Abstract
Introduction:
CD3+ γδ+ T cells comprise 2% to 5% of circulating T cells with Vγ9Vδ2+ cells the dominant circulating subtype. Vγ9Vδ2+ cells recognize non-peptide phosphoantigens and stress-associated NKG2D ligands expressed on malignant cells. Strategies that incorporate the tumoricidal properties of γδ T cells represent a promising immunotherapeutic strategy for treatment of solid malignancies including neuroblastoma (NB). In this prospective, non-randomized Phase I trial, we assessed whether circulating Vγ9Vδ2+ cells could be safely expanded using intravenous ZOL (Zoledronate [Zometa®]) and subcutaneous Interleukin-2 (IL-2) in patients with refractory NB.
Methods:
Patients 2 to 21 years of age with refractory neuroblastoma with no known curative therapeutic options received ZOL on day 1, and IL-2 on days 1 to 5 and 15 to 19 of each 28-day cycle (n = 4). Lymphocyte immunophenotyping was assessed weekly. Immunophenotyping studies from the treatment group were compared with healthy pediatric controls (n = 16; range, 5y–15y) and of untreated NB disease controls (n = 9; range, 4m–18y).
Results:
Treatment was well tolerated with no unexpected grade 3 and 4 toxicities. Lymphocyte subset counts did not differ significantly between volunteers and disease controls with the exception of γδ+ T cell counts that were significantly higher in healthy volunteers (212 + 93 vs. 89 + 42, P = 0.05). Study patients showed increases in circulating γδ+ T cell count (3–10 fold) after the first week, increasing into the range seen in healthy volunteers (125 + 37, P = 0.1940). Interestingly, all ZOL + IL-2 treated patients showed significant increases in CD3+CD4+CD27hiCD127dim T cells that rose weekly in 2 patients throughout the 4 weeks of observation (maximum 41% and 24% of total CD3+CD4+ T cells, respectively).
Conclusions:
In summary, combined ZOL and IL-2 is well tolerated and restored γδ+ T cell counts to the normal range with a moderate expansion of Natural Killer cells. Progressive increases in circulating CD4+ T cells with a regulatory phenotype cells may offset beneficial effects of this therapy.
Keywords: γδ+ T cells, cell therapy, neuroblastoma, regulatory T cell, zoledronic acid
1. Introduction
Neuroblastoma (NB) is the most common extracranial tumor diagnosed in children. Widely accepted standard therapy for high-risk NB includes 5 to 7 cycles of intensive cytotoxic chemotherapy, surgery, consolidative autologous stem cell transplantation (SCT), radiation therapy, and maintenance immunotherapy with anti-GD2 antibodies.[1] Such therapy carries considerable toxicity while survival remains generally poor as NB accounts for 15% of deaths attributable to cancer in childhood. Novel therapeutic approaches for NB are clearly needed.
CD3+ γδ+ T cells comprise 2% to 10% of circulating T cells and function in an Major Histocompatibility Complex (MHC)-independent manner that does not require antigen procession or presentation of antigen on antigen-presenting cells. Vγ9Vδ2+ cells are the dominant circulating γδ T cell subtype and recognize non-peptide phosphoantigens and stress-associated NKG2D ligands expressed on malignant cells. These γδ T cells show potent cytolytic activity against a wide variety of tumor types including neuroblastoma (NB).[2–4] Indeed, following expansion and activation in culture, γδ T cells from both healthy volunteers and NB patients exert a potent cytotoxic effect on NB cell lines and autologous NB in vitro. Vγ9Vδ2+ cells can be induced to proliferate in vivo by blocking farnesyl pyrophosphate synthase in the mevalonate pathway of isoprenoid synthesis in monocytes. Using this method, the aminobisphosphonate zoledronic acid (ZOL) promotes the accumulation of isopentenyl pyrophosphate that results in T cell receptor stimulation of Vγ9Vδ2 T cells. Interleukin-2 (IL-2) is also required for robust expansion of ZOL-activated γδ T cells.
Trials utilizing in vivo γδ T cell expansion with bisphosphonates in combination with IL-2 therapy have been undertaken in a variety of tumor types with therapeutic effects noted. Clinical responses to ZOL + IL-2 in patients with refractory metastatic prostate cancer have been demonstrated.[5] The bisphosphonate pamidronate in conjunction with low dose IL-2 has shown antitumor effect in patients with lymphoid malignancies[6] but the effect was only evident after selection of patients with marked γδ T cell activation in vitro. Among 9 patients selected in this manner, 5 of 9 patients demonstrated significant in vivo γδ T cell expansion/activation and 3 of 9 patients demonstrated objective tumor responses. Notably, γδ T cells have also been shown to penetrate the Central nervous System in response to developing brain tumors in an immunocompetent syngeneic mouse model.[7]
In this phase I trial, we studied the immunologic effects of 2 escalating doses of IL-2 in combination with ZOL in patients with recurrent/refractory NB. Four patients were enrolled on the trial and immunologic parameters and tumor response measured. We also quantified lymphocyte subtypes and functional parameters with respect to circulating γδ T cells in both newly diagnosed NB patients and healthy pediatric controls.
2. Materials and methods
2.1. Ethics, consent, and permissions
The University of Alabama at Birmingham (UAB) Institutional Review Board approved the study (protocol #F101013003) and registered the trial on 13 July 2011 (NCT01404702) under the Clinicaltrials.gov database. Informed consent was obtained from each patient or a designated family member. Each participant or their legally authorized relative (LAR) were given a minimum of 24 hours after receiving a copy of the consent document to consider the study and discuss with family/friends/other healthcare professions prior to signing the document. All participants or their LAR signed the Institutional Review Board approved informed consent document containing the sentence: “If information from this study is published or presented at scientific meetings, your name and other personal information will not be used.”
2.2. Study design and objectives
The trial was conducted as a prospective, non-randomized, single institution phase 1 trial of ZOL in combination with IL-2. Zometa was supplied as a gift from Novartis (Basel, Switzerland). The UAB Comprehensive Cancer Center Data Safety Monitoring Board undertook safety monitoring. All therapy and clinical care was provided at Children's of Alabama. The trial assessed 2 dose levels of recombinant IL-2 in combination with ZOL at 4 mg/m2 (maximum dose of 4 mg). ZOL was given intravenously on day 1, and IL-2 was given subcutaneously (SQ) on days 1 to 5 and 15 to 19 of every 28-day cycle (Fig. 1). The first dose level of IL-2 was 3 × 106 units/dose and the second dose level was 6 × 106 units/dose.
Figure 1.
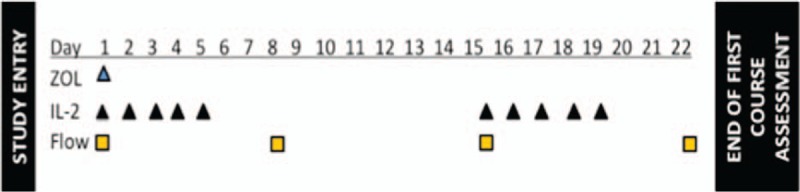
Schematic of the first course of therapy. Patients received Zoledronate (ZOL) at 4 mg/m2 with a maximum dose of 4 mg on day 1. Subcutaneous IL-2 (aldesleukin) was given at 3 × 106 IU daily on days 1 to 5 and days 15 to 19. Blood for flow cytometry was drawn prior to therapy on day 1 and again on day 8, day 15, and at the end of the course on day 22. Complete blood counts and blood chemistries including electrolytes were monitored weekly. Bone marrow disease assessments were also obtained at the beginning and end of the first course in patients with known bone marrow disease. End of course assessment included radiological scans to evaluate disease response. Patients with stable disease or better were allowed to proceed to the second course of therapy. IL-2 = Interleukin-2.
The primary objective of the study was to evaluate the safety and toxicity of Zoledronate (Novartis: Basel, HV) in combination with IL-2 (Aldesleukin; Prometheus Laboratories Inc.; San Diego, CA) as therapy for patients with refractory NB in a standard 3 × 3 dose escalation schema. Secondary objectives included evaluation of the biologic function of autologous in vivo expanded/activated γδ T cells in NB patients receiving therapy with ZOL + IL-2, monitoring of immune status of patients receiving ZOL + IL-2 therapy as compared with corresponding immunophenotypic data from healthy children and children with newly diagnosed NB receiving standard therapy, monitoring of antitumor cytotoxicity of expanded/activated autologous γδ T cells against established NB cell lines using in vitro cytotoxicity assays, determination of the ability of in vivo expanded/activated γδ T cells to infiltrate NB tissue using immunohistochemical techniques when post-therapy specimens were available, documentation of tumor response in patients with measurable disease. Volunteers were informed of the potential research studies for which their clinical specimens might be used and were provided with a check for participating in this one time assessment and data/specimen collection.
2.3. Patients and controls
Eligible patients were 2 to 21 years of age with refractory NB with no known curative therapeutic options. Minimum Lansky/Karnofsky scores were 60%. Between May 2012 and July 2013 a total of 4 patients diagnosed with Stage IV neuroblastoma were enrolled. Three had progressed on standard therapy and one has stable disease. No comorbidities were noted and all data points were obtained. Additionally, healthy pediatric siblings of general oncology patients (5–15y; n = 16) and NB patients at diagnosis and during standard therapy (4m–18y; n = 9) were also recruited for lymphocyte immunophenotyping. Patients and controls were excluded if they had been diagnosed with a coexisting immune system disorder; active viral, bacterial, or parasitic infection; or prior organ or bone marrow transplant.
2.4. Lymphocyte immunophenotyping
Peripheral blood was collected from study patients in evacuated tubes containing sodium heparin anticoagulant at 4 weekly time points. Blood was obtained once from healthy volunteers and disease controls. Single platform flow cytometric lymphocyte immunophenotyping was performed for each specimen using whole blood lysis technique after labeling with fluorochrome-conjugated monoclonal antibodies to CD3, CD4, CD8, CD16/56, CD19, CD27, CD28, CD45, CD45RA, CD25, CD57, CD127, CD197, and TCR γδ (BD Biosciences, San Jose, CA). The correlative biologic studies associated with this trial were designed for simple phenotyping in a setting that would be adaptable to a larger trial had the results been more favorable. In keeping with design, surface phenotyping was preferred if specimens were to be analyzed fresh at multiple centers. The BD Treg kit was specifically validated for the negative correlation of FOXP3 and CD127 (IL-7rα) expression and CD127 is a validated marker for this purpose as described by Simonetta.[8] Specimens were acquired in TruCount polystyrene tubes on a 3-laser 8-color FACS Canto II flow cytometer, stored as list mode files, and analyzed using FACS Canto and DiVa software (BD Biosciences, San Jose, CA).
2.5. Cytotoxicity assays
Target cell lines were labeled with the membrane dye PKH26 (Sigma; St. Louis, MO). Expanded/activated γδ T cells are then added to the tubes at ratios of 0:1 (Background), 5:1, and 10:1 effectors/NB targets, incubated for 4 hours at 37 °C and 5% CO2, washed 1 time and resuspended in 1 mL HBSS, and labeled with 20 μL membrane-permeable dye To-Pro Iodide (Molecular Probes; Eugene, OR) immediately prior to acquisition on the flow cytometer.
2.6. Immunohistochemistry
Paraffin embedded sections of neuroblastoma biopsy material were obtained for immunohistochemistry. Antibodies for detection of CD3 and NKG2D ligands were obtained from R&D Systems (Minneapolis, MN) and included major histocompatibility complex class-I polypeptide-related sequence A (MICA), major histocompatibility complex class-I polypeptide-related sequence B (MICB), UL-16 Binding Protein (ULBP)1, ULBP2, ULBP3, andULBP4 and matching isotype controls. Deparaffinized sections were post fixed in 4% neutral buffered formalin followed by antigen retrieval with Citra Plus (Biogenex Laboratories, Freemont, CA). Sections were block sequentially with avidin, biotin (Biogenex Laboratories, Freemont, CA) and FC receptor blocker (Innovex Biosciences, Richmond, CA) for 20 minutes at Room Temperature (RT). Primary antibodies (ULBP1-ULBP5), CD3, and MIC A were applied at 5 μg/mL overnight at 4 °C. Multilink secondary antibody (Biogenex Laboratories, Freemont, CA) was applied for 30 minutes at RT, followed by Streptavidin-labeled peroxidase (Biogenex laboratories, Fremont CA) for 30 minutes. For MICA staining rabbit anti-mouse secondary antibody were used (Biogenex Labs, Freemont, CA) for 30. The immunostaining was developed with Turbo DAB chromogen (Innovex Biosciences, Richmond, CA) for 2 minutes or until signal appeared.
2.7. Statistical considerations
The Biostatistics and Bioinformatics Core Facility for the UAB Comprehensive Cancer Center performed statistical analysis. This was a single-arm nonrandomized pilot study evaluating 2 dose levels of IL-2 when used in combination with the pediatric maximum tolerated dose (MTD) of ZOL. The sample size was determined based on the number of patients needed to evaluate 2 dose levels of IL-2. The primary end point was determination dose-limiting toxicities (DLTs) experienced by patients at each dose level of IL-2.
3. Results
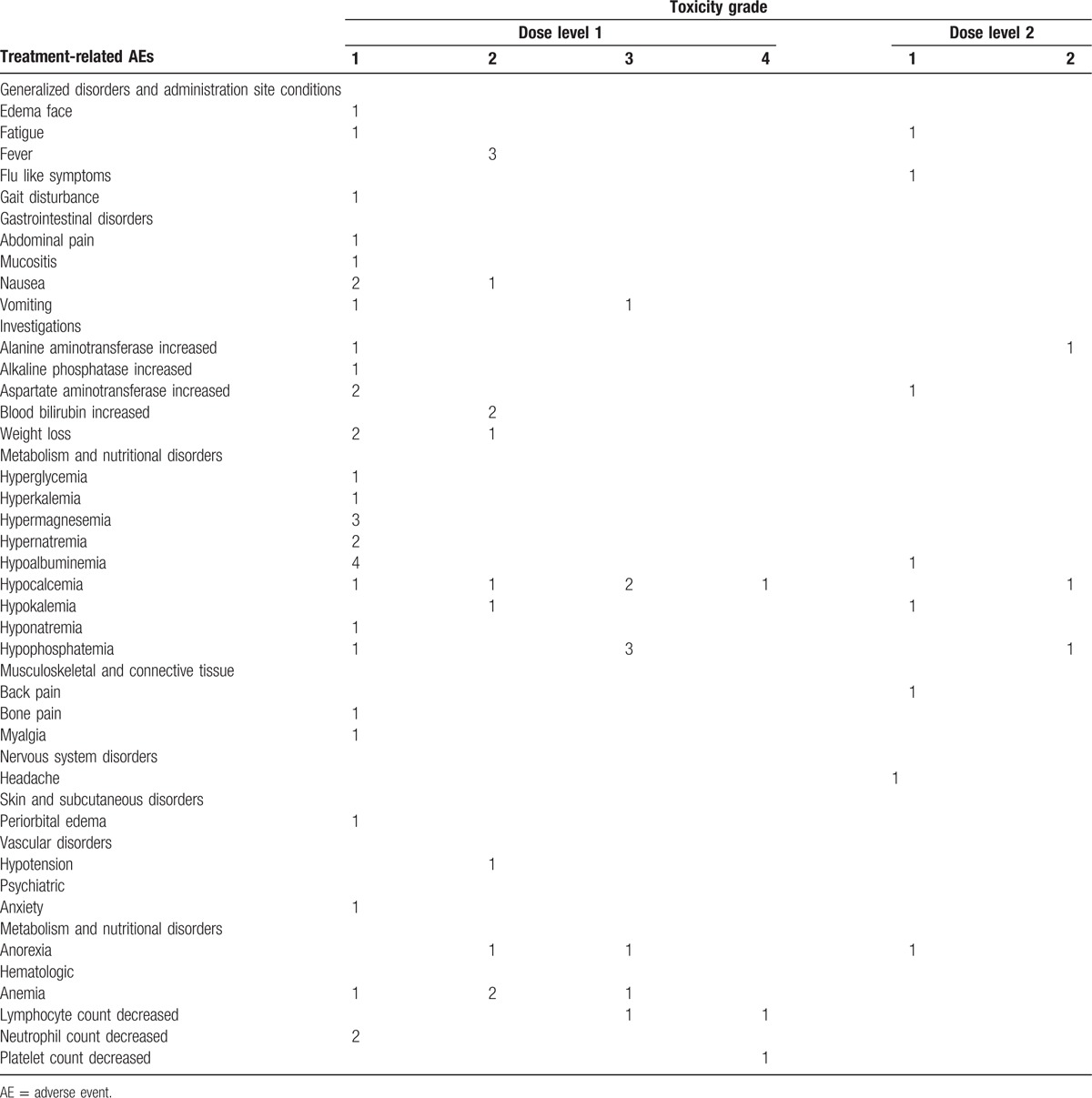
All enrolled patients met eligibility criteria for participation. The study included 3 males and 1 female. The age range at the time of study entry was 5 to 20 years with a median age of 13.5 years. The age at initial diagnosis ranged from 2 to 9 years with a median of 7 years. Patient age distribution was from 34 to 192 months (median 53 months) at the time of their original diagnosis. All patients classified as high-risk status at diagnosis and had progressed to stage 4 at enrollment. Tumor from 3 patients was MYCN non-amplified; the MYCN status of the remaining patient (patient A) was unknown. Three patients exhibited tumor metastases to the bone marrow (BM) (patient C did not have BM disease), and all were heavily pretreated at the time of study entry (Table 1). All patients previously received radiation therapy. No dose limiting toxicities or unexpected grade 3 or 4 toxicities occurred during the treatment phase. Hypocalcaemia, hypophosphatemia, and hypoalbuminemia were common adverse events with hypocalcaemia and hypophosphatemia being the most common grade 3 event (Table 2).
Table 1.
Patient Characteristics.
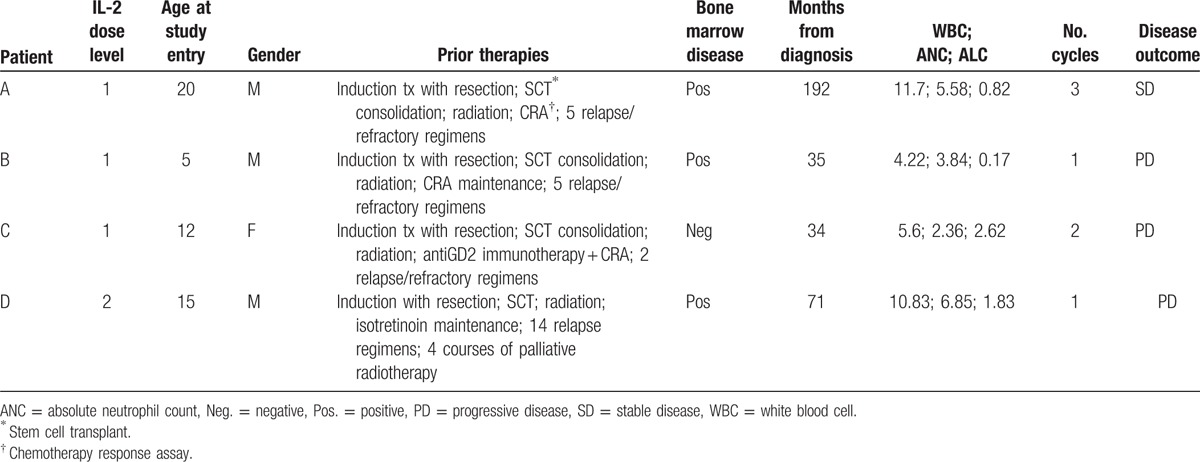
Table 2.
Adverse Events associated with Treatment.
Patient A was found to have stable disease at the end of course 1. During the third course of therapy due to persistent abdominal pain of uncertain etiology he was removed from study, which was deemed to be in his best interest by the treating physician. Patients B died as a result of disease progression during the first course after receiving all first cycle study therapy. Patient C progressed after 2 courses of therapy as evidenced by MIBG scan after initially demonstrating stable disease after course 1. Patient D also demonstrated progressive disease after course 1.
Flow cytometry revealed that γδ T cell absolute counts were significantly depressed in both newly diagnosed NB patients as well as recurrent/refractory patients enrolled on this trial (Fig. 2, bottom right) when compared with healthy controls (P = 0.05 and P < 0.001, respectively). Total T cell and CD4+ T cell counts were generally lower in NB patients as compared with controls as well with the exception of three individuals who showed increases in CD4+ T cell counts (Fig. 2). Natural Killer (NK) counts did not differ significantly. Treatment with ZOL and IL-2 resulted in an increase in the γδ T cell count into the range that we documented for healthy children (P = 0.1940), however, supranormal numbers thought to be required for a significant anti-tumor effect could not be achieved (Fig. 2). Additionally, γδ T cells from selected patients were found to proliferate in response to in vitro stimulation with ZOL + IL-2 (Fig. 3a) along with a more modest expansion of NK cells and were cytolytic against NB cell lines SKNAS and 1691 (Fig. 3b) expressing NKG2DL (Fig. 3c).
Figure 2.
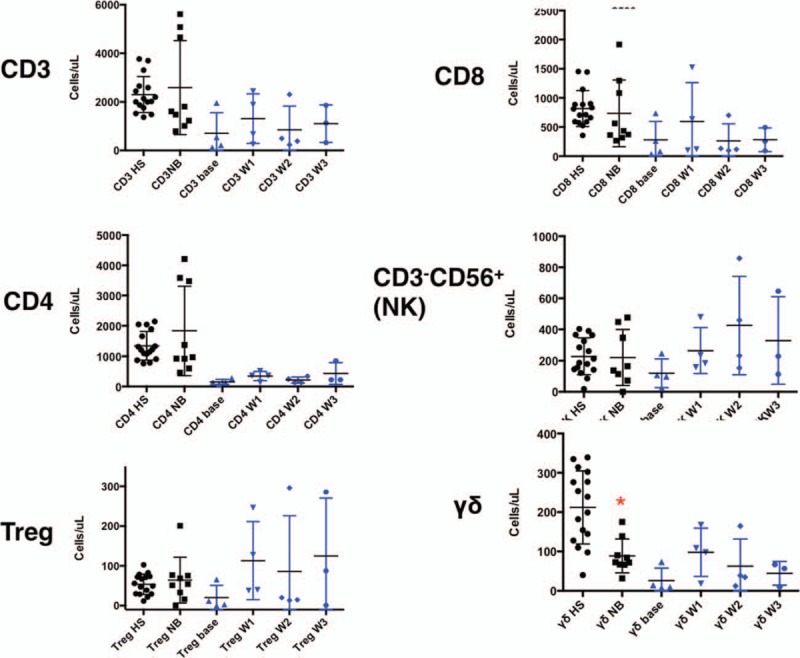
Comparison of major immune parameters between healthy children and newly diagnosed NB patients (black symbols, columns 1 and 2). A composite of the 4 treated patients at weekly time points in the trial is also shown (blue symbols). Three untreated NB controls showed a spontaneous proliferation of CD4+ T cells well above the range for the remaining patients that were generally lower than their healthy siblings. Circulating CD4+ T cells with a regulatory phenotype and NK counts did not differ between healthy siblings and untreated NB controls. A significant decline of γδ T cells in untreated, newly diagnosed NB patients (red asterisk) as well as treated patients prior to initiation of ZOL/IL-2 injections was seen when compared with healthy siblings. NK cells increased in treated patients, likely as a result of IL-2 therapy. IL-2 = Interleukin-2, NB = neuroblastoma, ZOL = Zoledronate.
Figure 3.
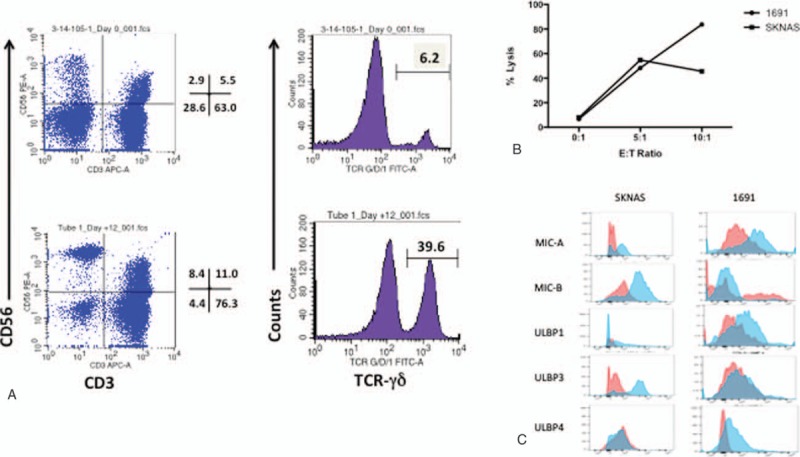
Cells were obtained from a Stage IV neuroblastoma patient prior to treatment with ZOL/IL-2 and expanded ex vivo for 14 days in media supplemented with 0.2 mM Zoledronate and 50 μg/mL IL-2. Media and cytokines were refreshed every 3 days. (A) Note that at day 14, γδ T cells had increased in proportion from 6.2% to 39.6% and (B) the γδ T cells exhibited incremental cytotoxicity against human neuroblastoma cell lines 1691 and SKNAS. (C) NKG2DL expression is shown on neuroblastoma cell lines SKNAS and 1691. MIC-A, MIC-B, and ULBP-3 are strongly expressed on SKNAS while 1691 shows no MIC-B expression but expresses ULBP-4. IL-2 = Interleukin-2, ZOL = Zoledronate.
Interestingly, regulatory T cell counts increased significantly in the treatment group (Fig. 2, bottom left) especially after week 3, a finding that was not encountered in newly diagnosed NB patients. Similar findings were made in the single patient enrolled in dose level 2. As regulatory T cell expansion can be associated with immunosuppression and consequent permissive tumor growth, this finding was considered a sufficient potential toxicity of the trial that ultimately led the investigators to suspend accrual.
We also compared cytotoxic T cell maturation profiles from patients treated with ZOL + IL-2 therapy with profiles obtained from healthy siblings (Table 3). The CD8+ T cell effector/memory status was generally uniform in healthy sibling controls with the majority showing a predominately naïve (CD27+CD45RA+, CD197+) T cell population (68.49% ± 7.89, n = 9) and less abundant central memory (C27+CD45RA-CD197+) populations (23.57% ± 9.47) and relatively few effector T cells (4.96% ± 8.56). Patients that received ZOL + IL-2 therapy showed greater variability in CD8+ effector/memory T cell status with Patients A and B showing a substantially lower pretreatment percentage of central memory cells and patients C and D showing a lower percentage of effector T cells. The percentage of effector cells diminished in patients A and B at Week 2 although the percentage of central memory T cells was generally level throughout the course of treatment.
Table 3.
Effector memory CD8+ T cell maturation profiles for patients the received ZOL/IL-2 therapy.

The expression of NKG2D ligands in two neuroblastoma excisional biopsy specimens was also evaluated. A representative specimen is seen in Fig. 4, where (Fig. 4a) is an H&E staining of tumor and (Fig. 4b) shows characteristic uniform expression of CD56 throughout the tumor. Expression of several of NKG2D ligands was noted on tumor cells with strong diffuse expression of ULBP3 and ULBP5 (Fig. 4c). Expression of ULBP1, ULBP2, and ULBP4 was not as strong, but still positive compared with control parenchyma. Indeed, the constitutive level of expression of NKG2D ligands on tumor was qualitatively higher compared with control, providing a clear potential for γδ T cell—mediated selective tumor killing.
Figure 4.
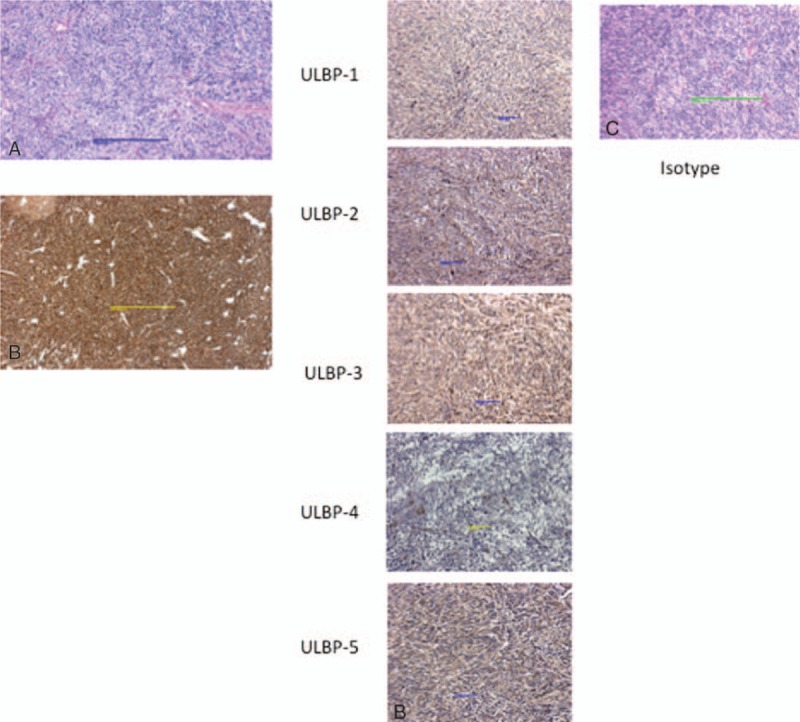
Representative excisional biopsy from Stage IV NB patient. Panel (A) shows H&E tissue staining and (B) shows characteristic expression of CD56. Stress-antigen expression is shown in (C). Note strong expression of ULBP-3 and ULBP-5 and mild expression of ULBP-1 and ULBP-4. NB = neuroblastoma.
4. Discussion
While the prognosis for high-risk NB remains poor, survival has improved with the advent of high dose chemotherapy with autologous SCT in patients who have previously responded to induction chemotherapy. In the setting of minimal residual disease following consolidative SCT and radiotherapy, maintenance immunotherapy with dinutuximab, a US Food and Drug Administration approved chimeric 14.18 anti-disialoganglioside GD2 antibody, given concurrently with IL-2 and granulocyte macrophage-colony stimulating factor has also improved outcome as demonstrated by the Children's Oncology Group.[9] Other investigators have demonstrated the promise of various antibody therapies in NB.[10] A variety of other immunologic therapies for NB have been studied or are in development in humans including allogeneic cord blood transplantation, tumor cell vaccine therapy, and treatment with chimeric antigen receptor T-cells.[11–13]
Trials utilizing in vivo γδ T cell expansion with bisphosphonates in combination with IL-2 therapy have been undertaken in a variety of tumor types with therapeutic effects noted. Clinical responses to ZOL + IL-2 in patients with refractory metastatic prostate cancer have been demonstrated.[5] The bisphosphonate pamidronate in conjunction with low dose IL-2 has shown antitumor effect in patients with lymphoid malignancies[6] but the effect was only evident after selection of patients with marked γδ T cell activation in vitro. Among 9 patients selected in this manner, 5 of 9 patients demonstrated significant in vivo γδ T cell expansion/activation and 3 of 9 patients demonstrated objective tumor responses. In both studies, evidence of γδ T cell expansion was sustained over several days especially ion cases where multiple infusions of bisphosphonate and IL-2 were undertaken.
γδ T cells exert potent anti-NB effects in vitro and in vivo.[2,3,14,15] Bisphosphonates such as ZOL may also exert anti-NB effects independent of γδ T cells. For instance, bisphosphonates are capable of eliciting potent differentiating and toxic effects on NB cells in vitro.[16] Bisphosphonates also function as adjuvants against skeletal metastases of various tumor types, inhibiting skeletal related events and reducing the need for radiotherapy.[17] In a mouse model of NB, ZOL effectively inhibited skeletal metastases when administered with cyclophosphamide and topotecan.[18] A similar approach has been assessed in a recent New Advances to Neuroblastoma Therapy trial of ZOL in combination with oral cyclophosphamide in refractory NB patients, although this trial did not study γδ T cell expansion.[19]
NKG2D ligands ULBP-3 and ULBP-5 were strongly expressed on tumor excisional biopsy specimens. Previous data from our laboratory and others indicate that the expression of stress associated ligands contributes to immune recognition and lysis by expanded/activated γδ T cells. Vδ1+ γδ T cells recognize some NKG2D ligands via the T cell receptor[20–24] and activated Vδ2+ T cells via NKG2D, both using mechanisms that are MHC-independent and require no prior antigen exposure or priming.[22,25,26] Indeed, Schilbach et al[2] has shown that Vδ1+ γδ T cells may have greater potential for NB therapy. Indeed, expansion protocols for clinical scale Vδ1+ γδ T cell stimulation and manufacturing are currently being explored[27,28]; approvable clinical implementation protocols have not been fully developed. Additionally, many aspects of γδ T cell function and potential ligand specificity remain unresolved,[29] and further studies will be necessary to define the interactions between NB and γδ T cells more completely.
Although we were able to expand γδ T cells to within the normal range and indirectly showed that the method results in production of anti-tumor cytotoxic γδ T cells, findings were variable and the potential utility of in vivo γδ T cell expansion appeared to be hindered by inadequate baseline levels. This phenomenon was particularly evident in heavily pretreated patients but was also detected in newly diagnosed NB patients, regardless of risk-status. We suggest that the presence of NB cells may co-opt immune checkpoint pathways, leading to suppressed proliferation or induced programmed death of γδ T cells. The mechanism of γδ T cell suppression in newly diagnosed NB patients in particular merits further exploration.
In vivo expansion of γδ T cells constitutes an attractive and logistically simple approach that has recently been reviewed by Fournie et al.[30] Transient results have been seen using coadministration of aminobisphosphonates in conjunction with IL-2 in low-grade non-Hodgkin lymphoma,[6] prostate cancer,[5] and renal cell carcinoma.[31,32] The best observed effect of ZOL + IL-2 therapy documented in the present study was to elevate γδ T cell levels to that of healthy children, but supra-physiologic numbers would presumably be necessary to promote a significant therapeutic effect. Other ZOL + IL-2 studies have demonstrated that patients with relatively high pretherapy γδ T cell levels were most likely to respond. Our study also showed that T cell effector/memory patterns can vary widely in Stage IV neuroblastoma patients and resulting outcomes in expansion and function may vary accordingly. The cohort described here was heavily pretreated and, even among high-risk NB patients, was found to have atypical clinical characteristics such as prolonged disease courses and advanced age. Accordingly, the patients studied may not well represent the NB patient population that would be targeted by such an approach.
A possible effect of ZOL + IL-2 therapy on our observations of increasing CD4+ T cells with a regulatory phenotype may also be detrimental to the strategy used here. It is known that Treg cell—derived soluble factors can suppress γδ T cell activity and numbers.[33] Exogenous IL-2 is necessary for in vivo expansion of ZOL-stimulated γδ T cells. ZOL-induced farnesyl pyrophosphate synthase inhibition has two major effects: upstream accumulation of the γδ T cell Ag isopentenyl pyrophosphate and downstream depletion of prenyl pyrophosphates. Even though stimulation occurs through the Vγ9Vδ2 TCR, inflammatory and proliferative effects require IL-2.[34] Indeed, the use of IL-2 designed to potentiate γδ T cell proliferation might have predicted the observed proliferation of regulatory T cells, a finding that was not explored in previous trials of in vivo bisphosphonate/IL-2 therapy.
With no published studies having measured the effect of IL-2 therapy on Tregs in NB patients, this finding may carry potentially critical implications for the use of IL-2 in maintenance NB therapy. Subcutaneous (SQ) IL-2 was chosen based on its effectiveness, tolerability, and relative ease of administration.[35] Whether continuous IL-2 infusion has different immunomodulatory effects than subcutaneous administration is not known. The small sample in this study does not permit specific analysis as to whether the decreasing γδ T cell counts following the initial rise was caused by the increase in Treg cells, γδ T cell activation induced cell death, or (most likely) a combination of both. In vivo Treg depletion and/or the use of a cytokine such as IL-7 that expands γδ T cells without the proliferative effects on Tregs that are seen with IL-2 may constitute an alternative approach. Additionally, strategies utilizing ex vivo γδ T cell expansion may circumvent the limitations of in vivo expansion in the present trial. Further, haploidentical donors such as a patient's parent would be capable of supplying a large cell dose without the risk of graft versus host disease (GVHD) that accompanies other allogeneic cellular therapies. Another potentially beneficial approach would include the enrichment of Vγ9Vδ1 cell that possess particularly potent tumoricidal properties.[2,27]
The ZOL + IL-2 regimen was found to be tolerable. A single patient experienced grade 3 pain that was attributed to tumor. Other grade 3 toxicities were expected including hypocalcemia, hypophosphatemia, and hypoalbuminemia. With improved compliance with supplementation, these electrolyte abnormalities could have potentially been lessened. Unexpected severe adverse effects were not encountered, and the safety and tolerability of the regimen would compare favorably with established therapies for NB.
5. Conclusions
The γδ T cell population is reduced in untreated newly diagnosed NB patients compared with healthy children (P < 0.001). Treatment with ZOL and SQ IL-2 increases the number of circulating γδ T cells and has an acceptable toxicity profile. An increase in the γδ T cell population is observed following ZOL + IL-2 administration that restores the count to the lower normal range but does not result in “supranormal” γδ T cell counts that would be considered therapeutic. An increase in Tregs, likely due to stimulation from low-dose IL-2, may offset any gains from increases in the γδ T cell population. The potential for IL-2 to suppress antitumor immunity by increasing the regulatory T cell population is concerning and suggests that the exploration of the potentially detrimental effects of IL-2 in maintenance NB therapy merits further study.
Acknowledgments
The authors wish to acknowledge the assistance of Samantha Langford and Mary Elizabeth Lamb for transcription and organization of the flow cytometry data and formatting for statistical analysis.
Footnotes
Abbreviations: DLT = dose-limiting toxicity, GVHD = graft versus host disease, MICA = major histocompatibility complex class-I polypeptide-related sequence A, MICB = major histocompatibility complex class-I polypeptide-related sequence B, MTD = maximum tolerated dose, NB = neuroblastoma, SCT = stem cell transplantation, SQ = subcutaneous, Treg = regulatory T cell (CD3+CD4+CD25brightCD127dim), UAB = University of Alabama at Birmingham, ULBP = UL-16 binding protein, y = year, ZOL = Zoledronate (Zometa).
Joseph G. Pressey: Present address: Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio.
JP and LL received funding from the Brendan Franco Foundation in Enterprise, AL for this work. The foundation had no role in the design, collection, analysis, or interpretation of the data.
The Brendan Franco Foundation, Enterprise, AL, supported this study.
ClinicalTrials.gov NCT01404702 Registered 13 July 2011.
The authors report no conflicts of interest.
References
- 1.Maris JM, Hogarty MD, Bagatell R, et al. Neuroblastoma. Lancet 2007; 369:2106–2120. [DOI] [PubMed] [Google Scholar]
- 2.Schilbach K, Frommer K, Meier S, et al. Immune response of human propagated gammadelta-T-cells to neuroblastoma recommend the Vdelta1+ subset for gammadelta-T-cell-based immunotherapy. J Immunother 2008; 31:896–905. [DOI] [PubMed] [Google Scholar]
- 3.Chargui J, Combaret V, Scaglione V, et al. Bromohydrin pyrophosphate-stimulated Vgamma9delta2 T cells expanded ex vivo from patients with poor-prognosis neuroblastoma lyse autologous primary tumor cells. J Immunother 2010; 33:591–598. [DOI] [PubMed] [Google Scholar]
- 4.Schilbach KE, Geiselhart A, Wessels JT, et al. Human gammadelta T lymphocytes exert natural and IL-2-induced cytotoxicity to neuroblastoma cells. J Immunother 2000; 23:536–548. [DOI] [PubMed] [Google Scholar]
- 5.Dieli F, Vermijlen D, Fulfaro F, et al. Targeting human {gamma}delta} T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res 2007; 67:7450–7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilhelm M, Kunzmann V, Eckstein S, et al. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood 2003; 102:200–206. [DOI] [PubMed] [Google Scholar]
- 7.Beck BH, Kim H, O’Brien R, et al. Dynamics of circulating gammadelta T cell activity in an immunocompetent mouse model of high-grade glioma. PLoS One 2015; 10:e0122387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simonetta F, Chiali A, Cordier C, et al. Increased CD127 expression on activated FOXP3+CD4+ regulatory T cells. Eur J Immunol 2010; 40:2528–2538. [DOI] [PubMed] [Google Scholar]
- 9.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010; 363:1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parsons K, Bernhardt B, Strickland B. Targeted immunotherapy for high-risk neuroblastoma--the role of monoclonal antibodies. Ann Pharmacother 2013; 47:210–218. [DOI] [PubMed] [Google Scholar]
- 11.Louis CU, Savoldo B, Dotti G, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 2011; 118:6050–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jubert C, Wall DA, Grimley M, et al. Engraftment of unrelated cord blood after reduced-intensity conditioning regimen in children with refractory neuroblastoma: a feasibility trial. Bone Marrow Transplant 2011; 46:232–237. [DOI] [PubMed] [Google Scholar]
- 13.Russell HV, Strother D, Mei Z, et al. Phase I trial of vaccination with autologous neuroblastoma tumor cells genetically modified to secrete IL-2 and lymphotactin. J Immunother 2007; 30:227–233. [DOI] [PubMed] [Google Scholar]
- 14.Nishio N, Fujita M, Tanaka Y, et al. Zoledronate sensitizes neuroblastoma-derived tumor-initiating cells to cytolysis mediated by human gammadelta T cells. J Immunother 2012; 35:598–606. [DOI] [PubMed] [Google Scholar]
- 15.Di Carlo E, Bocca P, Emionite L, et al. Mechanisms of the antitumor activity of human Vgamma9Vdelta2 T cells in combination with zoledronic acid in a preclinical model of neuroblastoma. Mol Ther 2013; 21:1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vorotnjak M, Boos J, Lanvers-Kaminsky C. In vitro toxicity of bisphosphonates on human neuroblastoma cell lines. Anticancer Drugs 2004; 15:795–802. [DOI] [PubMed] [Google Scholar]
- 17.Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer 2001; 91:1191–1200. [DOI] [PubMed] [Google Scholar]
- 18.Peng H, Sohara Y, Moats RA, et al. The activity of zoledronic Acid on neuroblastoma bone metastasis involves inhibition of osteoclasts and tumor cell survival and proliferation. Cancer Res 2007; 67:9346–9355. [DOI] [PubMed] [Google Scholar]
- 19.Russell HV, Groshen SG, Ara T, et al. A phase I study of zoledronic acid and low-dose cyclophosphamide in recurrent/refractory neuroblastoma: a new approaches to neuroblastoma therapy (NANT) study. Pediatr Blood Cancer 2011; 57:275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girardi M, Oppenheim DE, Steele CR, et al. Regulation of cutaneous malignancy by gammadelta T cells. Science 2001; 294:605–609. [DOI] [PubMed] [Google Scholar]
- 21.Kaminski MJ, Cruz PD, Jr, Bergstresser PR, et al. Killing of skin-derived tumor cells by mouse dendritic epidermal T-cells. Cancer Research 1993; 53:4014–4019. [PubMed] [Google Scholar]
- 22.Groh V, Steinle A, Bauer V, et al. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science 1998; 279:1737–1740. [DOI] [PubMed] [Google Scholar]
- 23.Wu J, Groh V, Spies T. T cell antigen receptor engagement and specificity in the recognition of stress-inducible MHC class I-related chains by human epithelial gamma delta T cells. J Immunol 2002; 169:1236–1240. [DOI] [PubMed] [Google Scholar]
- 24.Morita CT, Mariuzza RA, Brenner MB. Antigen recognition by human gamma delta T cells: pattern recognition by the adaptive immune system. Springer Semin Immunopathol 2000; 22:191–217. [DOI] [PubMed] [Google Scholar]
- 25.Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA [see comments]. Science 1999; 285:727–729. [DOI] [PubMed] [Google Scholar]
- 26.Groh V, Rhinehart R, Secrist H, et al. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci USA 1999; 96:6879–6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegers GM, Lamb LS., Jr Cytotoxic and regulatory properties of circulating Vdelta1+ gammadelta T cells: a new player on the cell therapy field? Mol Ther 2014; 22:1416–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegers GM, Ribot EJ, Keating A, et al. Extensive expansion of primary human gamma delta T cells generates cytotoxic effector memory cells that can be labeled with Feraheme for cellular MRI. Cancer Immunol Immunother 2013; 62:571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Brien RL, Roark CL, Jin N, et al. gammadelta T-cell receptors: functional correlations. Immunol Rev 2007; 215:77–88. [DOI] [PubMed] [Google Scholar]
- 30.Fournié JJ, Sicard H, Poupot M, et al. What lessons can be learned from gammadelta T cell-based cancer immunotherapy trials? Cell Mol Immunol 2013; 10:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lang JM, Kaikobad MR, Wallace M, et al. Pilot trial of interleukin-2 and zoledronic acid to augment gammadelta T cells as treatment for patients with refractory renal cell carcinoma. Cancer Immunol Immunother 2011; 60:1447–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennouna J, Bompas E, Neidhardt EM, et al. Phase-I study of Innacell gammadeltatrade mark, an autologous cell-therapy product highly enriched in gamma9delta2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother 2008; 57:1599–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunzmann V, Kimmel B, Herrmann Th, et al. Inhibition of phosphoantigen-mediated gammadelta T-cell proliferation by CD4+ CD25+ FoxP3+ regulatory T cells. Immunology 2009; 126:256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nussbaumer O, Gruenbacher G, Gander H, et al. Essential requirements of zoledronate-induced cytokine and gammadelta T cell proliferative responses. J Immunol 2013; 191:1346–1355. [DOI] [PubMed] [Google Scholar]
- 35.Ladenstein R, Pötschger U, Siabalis D, et al. Dose finding study for the use of subcutaneous recombinant interleukin-2 to augment natural killer cell numbers in an outpatient setting for stage 4 neuroblastoma after megatherapy and autologous stem-cell reinfusion. J Clin Oncol 2011; 29:441–448. [DOI] [PubMed] [Google Scholar]


