Supplemental Digital Content is available in the text
Keywords: cetuximab, cisplatin, locally advanced NPC, survival, toxicity
Abstract
To evaluate the treatment efficacies and toxicities of concurrent cetuximab-based bioradiotherapy (BRT) or cisplatin-based chemoradiotherapy (CRT) in locally advanced nasopharyngeal carcinoma. :Patients with previously untreated locally advanced nasopharyngeal carcinoma were matched into pairs, and enrolled into the study. All patients were given either BRT or CRT. Survival outcomes, toxicities, and prognostic factors were evaluated. :A total of 112 patients were enrolled. The 5-year overall survival was 79.3% and 79.5% in CRT and BRT arm, respectively (P = 0.797) and the 5-year DFS was 73.5% and 74.6%, respectively (P = 0.953). In toxicity analysis, CRT arm had more significant decrease in white blood cell, platelet, hemoglobin, and severe vomiting, while more severe skin reactions and mucositis were shown in BRT arm. :BRT was not less efficacious than traditional CRT. They lead to different aspects of toxicities. If patients cannot stand more severe toxicities caused by CRT, BRT could be an ideal alternative.
1. Introduction
Nasopharyngeal carcinoma (NPC) is a major part of tumors in head and neck region and the global incidence is increasing to half a million and causing more than 34.1 million death every year.[1] NPC has its distinct epidemiology and geographic distribution, where southern China and Southeast Asia are popular epidemic areas.
Patients with T1N0M0 NPC could achieve curable outcomes simply through radiotherapy, while patients with locally advanced NPC usually receive chemoradiotherapy with induction/concurrent chemotherapy with improved survival.[2,3] Studies showed prolonged loco-regional control interval and overall survival (OS), progression free survival (PFS) by concurrent chemoradiotherapy (CCRT). Among the concurrent platinum agents, single-agent cisplatin is superior to single-agent carboplatin and equivalent to carboplatin with 5-fluorouracil in retrospective analyses.[4,5] And cisplatin-based treatment has now been considered as the most common used, first-line treatment regimen to treat patients with recurrent metastatic NPC, for many large randomized studies have demonstrated that cisplatin-based regimen provided significantly higher response rates than radiotherapy alone in both locoregional advanced and recurrent NPC.[6,7] Chemotherapy with radiation therapy are recommended for locally advanced nasopharyngeal cancers, with acceptable cisplatin-based chemotherapy regimen.[8] Epidermal growth factor receptor (EGFR) seems to be critical to cancer cells growth and proliferation, but not normal cells, and the function of EGFR in these 2 settings seems to be different.[9,10] NPC showed EGFR functional difference compared with normal cells without exception.[11] EGFR expression level showed marked increase and overexpression in NPC, and it was shown to be an independent prognostic factor predicting poorer survival.[12] Thus, downregulating EGFR with EGFR inhibitors has become a burgeoning strategy in antitumor treatment. Cetuximab, an EGFR-targeting monoclonal antibody, is the first targeted therapy that showed therapeutic benefit in head and neck cancer,[13] and received FDA approval in the use of HNSCC in 2006.[14,15] Anti-EGFR treatment strategy with cetuximab has been conducted in NPC treatment by integrating cetuximab into traditional cisplatin-based CCRT[16,17] Up to date, improved locoregional control and prolonged survivals have been achieved in lung cancers, gastrointestinal cancers with addition of this anti-EGFR strategy into traditional chemoradiotherapy regimen.[18–20] However, this current combination of cetuximab and chemoradiotherapy would increase both treatment-related toxicity and cost at the same time, though the adverse events reported were generally acceptable.[21,22] Based on the demonstration that radiotherapy plus anti-EGFR cetuximab showed satisfying outcomes in HNSCC and the overexpression of EGFR in NPC, as with HNSCC and, we speculate that anti-EGFR cetuximab also benefit patients with NPC. We hypothesized that characteristics of these patient groups would be similar. Hence, we sought to compare the outcomes of concurrent IMRT with cisplatin or cetuximab in regard to survival results, and treatment-related adverse events in patients with NPC.
2. Methods and patients
2.1. Patient evaluation
Between January 1, 2008, and July 31, 2012, 56 patients with locally advanced nasopharyngeal carcinoma were enrolled in cetuximab-based bioradiotherapy (BRT) group, receiving concurrent IMRT plus cetuximab-based biotherapy from West China Hospital cancer center. In the same duration, 420 patients receiving concurrent IMRT cisplatin-based chemotherapy, and 56 of these patients were matched to BRT group according to age, gender, tumor staging, and Eastern Cooperative Oncology Group (ECOG) scoring.
A total of 112 patients who received radiotherapy combined with BRT or cisplatin-based chemoradiotherapy (CRT) with IMRT were enrolled into the study. All included patients had previously untreated, and pathologically proven squamous cell carcinoma of nasopharynx (T1-T4, N0-N3, M0, and no T1N0), which was suitable for chemoradiotherapy. The initial workup included staging the patient before treatment with a head and neck contrast-enhanced MRI, nasal fibroendoscopy, and a full clinical and biologic evaluation documenting the status of distant metastases, including a chest CT scan, abdominal color Doppler ultrasound, bone scan with or without a FDG-PET scan. Patients were preferred in a good performance scan, measured with ECOG scale score less than 2, as patients with unacceptable tumor burden or bad general condition might impair their complement of treatment regimen.
2.2. Treatment and follow-up
Eligible patients received 2 cycles of TPF induction regimen (paclitaxel 150–175 mg/m2 on day 1+ cisplatin 25 mg/m2 on days 1–3+ and fluorouracil 600 mg/m2 per through days 1–5) at Q21. Three weeks after the 2 induction cycles, patient started radiotherapy. All patients received intensity-modulated radiotherapy (IMRT) with variable 2.12 to 2.24 Gy fraction per day and 5 days per week up to a total of 70 to 74 Gy in 33 fractions. The delineation of target volumes was based on imaging (MRI or FDG-PET and were performed in the same series). Those patients in BRT received a loading dose of cetuximab 400 mg/m2 on day 1 of the week preceding RT and thereafter a weekly dose of 250 mg/m2 during RT till week 8 and those in CRT arm received 3 cycles of 25 mg/m2 cisplatin on days 1–3 every 3 weeks. Premedication consisted of oral dexamethasone (8 mg twice a day, which was 6 and 12 hours before paclitaxel administration, respectively). Granulocyte colony-stimulating factor was administrated in case of febrile neutropenia (150 μg/d), and platelet stimulating factor in grade 3 and 4 thrombocytopenia (15,000 U, i.h.). If the creatinine clearance decreased to 40 to 60 mL/min, the dose of cisplatin was reduced to 50 mg/m2.
After treatment regimen administration of patients, nasopharyngeal endoscopic evaluation, head and neck MRI scan, chest CT scan, and abdominal color Doppler ultrasound were scheduled per protocol at posttreatment every 3 months in the first 2 years and every 6 months after the first 2 years in case of suspected recurrence. Bone scan was scheduled once per year.
2.3. Statistical analysis
Treatment response and disease progression was analyzed 4 weeks after completion of radiotherapy (first f/u) and 3 months thereafter (second f/u). Treatment outcome/survival rates were evaluated using higher nonparametric statistics (Kaplan–Meier survival analysis/log-rank and Wilcoxon test, in which the log-rank test was used to compare survival curves). Progression-free survival was defined as time from start of radiation therapy until first event (ie, loco-regional relapse, distant metastases, and death). Accordingly, OS was calculated from the start of radiotherapy to the death event from any cause. All survival results were calculated from the day of the start of radiotherapy until the appearance of event or the time of last follow-up.
Categorical variables were compared between patients who received BRT or CRT using the Paired rank sum test, both univariate (using Kaplan–Meier survival analysis) and multivariate analyses (using Cox regression) to determine the potential prognostic risk factors associated with disease-free survival and OS, in which the statistical value indicate how many times of increased risk of the advent of events. The adverse events (toxicity) were assessed based on clinical judgment and was documented using CTCAE 4.02. P value less than 0.05 was regarded that there was statistically significant difference between analyzed groups in all those tests described above. All statistical analyses were performed using SPSS statistical software version 22 (SPSS, Chicago, IL).
2.4. Ethics statement
This study was approved by the Institutional Review Board of West China Hospital, Sichuan University, China. The institutional review board stated that the written consents of patients were not required, because personal information of theses participants was not included. All participants were protected by using anonymized patient identification numbers.
3. Results
3.1. Patients characteristics
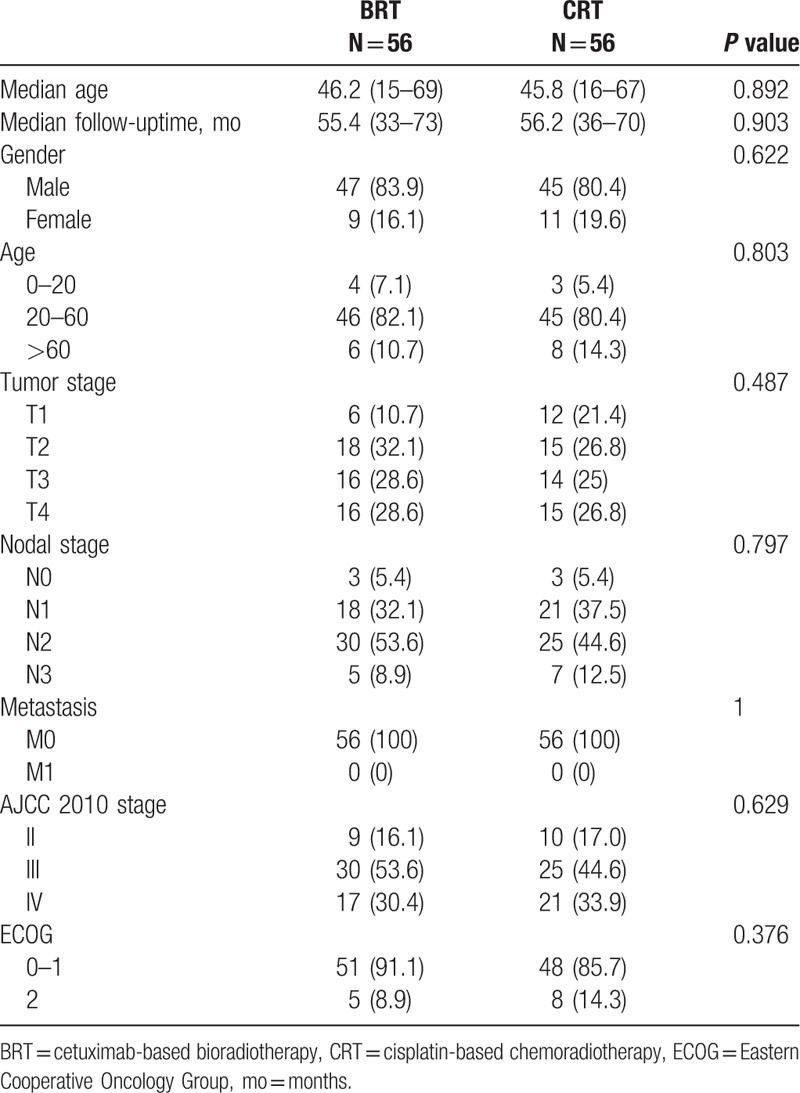
A total of 112 patients who received radiotherapy combined with cetuximab or cisplatin were enrolled into the study. In the 2 matched cohorts, there was no significant difference regarding the matched indicators, that is, age, tumor staging, gender, and ECOG scoring. The median ages of patients in the BRT and CRT groups were 46.2 (15–69) and 45.8 (16–67) months, respectively (P = 0.892). Patients in 2 comparison arms had a similar tumor stage and metastasis status of disease (P = 0.002). The median follow-up time was 55.4 (33–73)months in BRT arm and 56.2 (36–70)months in CRT arm, respectively. Patient basic characteristics were listed in Table 1.
Table 1.
Patient characteristics.
3.2. Survival outcomes
In all, 9 of 56 patients in patients receiving BRT died, compared with 10 of 56 in patients receiving CRT. Differences in OS were not statistically significant, with 5-year actuarial rates of 79.5% for BRT and 79.3% for CRT (log-rank P = 0.797; Fig. 1A) and 3-year survival for 2 arms were 92.9% and 92.8%, respectively. Median survival was 66.8 months for BRT and was 67.3 months for CRT patients.
Figure 1.
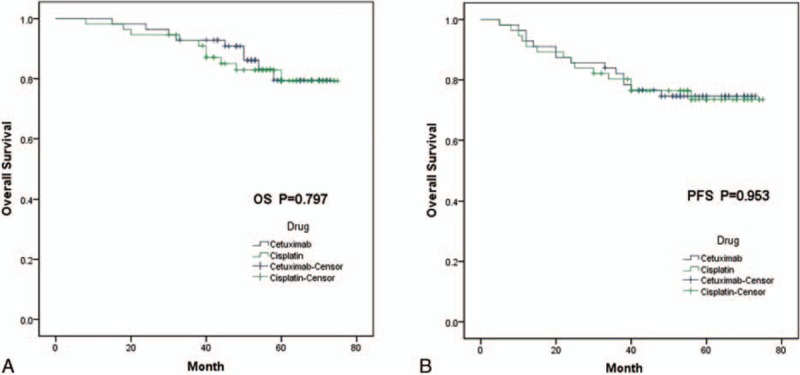
Kaplan–Meier survival curves. (A) Kaplan–Meier curves estimates for OS; (B) Kaplan–Meier curves estimates for PFS. OS = overall survival, PFS = progression-free survival.
For PFS outcomes, there were no significant differences between the 2 groups neither (log-rank P = 0.953; Fig. 1B). Median survival was 60.9 months for BRT and was 61.9 months for CRT patients. 3-year and 5-year PFS was 82.1%, 74.6% in patients receiving BRT and 80.3% and 73.5% for patients receiving CRT, respectively.
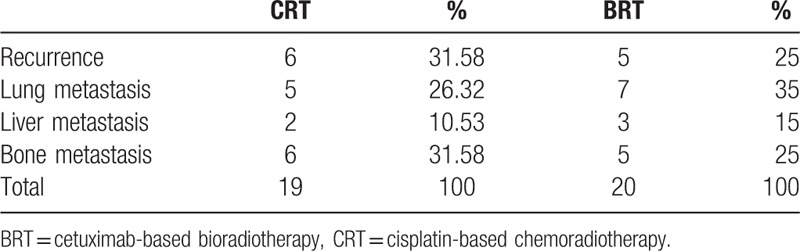
With regards to failure of treatment, 14 of 56 patients in patients receiving BRT had failure of treatment, compared with 14 of 56 in patients receiving CRT. Among these patients, 11 had recurrent disease, 12 had lung metastasis, 11 had bone metastasis, and 11 had liver metastasis. (Table 2).
Table 2.
Patterns of treatment failure.
3.3. Prognostic factors
OS and PFS were modeled using regression analysis with potential prognostic factors in both univariate and multivariate model. We analyzed sex, age, ECOG performance, T stage, N stage, tumor staging, treatment regimen, decreasing in white blood cell (WBC) count, change in hemoglobin, aminotransferase,gamma-glutamyl transpeptidase,blood urine nitrogen,rash, mucositis, and vomit as prognostic factors in all patients regarding survival.
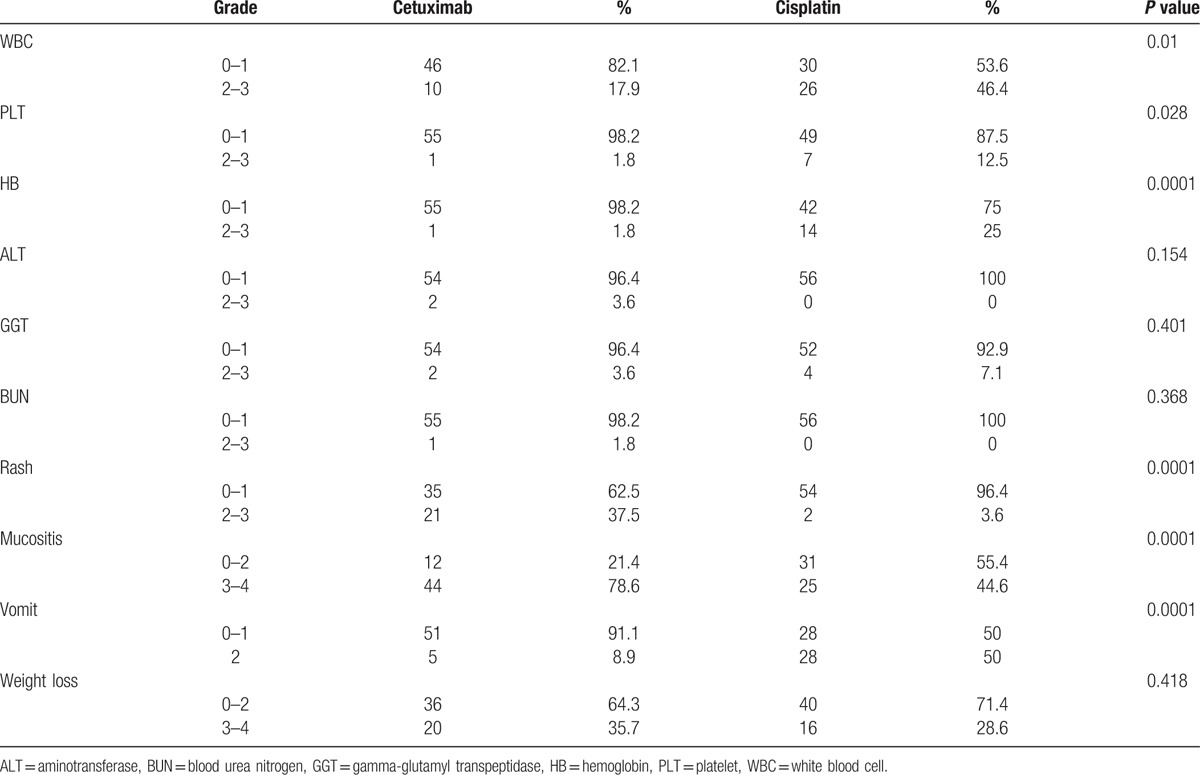
In univariate analysis, high ECOG scoring, advanced T stage, advanced N stage, advanced tumor grade, decreased WBC count, decreased platelet, decreased hemoglobin, and severe weight loss were independent prognostic factors predicting poorer OS (Fig. 2). In subgroup analysis that studied 2 cohorts individually, we found that though there was no significant difference of survivals observed regarding the severity of acute rash and/or mucositis, we could appreciate the tendency of separation of the 2 survival curves. In BRT arm, patients who showed grade 3 to 4 rash (Supplementary Figure S1) or grade 3 to 4 mucositis (Supplementary Figure S2), has a tendency showing better OS outcomes. In multivariate model,according to previous study and the univariate study, we chose sex,age, ECOG, tumor stage, regimen (CRT vs BRT) and the severity of WBC, HB, mucositis, vomit, and weight loss as candidates in this multivariate model. In result, no significant difference were found between BRT arm and CRT arm (P = 0.137), whereas age higher than 20 years old, worse ECOG performance, high tumor stage, high-grade toxicity on WBC, mucositis, and high grade of weight loss predicted poorer OS and PFS (Table 3). Toxicity Patients who received cisplatin-based chemotherapy had a greater percentage of grade III and IV toxicity of significant decreasing in WBC count (P = 0.01), significant decreased platelet (P = 0.028), significant decreased hemoglobin (P = 0.0001), and more severe vomiting in patients (P = 0.0001) than those who received BRT, but more severe acneiform skin reactions (P = 0.0001) and severe mucositis (P = 0.0001) were shown in BRT arm (Table 4).
Figure 2.
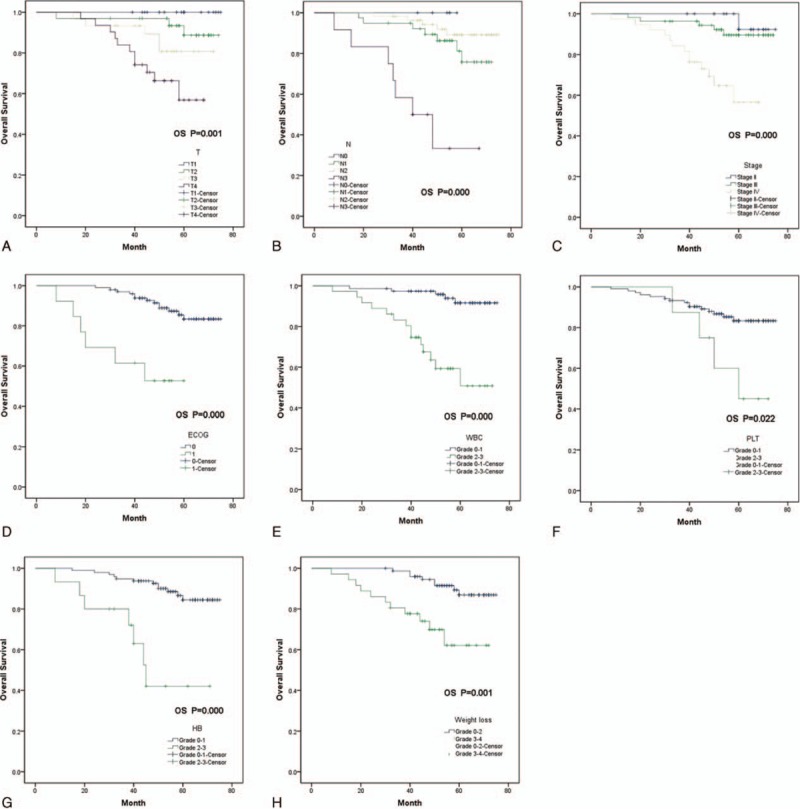
Kaplan–Meier survival curves analyze prognostic factors in univariate model.
Table 3.
Prognosis factors significantly associated in the multivariate analysis.
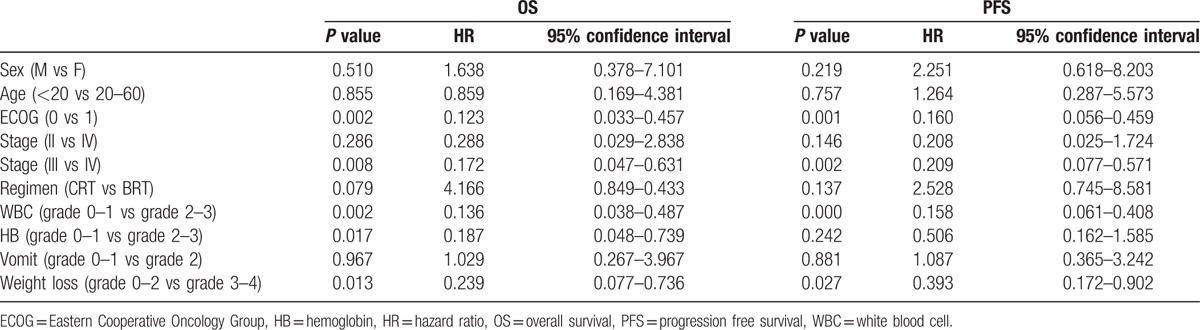
Table 4.
Treatment associated toxicity.
4. Discussion
In this study, we conducted a retrospective paired case study to compare the effect of cetuximab single agent plus radiotherapy versus cisplatin-based chemotherapy plus radiotherapy in controlling OS, progression-free survival and the tolerance of 2 treatment regimens. The key characteristics of patients in 2 cohorts including tumor T and N classifications, stage, gender, and age were balanced between the 2 treatment groups due to a relatively small sample size and pairing the patients could improve the statistical effect of these retrospective studies.
Our study demonstrated that the estimated OS and PFS rates were proportionately similar and no statistical difference was tested between the 2 CRT and BRT groups. Key prognostic factors predicting the poorer OS and PFS included older age, reduced performance, advanced T stage, advanced N stage, advanced tumor grade, decreased WBC count, decreased platelet, decreased hemoglobin, increasing of creatitine, and severe weight loss.
Concurrent cisplatin-based radiotherapy has been regarded as the standard treatment regimen for patients with NPC; however, cisplatin increases both immediate treatment-related adverse events and delayed toxicity, which hamper the quality of life in long-term usage compared with cetuximab.[23] Cetuximab, an emerging monoclonal antibody against EGFR, seemed helpful to provide patients an effect alternative with less toxic and improved quality of life.[13,24] Whether cetuximab could replace cisplatin in definitive chemoradiotherapy for HNSCC has not reached consistency, because cetuximab had superior and well-tolerated adverse event,[25] but the tumor control effect and survival benefit showed inconsistent results.
Adverse effect is an important parameter taken into consideration when comparing treatment regimens. In our study, we found that regimens comprised of cisplatin plus radiation caused more severe adverse events compared with cetuximab plus radiation, including decreased WBC count, platelet, and hemoglobin and severe vomiting. Concurrent cisplatin plus radiation is the standard treatment regimen with increased loco-regional control and prolonged survival outcomes[26,27] compared with chemotherapy or radiotherapy alone. However, CCRT, especially combined with high dose of radiotherapy, has been demonstrated to associate with significant toxicity and some mortal acute adverse events, and the intolerability has restricted the regimen use (discontinuation or reduction in dose) to some degree.[28] As comparison, cetuximab plus radiation displayed well tolerance among patients, though some adverse events could attribute to cetuximab, such as grade 3 to 4 acne-like rashes and severe mucositis, the severity showed mild to moderate without life-threatening event or impairment to the continuation of drug delivery.[29]
Recent studies on CRT versus BRT in patients with HNSCC showed controversial outcomes. It has been demonstrated by Vermorken et al[30] that significantly prolonged median OS has been seen in cetuximab plus concurrent cisplatin-based chemotherapy compared to chemotherapy alone. Two landmark studies, Erbitux trial and EXTREME trial, showed impressively improved survival outcomes and loco-regional control rates when comparing cetuximab plus radiation versus radiation alone[10] and comparing survival benefit of adding cetuximab to standard chemotherapy,[9] respectively. However, a following randomized phase III trial RTOG 0522 comparing concurrent cisplatin-based chemotherapy plus radiotherapy by Kian Ang et al[31] showed disappointing outcome with regard to both survival outcomes and recurrent rates. A recent randomized phase II study by Xu et al[35] showed that Concurrent cetuximab-based radiotherapy was not more efficacious than cisplatin but caused more likely to cause acute adverse events in LA NPC, which need further investigation to find out the toxicity profiles and time of occurrence of 2 different regimens.
In NPC, however, the combination of cetuximab with radiotherapy was worth trying in larger prospective clinical trials, as the difference in biologic behaviors and the responsive sensitivity of NPC to cisplatin-based regimens were not that much of HNSCC, which is why NPC is considered separately.[32] Previous studies had largely focused on the effect of combination of cetuximab with CCRT and the relevant adverse events. Ma et al[21] reported in a single arm retrospective study that the treatment safety was achieved when combining cetuximab with concurrent cisplatin and IMRT in locally advanced NPC; however, the incidence of moderate-to-severe acute skin and mucositis was proved to be much higher compared with concurrent cisplatin and radiotherapy. A similar result by Xu et al,[17] were shown in recurrent/metastatic NPC that combination of cetuximab to CCRT could be an alternative to whom the cisplatin plus radiation failed in. Thus, concurrent cisplatin combined with cetuximab plus radiation could potentially reach a good treatment outcome, the toxicity is left as a problem.
Giro et al[33] reported from community practice suggested an incidence of grade 3 to 4 skin toxicity encountered in up to 50% of patients in a questionnaire study carried out among the European Organization for Research and Treatment of Cancer Radiation Oncology Group and Head and Neck Group, and also exacerbation of acute radiation related skin and mucosal damage by the concomitant usage of cetuximab, cisplatin plus RT has been previously reported in several studies.[22,34] Therefore, to balance the effect and treatment-related adverse event and to get better quality of life and avoid these aggressive treatment regimens, concurrent cetuximab plus radiation versus cisplatin plus radiation has been compared in our study.
In our study, we looked at the effects of 2 regimens specifically on NPC and we found no statistical difference on OS and PFS. However, 2 regimens caused different aspects of adverse events, with CRT having more impact on digestive system and hematologic system. Thus our study showed that, BRT could be an alternative in patients who cannot tolerate the classic chemoradiotherapy regimen with equivalent therapeutic effect. As this is a retrospective study with a relative small sample size, larger prospective randomized clinical trials are warranted for further investigation.
5. Conclusion
In this retrospective case–control study, we evaluated the treatment efficacies and toxicities of induction chemotherapy followed by BRT or CRT in locally advanced nasopharyngeal carcinoma. We found that BRT was not inferior to traditional CRT. Two regimens lead to different aspects of toxicities-CRT arm had a greater percentage of grades III and IV toxicity of significant decrease in WBC, platelet, hemoglobin, and more severe vomiting, while more severe acneiform skin reactions and mucositis were shown in BRT arm. Thus, we carefully draw the conclusion that if patients cannot stand more severe toxicities caused by CRT, BRT could be an ideal alternative.
Supplementary Material
Acknowledgments
This study was supported by the National Natural Science Foundation of China, Beijing, China (Grant No. 81101991) and Research Award Fund for New Young Teachers in Higher Education Institutions, China (Grant No. 20120181120024). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the article.
Footnotes
Abbreviations: BRT = concurrent cetuximab-based bioradiotherapy, CCRT = concurrent chemoradiotherapy, CRT = cisplatin-based chemoradiotherapy, EGFR = epidermal growth factor receptor, IMRT = intensity-modulated radiotherapy, LA-NPC = locally advanced nasopharyngeal carcinoma, NPC = nasopharyngeal carcinoma, OS = overall survival, PFS = progression free survival.
This study was designed by LL and WX, and the article was written by HJ. Data were collected by HJ and HL. Statistical process was done by XL and ZJ. Figures and tables were made by HJ and LP.
The authors have no conflicts of interest to declare.
Supplemental Digital Content is available for this article.
Xin Wu and Jingwen Huang contribute equally to this study.
References
- 1.Chen W, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016. [DOI] [PubMed] [Google Scholar]
- 2.Hong RL, Ting LL, Ko JY, et al. Induction chemotherapy with mitomycin, epirubicin, cisplatin, fluorouracil, and leucovorin followed by radiotherapy in the treatment of locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol 2001; 19:4305–4313. [DOI] [PubMed] [Google Scholar]
- 3.Ma J, Mai HQ, Hong MH, et al. Results of a prospective randomized trial comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol 2001; 19:1350–1357. [DOI] [PubMed] [Google Scholar]
- 4.Rades D, Ulbricht T, Hakim SG, et al. Cisplatin superior to carboplatin in adjuvant radiochemotherapy for locally advanced cancers of the oropharynx and oral cavity. Strahlenther Onkol 2012; 188:42–48. [DOI] [PubMed] [Google Scholar]
- 5.Barkati M, Fortin B, Soulières D, et al. Concurrent chemoradiation with carboplatin-5-fluorouracil versus cisplatin in locally advanced oropharyngeal cancers: is more always better? Int J Radiat Oncol Biol Phys 2010; 76:410–416. [DOI] [PubMed] [Google Scholar]
- 6.Chan AT, Leung SF, Ngan RK, et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst 2005; 97:536–539. [DOI] [PubMed] [Google Scholar]
- 7.Lin JC, Jan JS, Hsu CY, et al. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol 2003; 21:631–637. [DOI] [PubMed] [Google Scholar]
- 8.Lee AW, Lau WH, Tung SY, et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol 2005; 23:6966–6975. [DOI] [PubMed] [Google Scholar]
- 9.Shin DM, Roy JY, Hong WK, et al. Dysregulation of epidermal growth factor receptor expression in premalignant lesions during head and neck tumorigenesis. Cancer Res 1994; 54:3153–3159. [PubMed] [Google Scholar]
- 10.Rubin Grandis J, Chakraboty A, Melhem MF, et al. Inhibition of epidermal growth factor receptor gene expression and function decreases proliferation of head and neck squamous carcinoma but not normal mucosal epithelial cells. Oncogene 1997; 15:409–416. [DOI] [PubMed] [Google Scholar]
- 11.Ke LD, Alder-Storthz K, Clayman GL, et al. Differential expression of epidermal growth factor receptor in human head and neck cancers. Head Neck 1998; 20:320–327. [DOI] [PubMed] [Google Scholar]
- 12.Ma X, Huang J, Wu X, et al. Epidermal growth factor receptor could play a prognostic role to predict the outcome of nasopharyngeal carcinoma: a meta-analysis. Cancer Biomark 2014; 14:267–277. [DOI] [PubMed] [Google Scholar]
- 13.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 2010; 11:21–28. [DOI] [PubMed] [Google Scholar]
- 14.Kono SA, et al. EGFR monoclonal antibodies in the treatment of squamous cell carcinoma of the head and neck: a view beyond cetuximab. Chemother Res Pract 2012; 2012:901320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen MH, Chen H, Shord S, et al. Approval summary: cetuximab in combination with cisplatin or carboplatin and 5-fluorouracil for the first-line treatment of patients with recurrent locoregional or metastatic squamous cell head and neck cancer. Oncologist 2013; 18:460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niu X, Hu C, Kong L. Experience with combination of cetuximab plus intensity-modulated radiotherapy with or without chemotherapy for locoregionally advanced nasopharyngeal carcinoma. J Cancer Res Clin Oncol 2013; 139:1063–1071. [DOI] [PubMed] [Google Scholar]
- 17.Xu T, Ou X, Shen C, et al. Cetuximab in combination with chemoradiotherapy in the treatment of recurrent and/or metastatic nasopharyngeal carcinoma. Anticancer Drugs 2016; 27:66–70. [DOI] [PubMed] [Google Scholar]
- 18.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009; 373:1525–1531. [DOI] [PubMed] [Google Scholar]
- 19.Lynch TJ, Patel T, Dreisbach L, et al. Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: results of the randomized multicenter phase III trial BMS099. J Clin Oncol 2010; 28:911–917. [DOI] [PubMed] [Google Scholar]
- 20.Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009; 360:1408–1417. [DOI] [PubMed] [Google Scholar]
- 21.Ma BB, Kam MK, Leung SF, et al. A phase II study of concurrent cetuximab-cisplatin and intensity-modulated radiotherapy in locoregionally advanced nasopharyngeal carcinoma. Ann Oncol 2012; 23:1287–1292. [DOI] [PubMed] [Google Scholar]
- 22.Feng HX, Guo SP, Li GR, et al. Toxicity of concurrent chemoradiotherapy with cetuximab for locoregionally advanced nasopharyngeal carcinoma. Med Oncol 2014; 31:170. [DOI] [PubMed] [Google Scholar]
- 23.Curran D, Giralt J, Harari PM, et al. Quality of life in head and neck cancer patients after treatment with high-dose radiotherapy alone or in combination with cetuximab. J Clin Oncol 2007; 25:2191–2197. [DOI] [PubMed] [Google Scholar]
- 24.Lang I, Köhne CH, Folprecht G, et al. Quality of life analysis in patients with KRAS wild-type metastatic colorectal cancer treated first-line with cetuximab plus irinotecan, fluorouracil and leucovorin. Eur J Cancer 2013; 49:439–448. [DOI] [PubMed] [Google Scholar]
- 25.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006; 354:567–578. [DOI] [PubMed] [Google Scholar]
- 26.Browman GP, Hodson DI, Mackenzie RJ, et al. Choosing a concomitant chemotherapy and radiotherapy regimen for squamous cell head and neck cancer: a systematic review of the published literature with subgroup analysis. Head Neck 2001; 23:579–589. [DOI] [PubMed] [Google Scholar]
- 27.Bernier J, Cooper JS. Chemoradiation after surgery for high-risk head and neck cancer patients: how strong is the evidence? Oncologist 2005; 10:215–224. [DOI] [PubMed] [Google Scholar]
- 28.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 2003; 21:92–98. [DOI] [PubMed] [Google Scholar]
- 29.Bernier J, Schneider D. Cetuximab combined with radiotherapy: an alternative to chemoradiotherapy for patients with locally advanced squamous cell carcinomas of the head and neck? Eur J Cancer 2007; 43:35–45. [DOI] [PubMed] [Google Scholar]
- 30.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008; 359:1116–1127. [DOI] [PubMed] [Google Scholar]
- 31.Ang KK, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol 2014; 32:2940–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsou YA, Hua CH, Tseng HC, et al. Survival study and treatment strategy for second primary malignancies in patients with head and neck squamous cell carcinoma and nasopharyngeal carcinoma. Acta Otolaryngol 2007; 127:651–657. [DOI] [PubMed] [Google Scholar]
- 33.Giro C, Berger B, Bölke E, et al. High rate of severe radiation dermatitis during radiation therapy with concurrent cetuximab in head and neck cancer: results of a survey in EORTC institutes. Radiother Oncol 2009; 90:166–171. [DOI] [PubMed] [Google Scholar]
- 34.Pfister DG, Su YB, Kraus DH, et al. Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: a pilot phase II study of a new combined-modality paradigm. J Clin Oncol 2006; 24:1072–8072. [DOI] [PubMed] [Google Scholar]
- 35.Xu T, Liu Y, Dou S, et al. Weekly cetuximab concurrent with IMRT aggravated radiation-induced oral mucositis in locally advanced nasopharyngeal carcinoma: results of a randomized phase II study. Oral Oncol 2015; 51:875–879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


