Abstract
Objective:
A meta-analysis was performed to investigate the effectiveness and safety of tranexamic acid (TXA) for the treatment of blood loss after a bilateral total knee arthroplasty (TKA).
Methods:
Patients prepared for bilateral TKA and intervention including TXA versus placebo were comprehensively retrieved from MEDLINE (PubMed), Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science from the time of the establishment of these databases to January 2016. The outcomes were all calculated by Stata 12.0 software. The continuous endpoints (total blood loss and blood loss in drainage) were calculated as mean difference (MD) and 95% confidence intervals (CIs). Binary variables (the need for transfusion, and the occurrence of deep venous thrombosis [DVT]) were calculated as relative risk (RR) with 95% CIs.
Results:
Pooled results revealed that treatment with TXA associated with less need for transfusion (P = 0.000) and the value of Hb drop postoperatively (P = 0.290) after bilateral TKA. The results also indicated that TXA can decrease the total blood loss and blood loss in drainage after bilateral TKA (P < 0.05). Meanwhile, TXA can decrease the blood units transfused per patient by 1.23 U (P = 0.001). There is no statistically significant difference in terms of the occurrence of DVT between the 2 groups (P = 0.461).
Conclusion:
Based on the current evidence, TXA can decrease the need for transfusion and the total blood loss without increasing the occurrence of DVT, and its administration is recommended routinely in bilateral TKA.
Keywords: blood loss, meta-analysis, total knee replacement, tranexamic acid
1. Introduction
Total knee arthroplasty (TKA) is one of the most effective and common surgeries for improving the quality of life of end-stage patients with osteoarthritis or rheumatic arthritis (RA) of the knee; however, TKA has always been associated with significant blood loss since a large area of bone is cut. It has been reported that ∼10% to 38% of patients need allogeneic blood transfusion.[1,2] The blood loss and subsequent blood transfusion can lead to many complications such as HIV infection, other infectious disease, fluid overload, and graft-versus-host disease.[3–5] Pharmacokinetic measures that include topical administration of tranexamic acid (TXA) and intravenous TXA have been identified as reducing blood loss, thus lowering the transfusion rate.[6,7] Tranexamic acid is a synthetic antifibrinolytic drug that can powerfully bind to the lysine-binding site of plasminogen because its chemical structure is similar to lysine but the binding capacity is more powerful than lysine and thus fibrinolysis is delayed.
Although bilateral TKA causes significant blood loss during the operation, there is no consensus about the efficacy and safety of TXA after TKA.[8] Thus, we carried out a meta-analysis to improve the evidence for understanding whether there is any difference between TXA and placebo in terms of (1) the need for transfusion, (2) the postoperative drop in hemoglobin (Hb) values and the number of blood units transfused per patient, (3) total blood loss and blood loss in drainage, and (4) thromboembolic complications including deep venous thrombosis (DVT) and pulmonary embolism (PE).
2. Material and methods
2.1. Search strategy
Electronic databases including MEDLINE (PubMed), Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science were searched for relevant studies published from the time of the establishment of these databases up to January 2016. In addition, the Google database was searched for additional literature. The reference lists of all the full-text articles were reviewed to identify any initially omitted studies without any restriction on the language of the publication. The search keywords were “tranexamic acid,” “total knee arthroplasty,” “total knee replacement,” “TKA,” and “TKR.” The relevant medical subject heading (MeSH) terms were used to maximize the specificity and sensitivity of the search. We did not include “bilateral TKA” to expand the scope of our search to ensure the accuracy of the literature research. These keywords and MeSH terms were combined with the Boolean operators AND or OR. The detailed search strategies can be seen in Appendix A. Since this is a meta-analysis, so no ethics committee or institutional review board to approve the study.
2.2. Eligibility criteria and study quality
Study selection was performed according to the following inclusion criteria: (1) published RCTs and non-RCTs about patients who underwent primary bilateral TKA; (2) intervention including tranexamic acid versus placebo or nothing; and (3) reported outcomes, including postoperative total blood loss, blood loss in drainage, the value of postoperative hemoglobin, blood units transfused per patient, the number of patients receiving blood transfusion, and the incidence of DVT or PE. All the studies must be clinical studies; trials with cadaver and artificial models or patients with bleeding disorders were excluded. Two reviewers independently scanned the quality of the eligible studies and discrepancies were solved by a senior reviewer. The Cochrane Handbook for Systematic Reviews of Interventions was used to evaluate the methodological quality and risk bias, which include (1) the randomization method; (2) allocation concealment; (3) blinding of participant, personnel, and assessor; and (4) complete outcome data and other bias.
2.3. Data extraction
The following data were extracted and recorded: (1) demographic data about the patients in the literature, author's name, publication date, the patient sample size, the ratio of male to female patients, and preoperative hemoglobin value; (2) the dose of TXA, whether the TXA was intravenous, topical, or topical combined with intravenous; the length of TXA use and the thromboprophylaxis to prevent the occurrence of DVT; and (3) postoperative total blood loss, blood loss in drainage, the value of postoperative hemoglobin, blood units transfused per patient, the number of patients receiving blood transfusion and the incidence of DVT or PE.
2.4. Outcome measures and statistical analysis
The main outcomes were the need for transfusion, a postoperative drop in Hb and the occurrence of DVT. Total blood loss, blood loss in drainage, and blood units transfused per patient were secondary outcomes. Continuous outcomes (total blood loss, blood loss in drainage, and the drop in Hb) were expressed as the mean differences (MD) and respective 95% confidence intervals (CIs). Dichotomous outcomes (the need for transfusion, DVT, and PE rates) were expressed as relative risks (RR) with 95% CIs. Statistical significance was set at P < 0.05 to summaries findings across the trials. Risk of bias assessment of each involved article was conducted according to the Cochrane Handbook for Systematic Reviews of Interventions and the using Review Manager (RevMan) software version 5.30 (The Cochrane Collaboration, Oxford, UK). The meta-analysis was performed using Stata software, version 12.0 (Stata Corp., College Station, TX). Statistical heterogeneity was tested using the chi-squared test and I2 statistic. In the absence of statistical evidence of heterogeneity (I2 < 50%, P > 0.1), a fixed-effects model was adopted; otherwise, a random-effects model was chosen. Publication bias was assessed by funnel plot and quantitatively assessed by Begg's test. Articles were considered to have no publication bias if the funnel plot was symmetrical and the P value was > 0.05.
3. Results
3.1. Search results
In the initial search, we identified 424 potentially relevant studies. Of these, 15 clinical trials with 679 patients (739 knees) were included in the meta-analysis.[9–23] Classification of the remaining studies resulted in a total of 8 RCTs,[12,14–16,19–22] 4 retrospective studies,[9,13,17,18] 1 prospective control study,[11] and 2 case–control studies.[10,23] As 1 study used topical and intravenous TXA to reduce blood loss during bilateral TKA, it was considered as 2 articles, compared with the placebo.[11] One study administered TXA at 10 mg/kg or 15 mg/kg, and this study was also considered as 2 articles for meta-analysis.[15]
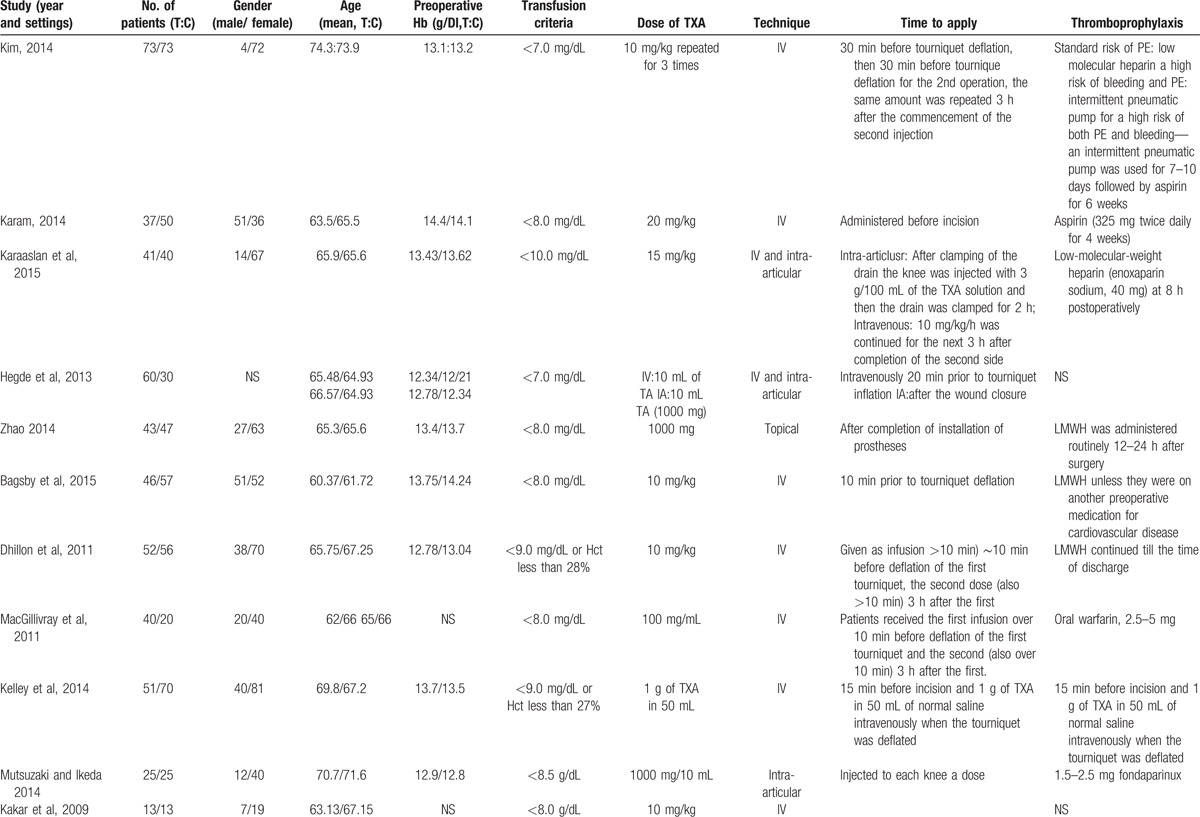
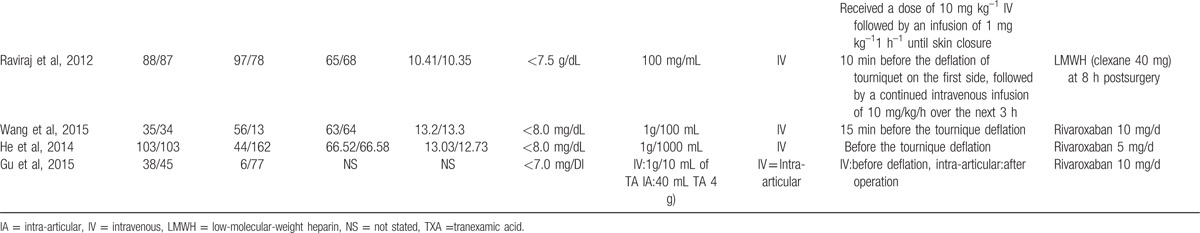
Of the included studies, 12 articles were in English[9–20] and 3 were in Chinese,[21–23] and all were published in 2009 or later. The characteristics of the studies that were included are shown in Table 1 . All participants in the included studies were elderly patients aged from 60.7 to 74.3 years, who had bilateral TKA. TXA administration was topical, intravenous, and combined topical with intravenous, and the dose ranged from 10 mg/kg to 20 mg/kg. All the included studies referenced different transfusion criteria. Detailed information on the transfusion criteria is given in Table 1 .
Table 1.
The general characteristic of the include studies.
3.2. Results of meta-analysis
3.2.1. Need for transfusion
A total of 12 included studies evaluated the need for transfusion after bilateral TKA. One study adopted different doses of TXA at the same time. We included the data from this study in the meta-analysis; therefore, a total of 1193 patients were included in the meta-analysis. There was no statistical heterogeneity (I2 = 29.7, df = 12, P = 0.147); so a fixed-effects model was applied to pool the effect size. The pooled RR showed that treatment with TXA can decrease the need for transfusion (RR = 0.58, 95%CI: 0.50–0.69, P = 0.000, Fig. 1).
Figure 1.
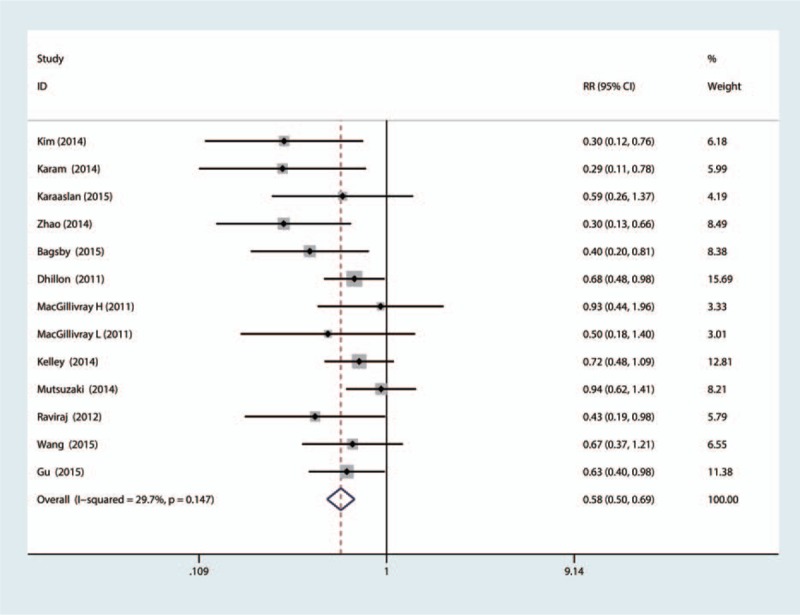
The forest plot compared the TXA versus placebo in terms of need for transfusion. TXA = tranexamic acid.
The funnel plot was symmetrical, as shown in Fig. 2, and the P-value calculated by Begg's test (Fig. 3) was 0.327. There was no publication bias among the 12 articles.
Figure 2.
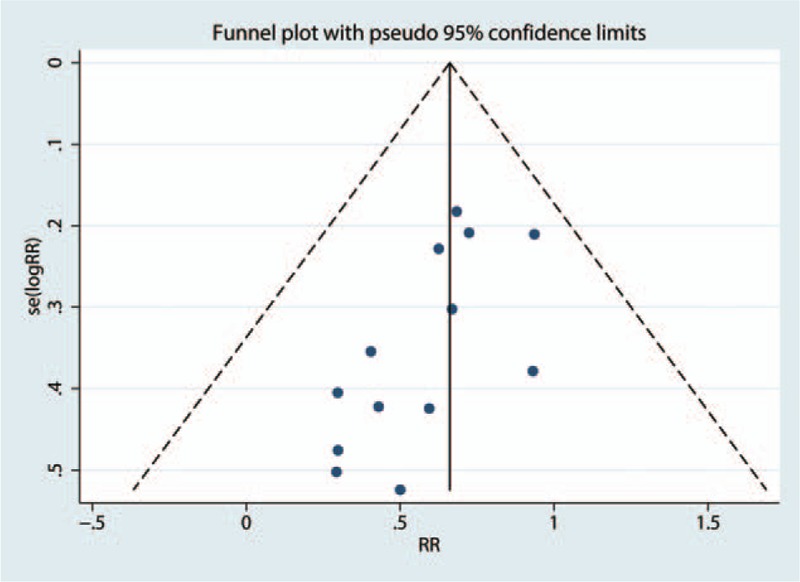
Funnel plot analysis of potential publication bias (for the 13 articles seen in Fig. 2).
Figure 3.
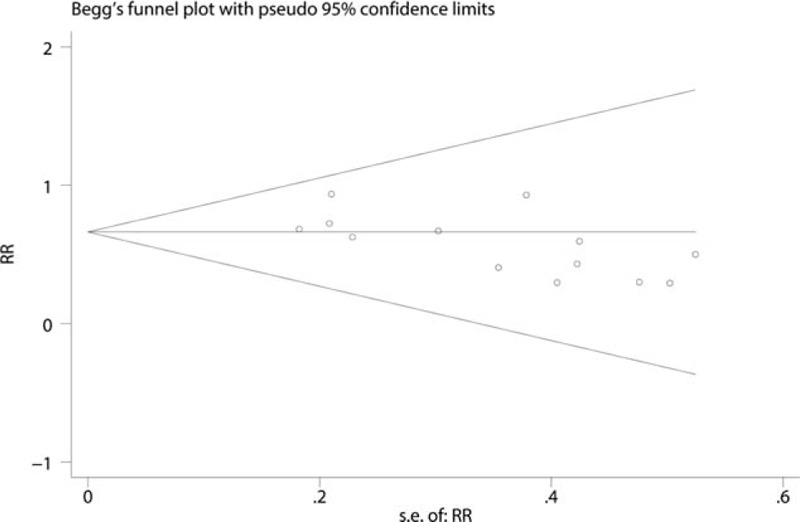
The funnel plot was symmetrical and the P-value calculated by Begg's test was 0.327.
3.2.2. Postoperative Hb drop
A total of 6 component studies (682 patients) provided the postoperative Hb drop value. There was a statistically significant difference between the groups with respect to postoperative drop in Hb (MD = −0.29; 95%CI −0.40 to −0.19; P = 0.29, Fig. 4). There was statistical heterogeneity (I2 = 84.8, df = 5, P = 0.000); hence, a random-effects model was performed.
Figure 4.

The forest plot compared the TXA versus placebo in terms of Hb drop. TXA = tranexamic acid.
3.3. Total blood loss, and blood loss in drainage
Eight articles (750 patients) reported total blood loss after TKA. Our meta-analysis indicated statistical heterogeneity in terms of total blood loss, so a random-effects model was used. The results indicated that TXA can decrease the total blood loss after bilateral TKA, and the difference was statistically significant (MD = −488.66; 95% CI −634.05 to −303.28; P = 0.000, Fig. 5). Eight studies (833 patients) reported blood loss in drainage after bilateral TKA, and meta-analysis indicated that TXA can decrease blood loss in drainage by 360.81 mL, and the difference is statistically significant (MD = −360.81; 95% CI −478.11 to −243.50; P = 0.000, Fig. 6).
Figure 5.
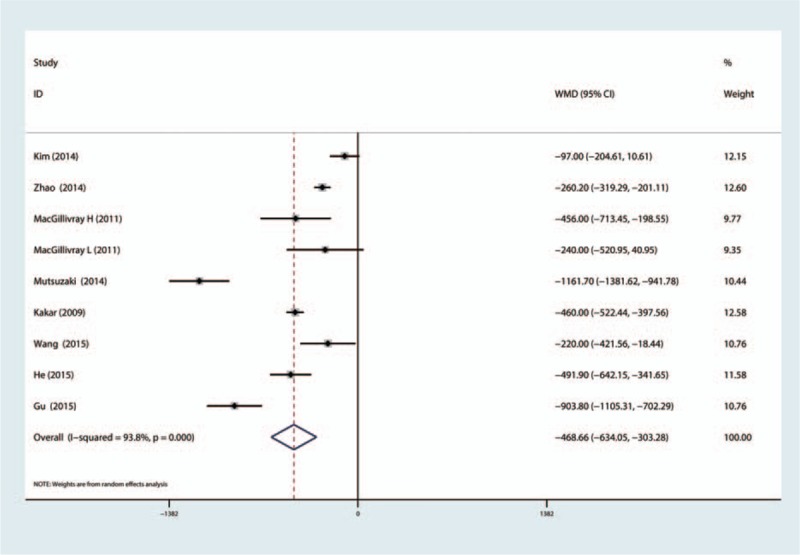
The forest plot compared the TXA versus placebo in terms of total blood loss. TXA = tranexamic acid.
Figure 6.
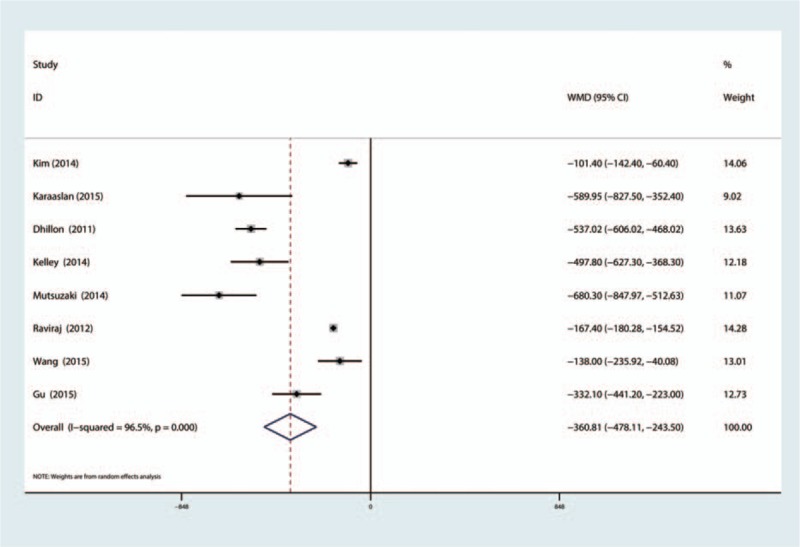
The forest plot compared the TXA versus placebo in terms of blood loss in drainage. TXA = tranexamic acid.
3.4. Blood units per patient transfused
A total of 8 papers (819 patients) reported the number of blood units transfused per patient. The meta-analysis indicated that TXA can decrease the number of blood units transfused per patient by 1.23 U, and the difference is statistically significant (MD = −1.23; 95% CI −1.94 to −0.52; P = 0.001, Fig. 7).
Figure 7.

The forest plot compared the TXA versus placebo in terms of blood unit per patient transfused. TXA = tranexamic acid.
3.5. The occurrence of DVT
Eleven studies reported the occurrence of DVT. The results of meta-analysis indicated that there is no statistically significant difference between the TXA and placebo in terms of the occurrence of DVT (RR = 0.68, 95%CI: 0.24–1.90, P = 0.461, Fig. 8).
Figure 8.

The forest plot compared the TXA versus placebo in terms of the occurrence of DVT and PE. DVT = deep venous thrombosis, PE = pulmonary embolism, TXA = tranexamic acid.
4. Subgroup analysis
The subgroup analysis was based on the RCTs and non-RCTs and whether TXA administration was topical, intravenous, or combined topical and intravenous. The outcomes of subgroup analysis are shown in Table 2.
Table 1 (Continued).
The general characteristic of the include studies.
5. Discussion
To our knowledge, this is the first meta-analysis of RCTs and non-RCTs comparing the efficacy and safety of TXA with placebo in the management of blood loss after bilateral TKA. The present meta-analysis was conducted on 8 randomized studies and 7 non-randomized studies and found better blood loss control and lower transfusion rates with TXA as compared to controls. There was no significant difference between TXA and the control in the occurrence of the DVT. To our knowledge, efficient blood loss control in perioperative bilateral TKA is more important than unilateral TKA, because bilateral TKA can cause more blood loss, leading to more patients requiring transfusion. All the included studies were published in 2009 or later, and most of the studies were from the year 2014. Four RCTs[12,14,19,20] were of high quality and 3 RCTs[15,16,22] did not state the random sequence generation. Two studies[16,22] did not involve allocation concealment, blinding of participants and personnel and blinding of the outcomes, and 1 RCT[21] is unclear concerning these criteria. All included studies showed comparable baseline data and provided the intention-to-treat analysis. The risk-of-bias graph and risk-of-bias summary are shown in Figs. 2 and 3. Methodological Index for Non-Randomized Studies (MINORS) quality scores for the 10 cohort studies ranged from 17 to 24 points, and all the included studies reported the baseline of the TXA group and control group. The MINORS quality score of each cohort is shown in Table 3.
Table 2.
Subgroup analysis about the total blood loss.
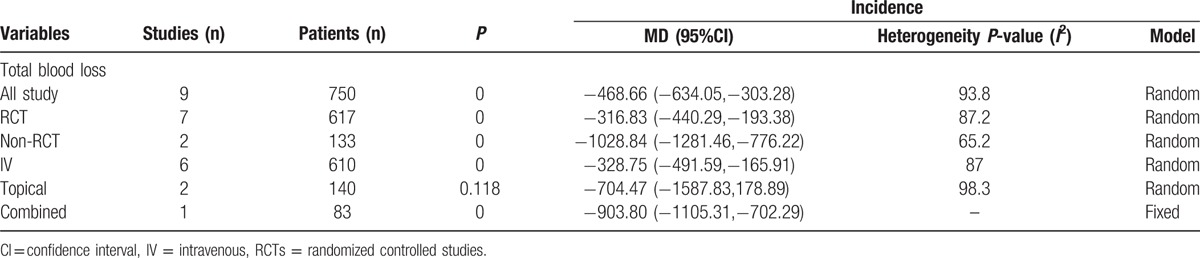
Table 3.
The Minors quality score of the non-RCTs. .

TXA has been used to reduce perioperative blood loss in many surgeries including cardiac surgery, caesarean section, TKA, and total hip arthroplasty (THA).[24–26] TXA can reduce blood loss by inhibiting fibrinolysis via combining with the lysine-binding sites of plasminogen.[27] Methods for application of TXA include topical, intravenous, and combined topical with intravenous.[28–30] These methods are efficient in reducing blood loss and the need for transfusion. It is still unclear whether or not TXA can decrease blood loss and the need for transfusion after bilateral TKAs; thus, published reports and meta-analysis are needed to identify the efficacy and safety of TXA in bilateral TKA.
The results of our meta-analysis indicate that TXA can decrease the need for transfusion. It is well known that transfusions are expensive and may bear the risk of infectious disease. Thus, a decrease in the need for transfusion can save blood product and avoid the risk of infectious disease. For transfusion rate, there is no significant heterogeneity between the groups. Kim et al[14] compared the efficacy of TXA in unilateral TKA versus bilateral TKA, and to our surprise, the transfusion reducing effects were more evident in simultaneous bilateral TKAs than in unilateral TKAs. The triggers for transfusion are different in all the studies, which may lead to bias in the overall results. Since there was no statistical heterogeneity among the studies, subgroup analysis was not conducted. Topical administration of TXA was as effective and safe in reducing blood loss and the need for transfusion as intravenous TXA.[31]
It is well established that TXA can decrease blood loss and blood loss in drainage in unilateral TKA. The results of the current meta-analysis indicated that TXA can decrease total blood loss, blood loss in drainage, and blood units transfused per patient. There is a controversy about the different administration routes for reducing blood loss after TKA. Wang et al[32] conducted a meta-analysis and found that no evidence to indicate topical administration of TXA is more effective than intravenous administration of TXA. Aggarwal et al[33] perform an RCT and found that topical TXA is better than IV TXA in reducing blood loss after TKA. Because the included studies used topical, intravenous, and combined administration of TXA and there was statistical heterogeneity, subgroup analysis was undertaken to try to diminish the heterogeneity. The result of this meta-analysis indicated that IV TXA can reduce total blood loss with a mean of 328.75 mL and topical TXA can save as 704.47 mL. Topical TXA may be better than IV TXA. Since this is an indirection comparison and need large sample direction RCT to draw a stable conclusion. A previous study has been identified that different doses of IV TXA were equally effective in reducing blood loss and transfusion when combined with autologous blood transfusion in bilateral TKA.[34] What is more, the different time to administration of TXA and doses of TXA is different in the included studies. All these factors influence the final results of total blood loss. Chen et al[35] conducted an RCT and results indicated that even fixed dose of TXA can reach better blood loss control. Levine et al[36] also concluded that weighted dose has equal efficacy versus uniform dose of TXA in patients undergoing primary TKA. Maniar et al[37] compared with different time to administration TXA for reducing blood loss in unilateral TKA and found that postoperative administration TXA has no benefit for controlling blood loss. In the included studies, no trials administrated TXA after surgery; thus, the time to administration TXA has no influence on the final results.
As for DVT, the pooled results indicated that there is no significant difference between the TXA group with control group. However, the short-term follow-up and the diagnostic techniques in each studies is not consistent.
This meta-analysis has several limitations: (1) only 8 RCTs and 7 non-RCTs were included, and the sample sizes in each trial were not large enough, which affects the final results; the small sample sizes can result in chosen publications bias to the final results; (2) the duration of follow-up in some studies was unclear, and long-term follow-up was needed for this analysis. As for DVT, the results of this meta-analysis indicated that TXA will not increase the occurrence of DVT. However, the short-term follow-up will omit the authentic occurrence of DVT; (3) the publication bias that existed in the meta-analysis influenced the results; (4) the dose and the method of TXA administration differ among the included trials, which affects the final conclusion; and (5) the heterogeneity among the studies will also affect the final conclusion, although we tried to use subgroup analysis to solve it.
6. Conclusion
In conclusion, administration of TXA can decrease the need for transfusion and, consequently, the economic cost. Total blood loss, blood loss in drainage, and postoperative Hb values also decrease through the administration of TXA. With respect to safety, TXA will not increase the occurrence of DVT. However, the TXA administration methods and dosage of TXA were not consistent; therefore, high-quality, well-designed RCTs are still warranted to identify the effect, safety, and optimal dose of TXA in bilateral TKAs.
Acknowledgments
The authors thank all the anonymous reviewers and editors for their suggestions.
Footnotes
Abbreviations: CIs = confidence intervals, DVT = deep venous thrombosis, Hb = hemoglobin, MD = mean differences, Mesh = medical subject heading, PRISMA = preferred reporting items for systematic reviews and meta-analyses, RCTs = randomized controlled studies, RR = relative risk, TKA = total knee arthroplasty, TXA = tranexamic acid.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Bong MR, Patel V, Chang E, et al. Risks associated with blood transfusion after total knee arthroplasty. J Arthroplasty 2004; 19:281–287. [DOI] [PubMed] [Google Scholar]
- 2.Hiippala S, Strid L, Wennerstrand M, et al. Tranexamic acid (cyklokapron) reduces perioperative blood loss associated with total knee arthroplasty. Br J Anaesth 1995; 74:534–537. [DOI] [PubMed] [Google Scholar]
- 3.Madjdpour C, Spahn D. Allogeneic red blood cell transfusions: efficacy, risks, alternatives and indications. Br J Anaesth 2005; 95:33–42. [DOI] [PubMed] [Google Scholar]
- 4.Bierbaum BE, Callaghan JJ, Galante JO, et al. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg 1999; 81:2–10. [DOI] [PubMed] [Google Scholar]
- 5.Cermakova Z, Simetka O, Kořístka M. [Transfusion-related acute lung injury (TRALI)-review]. Ceska gynekologie/Ceska lekarska Spolecnost J Ev Purkyne 2013; 78:211–215. [PubMed] [Google Scholar]
- 6.Martin JG, Cassatt KB, Kincaid-Cinnamon KA, et al. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty 2014; 29:889–894. [DOI] [PubMed] [Google Scholar]
- 7.Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, et al. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement. J Bone Joint Surg Am 2014; 96:1937–1944. [DOI] [PubMed] [Google Scholar]
- 8.Fu D, Li G, Chen K, et al. Comparison of clinical outcome between simultaneous-bilateral and staged-bilateral total knee arthroplasty: a systematic review of retrospective studies. J Arthroplasty 2013; 28:1141–1147. [DOI] [PubMed] [Google Scholar]
- 9.Bagsby DT, Samujh CA, Vissing JL, et al. Tranexamic acid decreases incidence of blood transfusion in simultaneous bilateral total knee arthroplasty. J Arthroplasty 2015; 30:2106–2109. [DOI] [PubMed] [Google Scholar]
- 10.Dhillon MS, Bali K, Prabhakar S. Tranexamic acid for control of blood loss in bilateral total knee replacement in a single stage. Ind J Orthop 2011; 45:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hegde C, Wasnik S, Kulkarni S, et al. Simultaneous bilateral computer assisted total knee arthroplasty: the effect of intravenous or intraarticular tranexamic acid. J Arthroplasty 2013; 28:1888–1891. [DOI] [PubMed] [Google Scholar]
- 12.Karaaslan F, Karaoğlu S, Mermerkaya MU, et al. Reducing blood loss in simultaneous bilateral total knee arthroplasty: combined intravenous–intra-articular tranexamic acid administration. A prospective randomized controlled trial. Knee 2015; 22:131–135. [DOI] [PubMed] [Google Scholar]
- 13.Karam JA, Bloomfield MR, DiIorio TM, et al. Evaluation of the efficacy and safety of tranexamic acid for reducing blood loss in bilateral total knee arthroplasty. J Arthroplasty 2014; 29:501–503. [DOI] [PubMed] [Google Scholar]
- 14.Kim TK, Chang CB, Kang YG, et al. Clinical value of tranexamic acid in unilateral and simultaneous bilateral TKAs under a contemporary blood-saving protocol: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc 2014; 22:1870–1878. [DOI] [PubMed] [Google Scholar]
- 15.MacGillivray RG, Tarabichi SB, Hawari MF, et al. Tranexamic acid to reduce blood loss after bilateral total knee arthroplasty: a prospective, randomized double blind study. J Arthroplasty 2011; 26:24–28. [DOI] [PubMed] [Google Scholar]
- 16.Zhao HL, Wan SG, Qi DZ, et al. Topical hemostatic procedures control blood loss in bilateral cemented single-stage total knee arthroplasty. J Orthop Sci 2014; 19:948–953. [DOI] [PubMed] [Google Scholar]
- 17.Kelley TC, Tucker KK, Adams MJ, et al. Use of tranexamic acid results in decreased blood loss and decreased transfusions in patients undergoing staged bilateral total knee arthroplasty. Transfusion 2014; 54:26–30. [DOI] [PubMed] [Google Scholar]
- 18.Mutsuzaki H, Ikeda K. Effect of injecting tranexamic acid from a drain to the joint and drain-clamping to reduce blood loss during bilateral cementless total knee arthroplasty. J Blood Disorders Transf 2014; 5:2. [Google Scholar]
- 19.Kakar P, Gupta N, Govil P, et al. Efficacy and safety of tranexamic acid in control of bleeding following TKR: a randomized clinical trial. Ind J Anaesthesia 2009; 53:667. [PMC free article] [PubMed] [Google Scholar]
- 20.Raviraj A, Anand A, Chakravarthy M, et al. Tranexamic acid reduces blood loss in simultaneous bilateral total knee arthroplasty: a randomized control trial. Eur J Orthop Surg Traumatol 2012; 22:381–386. [Google Scholar]
- 21.Wang R, Tian SQ, Ha CZ, et al. Efficacy and safety of tranexamic acid on reducing blood loss in bilateral total knee arthroplasty. Chin J Tissue Eng Res 2015; 19:3451–3456. [Google Scholar]
- 22.He Y, Wu H, Wu L, et al. Efficacy and safety of tranexamic acid in treating perioperative blood loss in bilateral total knee arthroplasty (TKA). J Xinjiang Med Univ 2014; 37:1425–1430. [Google Scholar]
- 23.Gu J, Du H, Jiang X, et al. Efficacy and safety of intravenous and intra-articular injection of tranexamic acid in reducing perioperative bleeding during simultaneous bilateral total knee arthroplasty. Shandong Med J 2015; 55:14–16. [Google Scholar]
- 24.Wesley MC, Pereira LM, Scharp LA, et al. Pharmacokinetics of tranexamic acid in neonates, infants, and children undergoing cardiac surgery with cardiopulmonary bypass. Anesthesiology 2015; 122:746–758. [DOI] [PubMed] [Google Scholar]
- 25.Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on transfusion rate in primary total hip arthroplasty. J Arthroplasty 2014; 29:387–389. [DOI] [PubMed] [Google Scholar]
- 26.Bhatia S, Deshpande H. Role of tranexamic acid in reducing blood loss during and after caesarean section. Med J Dr DY Patil Univ 2015; 8:21. [Google Scholar]
- 27.Roberts I, Prieto-Merino D, Manno D. Mechanism of action of tranexamic acid in bleeding trauma patients: an exploratory analysis of data from the CRASH-2 trial. Crit Care 2014; 18:685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alshryda S, Sukeik M, Sarda P, et al. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J 2014; 96:1005–1015. [DOI] [PubMed] [Google Scholar]
- 29.Soni A, Saini R, Gulati A, et al. Comparison between intravenous and intra-articular regimens of tranexamic acid in reducing blood loss during total knee arthroplasty. J Arthroplasty 2014; 29:1525–1527. [DOI] [PubMed] [Google Scholar]
- 30.Huang Z, Ma J, Shen B, et al. Combination of intravenous and topical application of tranexamic acid in primary total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty 2014; 29:2342–2346. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Shen B, Zeng Y. Comparison of topical versus intravenous tranexamic acid in primary total knee arthroplasty: a meta-analysis of randomized controlled and prospective cohort trials. Knee 2014; 21:987–993. [DOI] [PubMed] [Google Scholar]
- 32.Wang H, Shen B, Zeng Y. Comparison of topical versus intravenous tranexamic acid in primary total knee arthroplasty: a meta-analysis of randomized controlled and prospective cohort trials. Knee 2014; 21:987–993. [DOI] [PubMed] [Google Scholar]
- 33.Aggarwal AK, Singh N, Sudesh P. Topical vs intravenous tranexamic acid in reducing blood loss after bilateral total knee arthroplasty: a prospective study. J Arthroplasty 2016; 31:1442–1448. [DOI] [PubMed] [Google Scholar]
- 34.MacGillivray RG, Tarabichi SB, Hawari MF, et al. Tranexamic acid to reduce blood loss after bilateral total knee arthroplasty: a prospective, randomized double blind study. J Arthroplasty 2011; 26:24–28. [DOI] [PubMed] [Google Scholar]
- 35.Chen X, Cao X, Yang C, et al. Effectiveness and safety of fixed-dose tranexamic acid in simultaneous bilateral total knee arthroplasty: a randomized double-blind controlled trial. J Arthroplasty 2016; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 36.Levine BR, Haughom BD, Belkin MN, et al. Weighted versus uniform dose of tranexamic acid in patients undergoing primary, elective knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty 2014; 29 (9 suppl):186–188. [DOI] [PubMed] [Google Scholar]
- 37.Maniar RN, Kumar G, Singhi T, et al. Most effective regimen of tranexamic acid in knee arthroplasty: a prospective randomized controlled study in 240 patients. Clin Orthop Rel Res 2012; 470:2605–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]


