Abstract
Background & Objectives:
Telomere plays a critical role in the maintenance of genomic stability in eukaryotic chromosomes. More and more findings have shown that alteration in telomere length may involve in normal somatic cells and some diseases, however, whether the telomere length is associated with the development and/or progression of hepatic diseases remains poorly understood.
Methods:
A case–control study was employed to illustrate the correlation of relative telomere length (RTL) with chronic hepatitis B (CHB) and hepatocellular carcinoma (HCC). In this study, 152 patients with HCC, 212 patients with CHB, and 184 healthy controls were recruited. Genomic deoxyribonucleic acid (DNA) was extracted from the peripheral blood leukocytes, and fluorescence quantitative polymerase chain reaction (FQ-PCR) was used to detect telomere repeated numbers and 36B4 copy numbers. The RTL was calculated by telomere repeat copy number to single-copy gene number ratio in each sample compared with a reference DNA sample.
Results:
We found that the RTL in HCC group was the longest, followed by CHB group, and healthy control group was the shortest, showing significant statistical differences. When participants were categorized into longer and shorter group according to medium value in healthy controls, individuals who had longer RTL had a significant increased risk of CHB (odds ratio [OR]: 1.83, 95% confidence interval [CI]: 1.22–2.73) when the healthy control was used as the reference groups; furthermore, longer RTL also showed higher incidence of HCC (OR: 3.22, 95% CI: 2.01–5.17; OR: 1.58, 95% CI:1.03–2.41) when healthy control and CHB were used as the reference groups, respectively. When participants were categorized further into 4 groups according to quartile values of RTL in healthy controls, it showed that the longest RTL was also associated with an increased risk of CHB (OR: 2.09, 95% CI: 1.17–3.74) and HCC (OR: 4.31, 95% CI: 2.18–8.52; OR: 2.86, 95% CI: 1.53–5.34) when control and control/CHB group were used as the reference groups, respectively.
Conclusion:
Our results suggest that the alteration of telomere length in peripheral leukocytes might be involved in the hepatitis B virus infection and HCC events, and RTL might be a potential useful predictor of CHB and HCC.
Keywords: chronic hepatitis B, hepatocellular carcinoma, predictor, relative telomere length
1. Introduction
China is one of the highest hepatitis B virus (HBV)-infected countries worldwide[1]; about 9.75% of Chinese population are Hepatitis B virus surface antigen (HBsAg) carriers. HBV is a hepadnavirus which leads to chronic hepatitis B (CHB) and cirrhosis, and is associated with a high risk of hepatocellular carcinoma (HCC). HCC is a highly malignant cancer lacking of effective treatment methods, so early diagnosis and prevention are effective ways to improve the survival rate. In recent years, more and more researchers have paid attention to telomere length measurement and investigated the association between RTL and risk of many kinds of diseases, including cancer.[2–5]
Human telomeres complexes are composed of repetitive nucleotide sequences (TTAGGG) and nucleoproteins such as TRF1, TRF2, TPP1, POT1, TIN2, and RAP1 which protect chromosome ends from end-to-end fusions and degradation to maintain genomic integrity.[6] In human normal somatic cells, the length of telomeric TTAGGG is 10 to 15 kb, and shortened by 50 to 200 base pairs with each cell division cycle because of the end replication inefficiency of deoxyribonucleic acid (DNA) polymerase.[7] Besides the end replication problem, oxidative damage and chronic inflammation are also associated with telomere shortening.[8,9] When telomeres progresses shorten to a critical length, they may be recognized as double-strand breaks and activate the Rb and/or P53 pathway to initiate cellular senescence and apoptosis.[10,11] If cells escape from the apoptosis or senescence, the shortened telomeres lead to chromosome abnormality and active oncogenes to initiate carcinogenesis.
There are lots of studies focusing on the association between telomere length and cancer risk, while the results are inconsistent. Some findings showed that shorter telomere length not only increases the risk of gastric cancer,[12] but also decreases the survival rate of bladder cancer,[13] while in other epidemiologic investigates, longer telomere length was associated with the increased risk of cancers that were reported.[14–18] To date, little research has investigated the relationship between telomere length and the event of hepatocarcinogenesis (from normal, CHB to HCC) to our knowledge. In this study, the changes of leukocytes telomere length in different cohorts of diseases in a southwest Han Chinese population were investigated to try to understand the relationship of RTL in different stages of hepatocarcinogenesis.
2. Materials and methods
2.1. Study population
The study population was recruited from the affiliated hospital of North Sichuan Medical College from September 2013 to September 2014 (Nanchong, Sichuan, China), and the case group consists of 152 and 212 cases of newly diagnosed HCC patients and CHB patients, respectively. All the cases were confirmed by histology or imaging diagnosis or laboratory examinations, and 184 cases of controls were cancer-free individuals and were recruited from the physical examination cohorts at the same hospital. All the cases and controls employed in this study were Han Chinese, and all of the subjects were agreed to donate 2 mL peripheral blood for this study. General characteristics such as age, gender, alcohol consumption, and cigarette smoking were collected by querying medical records. This study obtained informed consents of all the subjects and was approved by the ethics committee of North Sichuan Medical College.
2.2. Telomere length measurement
Genomic DNA was extracted from peripheral blood leukocytes by using TIANamp Blood DNA Kit (Tiangen Biotech Co., Ltd., Beijing, China) according to the manufacturer's protocol. Telomere length was measured by quantitative polymerase chain reaction (PCR) technology as previous described by Cawthon.[19] In the protocol, 2 pairs of primers were used for measuring the copy numbers of telomere and single copy gene 36B4. Telomere forward primer (Tel F): 5′-ACACTAAGGTTTGGGTTTGGGTTTGGGTTTGGGTTAGTGT-3′, Telomere reverse primer (Tel R): 5′-TGTTAGGTATCCCTATCCCTATCCCTATCCCTATCCCTAACA-3′. 36B4 forward primer (36B4F): 5′-CAGCAAGTGGGAAGGTGTAATCC-3′, 36B4 reverse primer (36B4R): 5′-CCCATTCTATCATCAACGGGTACAA-3′. In brief, PCR (the total volume is 10 μL) comprised 2 × Master mix 5 μL (Roche, Basel, Switzerland), forward primer 0.4 μL (10 nM, Sangon, Shanghai, China), reverse primer 0.4 μL (10 nM, Sangon, China), template DNA 1 μL, and ddH2O 3.2 μL. The thermal cycling for telomere PCR is 95 °C for 10 minutes followed by 2 cycles of 95 °C for 15 seconds and 49 °C for 1 minute, the next step is 30 cycles of 95 °C for 15 seconds followed by 62 °C for 1 minute and 72 °C for 20 seconds. For 36B4 PCR, the thermal cycling is 95 °C for 10 minutes and followed by 40 cycles of 95 °C for 15 seconds and 58 °C for 1 minute. The copy numbers of telomere and 36B4 gene were run in separate 96 well plates on Roche Lightcycler 96 platform (Basel, Switzerland), and all the reactions were triplicate. In every single 96 well plate, negative control, positive control, and a standard curve were included. A 5-point standard curve was created by diluting the reference DNA sample using a 2-fold increment dilution in each reaction, only the result shown R2 > 0.99 is accurate for using 2−ΔΔCT method to calculate telomere and 36B4 gene copy number. The ratio of telomere/single copy gene copy numbers (T/S) is proportional to the average telomere length per cell as described by Cawthon.[19,20] Relative telomere lengths (RTLs) were measured as the ratio of patient T/S values to the reference DNA T/S values to eliminate plate-to-plate variation.
2.3. Statistical analysis
Chi-square and Student t test were used to evaluate the difference between categorical and continuous variables, respectively. RTL data were analyzed as categorical variable based on the cutoff points at the median and quartile value among controls. Odds ratio (OR) and 95% confidence interval (CI) were calculated by unconditional logistic regression model to evaluate the association between RTL and HCC risk. All tests were 2-sided, and P values below 0.05 were considered statistically significant. The SPSS software 11.5 (SPSS, Inc., Chicago, IL) was used to perform all of the analyses.
3. Results
3.1. Subject characteristics
The distributions of patient characteristics are summarized in Table 1. A total of 152 HCC patients (male 130 and female 22), 212 CHB patients (male 143 and female 69), and 184 healthy controls (male 100 and female 84) were recruited in this study. All participants were Han Chinese in southwest of China. The average age in patients with HCC was older than that of CHB and control, the differences were significant (P < 0.05). In addition, there were more male patients in HCC group than that of CHB and control. We also investigated cigarette smoking and alcohol consumption of all the subjects except CHB patients because of extramural hospital treatment.
Table 1.
Selected characteristics of all participants.
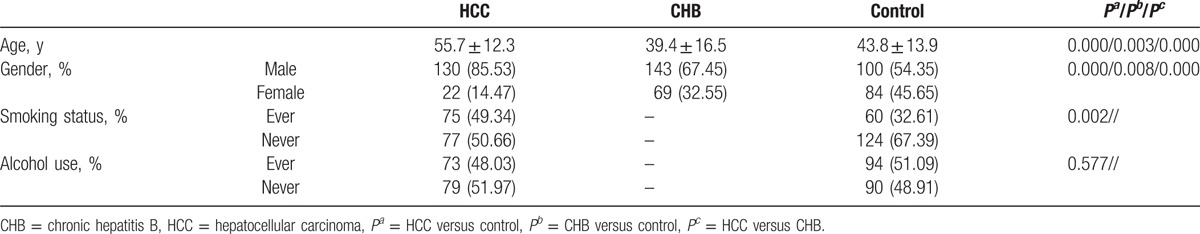
3.2. RTL distribution by age and gender in HCC, CHB and Controls
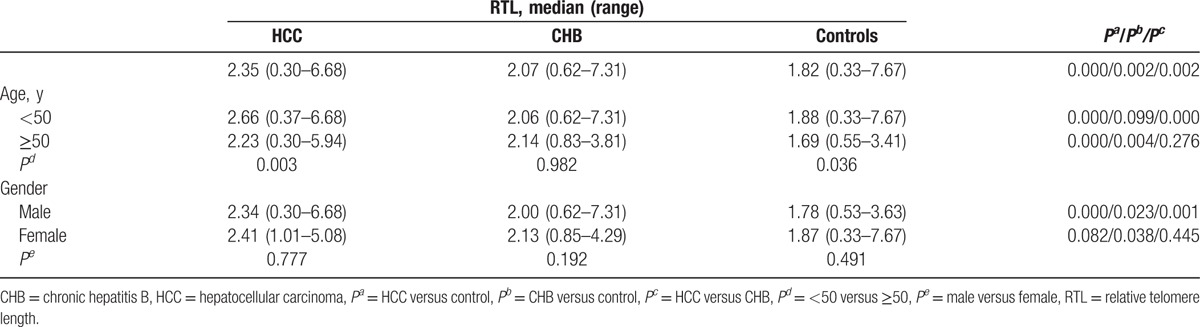
As shown in Table 2, the RTL in HCC group was the longest, followed by CHB group, and control group was the shortest, and the difference was statistically significant when CHB or control used as reference (P < 0.05). Participants who aged more than 50 years had shorter RTL in HCC and control group when compared with that in participants aged less than 50 years, while no significant difference was found in CHB group. For individuals who aged less than 50 years, RTL in HCC group was longer than that of in CHB and control group, while in individuals older than 50 years, the RTLs in both HCC and CHB groups were longer than that of in control. When stratified by gender, we found that RTL in male HCC was the longest, followed by CHB, and control was the shortest (P < 0.05), while in the female group, only RTL in CHB group was longer than that in control (P < 0.05). However, the differences of RTL in HCC, CHB, and control group were not significant in male and female participants, respectively.
Table 2.
RTL distribution by age and gender in HCC, CHB and Controls.
3.3. The correlations between RTL and risk of HCC and CHB
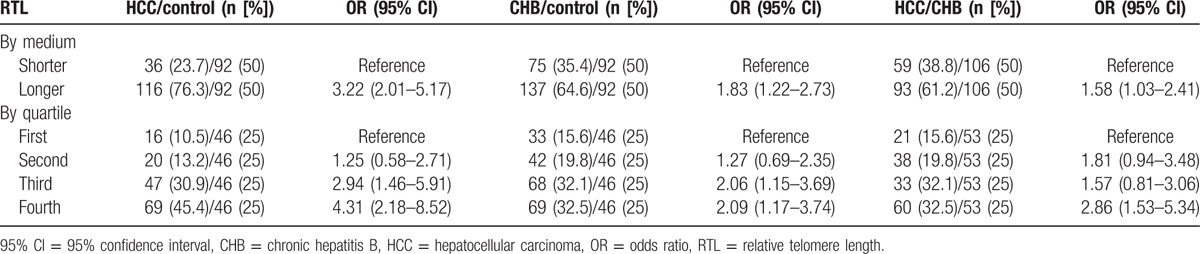
As shown in Table 3, when control was used as the reference group and RTL was dichotomized into long and short groups based on the median value in control group, individuals with longer RTL had a significantly increased risk of HCC (OR: 3.22, 95% CI: 2.01–5.17) and CHB (OR: 1.83, 95% CI: 1.22–2.73). When CHB was used as reference, longer RTL was also associated with an increased risk of HCC (OR: 1.58, 95% CI: 1.03–2.41). Furthermore, participants were further categorized into 4 groups according to quartile values of RTL in control individuals, and we also observed a significant relationship between the RTL and HCC and CHB risk. When the first quartile was used as the reference group, the ORs for the second, third, and fourth were 1.25 (95% CI: 0.58–2.71), 2.94 (95% CI: 1.46–5.91), and 4.31 (95% CI: 2.18–8.52) in HCC group, respectively. Similarly in the CHB group, the ORs for the second, third, and fourth were 1.27 (95% CI: 0.69–2.35), 2.06 (95% CI: 1.15–3.69), and 2.09 (95% CI: 1.17–3.74) when the first quartile was used as the reference group, respectively. Compared with CHB group, the risk of HCC was increased in the fourth quartile compared with the first quartile (OR = 2.86, 95% CI: 1.53–5.34).
Table 3.
The correlations between RTL and risk of HCC and CHB.
4. Discussion
In recent years, many researchers explored the association between RTL and cancer risk, while the results were controversial. In some types of cancers, such as gastric and bladder cancer, shorter RTL in leukocytes was a risk factor of tumorigenesis,[12,13,21] while it was opposite in lung and colorectal cancer.[17,18]
Up to now, there are several studies focusing on the association between RTL and hepatic diseases, including cirrhosis and HCC. Wan et al[22] demonstrated that cirrhotic cases had a significantly longer RTL in circulating serum DNA than that in noncirrhotic controls, and long RTL had a significantly increased risk of cirrhosis. In another study, researchers revealed that serum DNA RTL in HBV-relative HCC was longer than that in healthy control, and a dose–response relationship existed in the quartile analysis.[23] Plentz et al[24] measured RTL in HCC tissue, regenerative nodules, and surrounding noncancerous liver tissue, the results showed that RTL in HCC tissue was the shortest. In another study, RTL was gradually shortening during hepatocarcinogenesis (from low-grade dysplastic nodules, high-grade dysplastic nodules, HCC loci to HCC) and high-grade dysplastic nodules and HCC were the shortest, which was significantly positively correlated with chromosome instability.[25] Saini et al's[26] result revealed that patients with HCC had a shorter RTL in cancer tissue when compared with CHB and chronic hepatitis C. Besides these association studies, another functional study revealed that longer RTL was positively correlated with the invasive capacity of HCC cells, which indicated that telomere might play multiple roles in HCC initiation and progression.[27]
In the present study, we measured leukocytes RTL from peripheral blood using quantitative PCR technology and investigated the association between RTL and HCC risk in a southwest Han Chinese population. In the healthy controls, RTL in individuals aged more than 50 years was shorter than that in those aged less than 50 years, which was consistent with a previous study.[7] We also found that there was a significantly longer RTL in patients with HCC and CHB than that in healthy controls, which demonstrated that RTL in peripheral blood leukocytes might have gradually gotten lengthening from control, CHB to HCC. The similar results were obtained when stratifying analysis by age and gender, especially in male patients. When unconditional logistic regression was used to evaluate the association between RTL and the risk of HCC and CHB, it revealed that longer RTL was associated with an increased risk of HCC and CHB both by medium and quartile, which indicated that RTL in leukocytes might be a predictive biomarker of HCC as telomere lengthening gradually during the procedure of hepatocarcinogenesis.
In the previous studies, both shorter RTL in leukocytes and serum-free DNA and longer RTL in HCC tissue were associated with an increased risk of HCC risk. Although the conclusions were opposite, the detailed molecular mechanisms remain to be investigated further. Recent studies have suggested that telomere dysfunction had dual roles in cancer progression and carcinogenesis, including hepatocarcinogenesis.[28–30] Normal somatic cells need telomere length in a balance level to maintain cell proliferation, senescence, and apoptosis, excessively shortened telomere may give rise to more frequency of chromosome end-to-end fusion, while significant longer telomere delays cells senescence and apoptosis and more genetic lesions might occur during cell division, as a result, both exceed short and exceed long telomere could lead to chromosome instability and initiate tumorigenesis.[28,29] Recent studies, both in vivo and in vitro, suggested that HBx protein, encoded by HBV viral genome, increases both expression of telomere reverse transcriptase and telomerase activity, thus prolonging the lifespan of hepatocytes and contributing to malignant transformation.[31–34] Another 2 relative studies demonstrated that telomere length in leukocytes was positively correlated with normal liver tissue and cirrhotic liver tissue.[35,36] Here, from our study, we pointed out that longer RTL in leukocytes is associated with an increased risk of HCC, which is consistent with those findings.
There are a few limitations in this study. First, the characteristics of the subjects are not completely collected. The patients including those with HCC and control group had more characteristics such as cigarette smoking and alcohol consumption, while the individuals in CHB group were lacking of some information, because most of the CHB patients were treated outside the hospital and some patients were unwilling to provide their private information to doctors. Second, the average age in HCC was older than that in CHB and control, which should be more comparable and accordant. While in the present study, the result showed that RTL in HCC was the longest, which further demonstrated that the lengthened telomere may play a critical role in hepatocarcinogenesis. Third, the group of patients with cirrhosis should be recruited in this study as one of the diseases during HCC development. Because of the above limitations, more studies in different ethics and populations should be involved in the future experiments to further get more solid conclusion.
According to the results, whether telomere lengthening is a result of HCC or is an initiating factor for HCC remains edificatory. Nevertheless, we observed that the leukocytes RTL was longer in CHB and HCC patients, moreover RTL was longer in HCC than that in CHB, which suggests that RTL might have gotten gradually lengthening from CHB to HCC, indicating RTL might be involved in HBV infections and/or HCC events, and RTL could be served as a potential predictor for HCC.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, CHB = chronic hepatitis B, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, OR = odds ratio, RTL = relative telomere length, T/S = the ratio of telomere/single copy gene copy numbers.
QM and JC contributed equally to the article and should be considered co–first authors.
This study was supported by Sichuan Science and Technology Agency 2014JY0115.
The authors have no conflicts of interest to disclose.
References
- 1.Custer B, Sullivan SD, Hazlet TK, et al. Global epidemiology of hepatitis B virus. J Clingastroenterol 2004; 38:158–168. [DOI] [PubMed] [Google Scholar]
- 2.Alter BP, Giri N, Savage SA, et al. Telomere length in inherited bone marrow failure syndromes. Haematologica 2015; 100:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw JG, Vaughan A, Dent AG, et al. Biomarkers of progression of chronic obstructive pulmonary disease (COPD). J Thorac Dis 2014; 6:1532–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z, Tang J, Li H, et al. Shorter telomere length in peripheral blood leukocytes is associated with childhood autism. Sci Rep 2014; 4:7073–7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotsopoulos J, Prescott J, De Vivo I, et al. Telomere length and mortality following a diagnosis of ovarian cancer. Cancer Epidemiol Biomarkers Pre 2014; 23:2603–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shay JW, Wright WE. Role of telomeres and telomerase in cancer. Semin Cancer Biol 2011; 21:349–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klapper W, Parwaresch R, Krupp G. Telomere biology in human aging and aging syndromes. Mech Ageing Dev 2001; 122:695–712. [DOI] [PubMed] [Google Scholar]
- 8.Houben JM, Moonen HJ, van Schooten FJ, et al. Telomere length assessment: biomarker of chronic oxidative stress? Free Radic Biol Med 2008; 44:235–246. [DOI] [PubMed] [Google Scholar]
- 9.Gilley D, Tanaka H, Herbert BS. Telomere dysfunction in aging and cancer. Int J Biochem Cell Biol 2005; 37:1000–1013. [DOI] [PubMed] [Google Scholar]
- 10.Mathon NF, Lloyd AC. Cell senescence and cancer. Nat Rev Cancer 2001; 1:203–213. [DOI] [PubMed] [Google Scholar]
- 11.Gilley D, Herbert BS, Huda N, et al. Factors impacting human telomere homeostasis and age-related disease. Mech Ageing Dev 2008; 129:27–34. [DOI] [PubMed] [Google Scholar]
- 12.Qu F, Li R, He X, et al. Short telomere length in peripheral blood leukocyte predicts poor prognosis and indicates an immunosuppressive phenotype in gastric cancer patients. Mol Oncol 2015; 9:727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo A, Modica F, Guarrera S, et al. Shorter leukocyte telomere length is independently associated with poor survival in patients with bladder cancer. Cancer Epidemiol Biomarkers Pre 2014; 23:2439–2446. [DOI] [PubMed] [Google Scholar]
- 14.Kim ES, Ye Y, Vaporciyan AA, et al. Telomere length and recurrence risk after curative resection in patients with early-stage non-small-cell lung cancer: a prospective cohort study. J Thorac Oncol 2015; 10:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Julin B, Shui I, Heaphy CM, et al. Circulating leukocyte telomere length and risk of overall and aggressive prostate cancer. Br J Cancer 2015; 112:769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campa D, Martino A, Varkonyi J, et al. Risk of multiple myeloma is associated with polymorphisms within telomerase genes and telomere length. Int J Cancer 2015; 136:351–358. [DOI] [PubMed] [Google Scholar]
- 17.Seow WJ, Cawthon RM, Purdue MP, et al. Telomere length in white blood cell DNA and lung cancer: a pooled analysis of three prospective cohorts. Cancer Res 2014; 74:4090–4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segui N, Guino E, Pineda M, et al. Longer telomeres are associated with cancer risk in MMR-proficient hereditary non-polyposis colorectal cancer. PLoS One 2014; 9:86063–86066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res 2002; 30:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 2009; 37:21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mu Y, Zhang Q, Mei L, et al. Telomere shortening occurs early during gastrocarcinogenesis. Med Oncol 2012; 29:893–898. [DOI] [PubMed] [Google Scholar]
- 22.Wan S, Hann HW, Myers RE, et al. Telomere length in circulating serum DNA as a novel non-invasive biomarker for cirrhosis: a nested case-control analysis. Liver Int 2012; 32:1233–1241. [DOI] [PubMed] [Google Scholar]
- 23.Fu X, Wan S, Hann HW, et al. Relative telomere length: a novel non-invasive biomarker for the risk of non-cirrhotic hepatocellular carcinoma in patients with chronic hepatitis B infection. Eur J Cancer 2012; 48:1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plentz RR, Caselitz M, Bleck JS, et al. Hepatocellular telomere shortening correlates with chromosomal instability and the development of human hepatoma. Hepatology 2004; 40:80–86. [DOI] [PubMed] [Google Scholar]
- 25.Lee YH, Oh BK, Yoo JE, et al. Chromosomal instability, telomere shortening, and inactivation of p21(WAF1/CIP1) in dysplastic nodules of hepatitis B virus-associated multistep hepatocarcinogenesis. Mod Pathol 2009; 22:1121–1131. [DOI] [PubMed] [Google Scholar]
- 26.Saini N, Srinivasan R, Chawla Y, et al. Telomerase activity, telomere length and human telomerase reverse transcriptase expression in hepatocellular carcinoma is independent of hepatitis virus status. Liver Int 2009; 29:1162–1170. [DOI] [PubMed] [Google Scholar]
- 27.Ko E, Jung G. Positive association of long telomeres with the invasive capacity of hepatocellular carcinoma cells. Biochem Biophys Res Commun 2014; 447:358–363. [DOI] [PubMed] [Google Scholar]
- 28.Cosme-Blanco W, Chang S. Dual roles of telomere dysfunction in initiation and suppression of tumorigenesis. Exp Cell Res 2008; 314:1973–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hackett JA, Greider CW. Balancing instability: dual roles for telomerase and telomere dysfunction in tumorigenesis. Oncogene 2002; 21:619–626. [DOI] [PubMed] [Google Scholar]
- 30.Satyanarayana A, Manns MP, Rudolph KL. Telomeres and telomerase: a dual role in hepatocarcinogenesis. Hepatology 2004; 40:276–283. [DOI] [PubMed] [Google Scholar]
- 31.Xu C, Zhou W, Wang Y, et al. Hepatitis B virus-induced hepatocellular carcinoma. Cancer Lett 2014; 345:216–222. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Shi W, Luan F, et al. Hepatitis B virus X protein upregulates transcriptional activation of human telomerase reverse transcriptase. Virus Genes 2010; 40:174–282. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Dong N, Zhang H, et al. Effects of hepatitis B virus X protein on human telomerase reverse transcriptase expression and activity in hepatoma cells. J Lab Clin Med 2005; 145:98–104. [DOI] [PubMed] [Google Scholar]
- 34.Motavaf M, Safari S, Saffari Jourshari M, et al. Hepatitis B virus-induced hepatocellular carcinoma: the role of the virus × protein. Acta Virol 2013; 57:389–396. [DOI] [PubMed] [Google Scholar]
- 35.Dlouha D, Maluskova J, Kralova Lesna I, et al. Comparison of the relative telomere length measured in leukocytes and eleven different human tissues. Physiol Res 2014; 63:343–350. [DOI] [PubMed] [Google Scholar]
- 36.Liu J, Yang Y, Zhang H, et al. Longer leukocyte telomere length predicts increased risk of hepatitis B virus-related hepatocellular carcinoma: a case-control analysis. Cancer 2011; 117:4247–4256. [DOI] [PubMed] [Google Scholar]


