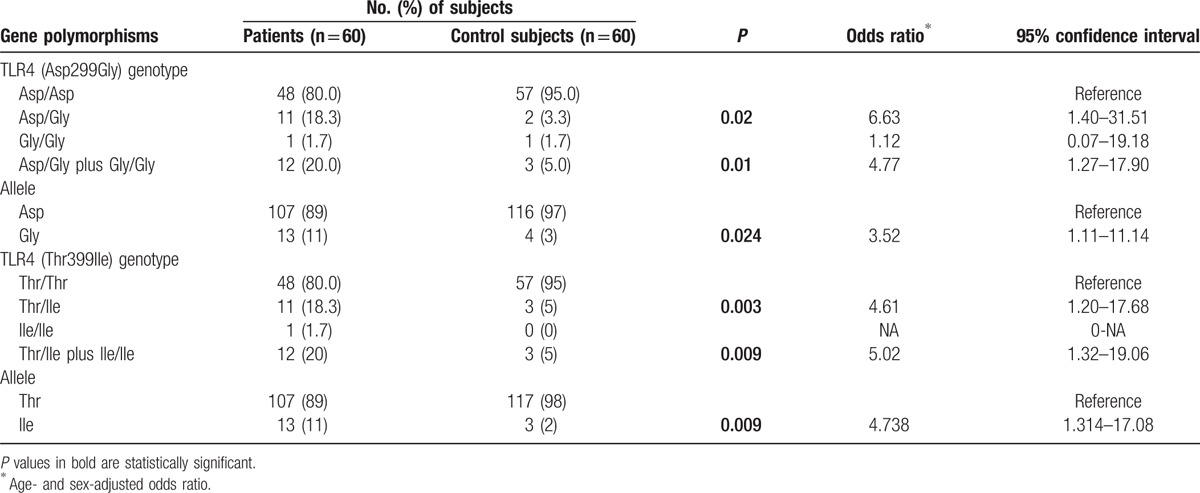
The Gly (Asp/Gly plus Gly/Gly) and Ile (Thr/Ile plus Ile/Ile) polymorphisms were found to be significantly associated with disseminated cysticercosis patients (P = 0.01, OR = 4.77, 95% CI = 1.27–17.90 and P = 0.009, OR = 5.02, 95% CI = 1.32–19.06, respectively) when compared with healthy controls. There was a significantly higher prevalence of the Gly (11% vs. 3%, P = 0.024, OR = 3.52, 95% CI = 1.11–11.14) and Ile alleles (11% vs. 2%, P = 0.009, OR = 4.738, 95% CI = 1.314–17.08) in disseminated cysticercosis patients as compared with healthy controls.
Haplotype analysis was also performed to analyze the additive effect of polymorphism. Haplotype frequency containing Gly/Thr was 0.074 in disseminated cysticercosis cases as compared with 0.017 in control group (P = 0.051, OR = 5.09, 95% CI = 1.01–25.67). Global haplotype association P value was 0.054.
3.7. Follow-up
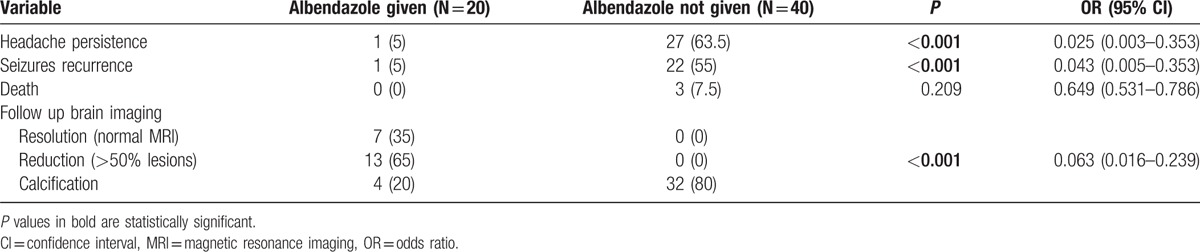
Twenty patients agreed to receive albendazole after informed consent. None of patients who received albendazole complained of any adverse reaction. After 6 months of treatment, in the albendazole group, seizures and headache were less frequent (P < 0.001, OR = 0.043, 95% CI = 0.005–0.353 and P < 0.001, OR = 0.025, 95% CI = 0.003–0.353, respectively). Of 20 patients who received albendazole, follow-up MRI became normal in 7 (35%) patients. In the remaining 13 patients a reduction in the lesion load was noted. In patients who did not receive albendazole follow up MRI remained unchanged (Table 3). Three deaths were recorded in patients who had not received antiparasitic treatment.
Table 3.
Response of albendazole therapy in disseminated cysticercosis after 6 months.
3.8. Cysticercal encephalitis
There were 8 patients of cysticercal encephalitis in our study. None of them received albendazole. Six patients improved with corticosteroids, one died, and one became corticosteroid-dependent.
3.9. Analysis of published cases
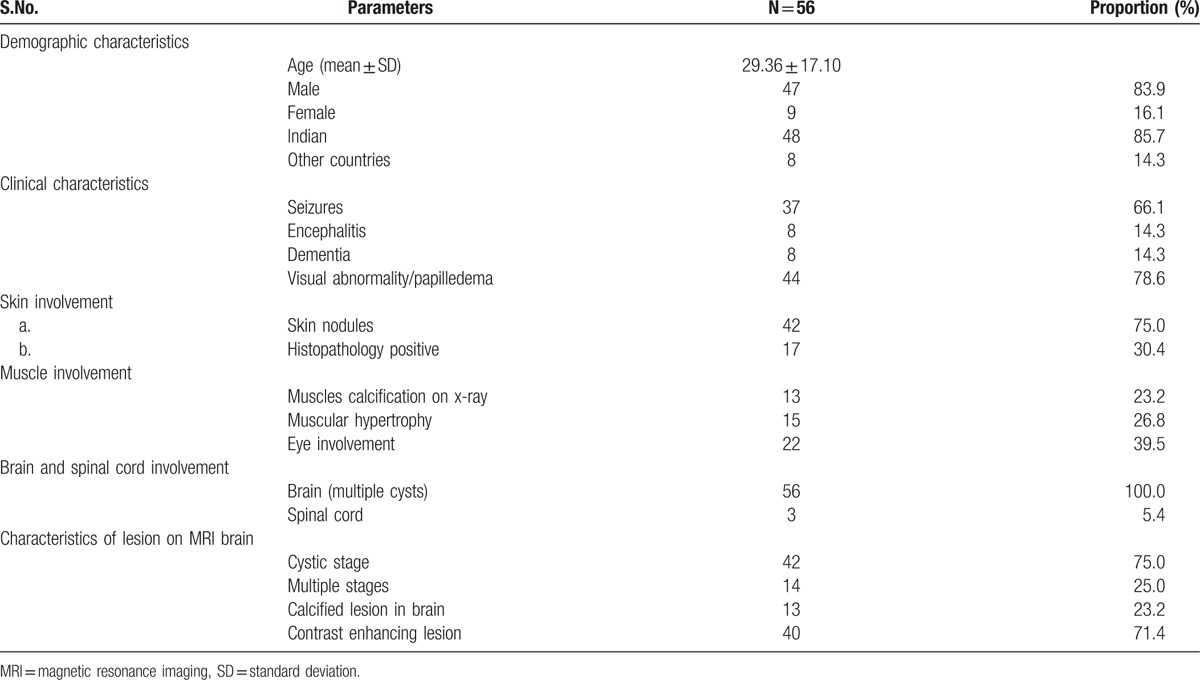
We were able to retrieve information of 56 published cases (Table 4, Supplementary material 2). In all these cases brain was unequivocally involved and at least one additional site was involved. Most (48/56, 85%) cases were Indian. Seizures, encephalopathy, and visual abnormality/papilledema were frequent clinical presentations. In 10 patients, numerous brain cysts imparted the classical “starry-night” appearance on computed tomography. Muscles (45/56, 80%) and skin (42/56, 75%) were the commonest extra-central nervous system sites of involvement. In muscles, x-rays showed multiple “cigar-shaped” calcifications in thigh and calf regions. Heart, lung, thyroid, pancreas, spleen, tongue, and salivary glands were other organs that were affected in various frequencies.
Table 4.
Demographic, clinical, and radiological characteristics of 56 published cases of disseminated cysticercosis.
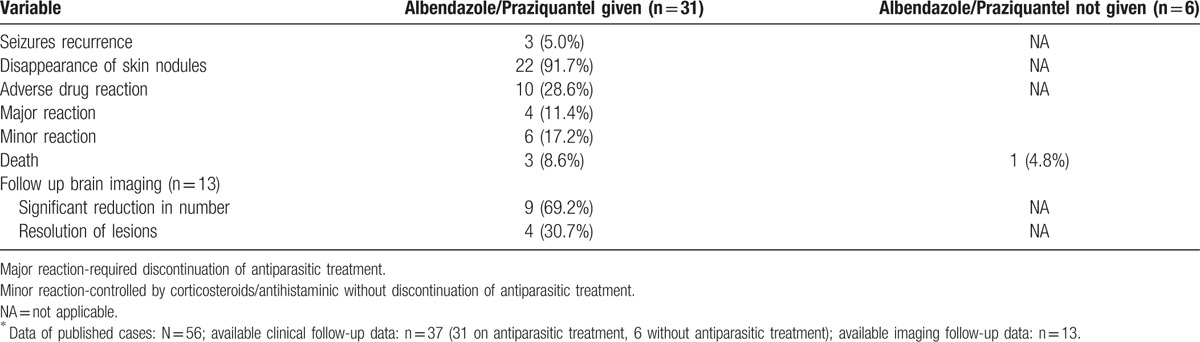
In the reported 56 cases, 33 patients received antiparasitic treatment with follow-up data available for 31 patients. In 4 patients praziquantel was used while 24 patients received albendazole. In 1 patient both praziquantel and albendazole was given. One patient received thiabendazole. In 3 patients antiparasitic therapy was given but specific drug was not mentioned. Follow-up data of only 6 patients, who did not receive any antiparasitic therapy, was available. Table 5 provides a comparison of follow-up data of patients receiving or not having received antiparasitic therapy. In majority of patients (37/56) corticosteroids (with antiparasitic therapy or without antiparasitic therapy) were used. In 11 cases, authors reported deterioration in the clinical condition following antiparasitic treatment, of whom a majority (8) improved subsequently. There were 4 deaths. Three patients with a massive parasitic load had severe clinical deterioration following praziquantel treatment. In 27 (out of 31) patients there was a significant clinical and/or imaging improvement. In 13 patients repeat neuroimaging was available, which revealed either a significant reduction in number (9) or complete resolution (4) of brain cysticercal lesions (Table 5).
Table 5.
Response of albendazole/praziquantel therapy in published cases of disseminated cysticercosis∗.
4. Discussion
We looked for an association of Toll-like receptor 4 gene polymorphisms and assessed the response of albendazole with supplemental corticosteroids in such patients. In order to provide an overview of the published literature, reports published since 1988 were compiled and analyzed.
The knowledge about disseminated cysticercosis is known since ages. There are numerous reports from earlier part of this century about disseminated cysticercosis in the published medical literature originating from countries that were earlier a part of British Empire. Ex-soldiers or persons who had resided in these countries were more affected.[14] Before the CT era, most cases with cysticercosis described in the literature were, in fact, disseminated cysticercosis because diagnosis was possible only with histopathology of a cutaneous or a muscular lesion. Many classical descriptions of disseminated cysticercosis belonged to this era. In one such description, Broughton-Alcock et al15, in 1928, described disseminated cysticercosis as follows “The brain is one of the situations most commonly affected.”
In 155 cases of cysticercosis compiled by Stiles, the brain was involved in 117, muscles in 32, the heart in 9, subcutaneous tissue in 5, and the liver in 2. In the Dresser's series of 87 cases, the brain was affected in 72 cases while muscles were involved in 13; while Muller in 36 cases found the parasite 21 times in the brain, 12 times in the muscles and thrice in the heart. In the Vosgien's series, following was the incidence of organs involved: the eyes and adjoining structure 46%, nervous system 40.9%, muscles 3.7%, and other organs 3.2%. Titu Vaisalu (1921), in an analysis of 330 cases of cysticercosis of the nervous system, described “brain and meninges as affected in 279, cerebral ventricle in 41, pons and medulla in 5, and spinal cord in 5 cases.”[15] Ewing, in 1941, described disseminated neurocysticercosis as follows “Numerous small cysts were seen scattered over the cortex. They were encapsulated and semi-transparent, ovoid in shape, and varied in size from that of a pea to a little larger. They were mostly single, occasionally in groups of 2 or 3. Their surface distribution appeared to bear a direct relation to the vascular supply, being most numerous in the area of the middle cerebral artery, and this may explain why the brain with its lavish vascular supply appears to be for the parasite a locuis minoris resistentiae. Most of the cysts made cup-like depressions in the cortex; some were buried in it. They were also present in the sulci and fissures, but were absent on the base.”[16] More than 100 years later, we noted that many things about disseminated cysticercosis have not changed. What has changed, since then, is ease of detection of cysticercal cysts in various organs by available neuroimaging methods; the rest, possibly, remains the same.
At times, heavy dissemination of larvae in muscles gives an appearance similar to that seen in pseudo-muscular hypertrophy, which in extreme cases imparts a characteristic “Herculean appearance.”[17] This phenomenon has been described in many earlier descriptions of disseminated cysticercosis. For example, Priest, in 1926, described it as follows “Nearly all muscles are enlarged, especially those of the shoulder girdles, and on contraction the affected muscles present a nodular appearance. He gives the impression of being a powerful man of ‘Sandow’ type development, but the muscle power is in fact very feeble.”[18]
Our analysis of all previous published cases suggests that disseminated cysticercosis is more prevalent in Indian population. Toll-like receptor 4 gene Asp299Gly and Thr399Ile polymorphisms were significantly associated with the occurrence of neurocysticercosis and, in particular, with symptomatic neurocysticercosis.[6] Toll-like receptor 4 gene polymorphisms, in a recent study, were found to be associated with the susceptibility to solitary cysticercus granuloma.[7] We noted that Toll-like receptor 4 gene abnormalities confer genetic susceptibility to disseminated cysticercosis as well. There was an increased risk (6.63 fold and 4.61-fold increased risk in the presence of Asp299Gly and Thr399Ile polymorphisms, respectively) of disseminated cysticercosis. Why some patients have massive lesion load in disseminated cysticercosis and why some patients have single lesion in solitary cysticercus granuloma, however, remains unresolved. Possibly, some other genetic factor or an unidentified environmental factors are responsible for different types of neurocysticercosis in different individuals.
In patients with countable number of brain cysts, albendazole has a definite role to play. Albendazole, in these patients, hastens the resolution of cysts and decreases the risk of seizure recurrence. The issue of antiparasitic treatment in disseminated, so far, has been controversial. After publication of a report by Wadia et al[4], that described 3 patients with numerous brain cysts treated with praziquantel, all of whom died, it was shown that praziquantel was “ineffective and hazardous”. Since then disseminated form of cysticercosis was considered a contraindication for antiparasitic treatment. On review of all published cases, we noted that albendazole and corticosteroids have been used frequently in disseminated cysticercosis but with variable results. Many patients showed remarkable resolution of brain cysts and symptomatic improvement. Some patients noticed transient exaggerated inflammation in subcutaneous cysts, muscles, and infrequently in brain. No death was reported. Review of other published reports also indicates that albendazole and/or praziquantel can safely be used with supplemental corticosteroids. It is the selection of patients that is of paramount importance and not the type of antiparasitic drug administered to patients with disseminated cysticercosis. Barring the events (death) reported by Wadia et al[4], deaths in disseminated cysticercosis are rare. In majority of our patients, albendazole led to symptomatic and/ or improvement in neuroimaging. Still, antiparasitic treatment has the potential for severe reactions leading to seizures, headache, and altered sensorium secondary to raised intracranial pressure. Massive release of cysticercal antigens possibly produce severe inflammatory changes in brain. Corticosteroids are often helpful in such a situation and should always be administered along with antiparasitic treatment. Another observation in our study was that of corticosteroid dependence and lends credence to the use of albendazole. Patients with a heavy load actually have a quasi-perpetual cycle of cystic degeneration and release of antigens. It appears that if this cycle is not blocked by an antiparasitic drug, it may at times be impossible to take the patient off corticosteroids, leading to corticosteroid dependence. Thus, antiparasitic drugs may help in prevention of such dependence.
Cysticercal encephalitis is considered a life threatening form of neurocysticercosis. Patients often present with acute encephalopathy and raised intracranial pressure. Treatment of cysticercal encephalitis includes corticosteroids and other edema reducing measures. If seizures are present, antiepileptic drugs are used. Antiparasitic drugs are usually considered a contraindication because of fear of aggravation of inflammation with further release of cysticercal antigens from dying parasites. However, there are isolated instances where albendazole led to a rapid clinical recovery.[19,20]
There were certain limitations to our study. Owing to the rarity of occurrence of disseminated cysticercosis, a fixed number of participants/patients necessary for the purpose of sample size calculation could not be attained to establish an association between the toll-like receptor polymorphisms and disseminated cysticercosis. Sample size may, thus, be calculated to better establish an association between the toll-like receptor polymorphisms and disseminated cysticercosis for future studies. Secondly, we did not include controls with more common forms of neurocysticercosis. For albendazole therapy, to prove or to disprove efficacy of albendazole a randomized controlled study would have been more desirable. A longer follow-up, preferably of more than 2 years, would have helped in delineating the natural course of disseminated cysticercosis more clearly. A special emphasis on knowing natural course in patient with heavy parasitic load (with or without albendazole) is also of paramount importance.
5. Conclusion
Toll-like receptor-4 gene polymorphisms are associated with susceptibility to disseminated cysticercosis, in India. Albendazole therapy leads to a reduction in lesion load and symptomatic improvement.
.
Supplementary Material
Footnotes
Abbreviations: CI = Confidence interval, DNA = deoxyribonucleic acid, MRI = magnetic resonance imaging, OR = odds ratio, PCR = polymerase chain reaction.
AQ, RKG, and HSM have contributed equally to the article.
This work was financially supported by the Council of Science and Technology Uttar Pradesh Lucknow.
The authors have no conflicts of interest to disclose..
Supplemental Digital Content is available for this article.
References
- 1.Garcia HH, Nash TE, Del Brutto OH. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol 2014; 13:1202–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia HH, Pretell EJ, Gilman RH, et al. Cysticercosis Working Group in Peru. A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. N Engl J Med 2004; 350:249–258. [DOI] [PubMed] [Google Scholar]
- 3.Garcia HH, Gonzales I, Lescano AG, et al. Efficacy of combined antiparasitic therapy with praziquantel and albendazole for neurocysticercosis: a double-blind, randomized controlled trial. Lancet Infect Dis 2014; 14:687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wadia N, Desai S, Bhatt M. Disseminated cysticercosis. New observations, including CT scan findings and experience with treatment by praziquantel. Brain 1988; 111:597–614. [DOI] [PubMed] [Google Scholar]
- 5.Sander HW, Castro C. Images in clinical medicine. Neurocysticercosis. N Engl J Med 2004; 350:266. [DOI] [PubMed] [Google Scholar]
- 6.Verma A, Prasad KN, Gupta RK, et al. Toll like receptor 4 polymorphism and its association with symptomatic neurocysticercosis. J Infect Dis 2010; 202:1219–1225. [DOI] [PubMed] [Google Scholar]
- 7.Singh A, Garg RK, Jain A, et al. Toll like receptor-4 gene polymorphism in patients with solitary cysticercus granuloma. J Neurol Sci 2015; 355:180–185. [DOI] [PubMed] [Google Scholar]
- 8.Del Brutto OH, Rajshekhar V, White AC, Jr, et al. Proposed diagnostic criteria for neurocysticercosis. Neurology 2001; 57:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg RK. Diagnostic criteria for neurocysticercosis: some modifications are needed for Indian patients. Neurol Ind 2004; 52:171–177. [PubMed] [Google Scholar]
- 10.Miller SA, Dykes DD, Polseky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988; 16:1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patton SJ, Wallace AJ, Elles R. Benchmark for evaluating the quality of DNA sequencing: proposal from an international external quality assessment scheme. Clin Chem 2006; 52:728–736. [DOI] [PubMed] [Google Scholar]
- 12.Underwood A, Green J. Call for a quality standard for sequence-based assays in clinical microbiology: necessity for quality assessment of sequences used in microbial identification and typing. J Clin Microbiol 2011; 49:23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sole X, Guino E, Valls J, et al. SNPStats: a web tool for the analysis of association studies. Bioinformatics 2006; 22:1928–1929. [DOI] [PubMed] [Google Scholar]
- 14.MacArthur WP. Cysticercosis as a cause of epilepsy in man. Trans R Soc Trop Med Hyg 1933; 26:525–528. [Google Scholar]
- 15.Broughton-Alcock W, Stevenson WE, Worster-Drought C. Cysticercosis of the brain: with report of a case. Br Med J 1928; 2:980–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ewing CW. Cysticercosis epilepsy. Br Med J 1941; 2:263–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chopra JS, Nand N, Jain K, et al. Generalized muscular pseudohypertrophy in cysticercosis. Postgrad Med J 1986; 62:299–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Priest R. A case of extensive somatic dissemination of cysticercus cellulosae in man. Br Med J 1926; 2:471–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Brutto OH, Campos X. Massive neurocysticercosis: encephalitic versus non-encephalitic. Am J Trop Med Hyg 2012; 87:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Río de la Loza LJ1, Meza EL. Cysticercotic encephalitis: case report of miliary infestation in an encephalopathic fashion. Arch Neurol 2008; 65:276–277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


